Abstract
Elephant limbs display unique morphological features which are related mainly to supporting the enormous body weight of the animal. In elephants, the knee joint plays important roles in weight bearing and locomotion, but anatomical data are sparse and lacking in functional analyses. In addition, the knee joint is affected frequently by arthrosis. Here we examined structures of the knee joint by means of standard anatomical techniques in eight African (Loxodonta africana) and three Asian elephants (Elephas maximus). Furthermore, we performed radiography in five African and two Asian elephants and magnetic resonance imaging (MRI) in one African elephant. Macerated bones of 11 individuals (four African, seven Asian elephants) were measured with a pair of callipers to give standardized measurements of the articular parts. In one Asian and three African elephants, kinematic and functional analyses were carried out using a digitizer and according to the helical axis concept. Some peculiarities of healthy and arthrotic knee joints of elephants were compared with human knees. In contrast to those of other quadruped mammals, the knee joint of elephants displays an extended resting position. The femorotibial joint of elephants shows a high grade of congruency and the menisci are extremely narrow and thin. The four-bar mechanism of the cruciate ligaments exists also in the elephant. The main motion of the knee joint is extension–flexion with a range of motion of 142°. In elephants, arthrotic alterations of the knee joint can lead to injury or loss of the cranial (anterior) cruciate ligament.
Keywords: Elephas maximus, Loxodonta africana, cruciate ligaments, gonarthrosis, Helical axis, kinematic analysis, stifle joint
Introduction
The knee joint of elephants, the largest land animal, plays a decisive role in transmitting thrust to the trunk (Schwerda, 2003). Although data on elephant osteology (Eales, 1929; Mariappa, 1955; Smuts & Bezuidenhout, 1994), myology (Weissengruber & Forstenpointner, 2004) as well as on limb posture and movements (Hutchinson et al. 2003; Schwerda, 2003) are available, anatomical and functional knowledge of hard and soft tissues forming the knee joint is sparse.
In standing elephants, the angle between femur and tibia, which is close to 180°, differs to the half-bent posture in most mammals. A similar ‘extended’ knee posture occurs only in the bipedal humans. In addition, the kinematic patterns of the graviportal hindlimb in elephants are more similar to those in humans than to those in cursorial quadrupeds (Schwerda, 2003). In a previous study (Sonnenschein, 1951), it was presumed that this posture of the knee corresponds to plantigrady (or even semi-plantigrady in elephants). Unlike those in in most other mammals (including humans), the articular surfaces of both tibial condyles are clearly concave in elephants (Smuts & Bezuidenhout, 1994). Beside other peculiarities in proboscidean limb design (e.g. prolongation of the femur instead of metatarsals or the occurrence of a sole cushion), the remarkable structure of the elephant knee joint seems to stand in close relationship with particular weight-bearing and locomotion patterns (Schwerda, 2003).
In elephants of greater age, the knee joint is affected frequently by osteoarthritis, degenerative joint disease or arthrosis (Ruthe, 1961; Salzert, 1972; Hittmair & Vielgrader, 2000; Forstenpointner et al. 2001; Hittmair et al. 2001). Owing to the sparse anatomical knowledge regarding this joint, a clear diagnosis of knee joint disorders in living elephants is not currently feasible (Hittmair & Vielgrader, 2000; Hittmair et al. 2001).
The aims of this study were to give a detailed anatomical overview of all structures forming the elephant knee joint, to analyse the morphology and function of the cruciate ligaments, to assess the kinematics of the knee by means of the helical axis concept, to determine how gonarthrosis affects the cruciate ligaments in elephants, and to compare the morphological and biomechanical properties of the elephant knee joint with the human knee.
Materials and methods
Specimens and preparation
The structures of the knee joint were examined in ten Asian elephants (Elephas maximus) and 12 African elephants (Loxodonta africana) either in fresh specimens, after formalin fixation or in macerated skeletons. The ages of the examined animals range from less than 1 year to more than 40 years, but in some cases, because of the origin of the specimens, data on age remained unclear or inaccurate. Therefore, we designated individuals with fused epiphyses as ‘adults’ and those with partly fused epiphyses as ‘subadults’. For the present study, African [four juvenile, eight (sub)adult] and Asian [all (sub)adult] elephants of both sexes were examined using gross anatomical methods [four juvenile and four (sub)adult African elephants, three (sub)adult Asian elephants], radiography [three juvenile and two (sub)adult African elephants, two (sub)adult Asian elephants] and magnetic resonance imaging (MRI; one adult African elephant). The macerated bones of 11 individuals from the Museum of Natural History Vienna and the University of Veterinary Medicine Vienna were measured with a pair of callipers to give the breadth of the articular surfaces and of the Fossa intercondylaris femoris (for data on species see Table 1). The specimens of the present study come from the Kruger National Park, South Africa, the Department of Anatomy and Physiology, Faculty of Veterinary Science, University of Pretoria, South Africa, the Museum of Natural History Vienna, Austria, the Vienna Zoo Schoenbrunn, Austria, the Safaripark Gaenserndorf, Austria, a private Austrian circus, and the Institute of Anatomy of the University of Veterinary Medicine Vienna, Austria. The animals were either shot during the regular elephant culling programme (South Africa) or euthanized by veterinarians due to various medical reasons (Austria).
Table 1.
Measurements of femur and tibia (macerated bones) in Elephas maximus (Em) and Loxodonta africana (La) (in mm)
| F | T | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Individual | Sex | Side | Epiphyses (F: distal, T: proximal) | Bd | Bm | Bl | Bi | Bp | Bm | Bl |
| Em 1 | Female | right | fused | 158 | 79 | 71 | 10.5 | |||
| left | fused | 160 | 86 | 81 | ||||||
| Em 2 | Male | right | partly fused | 161 | 153 | 86 | 71 | |||
| left | partly fused | 166 | 84 | 73 | 9.5 | 160 | 94 | 78 | ||
| Em 3 | Male | right | partly fused | 188 | 92 | 87 | 12.5 | 183 | 100 | 95 |
| Em 4 | ? | right | F: fused, T: partly fused | 172 | 93 | 75 | 9 | 171 | 97 | 84 |
| left | F: fused, T: partly fused | 179 | 95 | 78 | 7.5 | 171 | 97 | 82 | ||
| Em 5 | Male | right | not fused | 158.5 | 77 | 78 | 11.5 | 154 | 84 | 82 |
| left | not fused | 157 | 75.5 | 75.5 | 13 | 152 | 81 | 83 | ||
| Em 6 | Female | right | not fused | 147.5 | 66.5 | 62.5 | 19.5 | 148 | 80 | 79 |
| Em 7 | ? | right | not fused | 132 | 57 | 58 | 19 | 124 | 66 | 65 |
| left | not fused | 133 | 56 | 56 | 21 | 125 | 64 | 65 | ||
| La 1 (arthrotic joints) | Female | right | fused | 163 | 82 | 75 | 5 | 154 | 88 | 76 |
| left | fused | 165 | 85 | 79 | 0 | 159 | 85 | 82 | ||
| La 2 | Female | right | fused | 160 | 91 | 64 | 9.5 | 157 | 90 | 73 |
| left | fused | 159 | 90 | 61 | 12.5 | 154 | 90 | 74 | ||
| La 3 | Male | right | not fused | 221 | 116 | 100 | 10.5 | 211 | 122 | 105 |
| left | not fused | 221 | 120 | 97 | 12.5 | 210 | 114 | 109 | ||
| La 4 | Male | right | not fused | 62 | 22 | 140 | 85 | 74 | ||
F, femur; T, tibia. Femur: Bd, distal breadth of femur according to Von den Driesch (1976) (from the medial to the lateral edge of the articular surfaces of the condyli; the measuring points lie close to the area of insertion of the medial collateral ligament or to the area of origin of the M. popliteus, respectively); Bm, breadth of the medial condyle of the femur (all femoral measurements are co-planar with Bd); Bl, breadth of the lateral condyle; Bi, breadth of the Fossa intercondylaris between the medial edge of the articular surface of the lateral condyle and the lateral edge of the articular surface of the medial condyle. Tibia: Bp, proximal breadth of the tibia according to Von den Driesch (1976); Bm, breadth of the medial condyle; Bl, breadth of the lateral condyle (measurements of the separate tibial condyles exclude the breadth of the Eminentia intercondylaris but are co-planar with Bp). No measurements indicated = bone is damaged or missing.
During the dissections of arthrotic Elephas (one individual, one specimen) and Loxodonta (two individuals, four specimens) knee joints it was noticed that the anterior cruciate ligament (ACL) was missing. The knee structures and shapes of arthrotic knees were compared with non-arthrotic specimens used for the morphological and kinematic studies in order to understand the reason for the missing ACL. MR images of the left stifle joint specimen in one African elephant was acquired with a 0.23-T unit (Picker/Marconi) and surface body coil. Slices were generated with proton-density-weighted sequences (TR, 1600 ms; TE, 22 ms; 8-mm slice thickness) (Fig. 6g, dorsal oblique plane), T1-weighted spin-echo pulse sequences (TR, 380 ms; TE 18 ms; 5-mm slice thickness), and T2-weighted sequences (TR, 1600 ms, TE 100 ms; 8-mm slice thickness).
Fig. 6.
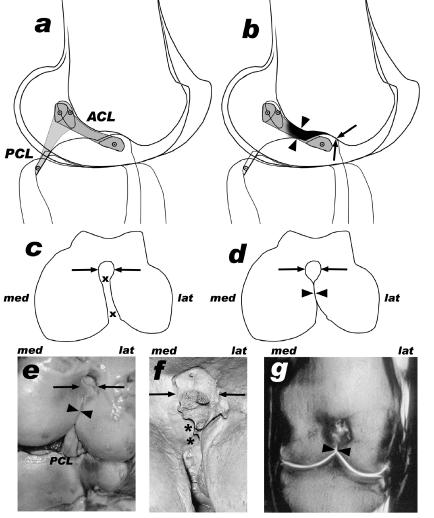
Intact (a,c) and arthrotic (b,d–g) knee joints, side view (a,b), distal view of femur (c–f) and dorsal oblique plane (MRI scan, g). In the specimens (both Loxodonta africana) of subfigures (e) and (g) the ACL was missing. Arrows: femoral notch cranial to the Fossa intercondylaris (the corresponding cranial ridge of the tibial intercondylar eminence is in contact with this notch), arrowheads: narrowing of the intercondylar fossa (med = medial, lat = lateral), x–x: passage for the ACL, *: osteophytic ridge.
Kinematic analysis
The kinematic analysis was carried out according to the helical axis concept (Fuss, 1993a, 1994, 2001, 2002; Fuss et al. 1997). In spatial kinematics, a rigid body rotates about and translates along an instantaneous helical axis. During motion, the helical axis moves along a regular surface, the so-called helical axis surface. The helical axes were generated for the mobile tibia with respect to the fixed femur and vice versa in four knee joints, one of Elephas maximus (fresh) and three of Loxodonta africana (embalmed). The unloaded knee joint was moved manually through extension and flexion. The relative bone positions during extension and flexion of the knees were tracked with a three-dimensional (3D) electromagnetic digitizer (Polhemus 3Space®, Isotrak® M100, McDonnell Douglas, Colchester, VT, USA), connected to an Apple workstation (Macintosh IIci). The sensor and the magnetic source were fixed to femur and tibia, respectively. The data provided by the digitizer were three point coordinates and three Euler angles. The software Tarsósβ 2.0 (Fuss, 1994, 2001) recorded the data and, upon calculation, displayed the helical axes. The axis data were imported into ACAD14 (Autodesk, San Rafael, CA, USA) via script-file for imaging purposes. Each motion was recorded three times for repeatability check. The coordinate system of the moving tibia is: horizontal, z-axis – leftward, tibial shaft axis (y-axis) – in proximal direction, x-axis – backward (towards the caudal side of the tibia). The positive motions (positive angular velocity vectors) consequently are: flexion, internal rotation and abduction. For the motion analysis, the helical axes were recorded with respect to the tibial coordinate system. The rotations (angular displacements, θz, θy, θx) about the three main axes were calculated according to Fuss (2001).
The velocity data of all tibial motions with respect to the helical angle were fitted by three different eighth-order polynomial functions. The three resulting polynomial functions delivered the individual mean velocities. The main motion is extension–flexion. Any other motions (external/internal rotation, ab/adduction) are compulsory motions and not additional, separate degrees of freedom. Such compulsory motions are typical for the knee joint, i.e. the so-called screw-home motion (automatic rotation).
The function of the cruciate ligaments was analysed according to the method described in previous publications (Fuss, 1989, 1991a,b, 1993b). The relative positions of the tibia with respect to the femur were recorded by a 3D electromagnetic digitizer (Polhemus 3Space®, Isotrak® M100, McDonnell Douglas), connected to an Apple workstation (Macintosh IIci), and the software Hyperspace™ Modeler 5.0 (Mira® Imaging Inc., Salt Lake City, UT, USA). This was performed in ten positions, whereby three points on both femur and tibia served as reference points for defining the positions. After the kinematic analysis, the knees were dissected and the fibre arrangement of the cruciate ligaments was determined, i.e. which points in the femoral and tibial footprints (attachment areas) of the cruciate ligaments are connected by fibres. After dissection, the three reference points on both femur and tibia were digitized again, together with the contours and several points in the footprints of the cruciate ligaments. Based on the reference points, the footprints were connected in HyperSpace Modeller 5.0 to the ten positions recorded previously. The distances between corresponding points (connected by fibres) were measured in HyperSpace Modeller 5.0. The behaviour of the distance between two corresponding attachment points defines the function of a ligament fibre (Fuss, 1989). The borders between functionally different areas within the footprints were determined according to Fuss (1991a).
Anatomical nomenclature
Anatomical names except the abbreviations for the cruciate ligaments and where indicated follow NAV (1994). The anterior (ACL) and posterior cruciate ligament (PCL) were named in accordance with the Terminologia Anatomica for humans, but correspond to the Ligamentum (Lig.) cruciatum craniale (= ACL) or caudale (= PCL), respectively, in quadrupeds.
Results
Morphology
Measurements of the articular surfaces and the Fossa intercondylaris are given in Table 1. Although insignificant differences between the species in the external morphology of the femur and the tibia were noticed (but not studied in detail here), soft tissues and articular surfaces revealed great similarity.
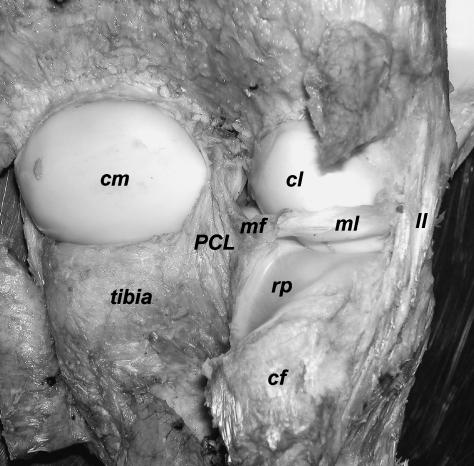
The knee joint consists of the Articulatio (Art.) femorotibialis and the Art. femoropatellaris. It is covered laterally by the Fascia lata and the distal end of the Musculus (M.) biceps femoris. Medially, a thick aponeurotic/fascial layer and the Mm. gracilis, semimembranosus and semitendinosus cover the knee. The heads of the M. gastrocnemius and the M. flexor digitorum superficialis are situated caudally of the knee joint. The tendon of origin of the M. popliteus arises laterally on the Condylus lateralis femoris and courses mediodistally over caudal parts of the lateral meniscus. Passing the caudal aspect of the lateral condyle of the tibia and the Caput fibulae, a synovial recess of the femorotibial joint lies below the M. popliteus (Fig. 1). The Mm. vastus medialis, intermedius and lateralis insert on the patella, whereas the distal tendon of the M. rectus femoris runs cranially of the other parts of the M. quadriceps femoris and inserts via a separate distal tendon on the Tuberositas tibiae. A Lig. patellae is present, whereas distinct Retinacula patellae are not discernible. The Lig. collaterale mediale and the smaller laterale are embedded within the thick fascial layers surrounding the knee. The Lig. collaterale laterale inserts on the Caput fibulae and the thicker mediale on the Condylus medialis tibiae.
Fig. 1.
Caudal aspect of the right knee joint in Loxodonta africana. Muscles, fasciae, joint capsule removed. cm: medial condyle of femur, cl: lateral condyle of femur, PCL: Lig. cruciatum caudale, mf: Lig. meniscofemorale, ml: Meniscus lateralis, ll: Lig. collaterale laterale, rp: synovial recess of the Art. femorotibialis for the M. popliteus, cf: Caput fibulae.
The lateral condyle of the femur is more slender than the medial condyle but projects further distally. The articular surfaces of the femoral condyles are continuous with the articular surface of the Trochlea femoris (Fig. 2). Each condyle of the femur and the proximal part of the tibia have an epiphyseal ossification centre (Fig. 3). The mineralized condyles of the femur first fuse with each other and later in life with the diaphysis. Owing to the marked concavity of the tibial condyles and the small menisci (see below), the articular surfaces of the tibia and the femur show a high degree of congruency (see also Fig. 6g).
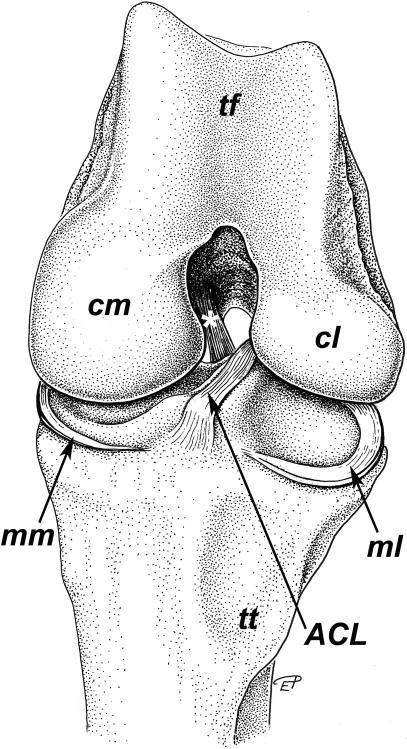
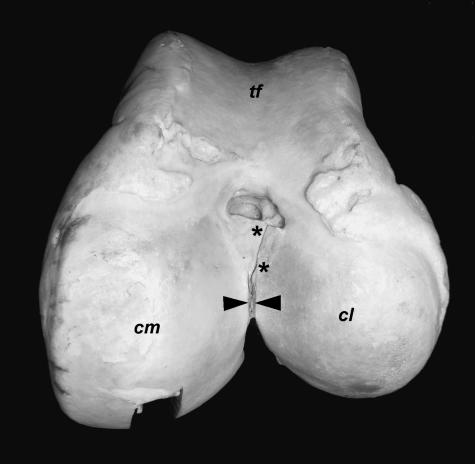
Fig. 2.
Cranial aspect of the left knee joint of Loxodonta africana. Joint in maximal passive flexion, surrounding structures and patella removed. tf: Trochlea femoris, cm: medial condyle of femur, cl: lateral condyle of femur, *: Lig. cruciatum caudale, ACL: Lig. cruciatum craniale, mm: Meniscus medialis, ml: Meniscus lateralis, tt: Tuberositas tibiae.
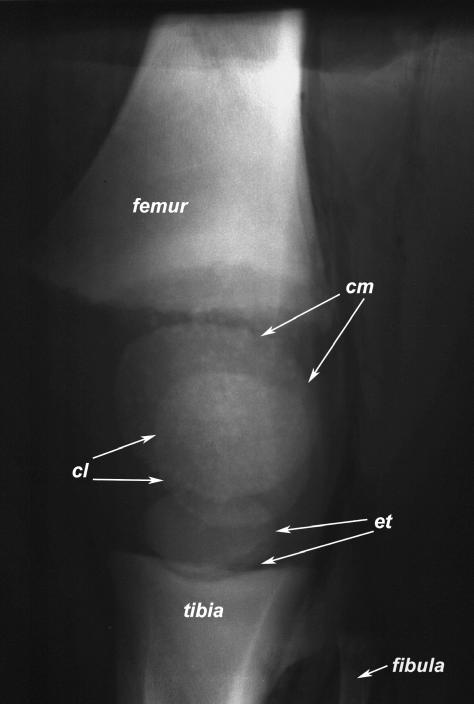
Fig. 3.
Lateromedial radiograph of a juvenile African elephant (< 1 year old). The patella is not mineralized at this age. cl: lateral mineralized epiphysis of femur (Condylus lateralis), cm: medial mineralized epiphysis of femur (Condylus medialis), et: mineralized proximal epiphysis of tibia.
The joint capsule of the knee joint encloses both the Art. femorotibialis and the Art. femoropatellaris. The capsule is tight with the exception of a recess proximal of the femoral trochlea and attaches also to the outer edges of the menisci. The Corpus adiposum infrapatellare is large and a Plica synovialis infrapatellaris and Plicae alares (Terminologia Anatomica; both terms not cited in the Nomina Anatomica Veterinaria) are present (Fig. 4).
Fig. 4.
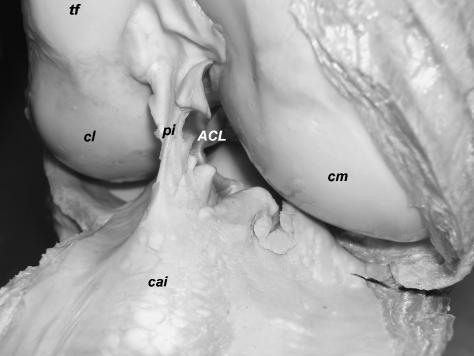
Craniomedial aspect of the right knee joint in Loxodonta africana. Joint capsule open-end, patella reflected. tf: Trochlea femoris, cl: lateral condyle of femur, pi: Plica synovialis infrapatellaris, ACL: Lig. cruciatum craniale, cm: medial condyle of femur, cai: Corpus adiposum infrapatellare.
The Meniscus lateralis and especially the Meniscus medialis are very narrow (lateral meniscus: breadth up to approximately 18 mm; medial meniscus: c. 9 mm) and on their medial or lateral edges, respectively, connected with the joint capsule. Both menisci are C-shaped and triangular in cross-section. The menisci are thicker on the outer border close to the joint capsule (lateral meniscus: c. 8 mm, medial meniscus: c. 5 mm) and thin out towards the longitudinal limb axis (Fig. 2). Especially the caudal part of the lateral meniscus resembles rather a fold of the joint capsule or a thin disc than a typical meniscus. Caudolaterally, the lateral meniscus forms a shallow groove for the M. popliteus (Fig. 1). Both menisci are attached cranially to the tibia (Fig. 2). The tibial attachment of the medial meniscus lies close to that of the cranial cruciate ligament (ACL). A Lig. meniscofemorale connecting the caudal part of the lateral meniscus with the inner surface of the medial femoral condyle is present (Fig. 1). The Lig. meniscofemorale fuses partly with the caudal cruciate ligament (PCL) and the joint capsule and resembles a ligamentous prolongation of the lateral meniscus towards the medial femoral condyle. Caudomedially, the medial meniscus merges with the joint capsule.
The Ligamentum cruciatum craniale (ACL) is flattened, its cross-section elliptical or approximately triangular and it courses sagittally between the vault of the Fossa intercondylaris femoris and the proximal surface of the flattened Eminentia intercondylaris tibiae (Figs 2 and 4). The ACL attaches caudally to the medial surface of the Condylus lateralis femoris and inserts on an impression at the craniolateral surface (corresponding to the Tuberculum intercondylare laterale) of the Eminentia intercondylaris. A fold of the synovial membrane attaches proximally to the ACL. The Ligamentum cruciatum caudale (PCL) courses from the caudal end of the lateral surface of the Condylus medialis femoris to the Area intercondylaris caudalis (Figs 1 and 2). Caudally, the PCL fuses with the joint capsule. The tendon of origin of the M. popliteus passes caudally over the Meniscus lateralis and a recess of the joint capsule lies between this muscle and the Condylus lateralis tibiae or the Caput fibulae, respectively (Fig. 1).
Pathological anatomy of arthrotic joints
Osteophytes or osteochondrophytes can appear in several positions such as at the borders of the articular surfaces (Fig. 5). The distal aspect of the femur shows a notch, which serves the purpose of receiving the cranial ridge of the tibial intercondylar eminence in extension (arrows in Fig. 6). This notch is continuous with the caudal intercondylar fossa. The cranial part of the intercondylar fossa near the transition region to the notch (arrowheads in Figs 5 and 6) can be narrow even in healthy joints but allows the passage of the ACL. In arthrotic elephant knees, the articular surfaces of the medial and lateral femoral condyles expand towards each other, therefore narrowing the intercondylar fossa continuously (see Table 1, individual La 1). This process compresses the ACL, which finally becomes worn out, once the passage is completely used up and closed. Additional osteophytic activity in the notch can injure the ACL.
Fig. 5.
Distal aspect of a left femur with arthrotic alterations after maceration, Loxodonta africana. Many osteophytes are visible in different positions. tf: Trochlea femoris, cm: medial condyle, cl: lateral condyle, *: osteophytic activity in the notch cranial to the Fossa intercondylaris, arrowheads: extreme narrowing of the Fossa intercondylaris.
In healthy joints the proximal edge of the intercondylar eminence is proximally flattened but it is sharp in arthrotic joints (Fig. 6e,g; below the arrowheads). Additionally, in healthy joints the intercondylar eminence is more flattened in younger or juvenile individuals than in older animals.
Kinematic analysis
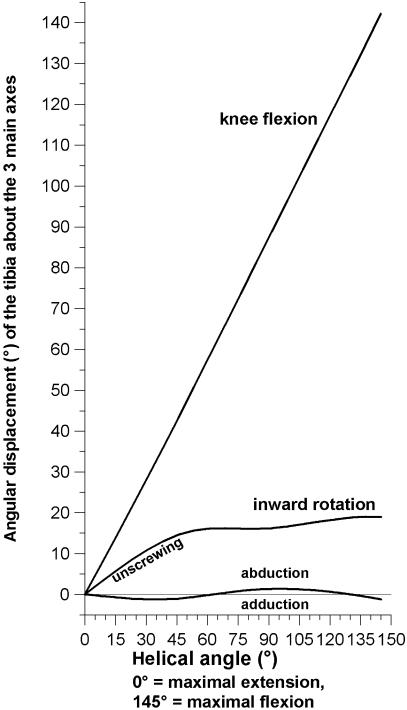
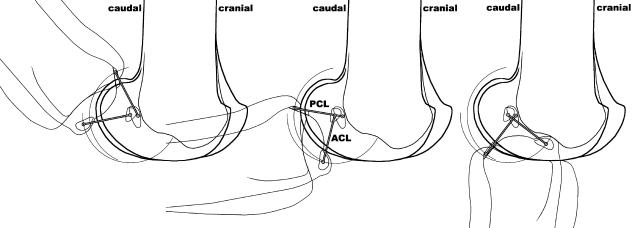
The helical axes surfaces are shown in Fig. 7 from different aspects. Between mid- and maximal flexion, the extension–flexion axis is almost horizontal, which indicates that the motion is a pure extension–flexion without any significant additional automatic motions (rotations, ab/adduction). Between a knee flexion angle of 60° and 0° (maximal extension), the helical axis becomes more and more inclined. Its direction is from medial–proximal to lateral–distal. This inclination indicates an automatic, compulsory rotation, namely a combination of extension and outward rotation of the tibia, or flexion and inward rotation, which corresponds to the so-called screw-home motion of the knee. The mean automatic rotation is 16° between a knee flexion angle of 0° and 60°, and 14° between 0° and 42° (Fig. 8).
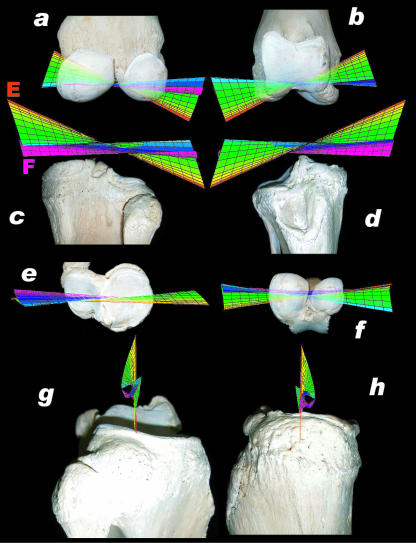
Fig. 7.
Helical axis surfaces projected on right femur and right tibia from different aspects (med = medial, lat = lateral, cran = cranial, caud = caudal). (a) Femur (caudal view), (b) femur (cranial view), (c) tibia (caudal view), (d) tibia (cranial view), (e) tibia (proximal view), (f) femur (distal view), (g) tibia, lateral view, (h) tibia (medial view). E = helical axis in maximal extension (red), F = helical axis in maximal flexion (purple); the colour code applies to all helical axes surfaces.
Fig. 8.
Angles of the three main motions vs. helical angle (unscrewing = reverse of screw-home motion); flexion, internal rotation and abduction have positive values.
Functional analysis of the cruciate ligaments
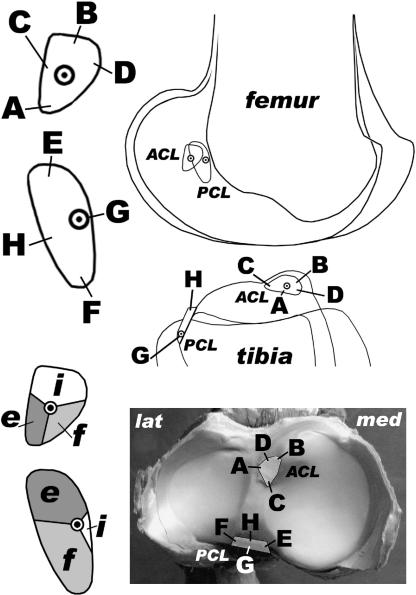
The fibre arrangement of the cruciate ligaments is shown in Fig. 9. The cruciate ligament fibres are slightly twisted about the ligament axis. Each cruciate ligament contains a guiding or isometric fibre bundle (Fig. 9), as well as flexion- and extension-constraining fibres and fibres taut in intermediate positions (Fig. 9). The isometric fibres constitute the side links of a four-bar linkage (Fig. 10), whereby femur and tibia serve as mutual coupler and ground link.
Fig. 9.
Position of the ligament attachments, fibre arrangement and functional subunits. ACL: cranial cruciate ligament, PCL: caudal cruciate ligament, A–D and E–H: four representative fibres of ACL and PCL, respectively. The origin of the isometric fibres is indicated within the footprints. e: fibres taut in maximal extension, f: fibres taut in maximal flexion, i: fibres taut in intermediate positions. Femoral attachments and femur in side view, tibia in side and proximal view (lat = lateral, med = medial).
Fig. 10.
Femur, tibia and the four-bar linkage (isometric bundles) in maximal and mid flexion and maximal extension.
Discussion
Although the locomotory system of humans and elephant are different, heavy and semi-plantigrade elephants have more column-like limbs compared with even-toed (including hippopotamuses) and odd-toed animals (including rhinoceroses), as the latter show a higher degree of flexion in the knee joint. Therefore, elephants and plantigrade humans are more comparable owing to the extended knee and evident similarities in muscular architecture (Weissengruber & Forstenpointner, 2004) as well as locomotion patterns (Schwerda, 2003).
Kinematics
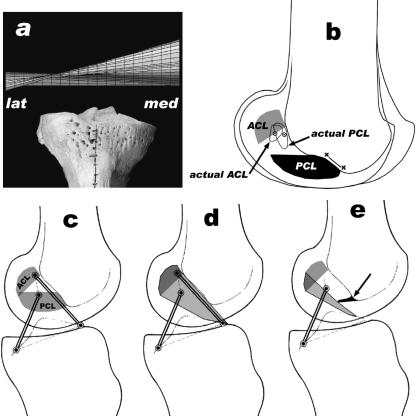
The kinematics of the elephant knee joint matches the human knee (Fig. 11a; Fuss, 1993a, 2001, 2002; Fuss et al. 1997). Any deviation of the helical axis from the horizontal flexion–extension axis indicates a compulsory rotation. This is true for the helical axis in extension, which runs from proximal–medial to distal–lateral, and represents the screw-home motion. The only difference between the elephant and human knee is that the human screw-home motion is smaller, i.e. 10° instead of 16° during flexion from 60° until maximal extension (Fuss, 2001, 2002). The cause of the screw-home motion is the oblique path of the PCL isometric bundle, which is attached to the inner side of the medial femoral condyle (Fuss, 1992). In higher mammals, the most anterior (cranial) PCL fibres (Fig. 11b, line x–x) usually originate from the roof of the intercondylar fossa, and hence are purely aligned in the sagittal plane. These fibres, however, are missing in the elephant knee. Thus, all PCL fibres are oblique, which explains the greater extent of screw-home rotation.
Fig. 11.
Comparison of human and elephant knee. (a) Human helical axis surface [tibia, front view, lat = lateral, med = medial; from Fuss et al. (1997) and Fuss (2001)]. (b) Actual and expected femoral footprints (cf.; Figs 9 and 10), the line x–x indicates PCL fibres which originate from the roof of the intercondylar fossa. (c,d) Human cruciate footprints, four-bar linkage, and shape of the ACL (modified from Fuss, 1989). (e) Arthrotic ridge (arrow) and reduction of the ACL.
Elephants mainly move at slow speeds but they can walk long distances and attain speeds greater than 6.0 m s−1 (Hutchinson et al. 2003; Schwerda, 2003). In slow-moving elephants, abduction of the femur and adduction of the tibia play a distinct role, whereas at the top-speed gait, which is not a clear ‘run’ following kinematic definitions, the main knee movement is flexion (Hutchinson et al. 2003; Schwerda, 2003). Following the study by Schwerda (2003) elephants seem to be rather ‘sprinters’ than other cursorial mammals, which are commonly but unlike elephants characterized by a narrow trunk, a long neck and elongated distal limb parts (Preuschoft et al. 1994).
Morphology
Bone measurements such as the breadth of femur and tibia (see Table 1) reveal a high degree of interindividual variation, which is related to body size (Christiansen, 2004) and probably to the age of the animal.
The fibre arrangement and twisting of the elephant cruciates is comparable with that of human cruciate ligaments. Equally, the four-bar mechanism, well known in the human knee (Strasser, 1917; Barnett et al. 1961; Kapandji, 1967; Huson, 1974; Menschik, 1974; Fuss, 1989, 1991a; O'Connor et al. 1989), exists in the elephant knee. In contrast to the human knee, the elephant ACL has more fibres taut in intermediate positions and in maximal flexion, and the PCL more fibres taut in maximal extension and fewer fibres taut in intermediate positions than the human ACL and PCL. The most striking difference, however, is the position of the femoral PCL footprint. In most species of higher mammals [Pteropus (Fuss, 1993b), Sus (Fuss, 1991b), Ruminantia, Camelus, Equus, Phoca, Carnivora and Papio (Fuss, unpublished data)] and in humans (Fuss, 1989; Fig. 11c), the PCL footprint is below (craniodistal) the ACL footprint. Figure 11(b) shows the actual and expected position of the femoral footprints of the elephant knee joint. The PCL footprint seems to have moved proximo-caudally and is almost superimposed with the ACL footprint. With regard to the cruciate ligaments, the knee of Hippopotamus amphibius exhibits the same morphology (Fuss, unpublished data).
Besides the position of the femoral PCL footprint, particular features of the elephant knee joint are the very small menisci, the markedly concave articular surfaces of the tibia and, subsequently, the high degree of congruency of the femoral and tibial articular surfaces.
In mammals, only certain bats (Parsons, 1900; Nauck, 1938; Sonnenschein, 1951; Fuss, 1993b) lack menisci. These animals do not use their hindlimbs primarily as locomotor organs, whereas the hindlimbs of elephants are important for supporting body weight and for locomotion (Schwerda, 2003). In humans (Boyd & Myers, 2003) and other mammals (e.g. Reinsfeld, 1932), the menisci have important biomechanical functions such as load transmission and acting as stability restraints. Because in elephants only small and narrow menisci are present and the corresponding articular surfaces of femur and tibia, especially at the periphery of the condyles, show a higher degree of congruency than in other mammals or humans, it is improbable that in elephants the menisci have a distinct functional value. Parsons (1900), however, established a causal connection between the inability of rotation and the absence of menisci. In comparison with other mammals or humans, in elephants larger sections of the femoral and tibial articular surfaces are in contact with each other and therefore the pressure is distributed directly to a greater area of articular cartilage. By contrast, in humans and other mammals the menisci are mobile structures, allowing maintenance of congruency throughout the range of flexion, and in humans up to 85% of the body weight is transmitted (Reinsfeld, 1932; Boyd & Myers, 2003). Herzmark (1938) assumed that the menisci are well developed in the human knee, because it bears weight in the fully extended position. In our opinion, the special locomotive feature of carrying weight in the fully extended knee posture is well supported by particular elements of the elephant knee joint morphology such as highly congruent articular surfaces and only small menisci which are less vulnerable to traumatic forces.
Considering the sparse and often incomplete palaeontological data (e.g. Court, 1994, 1995), it is not clear whether the early members of the African order Proboscidea, whose origin dates back at least to the Early Eocene (Gheerbrant et al. 2002), had a similar knee joint. Nevertheless, previous studies have revealed that the limb anatomy of virtually all proboscideans except Moeritherium and Numidotherium appears very similar (Christiansen, 2004) and that the gait in Numidotherium contrasts markedly with that of elephantiform proboscideans (Court, 1994). Thus, the peculiarities of extant elephants might have been adopted later and/or in sister groups of the mentioned taxa. Although not examined in detail, it is evident from the literature (e.g. Aouadi, 2001; Fladerer, 2001; Koenigswald, 2002) that the morphology of the femur and tibia of the Mammuthus group is very similar to that of extant elephant species and therefore a similar structure of the soft tissues can be assumed. Given that some early proboscideans such as Moeritherium and the extinct Desmostylidae were semi-aquatic and hippo-like (Domning et al. 1986; Beatty, 2004), that the Proboscidea and the aquatic Sirenia share a common ancestry (Court, 1995; Murata et al. 2003) and that extant elephants may have had aquatic ancestors (Gaeth et al. 1999; West, 2002), specific peculiarities of knee joint morphology in present-day terrestrial elephants might be traced to structural adaptations to a (semi)aquatic environment.
Pathological alterations
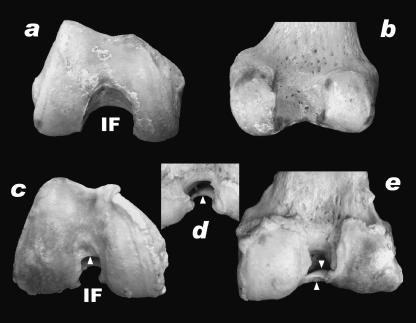
Gonarthrosis can result in loss of the ACL. This is due to the development of osteophytes or osteochondrophytes and/or the narrowing of the ACL outlet at the craniodistal end of the intercondylar fossa. There is a striking similarity with the arthrotic human knee (see also Hernigou & Garabedian, 2002). Furthermore, it has been shown in humans that the relatively small size of the intercondylar fossa creates a greater risk of injury of the ACL (Ireland et al. 2001). Figure 11(d) shows the path of the ACL. In human gonarthrosis, an osteophytic ridge (arrow in Fig. 11e, arrowhead in Fig. 12c–e) can develop at the anterodistal end of the intercondylar fossa (Fig. 12), which protrudes backwards (posteriorly). At the tip of the ridge, the ACL is subjected to wear, resulting into loss of the anterior part, which contains the isometric bundle (Fig. 11e). Once the osteophytic ridge has developed, the ACL consists only of the posterior fibres. In these cases, an artificial knee joint replacement justifies sacrificing of the ACL, as the isometric bundle not longer exists. In both the elephant and the human knee osteophytic activity and hence the restricted space in the intercondylar fossa is responsible for injury and the gradual reduction of the ACL. In the arthrotic human knee the ACL is attacked mainly from the anterior, whereas in the elephant knee the attack is from medial and lateral.
Fig. 12.
Intact (a,b) and arthrotic (c–e) right human knees, in distal (a,c), posterior (b,e) and proximodorsal (d) view. IF: intercondylar fossa, white arrowheads: the osteophytic ridge.
Conclusions
It is likely that in elephants and mammoths the distinct structure of the knee joint is closely related to the enormous weight of the animal, the ‘extended’ posture of the knee, the animals' graviportal stance (see Court, 1994) and the peculiarities of locomotion especially at higher speed. The present study gives the first description of the distinct morphological features of the elephant knee joint together with biomechanical analyses and pathological–anatomical discussion, but further work particularly on the structure and mechanical properties of the articular cartilage is necessary.
Acknowledgments
G.E.W. and G.F. thank Prof. H.B. Groenewald, Prof. J.T. Soley and Mr L. de Villiers, Department of Anatomy and Physiology, Faculty of Veterinary Science, University of Pretoria, RSA, for co-operation and hospitality, the Vienna zoo Schönbrunn for providing Loxodonta knees, Dr B. Herzig for access to the specimens of the Museum of Natural History, Vienna, Austria, Mr L. Habeler, Institute of Anatomy, Department of Pathobiology, University of Veterinary Medicine Vienna, Austria, for technical assistance, the staff members of the Diagnostic Imaging Section, Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, RSA, for radiography, Mag. med. vet. M. Konar, Radiology Clinic, University of Veterinary Medicine Vienna, Austria, for technical assistance with MRI, Mag. med. vet. E. Polsterer, Vienna, Austria, for graphical support, and the Gesellschaft der Freunde der Veterinärmedizinischen Universität Wien, Austria, for financial support. F.K.F. thanks Chris Gasser for organizing the Loxodonta knee joints from South Africa, Dr V. De Vos, Manager Scientific Services, National Parks Board, Skukuza, RSA, for providing the Loxodonta knees from Kruger National Park, Mr G.W. Haupt, Nestlé South Africa, Randburg, RSA, for organizing and shipping of the specimens, Dir. A. Ross, Nestlé South Africa, and V. Pres. R. Gasser, Nestlé S.A., Vevey, Switzerland, for the support by Nestlé S.A. in this matter, Dr med. vet. M. Antolini, Vienna, Austria, for providing one Elephas knee, Dir. Dr F. Weiss-Spitzenberger for arranging the dissection of two Elephas knee joints at the Museum of Natural History, Vienna, Austria, and the Austrian Science Foundation (Fonds zur Förderung der wissenschaftlichen Forschung) for financial support (project no. P7914-MED).
References
- Aouadi N. New data on the diversity of elephants (Mammalia, Proboscidea) in the Early and early Middle Pleistocene of France. In: Cavarretta G, Gioia P, Mussi M, Palombo MR, editors. Proceedings of the 1st International Congress ‘The World of Elephants’. Rome: Consiglio Nationals delle Ricerche; 2001. pp. 81–84. [Google Scholar]
- Barnett CH, Davies DV, MacConaill MA. Synovial Joints. London: Longmans; 1961. [Google Scholar]
- Beatty BL. Evidence for suction feeding in the Desmostylidae (Desmostylia, Mammalia) J Morphol. 2004;260:276–277. [Google Scholar]
- Boyd KT, Myers BT. Meniscus preservation; rationale, repair techniques and results. Knee. 2003;10:1–11. doi: 10.1016/s0968-0160(02)00147-3. [DOI] [PubMed] [Google Scholar]
- Christiansen P. Body size in proboscideans, with notes on elephant metabolism. Zool J Linn Soc. 2004;140:523–549. [Google Scholar]
- Court N. Limb posture and gait in Numidotherium koholense, a primitive proboscidean from the Eocene of Algeria. Zool J Linn Soc. 1994;111:297–338. [Google Scholar]
- Court N. A new species of Numidotherium (Mammalia: Proboscidea) from the Eocene of Libya and the early phylogeny of the Proboscidea. J Vert Paleont. 1995;15:650–671. [Google Scholar]
- Domning DP, Ray CE, McKenna MC. Two new Oligocene desmostylians and a discussion of tethyterian systematics. Smithson Contrib Paleobiol. 1986;59:1–56. [Google Scholar]
- Eales NB. Anatomy of a foetal african elephant, Elephas africanus (Loxodonta africana). Part III. The contents of the thorax and abdomen, and the skeleton. Trans Roy Soc Edin. 1929;56:203–246. [Google Scholar]
- Fladerer F. Die Faunenreste vom jungpaläolithischen Lagerplatz Krems-Wachtberg, Ausgrabung 1930. Wien: Österreichische Akademie der Wissenschaften; 2001. [Google Scholar]
- Forstenpointner G, Weissengruber G, Kübber-Heiss A, Hittmair K, Konar M. Morphological features of the stifle joint of the African elephant (Loxodonta africana, Blumenbach 1797) J Morph. 2001;248:230. [Google Scholar]
- Fuss FK. Anatomy of the cruciate ligaments and their function in extension and flexion of the human knee joint. Am J Anat. 1989;184:165–176. doi: 10.1002/aja.1001840208. [DOI] [PubMed] [Google Scholar]
- Fuss FK. The restraining function of the cruciate ligaments on hyperextension and hyperflexion of the human knee joint. Anat Rec. 1991a;230:283–289. doi: 10.1002/ar.1092300217. [DOI] [PubMed] [Google Scholar]
- Fuss FK. Anatomy and function of the cruciate ligaments of the domestic pig (Sus scrofa domestica): a comparison with human cruciates. J Anat. 1991b;178:11–20. [PMC free article] [PubMed] [Google Scholar]
- Fuss FK. Principles and mechanisms of automatic rotation during terminal extension in the human knee joint. J Anat. 1992;180:297–304. [PMC free article] [PubMed] [Google Scholar]
- Fuss FK. Helical axis surface of the knee joint. In: Bouisset S, Métral S, Monod H, editors. Proceedings of the XIVth Congress of the International Society for Biomechanics. Paris: International Society of Biomechanics; 1993a. pp. 438–439. [Google Scholar]
- Fuss FK. The knee joint of the flying fox (Pteropus rufus) Eur J Morph. 1993b;31:129–137. [PubMed] [Google Scholar]
- Fuss FK. A new method of clinical assessment of shoulder kinematics by means of the parameters of helical axes. Eur J Phys Med Rehabil. 1994;4:125–130. [Google Scholar]
- Fuss FK, Hamel G, Duval N, DeGuise J, Yahia LH. Altered knee kinematics after posterior cruciate ligament rupture and replacement by means of a ligament prosthesis. In: Yahia LH, editor. Proceedings of the 1st International Symposium on Advanced Biomaterials (SIBA 1997) Montréal: École Polytechnique; 1997. p. 136. [Google Scholar]
- Fuss FK. Helical axes – kinematic analysis, interpretation, and application. In: Magjarevic R, Tonkovic S, Bilas V, Lackovic I, editors. IFMBE Proceedings (Proceedings of the International Federation for Medical and Biological Engineering) Vol. 1. Zagreb: Croatian Medical and Biological Engineering Society; 2001. pp. 620–623. Part II. [Google Scholar]
- Fuss FK. Proceedings of the 2002 Combined Orthopaedic Meeting (25th Singapore Orthopaedic Association Meeting, 22nd Asean Orthopaedic Association Meeting, 5th Combined Meeting of Spinal and Pediatric Sections APOA, 7th Meeting of Knee and Sports Medicine Section APOA, 3rd Meeting of Asia Pacific Orthopaedic Society for Sports Medicine) Singapore: National University of Singapore; 2002. Kinematics of the knee after isometric single-bundle PCL reconstruction. [Google Scholar]
- Gaeth AP, Short RV, Renfree MB. The developing renal, reproductive, and respiratory systems of the African elephant suggest an aquatic ancestry. Proc Natl Acad Sci USA. 1999;96:5555–5558. doi: 10.1073/pnas.96.10.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheerbrant E, Sudre J, Cappetta H, Iarochène M, Amaghzaz M, Bouya B. A new large mammal from the Ypresian of Morocco: evidence of surprising diversity of early proboscideans. Acta Palaeontol Pol. 2002;47:493–506. [Google Scholar]
- Hernigou P, Garabedian JM. Intercondylar notch width and the risk for anterior cruciate ligament rupture in the osteoarthrotic knee: evaluation by plain radiography and CT scan. Knee. 2002;9:313–316. doi: 10.1016/s0968-0160(02)00053-4. [DOI] [PubMed] [Google Scholar]
- Herzmark MH. The evolution of the knee joint. J Bone Joint Surg. 1938;20:77–84. [Google Scholar]
- Hittmair KM, Vielgrader HD. Radiographic diagnosis of lameness in African elephants (Loxodonta africana) Vet Radiol Ultrasound. 2000;41:511–515. doi: 10.1111/j.1740-8261.2000.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hittmair K, Vielgrader H, Konar M, Weissengruber G, Forstenpointner G. Diagnostic imaging of the limbs of African elephants (Loxodonta africana) Vet Radiol Ultrasound. 2001;42:175. [Google Scholar]
- Huson A. Biomechanik des Kniegelenks. Orthopäde. 1974;3:119–126. [Google Scholar]
- Hutchinson JR, Famini D, Lair R, Kram R. Are fast moving elephants really running? Nature. 2003;422:493–494. doi: 10.1038/422493a. [DOI] [PubMed] [Google Scholar]
- Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9:200–205. doi: 10.1007/s001670100197. [DOI] [PubMed] [Google Scholar]
- Kapandji IA. Cited in. In: Kapandji IA, editor. Funktionelle Anatomie der Gelenke. Vol. 2. Stuttgart: Enke; 1967. [Google Scholar]
- Koenigswald Wv. Lebendige EiszeitKlima und Tierwelt Im Wandel. Darmstadt: Wissenschaftliche Buchgesellschaft; 2002. [Google Scholar]
- Mariappa D. The Anatomy of a foetal Indian Elephant. Part III. The bones and joints of the hind-limb. Indian Vet J. 1955;32:322–329. [Google Scholar]
- Menschik A. Mechanik des Kniegelenks, Teil 1. Z Orthop. 1974;112:481–495. [PubMed] [Google Scholar]
- Murata Y, Nikaido M, Sasaki T, et al. Afrotherian phylogeny as inferred from complete mitochondrial genomes. Mol Phylogenet Evol. 2003;28:253–260. doi: 10.1016/s1055-7903(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Nauck ET. Extremtitätenskelett der Tetrapoden. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere, 5. Band. Berlin: Urban & Schwarzenberg; 1938. pp. 71–248. [Google Scholar]
- NAV. Nomina Anatomica Veterinaria. 4. Zürich: World Ass. Vet. Anat; 1994. [Google Scholar]
- O'Connor JJ, Shercliff TL, Biden E, Goodfellow JW. The geometry of the knee in the sagittal plane. Proc Inst Mech Eng [H] 1989;203:223–233. doi: 10.1243/PIME_PROC_1989_203_043_01. [DOI] [PubMed] [Google Scholar]
- Parsons FG. The joints of mammals compared with those of man. Part II. Joints of the hind limb. J Anat. 1900;34:301–323. [PMC free article] [PubMed] [Google Scholar]
- Preuschoft H, Wille H, Christian A, Recknagel S. Körpergestalt und Lokomotion bei großen Säugetieren. Verh Dtsch Zool Ges. 1994;87:147–163. [Google Scholar]
- Reinsfeld R. Die Mechanik des Kniegelenkes vom Rinde. Z Anat Entwicklungsgeschichte. 1932;97:487–508. [Google Scholar]
- Ruthe H. Fußleiden der Elefanten. Wissenschaftliche Z Humboldt-Universität Berlin, Mathematisch-Naturwissenschaftliche Reihe. 1961;10:471–516. [Google Scholar]
- Salzert W. Tierärztliche Hochschule Hannover: 1972. Elefanten. Ihre Pathologie und den Tiergärtner interessierende physiologische Daten. Thesis. [Google Scholar]
- Schwerda D. Analyse Kinematischer Parameter der Lokomotion Von Loxodonta Africana (Proboscidea: Elephantidae) Diplomarbeit: Institut für spezielle Zoologie und Evolutionsbiologie mit Phyletischem Museum der Friedrich-Schiller-Universität Jena; 2003. [Google Scholar]
- Smuts MMS, Bezuidenhout AJ. Osteology of the pelvic limb of the African elephant. Onderstepoort J Vet Res. 1994;61:51–66. [PubMed] [Google Scholar]
- Sonnenschein A. Die Evolution des Kniegelenkes innerhalb der Wirbeltierreihe. Acta Anat. 1951;13:289–328. [PubMed] [Google Scholar]
- Strasser H. Lehrbuch der Muskel und Gelenkmechanik. Vol. 3. Berlin: Strasser; 1917. [Google Scholar]
- Terminologia Anatomica. Stuttgart: Thieme; 1998. [Google Scholar]
- Von den Driesch A. A guide to the measurement of animal bones from archaeological sites. Peabody Mus Bull. 1976;1 [Google Scholar]
- Weissengruber GE, Forstenpointner G. Musculature of the crus and pes of the African elephant (Loxodonta africana): insight into semiplantigrade limb architecture. Anat Embryol. 2004;208:451–461. doi: 10.1007/s00429-004-0406-1. [DOI] [PubMed] [Google Scholar]
- West JB. Why doesn't the elephant have a pleural space? News Physiol Sci. 2002;17:47–50. doi: 10.1152/nips.01374.2001. [DOI] [PubMed] [Google Scholar]