Abstract
Kager's fat pad is a mass of adipose tissue occupying Kager's triangle. By means of a combined magnetic resonance imaging, ultrasound, gross anatomical and histological study, we show that it has three regions that are closely related to the sides of the triangle. Thus, it has parts related to the Achilles and flexor hallucis longus (FHL) tendons and a wedge of fat adjacent to the calcaneus. The calcaneal wedge moves into the bursa during plantarflexion, as a consequence of both an upward displacement of the calcaneus relative to the wedge and a downward displacement of the wedge relative to the calcaneus. During dorsiflexion, the bursal wedge is retracted. The movements are promoted by the tapering shape of the bursal wedge and by its deep synovial infolds. Fibrous connections linking the fat to the Achilles tendon anchor and stabilize it proximally and thus contribute to the motility of its tip. We conclude that the three regions of Kager's fat pad have specialized functions: an FHL part which contributes to moving the bursal wedge during plantarflexion, an Achilles part which protects blood vessels entering this tendon, and a bursal wedge which we suggest minimizes pressure changes in the bursa. All three regions contribute to reducing the risk of tendon kinking and each may be implicated in heel pain syndromes.
Keywords: Achilles tendon, bursa, enthesis, enthesopathy, Haglund's deformity, Kager's triangle, retrocalcaneal bursitis
Introduction
Kager's (pre-Achilles) fat pad is a mass of adipose tissue lying within Kager's triangle that is well known to rheumatologists, radiologists, orthopaedic surgeons and sports medicine specialists alike. It appears in radiographs as a sharply marginated, radiolucent area bordered anteriorly by flexor hallucis longus (FHL), posteriorly by the Achilles tendon and inferiorly by the calcaneus (Ly & Bui-Mansfield, 2004). The ankle joint lies at the anteroinferior corner of the triangle and posterolaterally it is continuous with the retrocalcaneal bursa. Curiously, the triangle itself usually receives more attention than the fat within it, as it is a well-recognized radiological landmark that is commonly used as a reference frame in evaluating problems with the ankle joint and with the Achilles and FHL tendons (Ly & Bui-Mansfield, 2004). This lack of attention to the fat pad itself reflects an insufficient understanding of its functional significance and whether any alteration to its mechanics could bear on injury and pathology posterior to the ankle joint. It has been suggested that Kager's fat pad acts as a variable space filler and conveys a mechanical advantage on the Achilles tendon insertion, by moving into the retrocalcaneal bursa during plantarflexion (Canoso et al. 1988). By entering the bursa in this position, it is thought that the fat increases the lever arm of the tendon (Canoso et al. 1988). In the present paper, we have used a combined anatomical, ultrasound (US), magnetic resonance imaging (MRI) and histological study to explain how the tip of the fat pad moves into the bursa. We suggest that the presence of distinctive regions of fat within Kager's triangle provides a novel basis for understanding rheumatological disorders in the region.
Materials and methods
Macroscopic investigations
MRI study
MRI was performed at the University of California San Diego. The right foot of three normal male volunteers (age 31, 51 and 60 years) was imaged in three positions [maximum dorsiflexion, maximum plantarflexion and the anatomical (neutral) position] whilst the subjects were in the supine position and the images were acquired in three orthogonal planes. All the ankle movements were performed passively by strapping the forefoot to a stand. No volunteer had any history of significant injury to the Achilles tendon or ankle, and all three were asymptomatic. T1-weighted, non-fat-saturated conventional spin echo scans were obtained using a 1.5-T MR scanner (Siemens, Erlangen, Germany). The matrix size was 512 × 384, the field of view 18 cm, repetition time 480 ms, echo time 12 ms and slice thickness was 3 mm with zero gap.
US study
Ultrasound examination of the Achilles tendon region and Kager's fat pad was performed using a Toshiba Aplio scanning machine (Toshiba Medical Systems Europe, Zoetermeer, the Netherlands) at the University Hospital of Wales (Medical Physics and Clinical Engineering Directorate, UHW, Cardiff, UK) by an experienced sonographer (N.P.). One foot from each of four volunteers (three males, one female; age range 24–57 years) was scanned in the three different positions that are described above. Subjects were examined both when prone (non-load-bearing) and when standing (load-bearing). All ankle movements in the standing position were performed actively and under load. However, movements in the prone position were performed both actively without load and passively by an assistant moving the subject's foot. The degree of flexion was measured using a goniometer.
Gross anatomical examination
Six elderly perfusion-fixed dissecting room cadavers (three males, three females, ages 71–101 years) donated to Cardiff University for anatomical examination were used to study the gross anatomy of the fat pad.
Microscopic investigations
The central third of the Achilles tendon enthesis was removed from six formalin-fixed, dissecting room cadavers (three males, three females, ages 63–87 years) and processed for routine histology. The specimens were thus postfixed in 10% neutral buffered formal saline for 1 week, decalcified in 2% nitric acid, dehydrated in graded alcohols and embedded in paraffin wax. Serial sagittal sections were cut at 8 µm throughout each block and 12 sections were mounted on glass slides at 500-µm intervals. The sections were stained with Masson's trichrome, haematoxylin & eosin, or toluidine blue.
Results
Macroscopic studies
Three regions of Kager's fat pad could be discerned by combining the information gleaned from the gross anatomical, MRI and US studies. These are referred to as a large, superficial ‘Achilles-associated part’, a deep ‘FHL-associated part’ and a ‘calcaneal bursal wedge’ or tongue (Figs 1 and 2). The Achilles-associated part of Kager's fat pad lay immediately anterior to the tendon and the FHL-associated part was enclosed within the tendon sheath (Fig. 1b). In the sagittal plane, the FHL part of Kager's pad could be seen extending in an inverted J-shaped (dorsiflexed foot; Fig. 1b) or l-shaped (plantarflexed foot; Fig. 2a) fashion under the Achilles-associated fat, fusing with the latter and thus forming the calcaneal bursal wedge. This distal wedge was free of all connections to the Achilles tendon and extended into the retrocalcaneal bursa in a plantarflexed foot (Fig. 2). This bursal movement occurred during both active and passive plantarflexion, but was more pronounced when the foot was actively plantarflexed under load. Immediately proximal to the bursal wedge, the fat was firmly anchored to the Achilles tendon (Figs 2b and 3a). Small blood vessels passed in a tortuous fashion through the Achilles-associated fat (Figs 3b and 4a) and were identified in dissections as branches of the posterior tibial and peroneal arteries. The former lay superficially and were found in its inferior and medial aspects; the latter were deeper and typical of the superior and lateral regions. MRI in the axial plane revealed that the paratenon extends around the Achilles-associated part of Kager's fat pad, thereby holding the fat next to the tendon (Fig. 4b).
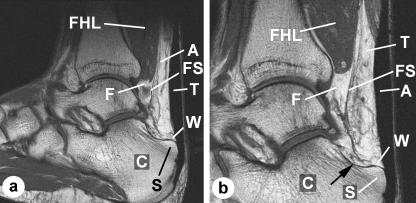
Fig. 1.
Macroscopic anatomy of Kager's fat pad – MRIs. (a) An MRI of a dorsiflexed foot imaged in the sagittal plane to show the three regions of the fat pad: the Achilles-associated part (A) lying immediately anterior to the Achilles tendon (T), the FHL-associated part (F) enclosed within the fascial sheath (FS), which extends from below the muscle belly of flexor hallucis longus (FHL), and the calcaneal bursal wedge (W). C, calcaneus; S, superior tuberosity. (b) A higher magnification view of an MRI adjacent to that featured in (a) showing the three regions of the fat pad. It demonstrates how the FHL-associated fat passes beneath the Achilles-associated fat (arrow) in a J-shaped curve to fuse with it and form the bursal wedge. Note how the FHL-associated fat is enclosed within an extension of the fascia that surrounds FHL and becomes its tendon sheath.
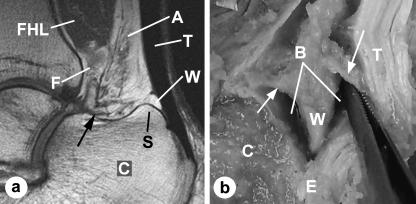
Fig. 2.
Macroscopic anatomy of Kager's fat pad. (a) An MRI of a plantarflexed foot (same subject as in Fig. 1) imaged in the sagittal plane. It shows how the bursal-protruding wedge of fat (W) has moved into the retrocalcaneal bursa, by passing over the superior tuberosity (S) of the calcaneus (C). Note how the tendon and muscle regions of the fat have been moulded into a new shape by foot movements. The FHL-associated fat (F) now makes a sharp inverted l-shaped bend (arrow) before fusing with the Achilles-associated fat (A). The Achilles fat is much wider distally than in a dorsiflexed foot. When the FHL and Achilles fat fuse together, they form the bursal wedge. T, Achilles tendon. (b) A hemisected Achilles tendon from a dissecting room cadaver, showing the bursal wedge of Kager's fat pad protruding into the retrocalcaneal bursa (B) near the tendon enthesis (E). The fat is anchored anteriorly and posteriorly by fibrous bands (arrows).
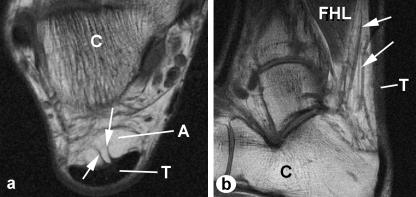
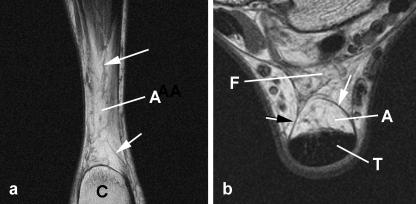
Fig. 3.
Macroscopic anatomy of Kager's fat pad – MRIs. (a) An axial plane image showing two conspicuous fibrous anchors (arrows) connecting the fat pad to the Achilles tendon (T) immediately proximal to the bursal wedge of fat. C, calcaneus; A, Achilles-associated fat. (b) A sagittal image to show the prominent blood vessels (arrows) passing through the Achilles-associated fat. FHL, flexor hallucis longus.
Fig. 4.
Macroscopic anatomy of Kager's fat pad – MRIs. (a) A coronal plane image through the Achilles-associated fat (A), immediately anterior to the Achilles tendon, showing blood vessels coursing through the fat (arrows). The tendon itself is not visible. (b) An axial plane image showing how Achilles-associated fat (A) is enclosed within the false tendon sheath (arrows) which surrounds the Achilles tendon (T). C, calcaneus; F, FHL-associated fat.
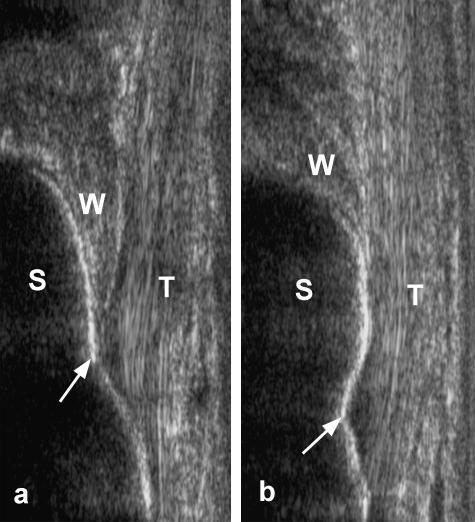
A change in the position of the calcaneal bursal wedge was evident ultrasonically with ankle movements (Fig. 5). On plantarflexion, the wedge of fat moved distally into the furthest extremities of the retrocalcaneal bursa, and this movement was further accentuated by an upward displacement of the superior tuberosity. The fat extended completely into the bursa, until it reached the enthesis itself (Fig. 5a). It retracted on returning the foot to the anatomical (neutral) position (Fig. 5b). The maximum range of excursion between full active plantarflexion and full dorsiflexion was approximately 4 mm in the non-load-bearing foot and 10–12 mm during load-bearing. By studying US movie files of active plantarflexion under load, it was clear that movements of the calcaneal bursal wedge also resulted from concentric contractions of FHL. As the muscle thickened, it moved (‘pumped’) the FHL-associated fat – which in turn moved the bursal wedge as the final link in a kinetic chain. However, the rapidity of the final phase of bursal-wedge movement suggested that the fat was also sucked into the bursa as the insertional angle between the Achilles tendon and the calcaneus increased on plantarflexion.
Fig. 5.
Ultrasound images of the Achilles tendon enthesis from a subject standing (a) on his toes in a load-bearing, plantarflexed foot and (b) in the anatomical (neutral) position. Note that in a plantarflexed foot, the bursal wedge of Kager's fat pad (W) extends completely into the bursa as far as the enthesis itself (arrows). Consequently, the superior tuberosity of the calcaneus (S) is in direct contact with the fat and this in turn directly touches the Achilles tendon (T). When the foot is returned to the neutral position, the fat pad is retracted and the tendon and bone now touch each other directly.
Microscopic studies
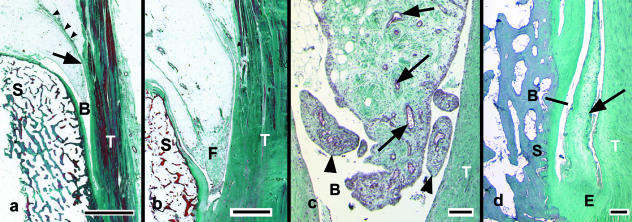
A posterior synovial invagination partly divided the calcaneal bursal wedge from the remainder (Fig. 6a). Fibrous strands anchoring the Achilles tendon to the fat were occasionally seen and attached to the pad in the region of the invaginations (Fig. 6a). The bursal wedge commonly had a fibrous leading edge (Fig. 6b,c) and occasional specimens showed evidence of inflammatory infiltrates. A meniscal fold of varying length was sometimes present between the Achilles tendon and the calcaneus (Fig. 6d). It originated from the most proximal part of the enthesis.
Fig. 6.
Sagittal histological sections of the bursal wedge of Kager's fat pad stained with Masson's trichrome. (a) A low-power view showing the tip of the fat pad protruding into the bursa (B) between the Achilles tendon (T) and the superior tuberosity of the calcaneus (S). Note the deep synovial invagination which promotes the mobility of this region of Kager's pad (arrow), and the fibrous tissue associated with it (arrowheads). Scale bar = 3 mm. (b) The fibrous tip (F) of the bursal wedge. Scale bar = 2 mm. (c) A higher power view of the fibrous tip of the bursal wedge than that shown in (b). Note the numerous small blood vessels (arrows) and dense fibrous connective tissue in its core, and the small synovial villi at its tip (arrowheads). Scale bar = 100 µm. (d) A tapering, fibrocartilaginous meniscal fold (arrow) protruding into the retrocalcaneal bursa (B) at the proximal end of the enthesis (E) of the Achilles tendon. Scale bar = 500 µm.
Discussion
We suggest that movement of the calcaneal bursal wedge of Kager's fat pad minimizes pressure changes in the retrocalcaneal bursa, when the insertional angle between the Achilles tendon and the calcaneus increases on plantarflexion. Because the deep fascia and paratenon in Kager's triangle create an air-tight seal, the pressure within the bursa would change, were it not for the fat pad acting as a ‘variable plunger’, moving in and out of the bursa with ankle movements (Canoso et al. 1988). As changes in pressure have been associated with synovial ischaemia and hypoxia in synovial joints (James et al. 1990), the lubricating function of the bursa could be affected by altered fat pad movements. The enclosure of much of the Achilles part of Kager's fat pad within the paratenon (i.e. the ‘false’ tendon sheath) directs movements of the calcaneal wedge of fat towards the bursa during plantarflexion, by restricting medial–lateral and anterior movements. The Achilles tendon itself prevents posterior movements and fibrous anchors stop the fat from moving proximally.
There would seem to be three mechanisms that could account for the movement of the bursal wedge as the insertional angle between the Achilles tendon and the bone increases during plantarflexion: (1) the movement is a passive consequence of an upward displacement of the calcaneus; (2) the fatty wedge is ‘sucked’ (i.e. pulled) into the bursa to minimize pressure changes; and (3) the fat is pushed into the bursa by muscle contraction, i.e. the muscle belly of FHL acts as a ‘fat pad motor unit’. Certainly, the upward motion of the calcaneus that occurs on plantarflexion and the opening of the insertional angle of the Achilles tendon must move the bursa towards the bursal wedge – rather than vice versa. The calcaneal movement lifts Kager's fat pad, so that it contacts both the hard posterior surface of the tibia and the rigid, contracted muscle belly of FHL. This increases the efficiency of this passive system. However, the US movie files showed that the upward movement of the calcaneus was also accompanied by a downward movement of the bursal wedge. Consequently, one or both of the other mechanisms must also occur. This idea is supported by the observation that the excursional range of the bursal wedge was greater during active plantarflexion under load. It is difficult to account for this by the calcaneal movement theory alone. The retraction of the bursal wedge during dorsiflexion is likely to be a passive consequence of a decreasing insertional angle. Either the fat gets squeezed out as the surfaces become apposed or it moves out as a consequence of increased bursal pressure.
The significance of the muscle belly of FHL to the mechanics of fat pad movement could explain the characteristic distal prominence of the muscle belly of FHL at a level where other muscles acting upon the foot (e.g. flexor digitorum longus and tibialis posterior) are tendinous (Standring, 2004). It also highlights an additional way in which tendon/tendon sheath disorders and anomalies of FHL could result in posteromedial heel pain (Narvaez et al. 2000) and emphasizes the importance during surgery within Kager's triangle of minimizing the risk of adhesions which would compromise fat pad movements. The role of FHL could be important to consider when evaluating injuries to this tendon that occur in ballet dancers en pointe, or in soccer players (ball-kicking results in forced plantarflexion) or in athletes engaged in downhill running (Lo et al. 2001). Although fluid accumulation is well recognized around the tendon of FHL either as a sign of pathology or as a consequence of a direct continuation between the synovial sheath of FHL tendon and the ankle joint cavity (Bureau et al. 2000; Lo et al. 2001; Lohman et al. 2001), the consequence of this on fat pad mechanics is currently overlooked. It is striking, for example, that the oedema illustrated by Lo et al. (2001) in a 38-year-old woman with FHL-entrapment associated with an enlarged os trigonum is precisely in the region of FHL-associated fat and this would be expected to influence the ultimate movements of the bursal wedge of Kager's pad.
The wedge shape of the tip of Kager's fat pad ensures that it moves progressively into the bursa as the foot is plantarflexed and its fibrous leading edge could minimize the risk of damage that accompanies the movement. This idea is supported by the observation that a long, thin wedge of fat with a fibrous tip was most typical of specimens with a prominent superior tuberosity. Where the tuberosity was less obvious, the fat was less fibrous. Equally, the length of meniscal folds also varied with the size of the superior tuberosity – folds were most prominent where the tuberosity was large. It is possible that such folds serve to modify contact stresses between tendon and bone when the surfaces are apposed in dorsiflexion. Whether the movements of Kager's fat pad are altered after resection of the calcaneal superior tuberosity in patients with Haglund's deformity is unclear. However, this could have a bearing on the poor outcome that has been reported with this procedure (Schneider et al. 2000) or emphasize the importance of choosing the most appropriate angle at which the calcaneus is resected (Sella et al. 1998). At least in the initial postoperative period, the synovial membrane which covers the bursal wedge may be in danger of being torn by exposed cancellous bone.
The partial enclosure of Kager's fat pad within the paratenon surrounding the Achilles tendon protects and stabilizes the blood vessels passing through the fat into the tendon and their tortuosity is a further adaptation to minimizing the effects of movement. This again emphasizes the importance of maintaining fat pad integrity at surgery and highlights the possibility that oedema within the fat (associated, for example, with inflammation) could temporarily diminish blood flow to the tendon. The confinement of the Achilles-associated fat within the tendon sheath accounts for the localized nature of the oedema reported by Ly & Bui-Mansfield (2004) in a patient with a partial Achilles tendon tear.
We suggest that Kager's fat pad as a whole is designed in a manner which promotes the movement of one part (its bursal wedge) while minimizing movement in another part (its Achilles-associated part). The synovial invaginations which partly isolate the bursal wedge from the rest are clearly an adaptation for encouraging the independent mobility of the wedge. Collectively, the variety of functions which may be associated with Kager's fat pad add fuel to the debate about the use of local corticosteroids to treat retrocalcaneal pain (Hugate et al. 2004), for one of the common side-effects of corticosteroids is fat atrophy (Cole & Schumacher, 2005). To what extent (if any) the mechanics of Kager's fat pad are altered in patients with an accessory soleus muscle belly, which may obliterate the fat pad in radiographs (Peterson et al. 1993), or altered by the surgical excision of this muscle (Brodie et al. 1997) is unclear.
In summary, we have shown that Kager's fat pad has three distinct regions that are associated with each of the three borders of Kager's triangle. The highly mobile, bursal-associated wedge of fat that is closely related to the calcaneus is moved both passively and actively into the retrocalcaneal bursa during plantarflexion. The more stable, Achilles-associated part protects blood vessels passing through it to supply the Achilles tendon, and all three regions of fat probably contribute to reducing the risk of tendon kinking on plantarflexion. Kager's fat pad should thus be seen as an important part of the Achilles tendon enthesis organ (Benjamin et al. 2004).
Acknowledgments
We thank Dr Simon Blease for his helpful comments on the manuscript.
References
- Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The ‘enthesis organ’ concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50:3306–3313. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- Brodie JT, Dormans JP, Gregg JR, Davidson RS. Accessory soleus muscle. A report of 4 cases and review of literature. Clin Orthop Relat Res. 1997;337:180–186. doi: 10.1097/00003086-199704000-00020. [DOI] [PubMed] [Google Scholar]
- Bureau NJ, Cardinal E, Hobden R, Aubin B. Posterior ankle impingement syndrome: MR imaging findings in seven patients. Radiology. 2000;215:497–503. doi: 10.1148/radiology.215.2.r00ma01497. [DOI] [PubMed] [Google Scholar]
- Canoso JJ, Liu N, Traill MR, Runge VM. Physiology of the retrocalcaneal bursa. Ann Rheum Dis. 1988;47:910–912. doi: 10.1136/ard.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Schumacher HR., Jr Injectable corticosteroids in modern practice. J Am Acad Orthop. 2005;13:37–46. doi: 10.5435/00124635-200501000-00006. [DOI] [PubMed] [Google Scholar]
- Hugate R, Pennypacker J, Saunders M, Juliano P. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg. 2004;86A:794–801. doi: 10.2106/00004623-200404000-00019. [DOI] [PubMed] [Google Scholar]
- James MJ, Cleland LG, Rofe AM, Leslie AL. Intraarticular pressure and the relationship between synovial perfusion and metabolic demand. J Rheumat. 1990;17:521–527. [PubMed] [Google Scholar]
- Lo LD, Schweitzer ME, Fan JK, Wapner KL, Hecht PJ. MR imaging findings of entrapment of the flexor hallucis longus tendon. Am J Roentgenol. 2001;176:1145–1148. doi: 10.2214/ajr.176.5.1761145. [DOI] [PubMed] [Google Scholar]
- Lohman M, Kivisaari A, Vehmas T, Kallio P, Malmivaara A, Kivisaari L. MRI abnormalities of foot and ankle in asymptomatic, physically active individuals. Skeletal Radiol. 2001;30:61–66. doi: 10.1007/s002560000316. [DOI] [PubMed] [Google Scholar]
- Ly JQ, Bui-Mansfield LT. Anatomy of and abnormalities associated with Kager's fat Pad. Am J Roentgenol. 2004;182:147–154. doi: 10.2214/ajr.182.1.1820147. [DOI] [PubMed] [Google Scholar]
- Narvaez JA, Narvaez J, Ortega R, Aguilera C, Sanchez A, Andia E. Painful heel: MR imaging findings. Radiographics. 2000;20:333–532. doi: 10.1148/radiographics.20.2.g00mc09333. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Stinson W, Carter J. Bilateral accessory soleus: a report on four patients with partial fasciectomy. Foot Ankle. 1993;14:284–288. doi: 10.1177/107110079301400509. [DOI] [PubMed] [Google Scholar]
- Schneider W, Niehus W, Knahr K. Haglund's syndrome: disappointing results following surgery – a clinical and radiographic analysis. Foot Ankle Int. 2000;21:26–30. doi: 10.1177/107110070002100105. [DOI] [PubMed] [Google Scholar]
- Sella EJ, Caminear DS, McLarney EA. Haglund's syndrome. J Foot Ankle Surg. 1998;37:110–114. doi: 10.1016/s1067-2516(98)80089-6. discussion 173. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy: the Anatomical Basis of Clinical Practice. Edinburgh: Elsevier/Churchill Livingstone.; 2004. [Google Scholar]