Abstract
Context
Primary care physicians provide care for the majority of patients with mild-to-moderate chronic obstructive pulmonary disease (COPD). Although clinical practice guidelines have been developed for COPD, their influence on primary care practice is unclear.
Objective
To examine primary care decision making, perceptions, and educational needs relating to COPD.
Design
A survey centered on COPD case-vignettes was developed and distributed to a random sample of physicians in adult primary care specialties.
Results
From 943 respondents, 784 practicing primary care physicians were used in analysis. On average, physicians estimated that 12% of their patients had COPD. Although 55% of physicians were aware of major COPD guidelines, only 25% used them to guide decision-making. Self-identified guidelines showed that users were more likely to order spirometry for subtle respiratory symptoms (74% vs 63%, P < .01), to initiate therapy for mild symptoms (86% vs. 77%, P < .01), and to choose long-acting bronchodilators for persistent dyspnea (50% vs 32%, P < .01).
Conclusions
Practice guidelines and CME programs are both valued resources, but have not yet adequately reached many physicians. Because guidelines appear to influence clinical decision-making, efforts to disseminate them more broadly are needed. Future education should present COPD assessment algorithms tailored to primary care settings, assess and strengthen spirometry interpretation skills, and discuss a reasoned approach to medication management. Patient-centered content that accurately reflects the nature of primary care practice may enhance physician's learning experience. Internet-based and distance learning formats may be essential for reaching physicians in many high-need areas.
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death and affects more than 1 in 20 adults in the United States.[1,2] Smoking is the most important cause but other factors may also be significant.[3,4] Despite its high prevalence, only a fraction of individuals with COPD have been diagnosed.[5,6] The slow progression of COPD and its early symptoms are often unrecognized by both patients and physicians despite substantial deterioration in health status and increased risk of mortality.[7] As a result, the initial diagnosis often occurs at an advanced disease stage.[8]
In 2001, the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Diseases (GOLD)[9] was published, marking a new generation of evidence-based guidelines. Jointly developed by the National Heart, Lung, and Blood Institute (NHLBI) and the World Health Organization (WHO), the guideline was designed to be a core resource for primary care physicians, who care for the majority of patients with COPD.
In response to findings suggesting underdiagnosis and underutilization of spirometry for detection and diagnosis, a series of campaigns to increase primary care physician awareness of COPD and best clinical practices have been conducted.[10] Despite these efforts, many primary care physicians are unaware of COPD guidelines[11] and the diagnosis of COPD on the basis of clinical findings alone remains common practice, leading to misdiagnosis, undertreatment, and inappropriate management.[12–16]
Interventions designed to enhance availability and comfort with spirometry in primary care settings have had variable long-term success[17–19] and have not been implemented broadly. This suggests that more effective approaches may be needed to effectively improve recognition and management of COPD. The present study was designed to further examine COPD from a primary care perspective. The study sought to examine the outpatient clinical choices of primary care physicians relative to guideline-based recommendations. Physicians' perceptions about COPD as well as their learning preferences were also assessed. Findings from this study are expected to provide an educational framework for creating more effective COPD programs.
Materials and Methods
Survey Development
To investigate the needs and practice patterns of primary care physicians in COPD management, a 30-item case-based survey was developed and validated for face validity with Family Medicine and Pulmonary Medicine experts. The first step in developing the survey items was a review of the scientific literature that examined gaps between actual clinical practice and guideline recommendations, using Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease, Updated 2005[9] as a benchmark. Practice issues that appeared important but were not well studied were also identified. A second step used a Nominal Group Technique to understand significant barriers to optimal COPD diagnosis and management from the perspective of primary care physicians. Finally, case writers used this information to create COPD case-vignettes with multiple-choice questions that examined diagnostic and management choices. This approach was chosen because clinical vignettes have shown good correlation with physicians' actual practice choices[20,21] and is cost-efficient. Additional questions examining perceptions about COPD, guideline awareness, and learning preferences were also included.
Survey Distribution
Surveys were distributed by e-mail and fax to a random sample of US primary care physicians during November and December 2006. Individuals who did not respond after 3 contacts were replaced by others drawn randomly until a usable sample of at least 700 respondents had been achieved. A small monetary incentive was offered for completing the survey.
Mapping
To visually assess factors relevant to COPD care, maps were created using ArcView v9.1 (Environmental Research Systems Institute [ESRI], Redlands, Calif.) to display COPD mortality rates, smoking prevalence, and pulmonologist practice locations. Crude county-level COPD mortality rates [ICD10: J40-J44] from the years 1999–2002 combined[22] were categorized and plotted as quartiles. Counties with fewer than 20 COPD deaths were considered to have unreliable rates, and were classified as ‘insufficient data.’ States with the highest smoking prevalence rates in the 2005 Behavioral Risk Factor Surveillance System (BRFSS)[23] were identified and indicated using cross-hatching. Pulmonologist office locations were acquired from the Physician Masterfile of the American Medical Association (AMA).[24] These practice locations were then geocoded using ArcView v9.1 and Streetmap USA (ESRI, Redlands, Calif.), a reference street network database derived from Census 2000.
Statistical Analysis
For categorical data, chi-square analyses were performed. T-tests were used to evaluate normally distributed continuous data. All analyses were performed using SAS System v9.1 (SAS Institute, Inc.; Cary, NC). The level of statistical significance was set at P < .05.
Results
A total of 943 physicians responded to the survey. For analysis, the sample was restricted to private and hospital-based primary care physicians currently engaged in direct patient care (N = 784). To determine the generalizability of results, demographic characteristics of respondents were compared to the AMA Physician Masterfile[24] and are presented in Table 1 . Although slight differences were noted in years since graduation, the overall sample was reasonably representative of US primary care physicians.
Table 1.
Demographics of Primary Care Respondents and AMA Registered Physicians
| Demographic | Primary Care Respondents | AMA Registered Physicians |
|---|---|---|
| Family and general practice physicians (N = 254) | ||
| Year of graduation | P < .01 | |
| < 1949 | 0.0% (0) | 1.9% (1686) |
| 1950–1969 | 7.9% (20) | 17.4% (15,584) |
| 1970–1989 | 58.3% (148) | 47.7% (42,643) |
| 1990 + | 33.9% (86) | 33.0% (29,444) |
| Gender | P = .40 | |
| Male | 74.0% (188) | 71.6% (64,491) |
| Female | 26.0% (66) | 28.4% (25,566) |
| Degree | P = .75 | |
| MD | 83.9% (213) | 83.1% (74,846) |
| DO | 16.1% (41) | 16.9% (15,211) |
| Internal medicine physicians (N = 208) | ||
|---|---|---|
| Year of graduation | P < .01 | |
| < 1949 | 0.5% (1) | 1.2% (1319) |
| 1950–1969 | 8.2% (17) | 12.2% (12,976) |
| 1970–1989 | 64.4% (134) | 46.8% (49,848) |
| 1990 + | 26.9% (56) | 39.8% (42,356) |
| Gender | P = .20 | |
| Male | 75.0% (156) | 71.0% (101,633) |
| Female | 25.0% (52) | 29.0% (41,558) |
| Degree | P = .02 | |
| MD | 93.3% (194) | 96.3% (137,883) |
| DO | 6.7% (14) | 3.7% (5308) |
Respondents were engaged in family practice (54%), internal medicine (41%), and general practice (5%), with 20% practicing in rural settings. On average, physicians had 17 years of practice experience and estimated that 12% of their patients had COPD . Spirometry was available in 64% of the physicians' practices; however 34% of physicians with a practice spirometer indicated that it was not routinely used.
Confidence and Optimism
Using a 10-point Likert scale (1 = Not at all confident; 10 = Very confident) to assess confidence in detecting, diagnosing, and providing up-to-date COPD management, 32%, 38%, and 30% of respondents, respectively, expressed high confidence (score ≥ 8). Fewer physicians (15%) expressed high optimism (score ≥ 8) about effectively preventing and treating COPD with current interventions.
Guideline Awareness and Exposure to CME
Fewer than half of physicians (45%) reported being aware of either the GOLD or ATS/ERS guidelines for COPD diagnosis and management. Among physicians who were aware of COPD guidelines, fewer than half (46%) reported using them to guide clinical decision-making. Physicians were also asked to rate their exposure to COPD-focused CME within the past year using a 10-point scale (1 = far too little; 5 = just right; 10 = far too much). Recent CME exposure was considered insufficient (score ≤ 4) by 60% of physicians.
Barriers to Optimal Chronic Obstructive Pulmonary Disease Care
Primary care physicians rated nonadherence (46%) and low awareness of COPD symptoms (25%) as the most important patient-related barriers to optimal COPD care. The tendency of smokers to avoid medical care (16%) and the presence of competing comorbid conditions (13%) were also considered important barriers by significant minorities. When asked about the most important practice-related barriers, 39% of respondents selected low physician suspicion of COPD in patients with minimal symptoms, and 25% selected insufficient resources for patient education and self-management skill training. The complexity and inconvenience of spirometry testing and low reimbursement for COPD patient care were chosen by 19% and 17% of respondents, respectively.
Differences Among Primary Care Specialties
Because differences between the adult primary care specialties may affect COPD management, the practice characteristics and perceptions of internal medicine physicians and those of family and general practitioners were compared (Table 2). Internal medicine physicians reported higher portions of patients with COPD in their practices (P < .01), were more frequently aware of COPD practice guidelines (P = .04), and had greater confidence in their ability to diagnose (P < .01) and manage COPD (P = .02). Internists also viewed impediments to optimal COPD care differently, expressing greater concern about low reimbursement and less concern about early recognition than family and general practice physicians (P = .01). No significant differences between the specialty groups were observed in access to spirometry or optimism about current COPD interventions.
Table 2.
Physician Characteristics Related to Chronic Obstructive Pulmonary Disease Management
| Parameter | Internal Medicine (n = 323) | Family and General Practice (n = 458) | P Value |
|---|---|---|---|
| Practice characteristic | |||
| Number of patients seen/day: Mean (SD) | 24.5% (12.70) | 26.0 (11.55) | .11 |
| Percent of patients with COPD | |||
| •Mean (SD) | 13.1% (10.45) | 10.6 (8.73) | < .01 |
| Spirometry in office: % (N) | |||
| •None | 36.6% (117) | 36.0% (164) | .49 |
| •Have, don't use routinely | 23.8% (76) | 20.7% (94) | |
| •Have, use routinely | 39.7% (127) | 43.3% (197) | |
| Physician perceptions | |||
|---|---|---|---|
| High confidence:* % (N) | |||
| •Detecting COPD | 35.8% (115) | 30.1% (137) | .09 |
| •Diagnosing COPD | 45.0% (144) | 33.9% (154) | < .01 |
| •Managing COPD | 34.4% (107) | 26.6% (120) | .02 |
| High optimism*: % (N) | 17.5 % (56) | 13.6% (62) | .14 |
| Inadequate exposure to COPD CME† | 54.2% (173) | 65.1 % (296) | < .01 |
| COPD guideline awareness: % (N) | |||
| •Unaware | 40.9% (131) | 48.2% (220) | .07 |
| •Aware; don't use | 33.8% (108) | 26.8% (122) | |
| •Aware; use | 25.3% (81) | 25.0% (114) | |
| Perceived barriers to COPD care | |||
|---|---|---|---|
| Most important patient-related barrier: % (N) | |||
| •Tendency of smokers to avoid medical care | 16.5% (52) | 15.6% (71) | .63 |
| •Patient nonadherence to recommended therapy | 43.3% (137) | 48.0% (218) | |
| •Presence of competing comorbid conditions | 14.2% (45) | 13.2% (60) | |
| •Low patient awareness of COPD symptoms | 26.0% (82) | 23.1% (105) | |
| Most important provider-related barrier: % (N) | |||
| •Complexity and inconvenience of spirometry testing | 19.1% (60) | 18.6% (84) | .01 |
| •Low reimbursement for COPD patient care | 21.3% (67) | 13.7% (62) | |
| •Low recognition of early COPD symptoms | 33.8% (106) | 43.4% (196) | |
| •Inadequate resources to educate and teach self-management skills | 25.8% (81) | 24.3% (110) | |
High confidence, high optimism = score ≥ 8 (scale: 1= not at all confident; 10 = very confident);
inadequate CME exposure = score ≤ 4 (scale: 1 = far too little; 5 = just right; 10 = far too much)
Chronic Obstructive Pulmonary Disease Detection and Diagnosis
Two patient scenarios were created to assess physicians' approaches to the evaluation of cardinal COPD symptoms in high-risk patients. Selected responses categorized by practice specialty and guidelines awareness are summarized in Table 3 . For a 58-year-old current smoker with persistent dyspnea and normal chest radiograph, 91% indicated that spirometry was the test they would choose to further evaluate symptoms. Notably, 6% of physicians selected peak flow measurement to assess this patient's symptoms, and 2% would not perform any additional diagnostic studies.
Table 3.
Selected Clinical Decision-Making Patterns of Primary Care Physicians When Evaluating and Treating Patients With COPD
| Pattern | Primary Care Specialty | P Value | Guideline Use | P Value | ||
|---|---|---|---|---|---|---|
| Internal Medicine | Family and General Practice | User | Non-User | |||
| Assessment | ||||||
| Test to evaluate persistent dyspnea in a smoker w/normal CXR: % (number) | ||||||
| •Spirometry | 89.3% (285) | 90.5% (411) | .71 | 92.4% (181) | 89.2% (513) | .13 |
| •Peak flow measurement | 6.9% (22) | 5.1% (23) | 3.6% (7) | 6.6% (38) | ||
| •ECG | 0.9% (3) | 1.8% (8) | 2.6% (5) | 1.0% (6) | ||
| •ECHO | 0.9% (3) | 1.1% (5) | 1.0% (2) | 1.0% (6) | ||
| •No additional tests | 1.9% (6) | 1.5% (7) | 0.5% (1) | 2.1% (12) | ||
| Would order spirometry for subtle respiratory symptoms in a high-risk patient: % (number) | 72.5% (234) | 60.8% (278) | < .01* | 73.5% (144) | 62.8% (365) | < .01 |
| Interpret FEV and FEV1/FVC consistently with GOLD classification: % (number) | 64.4% (208) | 67.5% (309) | .37 | 66.8% (131) | 66.0% (384) | .83 |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Initial therapy for a smoker with mild COPD: % (number) | ||||||
| •No treatment | 23.8% (77) | 18.2% (83) | .20 | 14.4% (28) | 22.7% (132) | .02 |
| •Short-acting bronchodilator | 22.3% (72) | 25.4% (116) | 26.8% (52) | 23.2% (135) | ||
| •Long-acting bronchodilator | 23.8% (72) | 22.6% (103) | 28.9% (56) | 21.3% (124) | ||
| •Inhaled corticosteroid | 30.0% (97) | 33.8% (154) | 29.9% (58) | 32.8% (191) | ||
| Therapy for a 58-year-old smoker with persistent dyspnea unresponsive to antibiotics and short-acting bronchodilator, before spirometry: % (number) | ||||||
| •Different antibiotic | 0.0% (0) | 3.2% (7) | .07 | 2.4% (2) | 1.9% (5) | < .01 |
| •Long-acting anticholinergic | 40.3% (54) | 33.0% (73) | 49.4% (41) | 32.1% (87) | ||
| •Inhaled corticosteroid | 30.6% (41) | 27.6% (61) | 15.7% (13) | 32.1% (87) | ||
| •Combination short-acting beta-agonist/anticholinergic | 29.1% (39) | 36.2% (80) | 32.5% (27) | 34.0% (92) | ||
| Risk reduction for COPD patients | ||||||
|---|---|---|---|---|---|---|
| Would offer detailed smoking cessation counseling and arrange follow-up (%) | 76.0% (244) | 77.2% (349) | .70 | 83.2% (163) | 74.6% (429) | .01 |
Difference between specialty groups remained significant after controlling for guideline use (P < .01)
A 61-year-old male who smokes cigarettes and has subtle respiratory symptoms as a secondary problem was also presented. When physicians were asked if they would order spirometry for this patient, 66% indicated that they would and were more likely to do so if they used COPD guidelines (P < .01), or routinely used a practice-based spirometer (75% vs 59%, P < .01). Among the 2 specialty groups, internists were significantly more likely to order spirometry (P < .01), a difference that remained significant after controlling for guideline use (P < .01).
Physicians were asked to make a diagnosis using history, physical examination, and information about forced expiratory volume in 1 second (FEV1) and FEV1/forced expiratory vital capacity (FVC) before and after bronchodilator administration. Eighty-eight percent of physicians interpreted the parameters as COPD and 65% diagnosed COPD and selected a stage consistent with GOLD classification.[9]
Chronic Obstructive Pulmonary Disease Management and Treatment
For the patient with subtle symptoms and spirometry-confirmed mild COPD, 20% of physicians elected not to treat further. When treatment was chosen, inhaled corticosteroids were preferred (32%), followed by a short-acting bronchodilator (24%), and a long-acting bronchodilator (23%). For a second patient with acute cough and dyspnea, most physicians chose to treat empirically using antibiotics with a short-acting bronchodilator (47%), without a bronchodilator (12%), or with a long-acting bronchodilator (9%), while 33% elected a chest radiograph to guide therapy. When this patient had persistent dyspnea, physicians who had initially chosen a short-acting bronchodilator had no clear preference in their choice of a long-acting bronchodilator (36%), combination short-acting beta-agonist/anticholinergic (33%), or an inhaled steroid (29%) as subsequent therapy. Within this subset, physicians who were COPD guideline users exhibited a distinct pattern, showing a significant preference for a long-acting bronchodilator rather than inhaled steroids (P < .01).
Smoking Intervention for Chronic Obstructive Pulmonary Disease
For a motivated patient with dyspnea, 91% of physicians indicated that they would provide detailed smoking cessation counseling and encourage establishment of a quit date. Consistent with the US Public Health Service's ‘5As’ approach,[25] 77% would also arrange follow-up by their office staff, with higher rates observed among guideline-using physicians (P = .01). Referral to a local smoking cessation program would also be consistent with the 5As and was chosen by 5% of physicians.
Learning Preferences
CME activities and clinical practice guidelines were identified by 34% and 30% of physicians as the 2 most important resources to help them provide optimal patient care. Expert opinion and articles describing new trial findings were considered to be of lesser importance. Physicians were then asked which of several CME delivery methods was most important, considering convenience, efficiency, and personal learning style. Live activities were selected as the most important delivery mechanism by 39% of physicians, but many physicians also favored educational activities that were available on-line (26%) or as printed materials (20%). Physicians were most interested in having CME provide more practical strategies that were relevant for daily practice (41%) and content that was more patient-centered (28%).
Mapping
COPD mortality data plotted by county (Figure 1) revealed several areas with the highest quartile of rates, notably the Appalachian Mountain region, Maine, Nevada, and north-central Florida. Smaller clusters were also identified in areas such as the Ozark Mountain region of Missouri and Arkansas, central Wyoming, northern California, and southwestern Oregon. Pulmonology practices were most commonly located in urban areas, with fewer located in the aforementioned higher burden areas. Kentucky, West Virginia, and Tennessee were among the 10 states with the highest current smoking prevalence rates, corresponding to the Appalachian COPD cluster pattern with pulmonologist practice locations. Ten highest state-level current smoking rates in cross-hatching.
Figure 1.
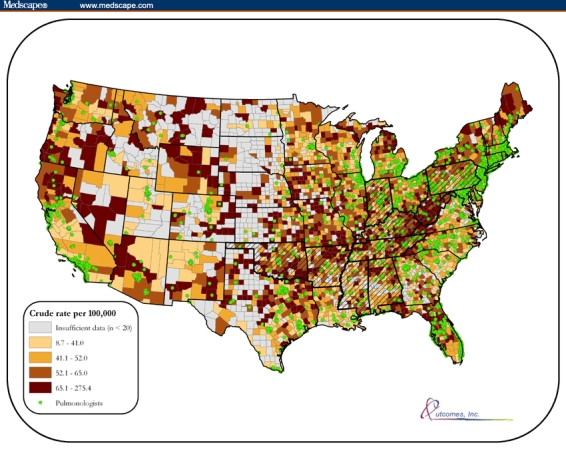
Crude COPD mortality rates per 100,000 population by county (1999–2002).
Discussion
Primary care physicians manage the majority of individuals in the United States with COPD.[10] In many areas of the country where COPD mortality is high such as Nevada and the Appalachian Mountain region, access to pulmonary specialists is limited. Consequently, primary care physicians skilled in detecting, diagnosing, and treating COPD are essential to address this major public health problem. Although one third of primary care physicians surveyed in this study were highly confident, findings from this study point to a desire and need for additional training to improve COPD management skills. Physicians indicated that CME programs and clinical practice guidelines were the most important mechanisms for improving their patient care skills; however, the majority indicated that they'd had insufficient exposure to CME activities related to COPD. Awareness of GOLD guidelines among primary care physicians has increased from 46%in 2003[11] to 60% in 2006 and is encouraging but suggests that dissemination efforts must continue and improve.
The findings from this study found significant differences between family practitioners and internists that may be relevant to COPD programs design. Family physicians were more likely to consider their CME exposure inadequate and were less frequently aware of COPD guidelines. Perhaps related to limited educational opportunities, family physicians also expressed lower confidence in their ability to detect and care for patients with COPD. Family medicine residencies provide greater focus on ambulatory care compared to the more hospital-based internal medicine residencies that may also include more extensive training in pulmonary medicine and spirometry. The observed differences may also reflect a lack of CME programs to improve COPD care that are specifically tailored for family physicians.
Improving COPD detection
In day-to-day practice, manifestations of COPD may be not be readily apparent. Patients often minimize, ignore, and defer medical attention for early COPD.[7] Physicians in this study seemed aware of this problem and ranked low patient and physician awareness of COPD as leading impediments to optimal care, but still appeared to rely on overt respiratory complaints to recognize COPD. COPD detection may be further hindered by common co-morbidities resulting from long-term smoking, chronic inflammation, and functional decline which may have similar manifestations or may distract attention from respiratory symptoms. To address these problems, CME programs should address not isolated COPD, but COPD in the more complex context of the whole person. Teaching physicians how to elicit early indices of COPD through strategic interview questions or the use of questionnaires such as that developed by Price and colleagues[26] may further improve detection rates, particularly if reinforced by periodic public and health professional awareness campaigns.
Improving COPD diagnosis
A sizeable number of physicians responding to this survey currently use on-site spirometry to evaluate suspected COPD and express high confidence in their diagnostic abilities. For the majority of physicians, however, considerable obstacles to obtaining and interpreting spirometry exist, limiting their ability to reliably establish a COPD diagnosis.
One third of surveyed physicians indicated that they would not order spirometry for a middle-aged smoking patient with chronic sputum production, but not dyspnea. According to GOLD guidelines, spirometry is indicated for this patient; however, an Agency for Healthcare Research and Quality (AHRQ) report suggests that reserving spirometry for patients with overt dyspnea is more cost-effective.[27] Whether physicians would be more likely to order spirometry for overt dyspnea was not clearly determined by this study, but would be useful to better understand determinants of spirometry testing.
A small number of surveyed physicians chose peak flow measurement as an initial assessment of dyspnea, but actual use may be more common. Although peak flow meters currently have no defined role in COPD assessment, they are inexpensive and readily available in many primary care practices. Continued education about their appropriate use and limitations in evaluating respiratory symptoms may be useful.
To reliably diagnose COPD, primary care physicians should be skilled in ordering spirometry appropriately and interpreting the results. A complete spirometry report contains numerous parameters, however GOLD definitions of COPD center on post-bronchodilator FEV1 and FEV1/FVC measurements. Accordingly, we examined physicians' ability to interpret these measures and found that two thirds made a diagnosis consistent with GOLD staging classification. Unexpectedly, guideline use and practice spirometry use did not improve physician's diagnostic accuracy relative to this benchmark. In fact, those with on-site spirometry were somewhat more likely to interpret the given data as asthma or normal lung function. Although the spirometry data presented showed a bronchodilator response that was not significant by GOLD criteria, they did highlight the challenges of interpreting bronchodilator responsiveness among patients with suspected COPD. If complete pre- and post-bronchodilator spirometry data had been presented, as would occur in a standard report, it seems likely that physicians would have had greater difficulty identifying the underlying diagnosis.
Currently, spirometry training is not a standard component of medical school training.[28] As a result, CME may be an important mechanism for solidifying pulmonary diagnostic skills. Since several hours of training may be optimal, tiered programming may be useful. One level might focus on hand-on experiences and emphasize obstructive lung disease patterns since these are prevalent in primary care practices. Advanced offerings at a subsequent date that examined more complex spirometry patterns would further refine and update skills. To help ensure the quality of primary care spirometry, access to experts who could review testing would also be beneficial.
Improving treatment
Inhaled bronchodilators are the cornerstone of COPD management and can increase exercise capacity and improve health status when used regularly. Primary care physicians in this study exhibited highly varied treatment preferences. A striking finding was the common selection of inhaled corticosteroids for both suspected and spirometry confirmed COPD. GOLD guidelines recommend using these for patients with recurrent exacerbations or moderate COPD adjunct therapy to a long-acting bronchodilator. Yet, more than one fourth of surveyed physicians chose inhaled steroids in contexts that did not meet these criteria. This may represent confusion with asthma management paradigms.
Physicians in this study also seemed unclear about the appropriate role of long-acting bronchodilators. In GOLD, these agents are the preferred initial therapy for individuals with persistent dyspnea, yet, only 35% of physicians chose a long-acting bronchodilator when a short-acting agent had failed. A third of physicians also chose a combination short-acting bronchodilator. Although this therapy may be more effective than a single short-acting agent and may be slightly less expensive, comparison trials with long-acting bronchodilators are lacking and this combination does not have a defined place in the GOLD treatment hierarchy. Continued education about the appropriate use of inhaled steroids and the relative advantages of available bronchodilator options may be helpful.
Patient education and rehabilitation
Patients with COPD typically need education to help them adequately understand their disease and develop effective self-management skills. Similarly patients with any degree of activity-limiting dyspnea are likely to substantially benefit from pulmonary rehabilitation referral. This study did not examine how physicians approach these important aspects of care, but results indicate that many physicians perceived that resources to support these tasks were inadequate. Providing physicians with patient-directed tools or services that help overcome this significant resource gap may help improve care.
Educational program delivery
Physicians in this study showed greatest preference for live CME programs to enhance their professional development. This option is often convenient for both educators and physicians located in metropolitan areas, but may be much less accessible in many key regions with a high COPD burden. The high ratings that were also given to online activities suggest that COPD education programs delivered through Internet or other distance learning technologies may be an important mechanism for reaching many primary care physicians.
Study limitations
This study used a survey as a surrogate measure of primary care physicians' actual practice patterns in the out-patient setting. Survey methodology using case-vignettes is both cost and time efficient and has been shown to provide good insight into physicians' actual practice patterns. On the other hand, the stated focus on COPD may have constrained this survey's ability to accurately gauge perceptions and clinical decisions, particularly those relating to COPD diagnosis. Relevant factors such as patients' health insurance status and medication costs on physician choices, were beyond the scope of this study, but may be strong determinants of clinical choices that merit further evaluation. This study was cross-sectional in nature, and while associations such as the one observed between self-identified guideline use and the use of long-acting bronchodilators were found, longitudinal observations are needed to draw causal inferences.
Finally, the 2005 GOLD guideline, which was used as a benchmark for this survey, is an evolving document and contains definitions and recommendations that have sometimes sparked controversy. During active data collection for this study, an updated edition of the guidelines was published with significant changes to disease definitions and diagnostic recommendations. This context should be kept in mind when evaluating the clinical decision patterns observed in this study.
Conclusion
This study presents a snapshot of primary care physician's clinical decision-making when caring for patients with mild-to-moderate COPD. The landmark GOLD guidelines are now 5 years old and may be an important mechanism for improving COPD care, but they have not yet reached many primary care physicians. Many physicians in this study made choices that were guideline-adherent, but discrepancies relating to COPD detection, diagnosis, and management were common and may point to important knowledge gaps. Both family medicine and internal medicine physicians reported that COPD was prevalent in their practices, but several distinctions were observed that may indicate somewhat different educational needs. Tiered educational programs that address critical knowledge gaps, simultaneously compare and contrast COPD with asthma and are well tailored to the primary care setting may lead to substantive improvements in COPD patient care. The broader use of the internet and other newer technologies may be important and can extend education to key primary care physicians practicing in less populous areas with high COPD mortality.
Acknowledgments
We are indebted to Collin Thompson of Outcomes, Inc., as well as John Walsh and Molly McGuire of the COPD Foundation for their instrumental support during this study.
Funding Information
This study was supported by an unrestricted educational grant from Pfizer and Boehringer Ingelheim. Maziar Abdolrasulnia had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Readers are encouraged to respond to the author at Jill.Foster@ceoutcomes.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication via email: pblumen@stanford.edu
Contributor Information
Jill A. Foster, Outcomes, Inc., Birmingham, Alabama Author's email address: Jill.Foster@ceoutcomes.com.
Barbara P. Yawn, Olmsted Medical Center, Rochester, MN.
Abdolrasulnia Maziar, Outcomes, Inc., Birmingham, Alabama.
Todd Jenkins, University of Birmingham, Alabama.
Stephen I. Rennard, Pulmonary and Critical Care Medicine Section, University of Nebraska Medical Center, Omaha, Nebraska.
Linda Casebeer, Outcomes, Inc., Birmingham, Alabama.
References
- 1.Lethbridge-çejku M, Rose D, Vickerie J. Vital and Health Statistics, Vol. 10. p 228. Hyattsville, MD: US Dept of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2006. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2004; pp. 19–21. [PubMed] [Google Scholar]
- 2.Minino AM, Heron MP, Smith BL. Deaths: Preliminary Data for 2004. National Vital Statistics Reports. 2006. p. 54. [PubMed]
- 3.Hnizdo E, Sullivan P, Bang K, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156:738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 4.Abbey D, Burchette R, Knutsen S, McDonnell W, Lebowitz M, Enright PL. Long-term particulate and other air pollutants and lung function in nonsmokers. Am J Respir Crit Care Med. 1998;158:289–298. doi: 10.1164/ajrccm.158.1.9710101. [DOI] [PubMed] [Google Scholar]
- 5.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. MMWR Surv Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 6.Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. COPD Screening efforts in primary care: what is the yield? Prim Care Respir J. 2007;16:41–48. doi: 10.3132/pcrj.2007.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Schayck CP, Chavannes CN. Detection of asthma and chronic obstructive pulmonary disease in primary care. Eur Respir J Suppl. 2003;39:16s–22s. doi: 10.1183/09031936.03.00040403. [DOI] [PubMed] [Google Scholar]
- 8.Calverley PM. COPD: early detection and intervention. Chest. 2000;117:365S–371S. doi: 10.1378/chest.117.5_suppl_2.365s. [DOI] [PubMed] [Google Scholar]
- 9.Global Initiative for Chronic Obstructive Lung Disease (GOLD), World Health Organization (WHO), National Heart, Lung and Blood Institute. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, MD: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization, National Heart, Lung and Blood Institute; 2005. [Google Scholar]
- 10.Ferguson GT, Enright PL, Buist S, Higgins MW. Office Spirometry for Lung Health Assessment in Adults: A Consensus Statement From the National Lung Health Education Program. Chest. 2000;117:1146–1161. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 11.Barr GR, Celli B, Martinez F, et al. Physician and patient perceptions in COPD: The COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.e9–1415.e17. doi: 10.1016/j.amjmed.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 12.Damarla M, Celli BR, Mullerova HX, Pinto-Plato VM. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120–1124. [PubMed] [Google Scholar]
- 13.Mapel DW, Hurley JS, Frost FJ, Petersen HV. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med. 2000;160:2653–2658. doi: 10.1001/archinte.160.17.2653. [DOI] [PubMed] [Google Scholar]
- 14.Lee TA, Bartle B, Weiss KB. Spirometry Use in Clinical Practice Following Diagnosis of COPD. Chest. 2006;129:1509–1515. doi: 10.1378/chest.129.6.1509. [DOI] [PubMed] [Google Scholar]
- 15.Anthonisen NR, Woodlrage K, Manfreda J. Use of spirometry and respiratory drugs in Manitobans over 35 years of age with obstructive lung diseases. Can Respir J. 2005;12:69–74. doi: 10.1155/2005/974678. [DOI] [PubMed] [Google Scholar]
- 16.Walker P, Mitchell P, Diamantea F, Warburton C, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006;28:945–952. doi: 10.1183/09031936.06.00019306. [DOI] [PubMed] [Google Scholar]
- 17.Kaminsky DA, Marcy TW, Bachand M, Irvin CG. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639–1648. [PubMed] [Google Scholar]
- 18.Lusuardi M, De Benedetto F, Paggiaro P, et al. A randomized controlled trial on office spirometry in asthma and COPD in standard general practice: data from spirometry in Asthma and COPD: a comparative evaluation Italian study. Chest. 2006;129:844–852. doi: 10.1378/chest.129.4.844. [DOI] [PubMed] [Google Scholar]
- 19.Schermer TR, Jacobs JE, Chavannes NH, Hartman J, Folgering HT, Bottema BJ, van Weel C. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disase (COPD) Thorax. 2003;58:861–866. doi: 10.1136/thorax.58.10.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–1722. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 21.Peabody JW, Luck J, Jain S, Hansen J, Spell M, Lee M. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141:771–1780. doi: 10.7326/0003-4819-141-10-200411160-00008. [DOI] [PubMed] [Google Scholar]
- 22.United States Department of Health and Human Services (U.S. DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Office of Analysis and Epidemiology (OAE), Compressed Mortality File (CMF) on CDC WONDER On-line Database. CMF 1999–2003, Series 20, No. 2I 2006. [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Ga: CDC; 2005. [Google Scholar]
- 24.Physician Characteristics and Distribution. 2006. Department of Data Quality and Measurement, Division of Data and Operations, American Medical Association; 2006. [Google Scholar]
- 25.A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. JAMA. 2000;283:3244–3254. [PubMed] [Google Scholar]
- 26.Price DB, Tinkelman DG, Halbert RJ, Nordyke RJ, Isonaka S, Nonikov D, Juniper EF, Freeman D, Hausen T, Levy ML, Ostrem A, van der Molen T, van Schayck CP. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73:285–295. doi: 10.1159/000090142. [DOI] [PubMed] [Google Scholar]
- 27.Wilt TJ, Niewoehner D, Kim C-B, et al. Rockville, Md: Agency for Healthcare Research and Quality; Use of Spirometry for Case Finding, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease (COPD) Evidence Report/Technology Assessment No. 121 (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-02-0009.) AHRQ Publication No. 05-E017-2. September 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yawn BP, Yawn RA. Spirometry testing education in medical schools: a missed opportunity. Prim Care Respir J. 2005;14:21–24. doi: 10.1016/j.pcrj.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolton C, Ionescu A, Edwards P, Faulkner T, Edwards S, Shale D. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005;99:493–500. doi: 10.1016/j.rmed.2004.09.015. [DOI] [PubMed] [Google Scholar]


