Abstract
Objective: To investigate the relationship between serum resistin level and acute coronary syndrome (ACS) or stable angina pectoris (SAP). Methods: Sixty-five patients, with coronary artery disease, were enrolled and divided into three subgroups: acute myocardial infarction (AMI), unstable angina pectoris (UAP) and SAP, and 26 healthy people were recruited as controls in the cross-sectional study. Serum resistin levels were determined by ELISA (enzyme-linked immunosorbent assay), and WBC (white blood cell count), hsCRP (high sensitive C-reaction protein), CKmax (maximum of creatinkinase), CK-MBmax (maximum of isozyme of creatinkinase) and cTnImax (maximum of troponin) were measured by standard laboratory methods. Results: The serum resistin levels were 4 folds higher in AMI patients, 2.43 folds in UAP patients and 1.12 folds in SAP patients than in the healthy controls (P<0.05). The resistin levels were also significantly different between AMI [(8.16±0.79) ng/ml], UAP [(5.59±0.75) ng/ml] and SAP [(3.45±0.56) ng/ml] groups (P<0.01); WBC, hsCRP, CKmax, CK-MBmax and cTnImax were significantly increased in AMI patients over UAP and SAP patients. Spearman analysis showed that serum resistin levels were positively correlated with WBC (r=0.412, P=0.046), hsCRP (r=0.427, P=0.037), CKmax, CK-MBmax and cTnImax (r=0.731, 0.678, 0.656; P<0.01). Conclusion: Serum resistin levels increased with inflammatory factors and myocardial impairment. The results suggest that human resistin might play an important role in the pathogenesis of atherosclerosis and AMI as an inflammatory factor.
Keywords: Resistin, Acute coronary syndrome (ACS), Stable angina pectoris (SAP)
INTRODUCTION
Adipose tissue is increasingly recognized to be not only a storage organ for lipids but rather a metabolically highly active endocrine organ. Studies have revealed that adipocytes synthesize and secrete a number of biologically active molecules (Lyon et al., 2003), so called adipokines, including tumor necrosis factor-α (TNF-α), leptin, interleukin-6 (IL-6), plasminogen activator inhibitor-1, adiponectin, and resistin (Hotamisligil and Spiegelman, 1994; Matsuzawa et al., 1999; Steppan et al., 2001a).
The discovery of resistin has directed intense research to fat-derived mediators in obesity-induced insulin resistance and type 2 diabetes (Steppan et al., 2001a). Thus, resistin, one amongst a family of three proteins, known as resistin-like molecules (RELMs) (Steppan et al., 2001b), may provide insight into links between obesity, inflammation and atherosclerosis.
Fat cells in mice secrete resistin, which causes tissues, especially the liver, to be less sensitive to the action of insulin (type 2 diabetes), and blood glucose levels to rise because of increased glycogenolysis and gluconeogenesis in the liver (Rajala et al., 2003). In humans, resistin is primarily a product of macrophages (Yang et al., 2003; Patel et al., 2003). Increased resistin levels have been established in response to the treatment with endotoxin and proinflammatory cytokines (Kaser et al., 2003). There is also interdependence in humans between elevated levels of resistin, obesity, and type 2 diabetes. Several follow-up studies have explored the cellular, physiological and clinical importance of resistin, but fundamental questions about the relationship between resistin, inflammatory processes, insulin resistance, and atherosclerosis remain still unclear, and several inconsistencies have emerged (Ohmori et al., 2005; Reilly et al., 2005; Pischon et al., 2005). The purpose of the present investigation is to assess the correlation of circulating resistin levels with other metabolic parameters and biomarkers in subjects with acute coronary syndrome (ACS) or stable angina pectoris (SAP). We hypothesize that the plasma level of resistin is a marker of vascular inflammation similar to those of high sensitive C-reaction protein (hsCRP) and white blood cell count (WBC).
MATERIALS AND METHODS
Study population
Sixty-five patients with coronary artery disease (CAD) were enrolled in this cross-section study. They were admitted to VIP and Cardiovascular Departments of the First Affiliated Hospital, School of Medicine, Zhejiang University, from September 2005 to September 2006. Coronary angiography was performed in all patients, and the relevant CAD was defined by >50% stenosis in at least one major coronary artery. We excluded patients without any evidence of CAD and patients with evidence of significant concomitant diseases, in particular hemodynamically significant valvular heart disease, surgery or trauma within the previous month, known cardiomyopathy, known malignant diseases, or febrile conditions. The medication data is not available. There were 24 subjects with acute myocardial infarction (AMI), 19 with unstable angina pectoris (UAP), and 22 with SAP. Twenty-six healthy individuals were selected as controls. Study group characteristics are presented in Table 1.
Table 1.
Clinic and laboratory variables of each group
| AMI | UAP | SAP | Control | |
| N (M/F) | 24 (18/6) | 19 (16/3) | 22 (17/5) | 26 (18/8) |
| Age (years) | 58.21±11.11 | 63.16±5.36 | 86.00±16.62 | 60.77±8.06 |
| BMI (kg/m2) | 23.96±3.35 | 24.74±4.26 | 23.79±3.34 | 22.96±2.76 |
| WBC (×109 L−1) | 9.87±2.06** | 7.22±2.33* | 7.00±1.75 | 5.99±1.93 |
| hsCRP (mg/L) | 21.60±9.20** | 13.27±4.13** | 5.57±3.75 | 4.95±1.97 |
| CKmax (U/L) | 1107.00±1022.00** | 195.84±189.46 | 122.68±74.85 | 68.12±43.10 |
| CK-MBmax (U/L) | 97.79±75.68* | 17.09±4.54 | 16.05±6.12 | 10.23±2.22 |
| cTnImax (μg/L) | 40.26±36.65** | 5.85±9.31 | 0.76±1.45 | 0.06±0.12 |
| TG (mmol/L) | 1.41±0.59 | 1.59±1.20 | 1.72±1.14 | 2.04±0.53 |
| CH (mmol/L) | 4.51±1.01 | 3.92±1.10 | 3.99±1.09 | 4.23±1.10 |
| HDL-C (mmol/L) | 1.26±0.35 | 1.14±0.27 | 1.28±0.44 | 1.13±0.42 |
| LDL-C (mmol/L) | 1.95±0.35 | 1.76±0.58 | 1.85±0.49 | 1.95±0.56 |
| FBS (mmol/L) | 5.94±2.02**∆● | 4.94±0.78 | 4.98±1.02 | 4.91±1.21 |
| SBP (mmHg) | 116.58±14.76* | 124.70±16.47 | 126.86±18.66 | 130.11±24.93 |
| DBP (mmHg) | 68.62±7.87 | 75.05±1.95 | 74.82±11.94 | 74.42±11.69 |
Data are expressed as mean±SD. BMI: Body mass index; WBC: White blood cell count; hsCRP: High sensitive C-reaction protein; CKmax: The maximum of creatinkinase; CK-MBmax: The maximum of isozyme of creatinkinase; cTnImax: The maximum of troponin; TG: Triglyceride; CH: Cholesterin; HDL-C: High density lipoprotein cholesterin; LDL-C: Low density lipoprotein cholesterin; FBS: Fasting blood sugar; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; AMI: Acute myocardial infarction; UAP: Unstable angina pectoris; SAP: Stable angina pectoris
P<0.05 meaning the significance when compared to control;
P<0.01 meaning the significance when compared to control;
P<0.05 compared to SAP;
P<0.05 compared to UAP.
Blood sample and laboratory methods
The serum samples of ACS (AMI and UAP) and SAP patients were collected on the day of admission or at the time of angina pectoris. The control serum samples were collected after a 12-hour fast. The serum samples were processed immediately, coded, and then stored at −70 °C until the blind analysis was conducted at the end of the study. Serum resistin concentrations were measured by using human resistin ELISA box (Biovender Company, German). WBC, hsCRP, creatinkinase (CK), isozyme of creatinkinase (CK-MB), and troponin (cTnI), and were measured by standard laboratory methods. CK, CK-MB and cTnI were monitored and the maximums of CK, CK-MB and cTnI (CKmax, CK-MBmax and cTnImax, respectively) were taken. Lipids and glucose were measured by routine methods.
Anthropometric data
After measuring the height and weight of patients, the body mass index (BMI) was calculated. Blood pressures were examined at least 30 min before vein blood taking at rest. Left ventricular ejection fraction (LVEF) was determined with echocardiography about (5±2) d after admission to hospital.
Statistical analysis
One-way ANOVA (analysis of variance) and Pearson correlation analysis were performed by SPSS 11.0 software. P<0.05 was considered the level of significant difference.
RESULTS
Serum resistin
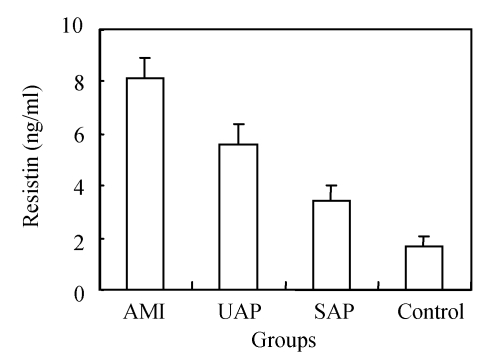
In the investigation of the relationship between the serum resistin level and ACS and SAP, we found that the serum resistin level was significantly elevated in AMI, UAP and SAP patients compared to the control group (Fig.1). In addition, the mean level of serum resistin in AMI patients was higher than those in UAP and SAP patients (P<0.01). There was also a significant difference in the serum resistin levels between the UAP and SAP groups (P<0.05). These results indicate that serum resistin level increases with the increasing pathogenic severity of the CAD.
Fig. 1.
Serum resistin levels in the acute myocardial infarction (AMI), unstable angina pectoris (UAP), stable angina pectoris (SAP) and control groups
The resistin level was higher in every patient group compared to the control group (P<0.05)
Data in AMI analysis
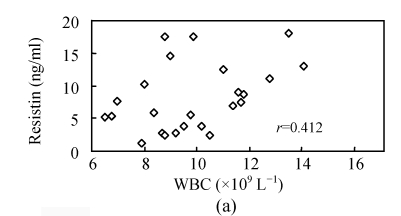
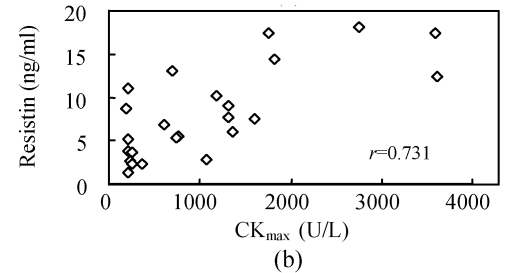
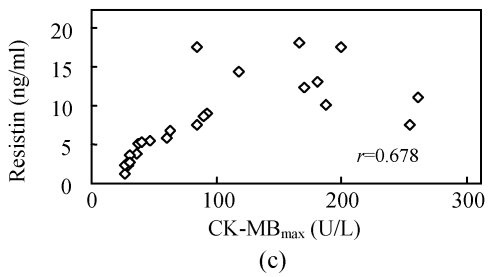
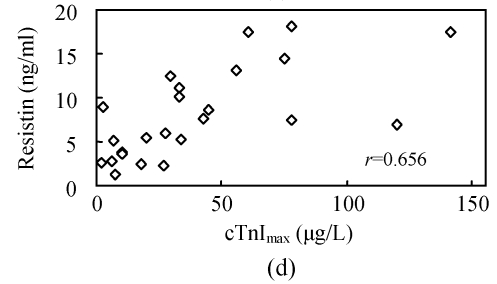
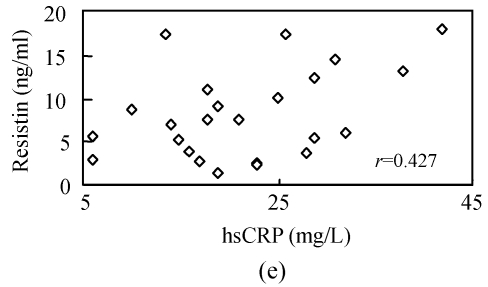
To investigate whether serum resistin is associated with inflammation, WBC, hsCRP, CKmax, CK-MBmax and cTnImax were measured. WBC, hsCRP, CKmax, CK-MBmax and cTnImax were significantly higher in AMI group compared to other groups. Correlation analysis demonstrated that serum resistin concentration was positively correlated with WBC, hsCRP, CKmax, CK-MBmax, cTnImax in AMI group (r=0.412, P<0.05; r=0.427, P<0.05; r=0.731, P<0.01; r=0.678, P<0.01; r=0.656, P<0.01, respectively) (Fig.2). In addition, there was no significant correlation between serum resistin and TG (triglyceride), CH (cholesterin), HDL-C (high density lipoprotein cholesterin), LDL-C (low density lipoprotein cholesterin), FBS (fasting blood sugar), SBP (systolic blood pressure) and DBP (diastolic blood pressure) in AMI group. In the other groups, however, no significant correlation was observed between serum resistin and WBC, hsCRP, CKmax, CK-MBmax, cTnImax.
Fig. 2.
Correlation of resistin with WBC (a), CKmax (b), CK-MBmax (c), cTnImax (d) and hsCRP (e)
DISCUSSION
CAD severely threats human’s health with increasing morbidity. Among its multiple risk factors, insulin resistance is considered as one of the new independent cardiovascular risk factors. Resistin first reported by Steppan et al.(2001a) extensively acts on the insulin targeted organs and influences the metabolisms of glucose and lipids via affecting signal transduction pathways and the transcription of the enzymes related to metabolism, resulting in insulin resistance (Steppan et al., 2005; Fan et al., 2007; Zhou et al., 2006). Insulin resistance may directly promote the development of CAD. Several studies proposed that resistin is the effector molecule that links the metabolic syndrome and insulin resistance to atherosclerotic burden, possibly through triggering inflammatory processes (Ohmori et al., 2005; Reilly et al., 2005; Pischon et al., 2005).
Resistin may contribute to the atherosclerotic process by activation of endothelial cells leading to endothelial dysfunction and thereby stimulating multiple pro-atherosclerotic pathways (Verma et al., 2003; Kawanami et al., 2004). The endothelium represents an important point of convergence of cardiovascular and metabolic pathways, since insulin resistance directly promotes endothelial dysfunction (Mather et al., 2001), which is considered as an early and integral step of atherosclerotic vascular disease (Verma and Anderson, 2002). Resistin promotes smooth muscle cell proliferation through activation of extra-cellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways (Calabro et al., 2004). Resistin induces endothelin-1 promoter activity via the AP-1 site, up-regulates adhesion molecules and chemokines, and down-regulates tumor necrosis factor receptor-associated factor-3 (Verma et al., 2003).
In this study we found that the serum resistin levels were significantly increased in AMI, UAP and SAP patients when compared with the controls. Previous studies have similarly reported an increased serum resistin level in the CAD. Reilly et al.(2005) discovered that circulating resistin level was independently associated with coronary artery calcification (CAC), a quantitative index of atherosclerosis. Burnett et al.(2005) reported that serum resistin levels of premature coronary artery disease (PCAD) patients were higher than normal controls. We also found that serum resistin levels increased with the pathagenic progress of CAD. The serum resistin level was elevated in UAP group compared to SAP group, while the level was highest in AMI patients (P<0.05). Lubos et al.(2007) reported that resistin levels were elevated in patients presenting with unstable angina, non-ST-elevation myocardial infarction and ST-elevation myocardial infarction, and might play a role as a diagnostic marker. These reports have demonstrated that the resistin level is related to the severity of CAD.
In the present investigation, CKmax, CK-MBmax, and cTnImax, the myocardial impairment markers, and WBC, hsCRP, the markers of inflammation, were significantly increased in AMI patients when compared with other CAD patients. Correlation analysis indicated that serum resistin levels in AMI patients were positively correlated with the markers of myocardial impairment and inflammation. The severer the myocardial impairment and inflammation, the higher the serum resistin level. Similarly, Kunnari et al.(2006) reported that resistin was associated with hsCRP and leukocytes and was considered as a pro-inflammatory factor.
It was reported that resistin levels are substantially higher in human inflammatory cells when compared with human adipocytes (Yang et al., 2003; Patel et al., 2003; Kaser et al., 2003). Elevation of resistin in the ACS might represent the presence of inflammatory process in mononuclear cells—precede myocardial necrosis. Resistin gene expression is regulated by multiple factors, for example, acute endotoxemia leading to dramatically (>7-fold) elevated serum level of resistin (Lehrke et al., 2004), which is associated with a state of insulin resistance in humans (Agwunobi et al., 2000). These findings may additionally support the hypothesis that in the conditions of the ACS resistin might represent inflammatory rather than a metabolic processes. Because of diverse myocardial ischemia and ischemic impairment in AMI\UAP\SAP patients, the inflammatory factors might be released in different degrees. It may explain why serum resistin levels in the AMI were significantly elevated when compared to UAP and SAP groups.
In conclusion, we found that serum resistin levels increased with the progress of pathogenic conditions and the severity of myocardial impairment, and the serum resistin level was positively correlated with CKmax, CK-MBmax, cTnImax, WBC and hsCRP. These observations suggest that resistin may participate in the process of atherosclerosis and AMI as an effector molecule of inflammatory reaction. Further studies are required in order to probe the molecule mechanism of resistin in cardiovascular diseases.
Footnotes
Project (No. 2003C33031) supported by the Science and Technology Department of Zhejiang Province, China
References
- 1.Agwunobi A, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85(10):3770–3778. doi: 10.1210/jc.85.10.3770. [DOI] [PubMed] [Google Scholar]
- 2.Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182(2):241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Calabro P, Samudio I, Willerson JT. Resistin promotes smooth muscle cell proliferation through activation of extra cellular signal regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110(21):3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 4.Fan HQ, Gu N, Liu F, Pan XQ, Guo M, Chen RH, Guo XR. Prolonged exposure to resistin inhibits glucose uptake in rat skeletal muscles. Acta Pharmacol Sin. 2007;28(3):410–416. doi: 10.1111/j.1745-7254.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43(11):1271–1278. doi: 10.2337/diabetes.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 6.Kaser S, Kaser A, Sandhofer A. Resistin messenger-RNA expression is increased by proinflammatory cytcokines in vitro. Biochem Biophys Res Commun. 2003;309(2):286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314(2):415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 8.Kunnari A, Ukkola O, Paivansalo M, Kesaniemi YA. High plasma resistin level is associated with enhanced highly sensitive C-reactive protein and leukocytes. J Clin Endocrinal Metab. 2006;91(7):2755–2760. doi: 10.1210/jc.2005-2115. [DOI] [PubMed] [Google Scholar]
- 9.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. Plos Med. 2004;1(2):e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubos E, Messow C, Schnabel R, Rupprecht H, Espinola-Klein C, Bickel C, Peetz D, Post F, Lackner K, Tiret L. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Athero-sclerosis. 2007;193(1):121–128. doi: 10.1016/j.atherosclerosis.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144(6):2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 12.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37(5):1344–1350. doi: 10.1016/S0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocytederived bioactive substances. Ann N Y Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohmori R, Momiyama Y, Kato R, Taniguchi H, Ogura M, Ayaori M, Nakamura H, Ohsuzu F. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46(2):379–380. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300(2):472–476. doi: 10.1016/S0006-291X(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 16.Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13(10):1764–1771. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 17.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111(2):225–230. doi: 10.1172/JCI200316521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111(7):932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 19.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 20.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98(2):502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25(4):1569–1575. doi: 10.1128/MCB.25.4.1569-1575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105(5):546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108(6):736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 24.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, Gong DW. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310(3):927–935. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Li Y, Xia T, Feng S, Chen X, Yang Z. Resistin overexpression impaired glucose tolerance in hepatocytes. Eur Cytokine Netw. 2006;17(3):189–195. [PubMed] [Google Scholar]