Abstract
Cyclooxygenase-2 (COX-2) and transforming growth factor-beta1 (TGF-beta1) were modulated in a variety of viral infections, but there is a paucity of data about their role in the pathologic process of cirrhosis and/or hepatocellular carcinoma (HCC) following chronic hepatitis C virus (HCV) infection. The material of the current study included 50 cases of chronic hepatitis C (CHC) without cirrhosis, 30 cases of CHC with cirrhosis, and 30 cases of HCC with HCV admitted to the Gastroenterology and Hepatology Department of Theodor Bilharz Research Institute, Giza, Egypt. Fifteen wedge liver biopsies, taken during laparoscopic cholecystectomy, were included in the study as normal controls. Laboratory investigations, serologic markers for viral hepatitis, and serum alpha fetoprotein levels (alpha-FP) were done for all cases of the study. Immunohistochemistry using primary antibodies against both factors revealed weak to faint immunoreactivity to COX-2 and TGF-beta1 in normal hepatic tissue (< 30% and < 50% of the cells, respectively). COX-2 expression was upregulated in patients with CHC with and without cirrhosis, yet 80% of positively stained cirrhotic cases showed marked staining intensity. Higher COX-2 expression was observed in well-differentiated HCC cases (80%) with marked staining intensity (75%) compared with advanced HCC tumors (P < .001). TGF-beta1 was expressed in the hepatocytes of all cases of CHC with and without cirrhosis as well as in 67% of HCC cases. Extensive cytoplasmic expression was detected in 52%, 93.3%, and 46.6% of CHC patients without cirrhosis, patients with cirrhosis, and patients with HCC, respectively. A positive correlation was observed between hepatic expression of COX-2 and TGF-beta1 (r = 0.67, P < .05); however, no correlation was detected between the latter and grade of HCC differentiation (r = 0.33, P > .05).
Conclusion
These findings may suggest that TGF-beta1 plays a role in hepatic cell damage following HCV infection thus stressing the usefulness of this cytokine as a prognostic marker for liver cell injury. However, COX-2 is a predictive marker for malignant transformation and has a role in the early stages of hepatocarcinogenesis, but not in the advanced stages. The combined expression of both factors in HCV-related HCC suggests their synergistic action in the pathophysiology of hepatocarcinogenesis.
Introduction
Hepatitis C virus (HCV) infection is a significant cause of morbidity and mortality, infecting more than 170 million people worldwide. Chronic infection with HCV can lead to serious liver disease, including cirrhosis and hepatocellular carcinoma (HCC).[1] An alarming increase in HCC cases has been projected over the next 10 years, mostly attributable to hepatitis C, although it is very common in all African countries, south of the Sahara, and in southeast Asia.[2] In response to viral infection, multiple signaling pathways are activated which participate in the regulation of gene expression related to inflammation.[3] Prostaglandins (PGs) play a central role in inflammation, and cyclooxygenase (COX) is the key enzyme in the conversion of arachidonic acid to prostaglandins.[4] Two isoforms of COX have been identified, COX-1 and COX-2.[5] Cyclooxygenase-2 is induced by a variety of factors such as cytokines, growth factors, and tumor promoters[6] and has been connected to inflammation and carcinogenesis.[7] It is involved in the inhibition of apoptosis[8] and promotion of angiogenesis.[9] It also modulates immune function and increases tumor cell invasiveness.[10] The HCV core protein was able to upregulate COX-2 expression in hepatocyte-derived cells, providing a potential mechanism for hepatic fibrosis during chronic HCV infection.[11] Recently, increased COX-2 expression has been documented in HCV-positive HCC.[1]
Hepatic fibrosis is characterized by abnormally excessive accumulation of extracellular matrix (ECM) accompanied by exaggerated cytokine releasing. HCV core protein is thought to produce reactive oxygen species through consequent derangement of lipid metabolism, leading to induction of proinflammatory cytokine TGF-beta1.[12] Transforming growth factor-beta (TGF-beta) is a member of the multifunctional cytokines and has been implicated in diverse cellular phenomena, including cell growth control, cell adhesion and motility, alteration of cellular phenotype, production and degradation of ECM protein, and apoptosis of hepatic cell lines.[13] TGF-beta signaling plays an important role in the pathogenesis of fibrosis in chronic hepatitis, and the hepatic stellate cells (HSCs) are the mediators of this protein.[14]
TGF-beta serves as either epithelial cell growth inhibitor or a tumor promoter, depending on the ECM context.[15] It activates a tumor-suppressor pathway by reversible arrest of cell proliferation.[16] Also, it inhibits cell-cycle progression during G1 phase through enhanced expression of cyclin-dependent kinase inhibitors such as p21.[13] Escape from TGF-beta-induced antiproliferative response plays a central role in development of many cancers,[17] and the lack of its growth inhibitory effect has also been associated with tumor progression and therapeutic resistance.[18]
This study aimed to assess the role of hepatic expression of COX-2 and TGF-beta 1 as early predictors of advancement of chronic hepatitis C disease. This can provide insight into the mechanisms by which HCV induces intracellular events relevant to complications associated with viral infection.
Subjects and Methods
Patients
A total of 125 cases were the subject of the current study. One hundred and ten patients (82 males and 28 females; mean age 45.6 ± 6.4, range 22–60 years) with circulating anti-HCV antibodies, and no serologic evidence of coinfection with hepatitis B virus, were selected from a group of patients admitted to the Department of Gastroenterology and Hepatology, Theodor Bilharz Research Institute, Giza, Egypt; for evaluation of their chronic liver disease. They included 50 cases of chronic hepatitis C virus infection (CHC) without cirrhosis, 30 cases of CHC with cirrhosis and 30 cases of HCC with HCV infection (HCC/HCV). Children, schistosomal patients, chronic viral diseases other than HCV, nonalcoholic steatohepatitis, biliary disorders, and malignancies other than HCC were excluded from the study. They all provided informed consent before inclusion. They were subjected to thorough clinical examination and were assessed by: (1) laboratory investigations; including urine and stool analysis, liver function tests, serologic diagnosis of schistosomiasis and hepatitis markers; (2) proctosigmoidoscopy with examination of rectal biopsies for Schistosoma ova by the transparency technique; (3) ultrasonography; and (4) liver biopsy using ultrasound-guided Menghini needle for histopathologic and immunohistochemical studies.
Fifteen control wedge liver biopsies were taken from age- and sex-matched individuals subjected to laparoscopic cholecystectomy after receiving their consent. They were 10 males and 5 females with a mean age of 42.8 ± 6.6 years. Their liver function tests were within normal range and had no serologic evidence of hepatitis B and/or C viruses.
Laboratory Investigations
Liver function tests were carried out using commercially available kits. Hepatitis B markers, including hepatitis B surface antigen and anti-HBs antibodies, total and IgM class antibodies against hepatitis B core antigen, hepatitis Be antigen, and anti-HBe antibodies, were tested using commercially available enzyme immunoassay kits (Abbott Laboratories; North Chicago, Illinois). Circulating anti-HCV antibodies were detected using Murex enzyme immunoassay kit (Murex anti-HCV, Version V; Murex Diagnostics; Dartford, England). The presence of HCV-RNA in patients' sera was detected by real-time polymerase chain reaction using the Amplicor test (Roche Diagnostic Systems; Meylan, France). Serum alpha-fetoprotein (alpha-FP) was tested using the Eurogenetics enzyme immunoassay kit (Eurogenetics, NV; Tessenderlo, Belgium).
Histopathologic Study
Thin serial sections (5 micrometers [mcm] thick) from formalin-fixed, paraffin-embedded blocks of core liver biopsies were stained with hematoxylin & eosin and Masson trichrome stains. Chronic hepatitis activity was scored as mild, moderate, and severe according to the degree of hepatic necroinflammation and the stage of hepatic fibrosis,[19] whereas HCC cases were classified into well-, moderately-, and poorly differentiated tumors.[20]
Immunohistochemical Study
Liver sections from formalin-fixed, paraffin-embedded blocks (3 mcm thick) were mounted on silanized slides (Dako Cytomation; Glostrup, Denmark) and deparaffinized with xylene. Following dehydration in absolute ethanol for 15 seconds, sections were covered with 2% H2O2 in methanol (vol/vol) and incubated for 45 seconds to inhibit endogenous peroxidase. The slides were then washed with 95% ethanol for 20 seconds, followed sequentially by 70% ethanol for 20 seconds, distilled water for 1 minute, and 10 mmol/L phosphate buffered saline (PBS; pH 7.4) for 5 minutes. Tissue sections were covered with 10 mmol/L citrate buffer, pH 6.0, and were microwaved at 120°C for 5 minutes. Sections were thoroughly washed with PBS and blocked for 45 minutes in PBS containing 2% (w/vol) bovine serum albumin (Sigma Chemicals; St. Louis, Missouri) and 1% normal rabbit serum (Vectastain; Vector Laboratories; Burlingame, California). Sections were covered with COX-2 goat polyclonal antibody (clone M 19: sc-1747; Santa Cruz Biotechnology Inc.; Santa Cruz, California), at 1:100 dilution and incubated at room temperature for 1 hour. After thorough washing in PBS, the sections were incubated with biotinylated secondary antibody at room temperature for 30 minutes and washed with PBS. Avidin-biotin-peroxidase complex solution was then added and incubated for 45 minutes at room temperature. The reaction products were visualized using 3–3′-diamino-benzidine-tetra-hydrochloride. The cell nuclei were counterstained with Mayer's hematoxylin. The internal positive control for COX-2 staining was the vascular smooth muscle tissue. Normal immunoglobulin G (Santa Cruz Biotechnology Inc.) substituted COX-2 to serve as negative control.
The scoring was based on intensity and extensiveness (by percentage population) of the positively stained cells. Both parameters were scored on a scale of 0–3 as follows: (a) intensity: 0 = negative staining (−), 1 = weakly positive staining (+), 2 = moderately positive staining (++), and 3 = strongly positive staining (+++); and (b) extensiveness (Frequency): 0 = negative, 1 = positive staining in < 30% of cells, 2 = 30%–70%, and 3 = > 70%. The score of each specimen was the sum of both parameters.[21]
Immunohistochemistry for TGF-beta1 was also performed on the consecutive formalin-fixed, paraffin-embedded tissue sections. After deparaffinization, rehydration, and endogenous peroxidase inactivation, antigen retrieval was done followed by incubation with TGF-beta1 mouse anti-human antibody (Clone TB21; BioSource International; Fleurus, Belgium) at 1:100 dilution in a humidified chamber at 4°C overnight. The biotinylated secondary antibody was applied and the reactivity was visualized with an avidin-biotin complex immunoperoxidase system, diamino-benzidine as the chromogen, and Mayer's hematoxylin as the counterstain. The positive control consisted of urinary bladder specimens with high immunoreactivity to TGF-beta1. Normal immunoglobulin G (Santa Cruz Biotechnology) substituted primary antibody to serve as negative control. Specimens were classified as altered for TGF-beta1 if more than 50% of the cells showed immunoreactivity to TGF-beta1, the cut-off point between the diseased and the normal tissue.[22]
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) for Windows (version 11) computer program was used for statistical analysis. Means of different groups were compared using one-way ANOVA. Comparison between percent positive cases was calculated by Chi-square test. A P value < .05 was considered statistically significant. Pearson correlation coefficient r was used to measure the relationship between 2 variables.
Results
Clinical Data
One hundred and ten HCV-infected patients together with 15 control cases were included in this study. Their clinical and laboratory data are shown in Table 1 .
Table 1.
Demographic and Laboratory Data of All Studied Cases
| Parameters | Controls (n = 15) | CHC Without Cirrhosis (n = 50) | CHC With Cirrhosis (n = 30) | HCC With HCV (n = 30) |
|---|---|---|---|---|
| Age | 42.8±6.6 | 42.4±9.3 | 45.1±5.9 | 48.9±7.2 |
| Male/female ratio | 2/1 | 4/1 | 17/13 | 5/1 |
| Pallor | 0 (0) | 2 (4) | 5 (16.5) | 8 (26.6) |
| Jaundice | 0 (0) | 3 (6) | 6 (20) | 13 (43.3) |
| Palmer erythema | 0 (0) | 0 (0) | 15 (50) | 17 (56.6) |
| Spider nevi | 0 (0) | 0 (0) | 14 (46.6) | 14 (46.6) |
| Lower-limb edema | 0 (0) | 0 (0) | 16 (53.3) | 9 (30) |
| Child-Pugh classification | ||||
| A | 10 (100) | 50 (100) | 8 (26.6) | 1 (3.3) |
| B | 0 (0) | 9 (30) | 11 (36.6) | |
| C | 0 (0) | 13 (43.3) | 18 (60) | |
| AST (U/L) | 8.09 ±2.11 | 14.91±5.32 | 11.42±4.12 | 18.90±6.47 |
| ALT (U/L) | 7.73±2.04 | 15.09±6.80 | 12.18±5.33 | 16.38±6.32 |
| Albumin (g/dL) | 4.01±0.38 | 3.60±0.85 | 3.80±0.72 | 3.08±0.48 |
| PT concentration | 97.6±3.4 | 89.7±5.8 | 41.4±11.2 | 68.5±3.8 |
| Alpha-fetoprotein (IU/mL) | 3.39±2.9 | 9.36±13.6 | 10.41±15.6 | 25.04±27.9 |
Data were expressed as means ± SD or as percent value (%).
Normal range for ALT and AST was up to 12 U/L.
Normal range for albumin was 3.5–5 g/dL.
Normal range of prothrombin concentration was 85%–100%.
Normal range for alpha-fetoprotein was 0.1–9.6 IU/mL.
Expression of COX-2
The pattern of COX-2 immunoreactivity in both nonneoplastic and neoplastic cells was that of diffuse cytoplasmic type with occasional perinuclear staining. Sections from histologically normal livers displayed weak cytoplasmic staining for COX-2 (< 30% of the cells) in 26.6% of cases. COX-2 immunoreactivity in CHC without cirrhosis was mainly localized to hepatocytes beside inflammatory cells, fibroblasts, vascular smooth muscle cells (Figure 1), and in the epithelial lining of bile ductules of the adjacent portal tracts (Figure 2). The histologic activity index (HAI) revealed more expression of COX-2 in liver tissues with active inflammation than in those without active inflammation (P < .01). However, in CHC with cirrhosis, hepatocytes which were strongly positive for COX-2 were small in size and mainly observed in the regenerative nodules (Figure 3). Eighty percent of positively stained cases showed marked staining intensity.
Figure 1.
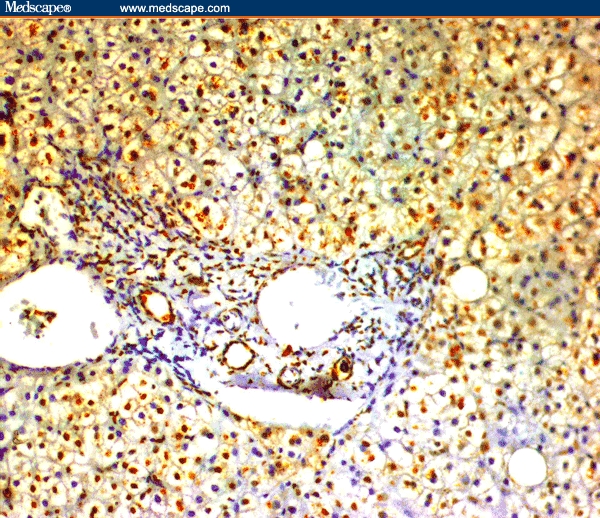
CHC without cirrhosis showing immunoreactivity for COX-2 in inflammatory cells, fibroblasts, and vascular smooth muscle cells of the portal tracts and in the cytoplasm of the hepatocytes (immunoperoxidase staining ×20).
Figure 2.
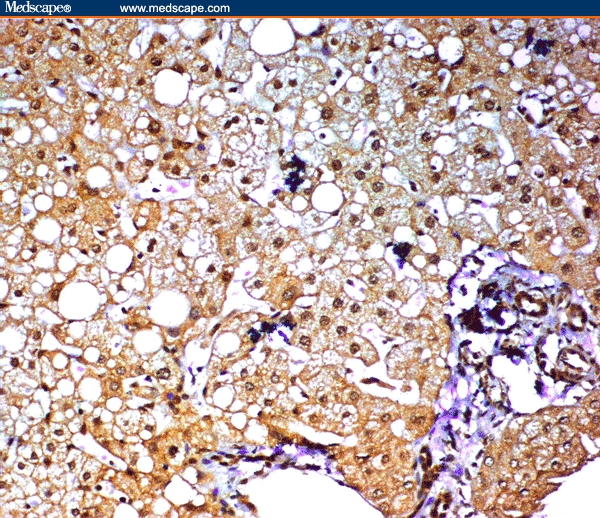
CHC without cirrhosis showing positive immunoreactivity for COX-2 in bile ductules of the portal tracts. Marked steatosis was observed in the hepatocytes (immunoperoxidase staining ×20).
Figure 3.
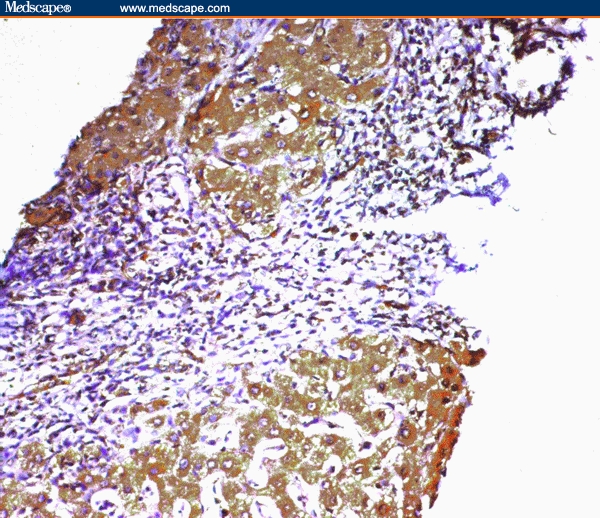
Marked staining of hepatocytes for COX-2 in a cirrhotic nodule with positively stained nearby bile ductules in the adjacent portal tract (immunoperoxidase staining ×20).
In HCC/HCV, COX-2 immunoreactivity was localized almost exclusively to hepatocytes whereas the stroma of the tumor was negative (Figure 4. The staining intensity was significantly higher in well-differentiated tumors compared with CHC with and without cirrhosis (P < .01) and vs moderately and poorly differentiated cancers (P < .01) (Table 2 and Table 3). The well-differentiated cancerous cells showed the highest extent of a homogeneous pattern of immunostain (> 70% of the tumor cells were positive) compared with the less differentiated tumors (Table 3). Moreover, patients with moderately and poorly differentiated HCC who displayed marked staining intensity represent lesser expression than those with CHC with and without cirrhosis (P < .01) (Table 2 and Table 3).
Figure 4.
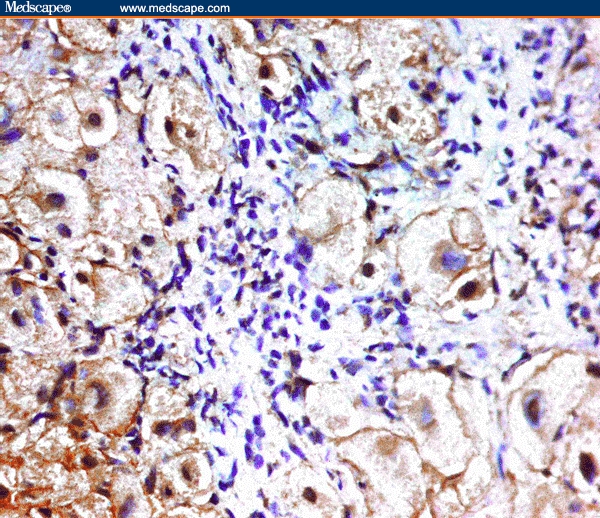
Cytoplasmic staining for COX-2 in the hepatocytes of HCC/HCV case (immunoperoxidase staining ×40).
Table 2.
Expression and Scoring of Cyclooxygenase-2 (COX-2) Immunostain in Liver Tissue of Different Studied Cases
| Histopathologic Diagnosis (n) | Percent (%) of Positive Cases | Frequency % | Intensity% | Score | ||||
|---|---|---|---|---|---|---|---|---|
| <30% | 30%–70% | >70% | + | ++ | +++ | |||
| Controls (15) | 26.6 | 26.6 | 0 | 0 | 20 | 6.6 | 0 | 4 |
| CHC without cirrhosis (50) | 32 | 0 | 10 | 22 | 0 | 8 | 24 | 4.5 |
| CHC with cirrhosis (30) | 33.3 | 3.3 | 6.6 | 23.3 | 0 | 6.7 | 26.6 | 5 |
| HCC with HCV (30) | 66.7a | 3.3 | 20.1 | 43.3 | 6.6 | 23.3 | 36.6 | 6.5 |
P < .01 relative to other groups
Table 3.
Immunohistopathologic Expression of Cyclooxygenase-2 (COX-2) in HCV-Positive Hepatocellular Carcinoma (HCC) Relative to the Grade of Tumor Differentiation
| Grade of HCC Differentiation (n) | Percent (%) of Positive Cases | Frequency % | Intensity% | Score | ||||
|---|---|---|---|---|---|---|---|---|
| <30% | 30%–70% | >70% | + | ++ | +++ | |||
| Well (10) | 80a | 0 | 0 | 80a | 0 | 20 | 60a | 9.5 |
| Moderate (11) | 63.7 | 27.3 | 18.2 | 18.2 | 45.5 | 0 | 18.2 | 5 |
| Poor (9) | 55.5 | 33.3 | 11.1 | 11.1 | 22.2 | 22.2 | 11.1 | 4.5 |
P < .01 relative to moderately and poorly differentiated tumors
Expression of TGF-Beta1
The TGF-beta1 immunoreactivity in nonneoplastic and neoplastic lesions was that of diffuse granular cytoplasmic staining (Figure 5).
Figure 5.
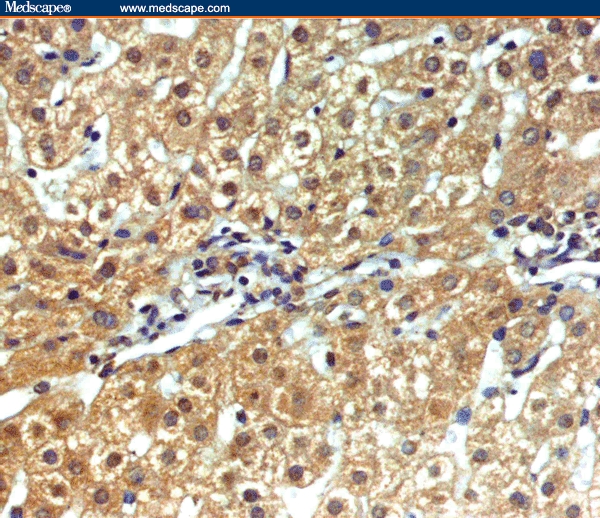
Marked granular cytoplasmic staining of hepatocytes for TGF-beta1 in a case of CHC without cirrhosis (immunoperoxidase staining ×20).
All sections from histologically normal livers displayed faint expression of TGF-beta1 in less than 50% of cells. Although TGF-beta1 was expressed in hepatocytes of all patients of CHC with and without cirrhosis, marked staining was detected in 52% and 93.3% of the specimens, respectively (Table 4). However, TGF-beta1 was expressed in 67% of patients with HCC/HCV and was ranked between high (46.6%) (Figure 6), and moderate (20%) frequency in positively stained cases (Table 4).
Table 4.
Immunohistochemical Expression of Transforming Growth Factor-beta1 (TGF-beta1) in Liver Tissue of All Studied Cases
| Histopathologic Diagnosis (n) | Percent (%) of Positive Cases | Frequency % of TGF-beta1 Expression | ||
|---|---|---|---|---|
| (50%–60%) | (61%–80%) | (>80%) | ||
| Controls (15) | 0 | 0 | 0 | 0 |
| CHC without cirrhosis (50) | 100 | 4 | 44 | 52 |
| CHC with cirrhosis (30) | 100 | 0 | 6.7 | 93.3 |
| HCC (30) | 66.6a | 0 | 20 | 46.6 |
*All sections from histologically normal livers displayed faint expression of TGF-beta1 in < 50% of the cells. P < .01 relative to CHC with and without cirrhosis
Figure 6.
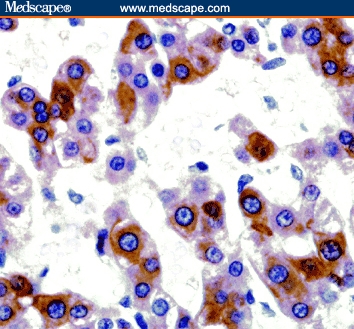
A case of HCC/HCV showing marked immunoreactivity for TGF-beta1 in the hepatocytes with intact intervening stroma (immunoperoxidase staining ×20).
A positive correlation was detected between hepatic expression of COX-2 and TGF-beta1 (r= 0.67, P < .05); however, no correlation was found between the latter and grade of HCC differentiation (r= 0.33, P > .05). Also, no correlation could be established between COX-2 and TGF-beta1 expression and serum alpha-FP level (r= 0.26 and r= 0.23, respectively, P > .05) (Table 5).
Table 5.
Correlation Analysis of the Patients of the Study
| Parameters | r | P |
|---|---|---|
| COX-2 vs alpha-fetoprotein | 0.26 | NS |
| TGF-beta1 vs alpha-fetoprotein | 0.23 | NS |
| COX-2 vs TGF-beta1 | 0.67 | .05 |
| COX-2 vs grade of HCC differentiation | 0.88 | .01 |
| TGF-beta1 vs grade of HCC differentiation | 0.33 | NS |
Discussion
COX-2 has been implicated in tissue injury and fibrogenesis in animal models but little is known regarding its role in HCV-related liver disease in humans.[11] HCV infection stimulates host inflammatory responses comprising cellular effectors and soluble factors. One such factor that has recently been implicated in the host response to a wide variety of viruses is COX-2-controlled prostaglandin E2 molecules.[1] Expression of COX-2 has been demonstrated in many contexts related to liver cell pathologies as regeneration after partial hepatectomy, animal models of cirrhosis, human hepatoma cell lines, chronic hepatitis C infection, and HCC.[23] In the current study, intrahepatic expression of COX-2 was increased in HCV patients with increased inflammatory activity and fibrotic stage. Also, overexpression was detected in cirrhotic patients, suggesting its pathogenic role in the process of fibrogenesis. This finding was in concordance with that of other authors[24] who found that the expression may be associated with high levels of necroinflammation in the background liver. However, stable expression of COX-2 in liver cells induced proliferation, with an increase in the proportion of cells in S-phase.[23]
HCC is unique in that its carcinogenesis is related to inflammatory changes and regenerative activities in the background liver.[20] Hepatocarcinogenesis is best described as a continuity of regeneration, proliferation, unregulated hyperplasia, dysplasia, and malignant transformation.[25] Selective inhibition of COX-2 may offer a therapeutic target and preventive potential in human hepatocarcinogenesis.[26] In this study, a profound expression of COX-2 was found in hepatocytes of HCC cases compared to noncancerous lesions, suggesting a role of COX-2 in malignant growth. Our results revealed increased COX-2 expression in well-differentiated tumor tissue, as found previously by Qiu and colleagues.[27] Because HCC progresses from a well-differentiated to a less-differentiated histopathologic grade as time passes,[28] the enhanced expression of COX-2 in well-differentiated HCC suggests its possible involvement in the early stages of hepatocarcinogenesis. Our data also showed that the less-differentiated HCC displayed downregulation of COX-2 expression. Liver-specific pathophysiology is characterized by dual blood supply – one portal and the other arterial.[29] In pathologic conditions, lipopolysaccharide (endotoxin) and proinflammatory cytokines, such as interleukin-1-beta and tumor necrosis factor-alpha, in portal blood, affect hepatic cells[30] and induce COX-2 expression.[31] It has been speculated that well-differentiated HCC with few arterial tumor vessels and preexisting portal tracts may be more influenced by these COX-2 inducers via portal blood flow, resulting in its strong expression, whereas the less-differentiated tumors lacking the preexisting portal tracts are less influenced.
TGF-beta1 is implicated in the pathogenesis of liver disease. It is involved in both liver regeneration and cirrhotic changes of hepatitis C virus infection.[32] The present study revealed that TGF-beta1 was expressed in hepatocytes of all patients with CHC with and without cirrhosis, as it is a multifunctional cytokine that promotes fibrogenesis through activation of HSCs and inhibition of hepatocyte proliferation and replication.[33] Sanz and coworkers.[34] suggested that interruption of TGF-beta1 signaling might improve liver fibrosis and stimulate liver regeneration because it may influence the biology of both HSCs and hepatocytes.
Liver sections from patients with HCC showed profound TGF-beta1 expression compared with histologically normal liver, suggesting a possible role of this cytokine in carcinogenesis. This may be attributed to either loss of TGF-beta tumor-suppressor activity or action of this protein as a tumor-promoting oncogene. Data from both experimental models and studies of human cancers show that not only the ligand itself but also its downstream elements, including its receptors and its primary cytoplasmic signal transducers, are important for suppressing primary tumorigenesis in many organs.[15] TGF-beta1 has the ability to induce malignant behavior of normal fibroblasts and is the key factor in uncoupling cells from normal growth control to become tumorigenic.[17] The tumor-promoting effect of TGF-beta1, enhancement of ECM deposition, degradation, angiogenesis, and conversion of transformed epithelial cells to more invasive mesenchymal phenotype are preserved even when growth inhibitory responses are lost, and this, in turn, may enhance tumor development.[35] A positive correlation was observed between immunohistochemical expression of both COX-2 and TGF-beta1 in this study, as described previously.[36] The authors found that COX-2-expressing cells induce upregulation of different growth factors, such as TGF-beta. Reactivation of alpha-FP gene expression is shown during massive hepatic necrosis and HCC.[37] Its use as a diagnostic marker for HCC became questionable and of limited value due to its limited sensitivity and specificity.[38] In the current study, 26% of cases of CHC with and without cirrhosis and 52.3% of HCC cases showed a remarkable increase in alpha-FP levels above the normal value. In accordance, Mohamedein and colleagues[39] also detected that estimation of alpha-FP alone can diagnose only 51% of HCC cases, and Garcia and coworkers[40] reported that 20% of HCC cases showed normal serum levels of alpha-FP. In the present study, no correlation was detected between COX-2 expression and serum alpha-FP. This result is in agreement with that of Bae and associates.[41]
Conclusion
In conclusion, our data reveal that HCV was able to upregulate COX-2 expression in hepatocytes, providing a potential mechanism for hepatic fibrosis during chronic HCV infection. COX-2 expression may be a useful predictive marker for malignant transformation in chronic HCV infection. Because profound COX-2 expression was associated with early stages of hepatocarcinogenesis only, this enzyme may consequently be related to HCC differentiation. Improvement in early diagnosis of HCC and regular screening of individuals at high risk for HCC provide the possibility of effective chemoprevention.
The upregulation of hepatic TGF-beta1 expression in CHC with and without cirrhosis suggests that this cytokine may be involved in pathologic process of inflammation and cirrhosis and stresses the importance of this mediator as a prognostic marker of hepatic damage in chronic HCV infection. Moreover, TGF-beta1 may play a crucial role in hepatocarcinogenesis. Regulation of TGF-beta1 activation and modulation of its signal transduction pathway may prevent the progression of hepatic damage and development of HCC in chronic HCV infection.
The combined expression of COX-2 and TGF-beta1 in HCC suggests a synergistic action of these factors in the pathophysiology of hepatocarcinogenesis. However, these are preliminary results based on a limited number of cases; further study of a larger cohort of patients is needed.
Footnotes
Reader Comments on: Expression of Cyclooxygenase-2 and Transforming Growth Factor-beta1 in HCV-Induced Chronic Liver Disease and Hepatocellular Carcinoma See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at azzaelbassiouny@yahoo.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication as an actual Letter in MedGenMed via email: pblumen@stanford.edu
Contributor Information
Azza E.I. El-Bassiouny, Theodor Bilharz Research Institute (TBRI), Giza, Egypt Author's E-mail: azzaelbassiouny@yahoo.com.
Mona M.K. Zoheiry, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Mona M.F. Nosseir, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Eman G. El-Ahwany, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Raafat A. Ibrahim, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Nora E.I. El-Bassiouni, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
References
- 1.Waris G, Siddiqui A. Hepatitis C stimulates the expression of cyclooxygenase-2 via oxidative stress: Role of prostaglandin E2 in RNA replication. J Virol. 2005;79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Rosai J. Liver tumors and tumor like conditions. In: Desmet VJ, Rosai J, editors. Surgical Pathology. 9th ed. Volume 2. London, Inc: Saunders, Elsevier Science; 2004. pp. 992–1017. [Google Scholar]
- 3.Steer SA, Corbett JA. The role and regulation of COX-2 during viral infection. Virol Immunol. 2003;16:447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- 4.Kmhoff M, Guan Y, Shappell HW. Enhanced expression of cyclooxygenase-2 in high grade human transitional cell bladder carcinomas. Am J Pathol. 2000;157:29–35. doi: 10.1016/S0002-9440(10)64513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hla T, Nilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberhat LE, Dubois RN. Eicosanoids and gastrointestinal tract. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 7.DuBois RN, Abramson SB, Cofford L, Gupta RN, Simon LS, Van de Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 8.Shiff SJ, Qiao L, Tsai LL. Rigas B: Sulindac sulfide an aspirin-like compound inhibits proliferation causes cell cycle quiescence and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tortora G, Capulo R, Damiano V, et al. Combination of selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD 1839 and protein kinase A antisense causes cooperative anti-tumor and anti-angiogenic effect. Clin Cancer Res. 2003;9:1556–1572. [PubMed] [Google Scholar]
- 10.Xu XC. Cyclooxgenase-2 inhibitors in cancer treatment and prevention: A recent development. Anticancer Drugs. 2002;13:127–137. doi: 10.1097/00001813-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nunez O, Frenandez-Martinez A, Majajno PL, et al. Increased intrahepatic cyclooxygenase-2, matrix metalloproteinase-2, matrix metalloproteinase-9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: Role of viral core and NS5A proteins. Gut. 2004;53:1665–1672. doi: 10.1136/gut.2003.038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 13.Alexandrow MG, Moses HL. Transforming growth factor-beta and cell cycle regulation. Cancer Res. 1995;55:1452–1457. [PubMed] [Google Scholar]
- 14.El-Youssef M, Mu Y, Huang L, Stellmach V, Crawford SE. Increased expression of TGF-beta1 and thrombospondin-1 in congenital hepatic fibrosis: Possible role of hepatic stellate cells. J Pediatr Gastroenterol Nutr. 1999;28:386–392. doi: 10.1097/00005176-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Roberts AB, Wakefield LM. The two faces of TGF-beta in carcinogenesis. Proc Natl Acad Sci USA. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Im YH, Markowitz SD, Bang YJ. Molecular mechanisms of inactivation of TGF-beta receptors during carcinogenesis. Cytokine Growth Factor Rev. 2000;11:159–168. doi: 10.1016/s1359-6101(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Mencn K, Alvarez E, Lu K, Teicher BA. Transforming growth factor beta and response to anti-cancer therapies in human liver and gastric tumors in vitro and in vivo. Inter J Oncol. 2000;16:599–610. doi: 10.3892/ijo.16.3.599. [DOI] [PubMed] [Google Scholar]
- 19.Ishak KG, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 20.Scheuer PJ. Neoplasms and nodules. In: Scheuer PJ, Lefkowitch JH, editors. Liver Biopsy Interpretation. 6th ed. London: Saunders, Elsevier Science, Inc; 2003. pp. 201–205. [Google Scholar]
- 21.Cheng AS-L, Chan H L-Y, Leung WK, et al. Expression of HBx and COX-2 in chronic hepatitis B, cirrhosis and hepatocellular carcinoma: implication of HBx in up-regulation of COX-2. Mod Pathol. 2004;17:1169–1179. doi: 10.1038/modpathol.3800196. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Shariat SF, Kim I, et al. Predictive value of expression of transforming growth factor-α1 and its receptors in transitional cell carcinoma of the urinary bladder. Cancer. 2001;92:1475–1483. doi: 10.1002/1097-0142(20010915)92:6<1475::aid-cncr1472>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Martinez A, Molla B, Mayoral R, Bosca L, Casado M, Martin-Sanz P. Cyclooxygenase-2 expression impairs serum-withdrawal-induced apoptosis in liver cells. Biochem J. 2006;398:371–380. doi: 10.1042/BJ20060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morinaga S, Yamamato Y, Noguchi Y, et al. Cyclooxygenase-2 mRNA is up-regulated in cirrhotic or chronic hepatitis liver adjacent to hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1110–1116. doi: 10.1046/j.1440-1746.2002.02836.x. [DOI] [PubMed] [Google Scholar]
- 25.Hu KQ. Rationale and feasibility of chemoprevention of hepatocellular carcinoma by cyclooxgenase-2 inhibitor. J Lab Clin Med. 2002;139:234–243. doi: 10.1067/mlc.2002.122281. [DOI] [PubMed] [Google Scholar]
- 26.Kern MA, Schubert D, Sahi D, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- 27.Qiu DK, Ma X, Peng YS, Chen XY. Significance of cyclooxygenase-2 expression in human primary hepatocellular carcinoma. World J Gastroenterol. 2002;8:815–817. doi: 10.3748/wjg.v8.i5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugihara N, Nakashima O, Kojiro M, Majima Y, Tanaka M, Tanikawa K. The morphologic transition in hepatocellular carcinoma: A comparison of the individual histologic features disclosed by ultrasound-guided fine-needle biopsy with those of autopsy. Cancer. 1992;70:1488–1492. doi: 10.1002/1097-0142(19920915)70:6<1488::aid-cncr2820700607>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Winter TC, III, Takayasu K, Muramatsu Y, et al. Early advanced hepatocellular carcinoma: Evaluation of CT and MR appearance with pathologic correlation. Radiology. 1994;192:379–387. doi: 10.1148/radiology.192.2.8029401. [DOI] [PubMed] [Google Scholar]
- 30.Crawford JM. Cellular and molecular biology of the inflamed liver. Curr Opin Gastroenterol. 1997;13:175–185. doi: 10.1097/00001574-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Belvisi MG, Saunders MA, Haddadel B, et al. Induction of cyclooxygenase-2 by cytokines in human cultured airway smooth muscle cells: The novel inflammatory role of this cell type. Br J Pharmacol. 1997;120:910–916. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi SH, Hwang SB. Modulation of TGF-beta signal transduction pathway by hepatitis C virus nonstructural 5A protein. J Biol Chem. 2006;281:7468–7478. doi: 10.1074/jbc.M512438200. [DOI] [PubMed] [Google Scholar]
- 33.Gressner A, Weistirchen R, Breitkopf K, Dooley S. Role of TGF-beta in hepatic fibrosis. Front Biosci. 2002;1:793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 34.Sanz S, Pucilowska JB, Liu S, et al. Expression of insulin-like growth factor-1 by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54:134–141. doi: 10.1136/gut.2003.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deckers M, van Dinther M, Buijs J, et al. The tumor suppressor Smad 4 is required for TGF-beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 36.Lara-Pezzi E, Gomez-Gaviro MV, Galvez BG, et al. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. Clin Invest. 2002;110:1831–1838. doi: 10.1172/JCI200215887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niwa Y, Matsumura M, Shiratori Y. Quantitation of alpha-FP and albumin messenger RNA in human hepatocellular carcinoma. Hepatology. 1996;23:1384–1392. doi: 10.1053/jhep.1996.v23.pm0008675155. [DOI] [PubMed] [Google Scholar]
- 38.Endo Y. Carcino-fetal antigens. Nippon-Rinsho. 1996;54:1499–1504. [PubMed] [Google Scholar]
- 39.Mohamedein A, Yousif-Kadaru AG, Ahmed SA. A carboxy prothrombin (PIVKA II) as a tumor marker for hepatocellular carcinoma and other liver diseases. East Afr Med J. 1995;72:584–587. [PubMed] [Google Scholar]
- 40.Garcia CD, Guzmande la FJ, Munoz ELE. Primary liver cancer. Its epidemiological, clinical and biochemical characteristics. Rev Gastroenterol Mex. 1994;59:17–22. [PubMed] [Google Scholar]
- 41.Bae SH, Jung ES, Park YM, et al. Expression of cyclooxygenase-2 in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7:1110–1115. [PubMed] [Google Scholar]


