Abstract
Acinetobacter species are becoming a major cause of nosocomial infections, including hospital-acquired and ventilator-associated pneumonia. Acinetobacter species have become increasingly resistant to antibiotics over the past several years and currently present a significant challenge in treating these infections. Physicians now rely on older agents, such as polymyxins (colistin), for treatment. This paper reviews the epidemiology, treatment, and prevention of this emerging pathogen.
Introduction
Pneumonia caused by Acinetobacter species can present major challenges for physicians. Outbreaks of Acinetobacter infection, including pneumonia, have occurred in healthcare facilities worldwide, including military treatment facilities caring for troops serving in Southwest Asia in support of Operation Iraqi Freedom (OIF) and in Afghanistan in support of Operation Enduring Freedom (OEF).[1–14] Acinetobacter species were responsible for 6% of cases of ventilator-associated pneumonia between 1992 and 1997 in the United States.[15] According to National Nosocomial Infections Surveillance data, Acinetobacter species caused 7% of intensive care unit (ICU) nosocomial cases of pneumonia in 2003 compared with 4% in 1986.[16] Community-acquired Acinetobacter pneumonia can also occur among certain at-risk populations. Of growing concern is the increase in multidrug resistance exhibited by clinically relevant species. Physicians are now relying on such antibiotics as the polymyxins (colistin) for treatment. In this article, we will review the epidemiology, treatment, and prevention of this emerging pathogen, with a focus on Acinetobacter pneumonia.
Taxonomy/Microbiology
Acinetobacter as a genus was not definitively established until 1971, although today more than 25 species have been identified via DNA-DNA hybridization.[11,17,18] To date, only 10 of these species have been named and identification of specific species in the laboratory using phenotypic tests can be very difficult. In most circumstances, species identification requires advanced molecular techniques. The term Acinetobacter calcoaceticus-A baumannii complex (Abc) has been given to 4 phenotypically similar strains (strains 1, 2, 3, and 13) that account for 80% of clinical infections. Consequently, most laboratories will report isolation of Abc, which is sufficient to direct clinical decision-making. Other species, however, can cause disease and should not be considered contaminants, especially in the setting of repeatedly positive cultures.[18]
Acinetobacter species are gram-negative, nonfermentative, non-spore-forming, nonmotile, aerobic coccobacillary organisms. However, they can be gram-variable and occasionally gram-positive on initial gram stain (Figure). Morphologic characteristics may change depending on the growth phase, resulting in a rod-shaped appearance during rapid growth but a coccobacillary appearance during the stationary phase. Acinetobacter species are oxidase-negative, which helps distinguish them from other gram-negative organisms, such as Pseudomonas, Neisseria, and Moraxella.[18]
Figure.
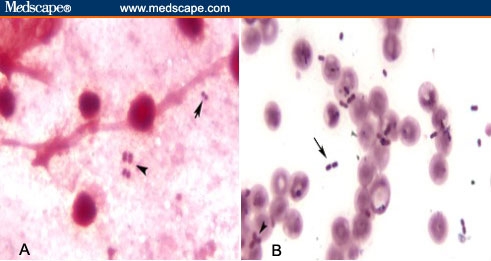
A. Gram-variable cocci in sputum. B. Gram-variable rods in blood culture (arrow = gram-positive; arrowhead = gram-negative).
Epidemiology
Acinetobacter grow easily in nature and have been isolated from many sources throughout the world.[12] In nature, Acinetobacter are most commonly found in soil and water, but have been isolated from animals as well.[5, 18, 19] It was recently found in human body lice of homeless people in France.[20] It has also been isolated from food (including hospital food), ventilator equipment, suctioning equipment, infusion pumps, sinks, stainless steel trolleys, pillows, mattresses, tap water, bed rails, humidifiers, soap dispensers, and other sources.[11, 18, 21–29]
In humans, Acinetobacter have been isolated from all culturable sites.[18] Up to 40% of healthy adults can have skin colonization, with higher rates among hospital personnel and patients.[18, 30, 31] Skin colonization is common among injured military personnel returning from OIF – an average of 15% are colonized on admission to Walter Reed Army Medical Center (WRAMC). At Brooke Army Medical Center, skin, soft tissue, and orthopedic infections caused by Acinetobacter are 10 times more common than bacteremia.[32] A study of healthy troops, most of whom had not deployed, revealed colonization rates of 17%, with Acinetobacter strains genotypically and phenotypically different from those of infected or colonized soldiers returning from OIF.[33]
The respiratory tract is an important site of colonization and is the most frequent site of infection.[18, 34] Acinetobacter colonization has been reported from the nares, nasopharynx, and tracheostomy sites.[34–35] Rates of colonization increase during ICU stays.[35–36] The frequency of nasal colonization among healthy persons has been variable. A recent study showed no nasal colonization among 293 healthy, nondeployed US soldiers.[37] Ongoing studies in soldiers returning from OIF and OEF may show different results. Whether nasal colonization is a risk factor for pneumonia remains to be determined.
The prevalence of Abc infections is increasing.[16] The risk factors for Acinetobacter hospital-acquired pneumonia (AHAP) and Acinetobacter ventilator-associated pneumonia (AVAP) published in the literature are derived from studies with small sample sizes. Husni and colleagues assessed 15 patients in a case-control study and identified prior ceftazidime treatment as a risk factor by univariate analysis.[38] Baraibar and coworkers reported on 12 intubated patients and, using logistic regression, identified risk factors for AVAP, including previous neurosurgery, head trauma, and large-volume pulmonary aspiration.[39]
A recent study by Garnacho-Montero and coworkers has better characterized risk factors for AVAP and imipenem-resistant AVAP.[40] This study compared 41 cases of AVAP with 40 cases of VAP secondary to other pathogens. The univariate analysis showed prior sepsis, previous antibiotic use, reintubation, length of hospital stay, length of mechanical ventilation, imipenem exposure, and fluoroquinolone exposure as potential risk factors for AVAP. The multivariate analysis, however, only showed prior antibiotic exposure as a risk factor. Patients with a history of antibiotic use were more likely to develop AVAP (odds ratio [OR], 14; 95% confidence interval [CI], 4.1–91; P < .0001). The authors reported that previous exposure to imipenem was the only variable by multivariate analysis that was associated with AVAP secondary to imipenem-resistant Acinetobacter (OR, 4; CI, 1.1–29.8; P < .005).[40] This study and others have shown that inappropriate empirical use of antibiotics results in worse outcomes in AVAP cases, which has implications for treatment.[40–41]
Pathogenesis
Abc remains an opportunistic pathogen that typically causes serious infection in immune-compromised hosts. The organism is encapsulated and has a cell wall containing lipopolysaccharides, but the effect of the lipopolysaccharides in humans is not well-understood.[42] There are few published studies that have examined the potential virulence factors of Abc. It is likely that several factors contribute to the transition from colonizer to invasive bacteria.
A recent review by Jolly-Guillou described the current literature regarding this topic.[42] One highlighted virulence factor was exopolysaccharide production, which is known to protect bacteria from the host's immune response.[42–43] Thirty percent of Abc strains produce exopolysaccharides, and studies in mice have demonstrated that strains producing exopolysaccharides are more virulent than nonproducing strains.[42–43] However, their role in human infection remains unknown.
Mechanisms of Antibiotic Resistance
A unique feature of Abc is rapid acquisition of antibiotic-resistance mechanisms. In the human host, these bacteria have transformed from largely susceptible 30 years ago to multidrug-resistant today.[44] Abs species utilize multiple mechanisms of resistance, and it is not uncommon for several mechanisms to exist within the same isolate (Table 1). This has created new challenges for managing these infections. A recent review of this topic has been published.[45]
Table 1.
Mechanisms of Antibiotic Resistance Found in Acinetobacter Species
| Mechanism of Action | Antibiotic |
|---|---|
| Beta-lactamases (AmpC cephalosporinase) | Ceftazidime and extended spectrum cephalosporins |
| DNA topoisomerase mutations | Quinolones |
| Aminoglycoside-modifying enzyme | Aminoglycosides |
| Efflux pumps | Aminoglycosides, quinolones, tetracyclines, trimethoprim |
| Mobile genetic elements | Contains genes with resistance to multiple classes |
| Outer membrane protein changes | Imipenem |
AmpC cephalosporinases, which are chromosomally encoded, are present in all strains of Abc.[46] AmpC is responsible for resistance to ceftazidime and other extended-spectrum cephalosporins. Multiple other beta-lactamases have also been reported in Abc and are described in detail in a recent paper.[45]
Integrons are mobile genetic elements that can carry multiple genes encoding for antibiotic resistance. It is important to note that they carry OXA beta-lactamases that confer resistance to carbapenems. They are also known to carry metalol-beta-lactamases, but these have been described mainly in Europe and Asia.[47–50] In addition to integrons, outer membrane protein changes can confer resistance to carbapenems.[45]
Molecular genetics now allows comparison of resistance phenotypes with genetic resistance markers.[46] Two studies illustrate the complicated genetic make-up responsible for the antibiotic resistance in Acinetobacter species. Hujer and colleagues provided genetic and phenotypic data on 75 distinct isolates from WRAMC.[46] They reported that 89% of the isolates were resistant to 3 or more classes of antibiotics. A total of 15% of isolates was resistant to 9 antibiotics (5 classes) tested. Over 90% of isolates were resistant to ciprofloxacin, and at least 80% were resistant to extended-spectrum cephalosporins. Twenty percent of isolates were resistant to imipenem and 24% to meropenem. This discordance between imipenem and meropenem sensitivity has been reported previously.[51] The Hujer study identified 16 resistance genes and 4 mobile genetic elements.[46]
Fournier and coworkers examined the genomic make-up of Acinetobacter resistance.[44] A study of a multidrug-resistant A baumannii strain (AYE) was compared with a fully susceptible strain (SDF),which identified the presence of an 86-kb resistance island that contained a cluster of 45 resistance genes in the AYE strain. The authors suggested that this island could be a “hotspot” that allows Acinetobacter to acquire resistance genes rapidly when under antibiotic pressure. Based on gene sequencing of the AYE strain, it appears that the organism has acquired most of its resistance genes from Pseudomonas, Salmonella, and Escherichia species.[44] The ability to develop multidrug resistance is becoming increasingly concerning because treatment options are limited.
Colistin is being used with rising frequency with the emergence of multidrug-resistant Abc. Not surprisingly, antibiotic resistance to colistin has been reported as well.[52–54] Beno and coworkers recently reported resistance data on 10 patients infected with 18 gram-negative bacilli isolates (2 with Abc) that were resistant to colistin.[52] They postulated that previous exposure to colistin and ciprofloxacin may have influenced the development of resistance. A previous abstract on an Israeli cohort of patients presented by Gilad and colleagues in 2005 also reported an association with colistin use and development of resistance.[53] While additional studies are needed, these trials suggest that using colistin alone may lead to future resistance.
Clinical Features
Patients with hospital-acquired or ventilator-associated Abc pneumonia present similarly to patients with VAP or HAP caused by other nosocomial pathogens. Community-acquired Acinetobacter pneumonia is unusual but has been reported in the literature, mainly from Australia and Asia.[55–66] Cases tend to occur in patients who are smokers, have diabetes, or have chronic obstructive pulmonary disease.[55–57] However, cases have been reported in healthy individuals.[55–59]
In a case-control study of A baumannii community-acquired pneumonia (ACAP) vs AHAP (19 and 74 cases, respectively),[55] fever was present in over 80% of cases in both groups. Cough was more common in outpatients (84% vs 49%; P = .008), although this may be result from inpatients being mechanically ventilated. Sputum production was more common in inpatients (93 vs 68%; P = .008). Sputum or tracheal cultures were positive in 95% of outpatients and 100% of inpatients in this cohort. Limited data were presented on findings on chest radiography, but lobar consolidation was found more consistently in outpatients (68% vs 16%; P = .013.). Pulmonary cavitations were absent in both groups, and only AHAP was associated with small pleural effusions (12%). The ACAP cases had a higher incidence of bacteremia, acute respiratory distress syndrome, and death. All ACAP isolates were sensitive to amikacin, ticarcillin/clavulanate, and ampicillin/sulbactam, whereas only 9 of 19 (47.4%) were sensitive to ciprofloxacin.[55] These results differ strikingly from the higher levels of resistance seen in hospital-acquired Acinetobacter profiles.
Rates of bacteremia associated with Abc pneumonia are inconsistent in the literature.[40, 55] Leung and colleagues stated that bacteremia was more commonly associated with ACAP than with AHAP (32% vs 0%).[55] Garnacho-Montero and coworkers found a 12% incidence of bacteremia in 41 cases of AHAP.[40] Another study reported that only 1 of 8 patients with AVAP had bacteremia.[67]
Diagnosis
The diagnosis of VAP is generally complicated, and it can be difficult to discriminate between true infection vs colonization. The diagnosis of AHAP and VAP should be based on clinical suspicion supported by radiographic and microbiologic data. Bronchoscopy, when available, is a useful adjunct to provide both diagnostic information as well as culture data for sensitivities to guide therapy. The American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) guidelines provide a concise evidenced-based review on the diagnostic work-up of HAP and VAP.[68]
Acinetobacter will grow on routine laboratory media, including sheep blood agar; colony growth appears smooth, mucoid, and nonpigmented (yellowish to gray).[69] The classic gram stain appearance of Abc is a gram-negative coccobacilli but can vary, as previously mentioned. The organism is described as aerobic, but it can grow under anaerobic conditions, such as in blood culture media.
Treatment
There are no randomized, controlled trials (RCTs) comparing antimicrobial therapy for pneumonia caused by Acinetobacter species, but retrospective and prospective therapeutic observational studies have been published. Most recent literature on the topic describes use of polymyxin B or E (colistin) (intravenous, intramuscular, or inhaled) for treatment of Abc. This is largely because some nosocomial isolates are only susceptible to colistin due to increasing resistance. A prospective study comparing the efficacy of treatment of multidrug-resistant A baumannii VAP with intravenous colistin vs imipenem showed no differences in clinical cure, in-hospital mortality rates, or toxicity.[70] In a comprehensive review of the published literature on use of polymyxins to treat critically ill patients, Falagas and colleagues showed the utility of these agents; however, they emphasized judicious use of polymyxins to prevent drug resistance.[71] Risk for nephrotoxicity was also highlighted. Several studies have found less colistin-associated nephrotoxicity than originally reported; nevertheless, focus should remain on appropriate dosing and close monitoring of renal function due to this potential complication.[72–76]
Use of inhaled colistin has been reported for over 30 years, but interest in its use has dramatically increased along with emergence of multidrug-resistant Abc and Pseudomonas aeruginosa.[77–81] Current knowledge about inhaled colistin derives mainly from use in patients with cystic fibrosis. More recent reports have examined the benefit of this therapy in patients without cystic fibrosis, and despite a clear lack of data regarding dosing, efficacy, and safety from RCTs, colistin is being used clinically for treatment of multidrug-resistant Abc.[82–84]
Small case series have demonstrated clinical improvement when inhaled colistin has been used as adjunctive therapy for multidrug-resistant Abc HAP or VAP.[67, 82–84] The largest retrospective series to date reported 71 patients, including 47 with Abc pneumonia, treated with inhaled colistin (monotherapy in 9 cases).[84] The mean duration of therapy was 12 ± 8 days. None of the 33 patients with Abc pneumonia assessed for microbiologic cure had positive follow-up cultures.
The exact pharmacokinetics, pharmacodynamics, and optimal dosing regimens for aerosolized colistin remain unclear. Manufacturer recommended doses range from 40–80 mg (500,000–1 million units) every 12 hours depending on weight, but in published reports providers have used varied dosing and frequency regimens.[67, 82–84]
Administration of inhaled aminoglycosides as an adjunct to systemic treatment for gram-negative VAP has not been extensively considered in the literature, despite favorable pharmacokinetic and patient tolerance profiles.[85] In a recent pilot study, Hallal and colleagues compared a 14-day course of aerosolized tobramycin plus an intravenous beta-lactam antibiotic with a 14-day course of an intravenous beta-lactam plus intravenous tobramycin for treatment of P aeruginosa or Abc VAP; they found a survival benefit in the inhaled tobramycin group.[86] To date, there are no published data on inhaled aminoglycosides for multidrug-resistant Abc VAP.
Because drug resistance is increasing and there are no new antibiotics in development for treatment of multidrug-resistant gram-negative bacteria, there is growing interest in pharmacokinetic and pharmacodynamic principles to combat these pathogens and prevent further development of resistance. Mattoes and coworkers, following an extensive review, reported that meropenem pharmacodynamics for susceptible pathogens could be optimized by using higher doses, increasing dosing frequency, or prolonging the duration of infusion.[87] Li and colleagues used a population pharmacokinetic model to demonstrate that prolonged meropenem infusion time (3 hours) resulted in increased probability of achieving the target mean inhibitory concentration or Enterobacteriaceae, Acinetobacter species, and P aeruginosa.[88] Novel strategies, including increased dosing and infusion times of available time-dependent killing antibiotics, must be considered to optimize therapeutics for multidrug-resistant bacteria.
Combination therapy for treatment of multidrug-resistant Abc pneumonia remains controversial. Saballs and colleagues evaluated the efficacy of combination therapy with rifampin and imipenem in carbapenem-resistant Abc infections based on success in a mouse model.[89] However, because of therapeutic failures, the authors recommended against this combination for carbapenem-resistant Abc. Lee and coworkers retrospectively compared combination therapy of “pan-drug resistant” A baumannii with a carbapenem plus sulbactam vs other antibiotics (eg, cephalosporins, fluoroquinolones) plus an aminoglycoside.[90] The combination of carbapenem and sulbactam lowered mean inhibitory concentrations for many of the isolates; however, no significant difference in outcomes was seen between the 2 groups. Falagas and colleagues retrospectively compared treatment of multidrug-resistant gram-negative infections with colistin alone vs colistin combined with meropenem.[76] No significant differences in clinical response or nephrotoxicity were observed. In vitro and animal studies have shown synergistic antibiotic combinations against Abc; however, no formal RCTs to assess clinical efficacy have been done.[91–93]
Therapy for pneumonia caused by Abc should be based on susceptibility results. However, pending microbiologic data, empirical antibiotic therapy should be chosen based on the most likely offending pathogens and the local antibiogram. Dual empirical therapy (2 parenteral agents or 1 parenteral agent and 1 inhaled agent) should be considered in critically ill patients who are likely to be infected with a multidrug-resistant pathogen.
Mortality
There is debate in the literature on the impact of Acinetobacter species infection on crude and attributable mortality.[11,94,95] A recent review and editorial highlighted the complexity surrounding this issue. In the editorial,[94] the authors cited a systematic review of 6 case-control studies of A baumannii infections, with an in-hospital and ICU attributable mortality of 7.8% to 23% and 10% to 43%, respectively. The authors concluded, however, that the heterogeneity of the studies precluded a definitive answer.[94]
Two more recent studies have been published. Investigators from Brooke Army Medical Center examined the effect of Abc infection on mortality in a cohort of burn patients.[96] Abc infection was associated with an increase in burn-related mortality in the univariate analysis but was no longer statistically significant in the multivariate analysis.
A retrospective, matched cohort study examined the mortality and length of hospital stay in patients infected with Acinetobacter.[97] Patients with multidrug-resistant Abc were compared with patients with susceptible Abc and those without Abc. A significant increase in length of both ICU and hospital stay was found in patients with multidrug-resistant Abc. There was a trend toward increased mortality but it failed to reach statistical significance after severity of illness was controlled for.
Prevention
The ability of Abc to survive for long periods in the hospital environment contributes to its ability to cause outbreaks. Jawad and colleagues demonstrated that under simulated hospital conditions, Abc can survive an average of 20 days.[98] Another study reported that it can survive on hospital surfaces for up to 4 months.[99] A study by Hujer and coworkers suggested that failure to completely disinfect treatment facilities contributed to nosocomial spread of Abc. In that study, 37% of isolates were acquired via nosocomial transmission.[46] At least 1 study has reported that hypochlorite solutions may be effective in disinfecting the hospital environment when other solutions have failed.[100]
The American Thoracic Society (ATS)/Infectious Disease Society of America (IDSA) guidelines provide an overview of available methods for preventing HAP and VAP.[68] These methods have proven efficacy in reducing these disorders, but less information is available about controlling outbreaks of Abc. Previous outbreaks have been controlled by education on hand washing, patient and staff cohorting, implementing contact precautions, minimizing use of broad-spectrum antibiotics, closing hospital units, discharging colonized patients, and decontaminating the environment.[11, 29, 101] However, these practices do not always control the outbreak. The authors of a recent review concluded that data on effectively controlling multidrug-resistant gram-negative bacteria are insufficient.[102] Consequently, each institution is responsible for individual decisions based on the most likely source of the outbreak. Regardless, strict adherence to infection control policies by physicians, nurses, ancillary support staff, patients, and their families is crucial to the success of any program.
Conclusion
Abc pneumonia is an emerging cause of nosocomial pneumonia, and multidrug resistance makes treatment a major challenge. Early recognition and appropriate antibiotic therapy based on culture and susceptibility data are necessary to obviate poor outcomes and prevent increasing resistance. There are several areas where future research is needed (Table 2), including the pressing need for effective infection control strategies and development of new antimicrobials against gram-negative bacteria.
Table 2.
Future Areas of Research for Acinetobacter
| Virulence factors |
| Mechanisms of antibiotic resistance |
| Colistin |
| Optimal timing and dosing for intravenous and inhaled |
| Outcome data, including adverse events |
| Role of new and alternative antimicrobials |
| Tigecycline, minocycline, rifampicin |
| Combination therapy |
| Mortality data (attributable and overall) |
| Infection control |
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of Walter Reed Army Medical Center, the Department of the Army, or the Department of Defense.
Footnotes
Readers are encouraged to respond to the author at Joshua.Hartzell@na.amedd.army.mil or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
Contributor Information
Joshua D. Hartzell, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Department of Internal Medicine, Walter Reed Army Medical Center, Washington, DC Author's email: Joshua.Hartzell@na.amedd.army.mil.
Andrew S. Kim, Walter Reed Army Medical Center, Washington, DC.
Mark G. Kortepeter, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Department of Medicine, Walter Reed Army Medical Center, Washington, DC.
Kimberly A. Moran, Tropical Public Health, Uniformed Services University of the Health Sciences, Bethesda, Maryland; Infectious Diseases, Uniformed Services University of the Health Sciences, Bethesda, Maryland.
References
- 1.Jones A, Morgan D, Walsh A. Importation of multidrug-resistant Acinetobacter spp. infections with casualties from Iraq. Lancet Infect Dis. 2006;6:317–318. doi: 10.1016/S1473-3099(06)70471-6. [DOI] [PubMed] [Google Scholar]
- 2.Zapor MJ, Moran KA. Infectious diseases during wartime. Curr Opin Infect Dis. 2005;18:395–399. doi: 10.1097/01.qco.0000182102.50430.2c. [DOI] [PubMed] [Google Scholar]
- 3.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb Mortal Wkly Rep. 2004;53:1063–1066. [PubMed] [Google Scholar]
- 5.Jain R, Danziger L. Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann Pharmacother. 2004;38:1449–1459. doi: 10.1345/aph.1D592. [DOI] [PubMed] [Google Scholar]
- 6.Rello J, Sa-Borges M, Correa H, Leal SR, Baraibar J. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Repsir Crit Care Med. 1999;160:608–613. doi: 10.1164/ajrccm.160.2.9812034. [DOI] [PubMed] [Google Scholar]
- 7.Gales AC, Jones RN, Forward KR, et al. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999) Clin Infect Dis. 2001;32(suppl 2):S104–S113. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- 8.Wood GC, Hanes SD, Croce MA, Fabian TC, Boucher BA. Coparison of ampicillin-sulbactam and imipenum-cilastin for treatment of Acinetobacter ventilator-associated pneumonia. Clin Infect Dis. 2002;34:1425–1430. doi: 10.1086/340055. [DOI] [PubMed] [Google Scholar]
- 9.Erbay H, Yalcin AN, Serin S, et al. Nosocomial infections in intensive care unit in Turkish university hospital: a 2-year survey. Intensive Care Med. 2003;29:1482–1488. doi: 10.1007/s00134-003-1788-x. [DOI] [PubMed] [Google Scholar]
- 10.Landman D, Quale JM, Mayorga D, et al. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: the preantibiotic era has returned. Arch Intern Med. 2002;162:1515–1520. doi: 10.1001/archinte.162.13.1515. [DOI] [PubMed] [Google Scholar]
- 11.Fournier P, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. Epub 2006 Jan 26. [DOI] [PubMed] [Google Scholar]
- 12.Patterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(suppl 2):S43–S48. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- 13.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum beta lactamase VEB-1-producing isolates of Acinetobacter baumannii in French hospital. J Clin Microbiol. 2003;41:3542–357. doi: 10.1128/JCM.41.8.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turton JF, Kaufmann ME, Warner M, et al. A prevalent, multiresistant clone of Acinetobacter baumannii in Southest England. J Hosp Infect. 2004;58:170–179. doi: 10.1016/j.jhin.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Richards MJ, Edwards JR, Culver DH, Gayenes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infection Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 17.Juni E. Interspecies transformation of Acinetobacter, genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen DM, Hartman BJ. Acinetobacter species. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's Principles and Practice of Infectious Disease. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 2632–2626. [Google Scholar]
- 19.Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–165. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Scola B, Raoult D. Acinetobacter baumannii in human body louse. Emerg Infect Dis. 2004;10:1671–1673. doi: 10.3201/eid1009.040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerner-Smidt P. Acinetobacter: epidemiological and taxonomic aspects. APMIS Suppl. 1994;47:1–41. [PubMed] [Google Scholar]
- 22.Gervich DH, Grout CS. An outbreak of nosocomial Acinetobacter infections from humidifiers. Am J Infect Control. 1985;13:210–215. doi: 10.1016/0196-6553(85)90059-8. [DOI] [PubMed] [Google Scholar]
- 23.Cefai C, Richards J, Gould FK, McPeake P. An outbreak of Acinetobacter respiratory tract infection resulting from incomplete disinfection of ventilatory equipment. J Hops Infect. 1990;15:177–182. doi: 10.1016/0195-6701(90)90128-b. [DOI] [PubMed] [Google Scholar]
- 24.Adams BG, Marrie TJ. Hand carriage of aerobic gram-negative rods by health care personnel. J Hyg. 1982;89:23–31. doi: 10.1017/s0022172400070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guenthner SH, Hendley JO, Wenzel RP. Gram-negative bacilli as non-transient flora on the hands of hospital personnel. J Clin Microbiol. 1987;25:488–490. doi: 10.1128/jcm.25.3.488-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherertz RJ, Sullivan ML. An outbreak of infections with Acinetobacter calcoaceticus in burn patients: contamination of patients' mattresses. J Infect Dis. 1985;151:252–258. doi: 10.1093/infdis/151.2.252. [DOI] [PubMed] [Google Scholar]
- 27.Weernik A, Severin WP, Tjernberg I, Dijkshoorn L. Pillows, an unexpected source of Acinetobacter. J Hosp Infect. 1995;29:189–199. doi: 10.1016/0195-6701(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 28.Neely AN, Maley MP, Warden GD. Computer keyboards as reservoirs for Acinetobacter baumannii in a burn hospital. Clin Infect Dis. 1999;29:1358–1360. doi: 10.1086/313463. [DOI] [PubMed] [Google Scholar]
- 29.Catalano M, Quelle LS, Jeric PE, DiMartino A, Maimonet SM. Survival of Acinetobacter baumannii on bed rails during an outbreak and during sporadic cases. J Hosp Infect. 1999;42:27–35. doi: 10.1053/jhin.1998.0535. [DOI] [PubMed] [Google Scholar]
- 30.Al-Khoja MS, Darrell JH. The skin as the source of Acinetobacter and Moraxella species occurring in blood cultures. J Clin Pathol. 1979;32:497–499. doi: 10.1136/jcp.32.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, et al. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol. 2004;25:819–824. doi: 10.1086/502302. [DOI] [PubMed] [Google Scholar]
- 32.Murray CK, Hospenthal DR. Treatment of multidrug resistant Acinetobacter. Curr Opin Infect Dis. 2005;18:502–506. doi: 10.1097/01.qco.0000185985.64759.41. [DOI] [PubMed] [Google Scholar]
- 33.Griffith ME, Ceremuga JM, Ellis MW, et al. Acinetobacter Skin Colonization of US Army Soldiers. Infect Control Hosp Epidemiol. 2006;27:659–661. doi: 10.1086/506596. [DOI] [PubMed] [Google Scholar]
- 34.Glew RH, Moellering RC, Jr, Kunz LJ. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): Clinical and laboratory studies. Medicine. 1977;56:79–797. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Ayats J, Corbella X, Ardanuy C. Epidemiological significance of cutaneous, pharyngeal, and digestive tract colonization by multiresistant Acinetobacter baumannii in ICU patients. J Hosp Infect. 1997;37:287–295. doi: 10.1016/s0195-6701(97)90145-6. [DOI] [PubMed] [Google Scholar]
- 36.Peacock JE, Jr, Sorrell L, Sottile FD, Price LE, Rutala WA. Nosocomial respiratory tract colonization and infection with aminoglycoside-resistant Acinetobacter calcoaceticus var anitratus: epidemiologic characteristics and clinical significance. Infect Control Hosp Epidemiol. 1988;9:302–308. doi: 10.1086/645859. [DOI] [PubMed] [Google Scholar]
- 37.Griffith ME, Ellis MW, Murray CK. Acinetobacter nares colonization of healthy US soldiers. Infect Control Hosp Epidemiol. 2006;27:787–788. doi: 10.1086/505923. [DOI] [PubMed] [Google Scholar]
- 38.Husni RN, Goldstein LS, Arroliga AC, et al. Risk factors for an outbreak of multi-drug-resistant Acinetobacter nosocomial pneumonia among intubated patients. Chest. 1999;115:1378–1382. doi: 10.1378/chest.115.5.1378. [DOI] [PubMed] [Google Scholar]
- 39.Baraibar J, Correa H, Mariscal D, et al. Risk factors for infection by Acinetobacter baumannii in intubated patients with nosocomial pneumonia. Chest. 1997;112:1050–1054. doi: 10.1378/chest.112.4.1050. [DOI] [PubMed] [Google Scholar]
- 40.Garnacho-Montero J, Ortiz-Leyba C, Fernandez-Hinojosa E, et al. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med. 2005;31:649–655. doi: 10.1007/s00134-005-2598-0. [DOI] [PubMed] [Google Scholar]
- 41.Iregui M, Ward S, Sherman G, et al. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 42.Jolly-Guillou M. Clinical impact and pathogenicity of Acinetobacter. Clin Microbiol Infect. 2005;11:868–873. doi: 10.1111/j.1469-0691.2005.01227.x. [DOI] [PubMed] [Google Scholar]
- 43.Obana Y. Pathogenic significance of Acinetobacter calcoaceticus: analysis of experimental infection in mice. Microbiol Immunol. 1986;30:645–647. doi: 10.1111/j.1348-0421.1986.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 44.Fournier PE, Vallenet D, Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genetics. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. Epub 2006 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonomo R, Szabo D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl 2):S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 46.Hujer K, Hujer A, Hulten E. Multi-drug resistant Acinetobacter spp. isolates from military and civilian patients treated at Walter Reed Army Medical Center: analysis of antibiotic resistance genes. Antimicrob Agents Chemother. 2006;50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh TR, Toleman MA, Poirel L, et al. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yum JH, Yi K, Lee H, et al. Molecular characterization of metallo-beta-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the bla(VIM-2) gene cassettes. J Antimicrob Chemother. 2002;49:837–840. doi: 10.1093/jac/dkf043. [DOI] [PubMed] [Google Scholar]
- 49.Lee K, Ha G, Shin B, et al. Metallo-beta-lactamase-producing Gram-negative bacilli in Korean Nationwide Surveillance of Antimicrobial Resistance group hospitals in 2003: continued prevalence of VIM-producing Pseudomonas spp. and increase of IMP-producing Acinetobacter spp. Diagn Microbiol Infect Dis. 2004;50:51–58. doi: 10.1016/j.diagmicrobio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Da Silva G, Correria M, Vital C, et al. Molecular characterization of bla(IMP-5), a new integron-borne metallo-â-lactamase gene from an Acinetobacter baumannii nosocomial isolate in Portugal. FEMS Microbiol Lett. 2002;215:33–39. doi: 10.1111/j.1574-6968.2002.tb11366.x. [DOI] [PubMed] [Google Scholar]
- 51.Lesho E, Wortmann G, Moran K, et al. Fatal Acinetobacter baumannii infection with discordant carbapenem susceptibility. Clin Infect Dis. 2005;41:758–759. doi: 10.1086/432623. [DOI] [PubMed] [Google Scholar]
- 52.Beno P, Krcmery V, Demitrovocova A. Bacteraemia in cancer patients caused by colistin-resistant Gram-negative bacilli after previous exposure to ciprofloxacin and/or colistin. Clin Microbiol Infect. 2006;12:497–498. doi: 10.1111/j.1469-0691.2006.01364.x. [DOI] [PubMed] [Google Scholar]
- 53.Gilad J, Eskira S, Riesenberg K, et al. Emergence of nosocomial colistin-resistant Acinetobacter baumannii. Program and abstracts of the 45th Interscience Conference of Antimicrobial Agents and Chemotherapy; December 16–19, 2005; Washington, DC. [Google Scholar]
- 54.Hawley JS, Murray CK, Griffith ME, et al. Susceptibility of Acinetobacter strains isolated from deployed U.S. military personnel. Antimicrob Agents Chemother. 2007;51:376–378. doi: 10.1128/AAC.00858-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leung W, Chung-Ming C, Kay-Yang T, et al. Fulminant community-acquired Acinetobacter pneumonia as a distinct clinical syndrome. Chest. 2006;129:102–109. doi: 10.1378/chest.129.1.102. [DOI] [PubMed] [Google Scholar]
- 56.Anstey NM, Currie BJ, Withnall KM. Community-acquired Acinetobacter pneumonia in the northern territory of Australia. Clin Infect Dis. 1992;14:83–91. doi: 10.1093/clinids/14.1.83. [DOI] [PubMed] [Google Scholar]
- 57.Chen MZ, Hsueh PR, Lee LN, et al. Severe Community-acquired pneumonia due to Acinetobacter baumannii. Chest. 2001;120:1072–1077. doi: 10.1378/chest.120.4.1072. [DOI] [PubMed] [Google Scholar]
- 58.Achar KN, Johny M, Achar MN, et al. Community-acquired Acinetobacter pneumonia with survival. Post Grad Med J. 1993;69:934–937. doi: 10.1136/pgmj.69.818.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bick JA, Semel J JD. Fulminant Community-acquired Acinetobacter pneumonia in a healthy woman. Clin Infect Dis. 1993;17:820–821. doi: 10.1093/clinids/17.4.820. [DOI] [PubMed] [Google Scholar]
- 60.Bilgic H, Akin ES, Tasan Y, et al. A case of Acinetobacter calcaoceticus pneumonia. Thorax. 1995;50:315–316. doi: 10.1136/thx.50.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang CH, Chen KJ, Wang CK. Community-acquired Acinetobacter pneumonia: a case report. J Infect. 1997;35:316–318. doi: 10.1016/s0163-4453(97)93574-x. [DOI] [PubMed] [Google Scholar]
- 62.Goodhart GL, Abrutyn E, Watson R, et al. Community-acquired Acinetobacter calcaoceticus var anitratus pneumonia. JAMA. 1977;238:1516–1518. [PubMed] [Google Scholar]
- 63.Rudin ML, Michael JR, Huxley EJ. Community-acquired Acinetobacter pneumonia. Am J Med. 1979;67:39–43. doi: 10.1016/0002-9343(79)90071-8. [DOI] [PubMed] [Google Scholar]
- 64.Vathesatogkit P, Charoenphan P, Saenghirunvattana S, et al. Community-acquired Acinetobacter pneumonia in Thailand. Report of 5 cases. J Med Assoc Thai. 1987;70:96–101. [PubMed] [Google Scholar]
- 65.Gottlieb T, Barnes DJ. Community-acquired Acinetobacter pneumonia. Aust N Z J Med. 1989;19:259–260. doi: 10.1111/j.1445-5994.1989.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 66.Krisanapan S, Naphathorn P, Kaewprom P. Community acquired Acinetobacter pneumonia: report of two cases. Southeast Asian J Trop Med Public Health. 1989;20:497–498. [PubMed] [Google Scholar]
- 67.Michalopoulos A, Kasiakou S, Mastora Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005;9:R53–R59. doi: 10.1186/cc3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American Thoracic Society Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 69.Bergogne-Berezin EB, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–65. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, et al. Treatment of multidrug-resistant Acinetobacter baumanii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36:1111–1118. doi: 10.1086/374337. [DOI] [PubMed] [Google Scholar]
- 71.Falagas ME, Kasiakou SK, Tsiodras S, Michalopoulos A. The use of intravenous and aerosolized polymyxins for the treatment of infections in critically ill patients: a review of the recent literature. Clin Med Res. 2006;4:138–146. doi: 10.3121/cmr.4.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berlana D, Llop JM, Fort E, et al. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am J Health Syst Pharm. 2005;62:39–47. doi: 10.1093/ajhp/62.1.39. [DOI] [PubMed] [Google Scholar]
- 73.Falagas ME, Kasiakou SK, Kofteridis DP, et al. Effectiveness and nephrotoxicity of intravenous colistin for treatment of patients with infections due to polymyxin-only-susceptible (POS) gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 2006;25:596–599. doi: 10.1007/s10096-006-0191-2. [DOI] [PubMed] [Google Scholar]
- 74.Kasiakou SK, Michalopoulos A, Soteriades ES, et al. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Antimicrob Agents Chemother. 2005;49:3136–146. doi: 10.1128/AAC.49.8.3136-3146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Motaouakkil S, Charra B, Hachimi A, et al. Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii. J Infect. 2006;53:274–278. doi: 10.1016/j.jinf.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Falagas ME, Rafailidis PI, Jasiakou SK, et al. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect. 2006;12:1227–1230. doi: 10.1111/j.1469-0691.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 77.Pino G, Contero G, Colongo PG. Clinical observations on the activity of aerosol colimycin and of endobronchial instillations of colimycin in patients with pulmonary suppurations. Minerva Med. 1963;54:2117–2122. [PubMed] [Google Scholar]
- 78.Marschke G, Sarauw A. Polymyxin inhalation therapeutic hazard. Ann Intern Med. 1971;74:144–145. doi: 10.7326/0003-4819-74-1-144. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Nation R, Turnidge J, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 80.Falagas ME, Kasiakou SK. Colistin: The revival of polymyxins for the management of multi-resistant Gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 81.Linden PK, Paterson DL. Parenteral and inhaled colistin for treatment of ventilator-associated pneumonia. Clin Infect Dis. 2006;48:S89–S94. doi: 10.1086/504485. [DOI] [PubMed] [Google Scholar]
- 82.Kwa AL, Loh C, Low JG, Kurup A, Tam VH. Nebulized colistin in the treatment of pneumonia due to multi-drug resistant Acinetobacter baumanii and Pseudomonas Aeruginosa. Clin Infect Dis. 2005;41:754–757. doi: 10.1086/432583. [DOI] [PubMed] [Google Scholar]
- 83.Hamer DH. Treatment of nosocomial pneumonia and tracheobronchitis caused by multidrug-resistant Pseudomonas Aeruginosa with aerosolized colistin. Am J Respir Crit Care Med. 2000;162:328–330. doi: 10.1164/ajrccm.162.1.9910071. [DOI] [PubMed] [Google Scholar]
- 84.Berlana D, Llop J, Fort E, et al. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am J Health-Syst Pharm. 2005;62:39–47. doi: 10.1093/ajhp/62.1.39. [DOI] [PubMed] [Google Scholar]
- 85.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 86.Hallal A, Cohn SM, Namias N, et al. Aerosolized tobramycin in the treatment of ventilator-associated pneumonia: a pilot study. Surg Infect. 2007;8:73–81. doi: 10.1089/sur.2006.051. [DOI] [PubMed] [Google Scholar]
- 87.Mattoes HM, Kuti JL, Drusano GL, Nicolau DP. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin Ther. 2004;26:1187–1198. doi: 10.1016/s0149-2918(04)80001-8. [DOI] [PubMed] [Google Scholar]
- 88.Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in Adult Patients. J Clin Pharmacol. 2006;46:1171–1178. doi: 10.1177/0091270006291035. [DOI] [PubMed] [Google Scholar]
- 89.Saballs M, Pujol M, Tubau F, et al. Rifampicin/imipenem combination in the treatment of carbapenem-resistant Acinetobacter baumannii infections. J Antimicrob Chemother. 2006;58:697–700. doi: 10.1093/jac/dkl274. [DOI] [PubMed] [Google Scholar]
- 90.Lee CM, Lim HK, Liu CP, Tseng HK. Treatment of pan-drug resistant Acinetobacter baumannii. Scand J Infect Dis. 2005;37:195–199. doi: 10.1080/00365540510026869. [DOI] [PubMed] [Google Scholar]
- 91.Sader H, Jones R. Comprehensive in vitro evaluation of cefepime combined with aztreonam or ampicillin/sulbactam against multi-drug resistant Pseudomonas aeruginosa and Acinetobacter spp. Int J Antimicrob Agents. 2005;25:380–384. doi: 10.1016/j.ijantimicag.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Marques MB, Brookings ES, Moser SA, et al. Comparative in vitro antimicrobial susceptibilities of nosocomial isolates of Acinetobacter baumannii and synergistic activities of nine antimicrobial combinations. Antimicrob Agents Chemother. 1997;41:881–885. doi: 10.1128/aac.41.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montero A, Ariza J, Corbella X, et al. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J Antimicrob Chemother. 2004;54:1085–1091. doi: 10.1093/jac/dkh485. [DOI] [PubMed] [Google Scholar]
- 94.Falagas M, Kopterides P, Siempos I. Attributable mortality of Acinetobacter baumannii infection among critically ill patients. Clin Infect Dis. 2006;43:389. doi: 10.1086/505599. author reply 389–390. [DOI] [PubMed] [Google Scholar]
- 95.Falagas M, Bliziotis I, Siempos I. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10:R48. doi: 10.1186/cc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Albrecht M, Griffith M, Murray C, et al. Impact of Acinetobacter infection on the mortality of burn patients. J Am Coll Surg. 2006;203:546–550. doi: 10.1016/j.jamcollsurg.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 97.Sunenshine R, Wright M, Maragakis L. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jawad A, Seifert H, Snelling AM, et al. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wendt C, Dietze B, Dietz E, Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aygun G, Demirkirian O, Utku T, et al. Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J Hosp Infect. 2002;52:259–262. doi: 10.1053/jhin.2002.1300. [DOI] [PubMed] [Google Scholar]
- 101.Wagonvoort JHT, DeBrauwer EIGB, Toenbreker HMJ, van der Linden CJ. Epidemic Acinetobacter baumannii stain with MRSA-like behavior carried by healthcare staff. Eur J Clin Microbiol Infect Dis. 2002;21:326–327. doi: 10.1007/s10096-002-0716-2. [DOI] [PubMed] [Google Scholar]
- 102.Harris A, McGregor J, Furuno J. What infection control interventions should be undertaken to control multi-drug resistant gram-negative bacteria? Clin Infect Dis. 2006;43:S57–S61. doi: 10.1086/504479. [DOI] [PubMed] [Google Scholar]


