Abstract
Histological analyses of dental development have been conducted for several decades despite few studies assessing the accuracy of such methods. Using known-period incremental features, the crown formation time and age at death of five pig-tailed macaques (Macaca nemestrina) were estimated with standard histological techniques and compared with known ages. Estimates of age at death ranged from 8.6% underestimations to 15.0% overestimations, with an average 3.5% overestimate and a 7.2% average absolute difference. Several sources of error were identified relating to preparation quality and section obliquity. These results demonstrate that histological analyses of dental development involving counts and measurements of short- and long-period incremental features may yield accurate estimates, particularly in well-prepared material. Values from oblique sections (or most naturally fractured teeth) should be regarded with caution, as obliquity leads to inflated cuspal enamel formation time and underestimated imbricational formation time. Additionally, Shellis's formula for extension rate and crown formation time estimation was tested, which significantly overestimated crown formation time due to underestimated extension rate. It is suggested that Shellis' method should not be applied to teeth with short, rapid periods of development, and further study is necessary to validate this application in other material.
Keywords: age at death, crown formation time, dental development, extension rate, incremental features
Introduction
Physical anthropologists have placed special emphasis on the study of human and non-human primate dentitions, due in large part to the relative abundance of teeth in fossil assemblages. Specialists have characterized aspects of dental morphology, tooth wear, tissue distributions and patterns of development, providing insight into evolutionary relationships, functional morphology, behavioural ecology and even primate life histories. Teeth are believed to be particularly rich sources of data on the pace of somatic development and the environment in which the dentition was formed. Specific information on developmental rate has been inferred from incremental features in enamel and dentine, which show well-established periodicities (FitzGerald, 1998; Smith, 2004, in press). Using counts or measurements of these features, the developmental chronology of a tooth may be mapped from the initial deposition of hard tissue to the completion of root formation. Life history events such as the day of birth, or growth disruptions due to illness or environmental stress, may be identified (e.g. Dirks et al. 2002; Schwartz et al. in press). Additionally, the age at death also may be determined from dentitions that were still developing at time of death (e.g. Boyde, 1963; Stringer et al. 1990; Kelley & Smith, 2003).
In primates, the first molar is the first permanent tooth to begin crown formation prior to birth, which often registers the event as an accentuated line known as the neonatal line. The neonatal line permits registration between developmental time and chronological age; subsequent formation time can be added for estimation of chronological age. In developing material, it is possible to use this information and the principles of incremental development to determine the individual's age at death. Several aspects of dental development are critical for this assessment: the neonatal line must be identified, enamel crown formation time must be determined (or the corresponding dentine formation) and the duration of root formation must be established (Fig. 1). However, it has recently been suggested that estimates of crown formation time derived from counts and measurements of incremental features may be invalid (Macho et al. 2003). That study implied that recent histological analyses of dental development and age at death may be erroneous, as well as the resulting insights into life history in fossil apes and humans.
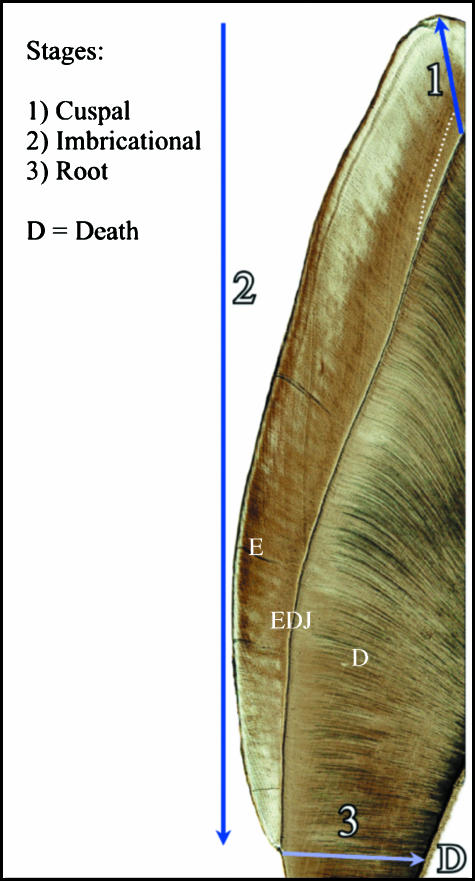
Fig. 1.
Stages of dental development analyzed in this study. Enamel is signified by E, dentine by D, and the dark line between the two is the enamel dentine junction (EDJ). (1) Cuspal enamel formation begins over the tip of the dentine horn, where enamel is secreted outward in an appositional manner until the full thickness of cuspal enamel is produced. The neonatal line (day of birth) is indicated by a dotted white line, which distinguishes pre- and postnatal formation. (2) Imbricational enamel formation is represented by Retzius lines running from the EDJ to the tooth surface. In this region, enamel grows by extension along the EDJ down towards the future cervix, and by apposition from the EDJ to the tooth surface. (3) Root dentine grows inwards towards the future pulp cavity, and ceases at the time of death (when death occurs prior to developmental completion).
The aims of this study are to assess the accuracy of the ‘standard methodology’ for determining crown formation time (recently reported in Smith et al. 2003, 2004) and age at death, and to identify the factors that reduce the accuracy of these methods. In addition, a method for crown formation time estimation proposed by Shellis (1984a,b, 1998) is tested. The first aim is accomplished using first molars from five individual pig-tailed macaques (Macaca nemestrina). Three aspects of dental development are quantified to assess crown formation time and age at death (described below). Estimated age at death is then compared with the known age at death to determine the degree of methodological accuracy. Subsequently, adjusted crown formation times are used to assess the accuracy of Shellis' method. Additional experimentally labelled deciduous and permanent teeth are also used to test Shellis' formula for predicting the local extension rate and local formation time. The results of this study permit assessment of the nature and degree of error in histological analyses of dental development, as well as greater confidence in estimates of crown formation time and/or age at death in material that is of unknown age.
Crown formation time determination and age at death assessments
Crown formation time is the product of cuspal and imbricational (lateral and cervical) enamel formation, which are generally determined individually and then combined to yield a total time of formation. Assessment of cuspal enamel formation, which occurs as enamel- forming cells (ameloblasts) move in a three-dimensional fashion away from the dentine horn, frequently depends on identification and counts or measurements of daily lines known as cross-striations. Cuspal enamel formation may be quantified by a number of different methods (reviewed in Dean, 1998; Smith, 2004), although it may be complicated by misidentification of daily lines or imprecise preparation (Smith et al. 2004). Imbricational enamel formation is typically quantified by counting Retzius lines, or the external manifestations of these lines known as perikymata, and then by multiplying this by the Retzius line periodicity (Fig. 2a). Provided that a complete series of Retzius lines and an area of cross-striations facilitating periodicity determination are both clear, imbricational enamel formation is typically easier to determine than cuspal formation time. If these conditions are all satisfied, crown formation time is predicted to be highly accurate.
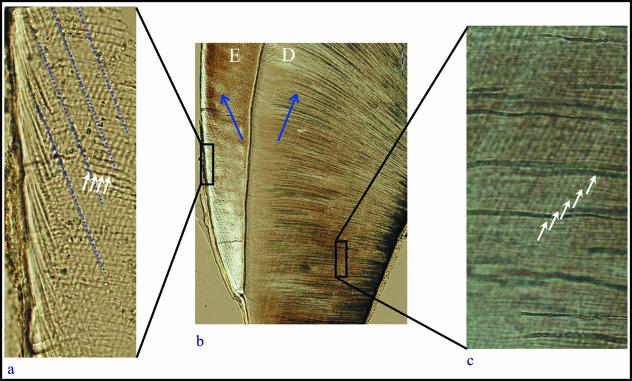
Fig. 2.
(a–c) Transmitted light micrographs of long- and short-period incremental lines in the enamel and dentine from a mesial section of an upper first molar.(a)High-magnification image of outer enamel showing Retzius lines (dotted lines) running diagonally to the tooth surface (on the left). Daily cross-striations (white arrows) can be seen; four cross-striations may be counted between pairs of Retzius lines, representing the Retzius line periodicity. (b)Low-magnification overview of the cervical region of the crown, with enamel (E) on the left and dentine (D) on the right. Long-period lines may be faintly seen in both tissues, running in opposite directions (indicated by arrows) from the enamel dentine junction. A well-defined line to the right of the arrow in the dentine represents an accentuated line formed just prior to enamel completion. (c)High-magnification image of the daily von Ebner's lines in the dentine (white arrows), which are generally difficult to image between long-period (Andresen) lines.
Root formation is represented by dentine formed after completion of the cervical enamel, which grows by extension and apposition, resulting in progressive lengthening and thickening of the root (towards the pulp). Dentine formation is also characterized by long- and short-period incremental lines, which represent the regular secretion of the odontoblasts (dentine-forming cells), similar to the process of enamel formation. Long-period lines are known as Andresen lines (Fig. 2b), which are equivalent to Retzius lines; short-period lines are known as von Ebner's lines, which are equivalent to cross-striations (Fig. 2c) (Bromage, 1991; Dean et al. 1993a; Dean, 1995; Dean & Scandrett, 1996). The duration of root formation may be assessed by several methods: counting Andresen lines, which are multiplied by the periodicity to yield the time in days; division of the length of the root along the cementum–enamel junction by the average extension rate; or division of the thickness of the root (parallel to the dentine tubules) by the average dentine daily secretion rate (DSR). In teeth that are still developing, this information may be added to the postnatal crown formation time to assess the age at death.
An additional aspect of enamel and dentine formation is that under certain conditions, specific points in time may be represented by accentuated lines, which are generally believed to result from pronounced developmental disruptions due to physiological stress or illness (e.g. Dirks et al. 2002; Schwartz et al. in press; also see images of accentuations related to injections of fluorescent labels in Smith, in press). The neonatal line is often regarded as an accentuated line. Teeth (or individual cusps) that do not show a neonatal line must be registered with other teeth (or cusps) in order to relate formation time to chronological age. This is generally accomplished by precisely matching a series of accentuated lines (between teeth or cusps), and may also permit registration of enamel and dentine formation. When matched accurately, they allow demarcation of periods of formation, and may permit counts to be continued between teeth, cusps or tissues (Boyde, 1963).
Assessments of accuracy and extension rate/formation time determination
Although the periodic nature of short- and long-period incremental features in enamel and dentine has been well established (reviewed in FitzGerald, 1998; Smith, in press), it is less common that histological methods used to determine crown formation time have been tested (Stringer et al. 1990; Dean, 1998; Antoine, 2000; Smith et al. 2004). Because it is unusual for histological material to preserve a complete, clearly visible succession of increments from the beginning to end of enamel (or dentine) formation, some estimation is generally required. Two main types of methods have been proposed for crown formation time reconstruction: (1) assessment of the cuspal and imbricational components of appositional growth from counts and measurements of short- and long-period increments (detailed above); and (2) estimation of the duration of extension from measurements of the short-period features, developing enamel front and enamel prisms at the enamel dentine junction (EDJ) (detailed below). Appositional growth occurs as ameloblasts progressively secrete enamel from the EDJ to the surface of the tooth, while extension occurs by the progressive activation of secretory ameloblasts along the EDJ from the cusp to the cervix.
Shellis (1984a,b) suggested that the extension rate of enamel formation may be understood as a trigonometric model of enamel development based on the angle of the developing enamel front at the EDJ, the angle of an enamel prism at the EDJ and the DSR at the EDJ (illustrated in Shellis, 1984b, fig. 1, p. 698). From knowledge of this growth parameter, he suggested that it is possible to determine crown formation time by dividing EDJ length by extension rate in successive increments. He also noted several limitations of this method: it was not possible to use it for teeth that preserved few incremental lines; was less accurate in areas with low angles of intersection between the developing enamel front and EDJ; and that it is essential that measurements of extension rate are derived from all levels of the crown, as extension rates vary from cusp to cervix. Shellis (1998) noted that additional investigation is necessary to determine the accuracy of this formula for total crown formation time determination, particularly in teeth that are relatively fast-forming.
The first method (appositional approach) requires histological sections from teeth that are not missing enamel, which should ideally be derived from the plane of section preserving the tips of the dentine horns (non-oblique section). The second method (extension approach) may be used on partially worn material, as long as the entire EDJ is preserved. The effect of obliquity on this method is unknown. There are three independent ways of determining the accuracy and/or precision of these methods: comparison with a known period of formation (potentially in teeth that were developing at the time of death), comparison with enamel in other cusps or teeth forming simultaneously, or comparison with a corresponding amount of dentine formation. Of these, only the first method may yield a direct assessment of accuracy. It is suggested that studies of incremental development would benefit from an empirical test of these methods, as well as an examination of the effects of obliquity, which may serve to counter criticisms of the validity of results derived from these types of analyses (e.g. Macho et al. 2003).
Materials and methods
Material
Six unerupted first molars (two maxillary and four mandibular) from five macaque (Macaca nemestrina) dentitions were examined. The individuals were all of known age at death, between 146 and 458 days, with molars that had yet to complete crown and/or root formation. A total of 14 mesial and distal thin sections were prepared according to established histological procedures (Reid et al. 1998a,b), although some sections were observed to be partially oblique (not preserving the true profile of the dentine horns) (Fig. 3). Cusps that were deemed markedly oblique were excluded from analysis, and cusps that were slightly oblique were noted and included.
Fig. 3.
(a–j) Transmitted light microscope overviews of histological sections used in this analysis. (a) Mesial section of Specimen 1, metaconid on left, protoconid on right. (b) Mesial section of Specimen 2, metaconid on left, protoconid on right. (c) Mesial section of Specimen 3, paracone on left, protocone on right. (d) Mesial section of Specimen 4a, metaconid on left, protoconid on right. (e) Mesial section of Specimen 4b, paracone on left, protocone on right. (f) Distal section of Specimen 4b, metacone on left, hypocone on right. (g) Specimen 5, section 5.1, metaconid on left, protoconid on right. (h) Specimen 5, section 5.2, metaconid on left, protoconid on right. (i) Specimen 5, section 5.3, entoconid on left, hypoconid on right. (j) Specimen 5, section 5.4, entoconid on left, hypoconid on right. (Overviews of the four additional sections may be obtained from the first author.)
In addition, a single deciduous canine, four deciduous premolars and two permanent molars from five experimentally labelled individuals were used to test the accuracy of local extension rate determination. During the original study on bone growth rates, all individuals were injected 3–5 times with 1–3 fluorescent labels: minocycline hydrochloride, xylenol orange and DCAF (2,4-Bis [N,N′ Di (carbomethyl) aminomethyl] fluorescein) during the final 2 months of life (Newell-Morris & Sirianni, 1982; Sirianni, 1985). The labels established known-period intervals of crown formation that ranged from 6 to 28 days of pre- and/or postnatal crown growth. Additional details of the preparation of this material are given in Smith (2004, in press).
Crown formation time and age at death
Cuspal formation time was generally determined by dividing the cuspal enamel thickness (measured from the dentine horn to the position of the first imbricational Retzius line at the tooth surface) by the average cuspal DSR, determined from an average of measurements of cross-striations in inner, middle and outer cuspal enamel (Beynon et al. 1991; Reid et al. 1998a,b). The thickness was not corrected for prism decussation as suggested by Risnes (1986); several studies have suggested that this correction may not be necessary in non-human primate teeth (discussed below). In the majority of sections, the neonatal line was identified, and formation times were determined separately for the enamel formed before and after this line. When multiple planes of section were available, the minimum cuspal thickness and minimum DSR were used, as these may be inflated by section obliquity (Risnes, 1999; Smith et al. 2004). In three instances, it was necessary to estimate cuspal formation time using accentuated lines in the enamel lateral to the cusp tip, which appeared to represent the neonatal line and the approximate end of cuspal formation, and allowed this period to be estimated from counts and measurements of cross-striations.
Imbricational enamel formation time was determined by counting Retzius lines from the first line at or near the cusp tip to the cervical tip, and multiplying this number by the periodicity, or number of cross-striations between Retzius lines (determined from direct counts in each section) (e.g. Fig. 2a). Total (cusp-specific) crown formation time was estimated by combining cuspal and imbricational enamel formation times. Additionally, well-marked accentuated lines were identified in the enamel and dentine of each cusp in Specimens 3–5, which allowed registration between cusps and assessment of the consistency of estimates.
Estimation of the period of root formation prior to death was accomplished by two methods whenever possible. First, an accentuated dentine line (contour line of Owen: see Dean et al. 1993a) was chosen at or near the point of crown completion (e.g. Fig. 2b), and Andresen lines were counted between this line and the end of dentine formation. This number was multiplied by the periodicity (determined by counts of cross-striations between Retzius lines in enamel) to yield the time of root formation. Additionally, the path length of a dentine tubule was measured from the EDJ at the tip of the cervix to the end of dentine formation, and this was divided by the average local dentine DSR. The DSR was determined in two ways: from measurements of several successive Andresen lines divided by the number of days of formation (number of lines times the periodicity), or by measurements of the daily von Ebner's lines under high magnification (40–60× objectives) (e.g. Fig. 2c). The age at death was determined by combining the time of postnatal crown formation and the duration of root formation for each individual cusp. These estimates were compared with the known ages at death to determine the methodological accuracy.
Extension rate
The application of Shellis's (1984a,b) extension rate formula for crown formation time estimation was tested against the actual or adjusted crown formation time of each cusp using the Wilcoxon signed ranks test. This formula, extension rate (c) = d[(sin I/tan D) − cos I], requires measurements of the angle of intersection of the enamel prisms with the EDJ (I), the angle of the developing enamel front with the EDJ (D) and the spacing of the cross-striations (d). As noted above, Shellis (1984a, 1998) suggested that crown formation time could be estimated by dividing EDJ length by the calculated extension rate and summing times derived from successive segments of the EDJ. This was done using calculated rates derived from a minimum of four areas along the EDJ. For comparison, adjusted formation time was determined as the sum of the known age at death and estimated prenatal enamel formation in Specimens 1 and 2. In Specimens 3–5, the average root formation times were subtracted from the known age at death, and the prenatal enamel estimate was added to yield adjusted crown formation time. From these times, the average coronal extension rate was determined by dividing the cusp-specific EDJ length (tip of the dentine horn to the tip of the cervix) by the adjusted time of formation. The length was derived from the section of each tooth that showed the longest EDJ, assumed to indicate minimum obliquity (excluding a single very oblique section). Lengths were measured from overviews with Sigma Scan software and a SummaSketch III digitizing tablet.
In addition to testing the crown formation time predicted from Shellis' formula, the calculated local extension rate/formation time was also examined in sections from several teeth that had been experimentally labelled. Extension rate was calculated as described above at the intersection of accentuated lines (related to experimental labels or the neonatal line) with the EDJ, and an average local (segment) extension rate was determined as the average of upper and lower boundaries of each segment along the EDJ. To test the accuracy of this formula, the length of the EDJ was measured between lines, which was then divided by the injection interval or known period of formation. The calculated values from Shellis' formula were compared with the empirically derived extension rates and times, and the Wilcoxon signed ranks test was used to examine differences.
Results
Crown formation time and age at death
The estimated prenatal, cuspal and imbricational enamel formation times, root formation time, and age at death are given in Table 1. Total crown formation time represents the sum of prenatal and postnatal enamel formation, which are listed separately under prenatal time and age at crown completion (Age CC). The estimated chronology of dental development and age at death for Specimens 3–5 are shown in Fig. 4. In general, calculated ages at death tended to be overestimates of the actual age (in five of six teeth, or in nine of 13 cusps), which suggests that crown formation time and/or root formation was overestimated. The average error was 3.5% more than the known age, and the average absolute error was 7.2% (under- or overestimate). A few of the cusps with the highest overestimates were known to be oblique a priori based on the morphology of the dentine horns, which did not appear to show a true ‘horn’ in the available planes of section, and led to inflated values.
Table 1.
Estimated crown formation and age at death in six Macaca nemestrina first molars
| Cusp | Prenatal | Postnatal–Cuspal | Imbricational | Age | Dentine | Age at death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cusp | Thick. | Rate | Time | Thick. | Rate | Time | Ret. * Per. | Time | CC | Thick. | Rate | Time | Andr. | Min. − Max. | Known | Diff. | % Error |
| Specimen 1 (lower) | |||||||||||||||||
| Meta | 170 | 4.50 | 38 | 4601 | 5.921 | 78 | 19 * 4 | 76 | n/a | n/a | 154 | 146 | 8 | +5.48 | |||
| Specimen 2 (lower) | |||||||||||||||||
| Proto | ∼200 | 4.30 | 47 | 5751,3 | 5.781 | 99 | 25 * 4 | 100 | n/a | n/a | 199 | 173 | 26 | +15.03 | |||
| Specimen 3 (upper) | |||||||||||||||||
| Para | 1452 | 4.242 | 34 | 300 | 4.792 | 63 | 62 * 4 | 248 | 311 | 620 | 3.40 | 182 | 43 | 483–493 | 438 | 50 | +11.42 |
| Proto | 150 | 3.90 | 38 | 5803 | 5.13 | 113 | 59 * 4 | 236 | 349 | 390 | 3.60 | 108 | 457 | 19 | +4.34 | ||
| Specimen 4a (lower) | |||||||||||||||||
| Meta | 150 | 4.70 | 32 | 5053 | 5.45 | 93 | 464 * 4 | 184 | 277 | 765 | 3.45 | 222 | 47 | 469–499 | 458 | 26 | +5.68 |
| Proto | 245 | 5.25 | 47 | 3953 | 4.83 | 82 | 70 * 4 | 280 | 362 | 550 | 3.20 | 172 | 25–304 | 462–534 | 40 | +8.73 | |
| Specimen 4b (upper) | |||||||||||||||||
| Para | 175 | 5.05 | 35 | 5001 | 4.551 | 110 | 505 * 4 | 200 | 310 | 760 | 3.90 | 195 | 54 | 505–526 | 458 | 58 | +12.66 |
| Proto | 195 | 4.58 | 43 | 485 | 4.35 | 111 | 65 * 4 | 260 | 371 | 270 | 3.506 | 77 | 448 | −10 | −2.18 | ||
| Meta | 130 | 4.38 | 30 | 380 | 4.33 | 88 | 47 * 4 | 188 | 276 | 750 | 3.90 | 192 | 53 | 468–488 | 20 | +4.37 | |
| Hypo | 165 | 3.78 | 44 | 500 | 4.78 | 105 | 71 * 4 | 284 | 389 | 240 | 3.50 | 69 | 21 | 458–473 | 8 | +1.75 | |
| Specimen 5 (lower) | |||||||||||||||||
| Meta | 110 | 4.52 | 24 | 335 | 4.75 | 71 | 504 * 4 | 200 | 271 | 280 | 3.50 | 80 | 20 | 351 | 374 | −23 | −6.15 |
| Proto | 185 | 4.35 | 43 | 400 | 5.12 | 78 | 584 * 4 | 232 | 310 | 140 | 3.65 | 38 | 10 | 348–350 | −25 | −6.68 | |
| Ento | n/a | 5703 | 5.03 | 113 | 304 * 4 | 120 | 233+ | 360 | 3.40 | 106 | 26 | 337–339+ | n/a | ||||
| Hypo | 155 | 4.45 | 35 | 460 | 4.98 | 92 | 604 * 4 | 240 | 332 | 25 | 2.10 | 12 | 2–34 | 340–344 | −32 | −8.56 | |
Cusp values for lower first molars: meta, metaconid (mesiolingual cusp); proto, protoconid (mesiobuccal cusp); ento, entoconid (distolingual cusp); hypo, hypoconid (distobuccal cusp). For the upper molars: para, paracone (mesiobuccal cusp); proto, protocone (mesiopalatal cusp); meta, metacone (distobuccal cusp); hypo, hypocone (distopalatal cusp). For prenatal enamel, values were derived from enamel formed prior to the presumed neonatal line: Thick., linear thickness from dentine horn to neonatal line (µm); Rate, local daily secretion rate (µm day−1); Time, thickness divided by rate (days). Postnatal values are given for cuspal formation after birth: Thick., linear thickness from neonatal line to cusp tip (µm); Rate, daily secretion rate (µm day−1); Time, thickness divided by rate (days). For imbricational enamel: Ret, number of Retzius lines; Per., periodicity of Retzius lines; Time, number of lines multiplied by the periodicity (days). For age: CC, age at crown completion (days), determined by adding the postnatal cuspal time and the imbricational time. Specimens 1 & 2 were still forming their crowns at the time of death, so data on age at crown completion or duration of root formation were not available. For dentine: Thick., linear thickness along a tubule from the enamel dentine junction at the cervix to the end of dentine formation (µm); Rate, local daily secretion rate (µm day−1); Time, thickness divided by rate (days); Andr., number of Andresen lines counted in the same area, which was multiplied by the periodicity for a separate estimate of dentine formation time (not shown but included in the following column). For age at death: Min. − Max., range of times of estimated age at death (days), calculated by adding the age at crown completion to the minimum and maximum estimates of root formation; Known, actual age at death; Diff., difference score, calculated by subtracting the average calculated age at death from the actual age at death; % Error, difference score divided by the known age × 100. Direction of estimation is indicated as +, overestimate; –, underestimate.
Derived from the lateral enamel corresponding to the approximate period of cuspal formation. Values are likely to be higher than the actual cuspal thickness and rate, but it was not possible to calculate these.
Represents an average of two sections, due to missing cuspal enamel.
Believed to be slightly oblique, which may result in inflated cuspal formation time.
Includes slight estimate due to the lack of clarity of a few long-period lines.
Incomplete count due to the lack of clarity of a few long-period lines, cuspal formation estimated from lateral aspect.
Estimated value derived from hypocone root.
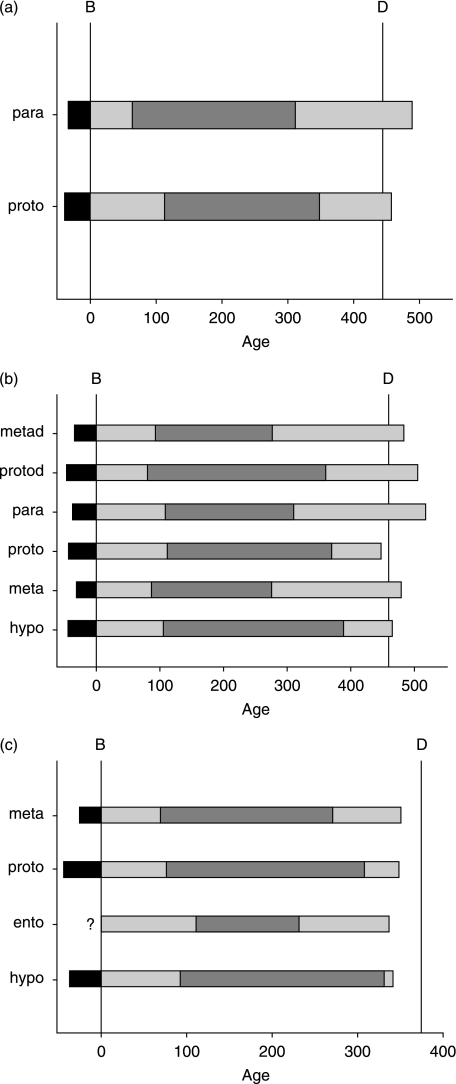
Fig. 4.
(a–c) Developmental chronology of individual cusps of four first molars (Specimens 3–5). Prenatal enamel formation is shown in black, followed by postnatal cuspal formation in grey, imbricational formation in dark grey, and root formation in light grey. Birth (B) and age at death (D) are approximated by vertical lines. (a) Specimen 3, para and proto represent the paracone and protocone. (b) Specimen 4a, metad and protod represent the metaconid and protoconid; Specimen 4b, para, proto, meta and hypo represent the paracone, protocone, metacone and hypocone, respectively. (c) Specimen 5, meta, proto, ento and hypo represent the metaconid, protoconid, entoconid and hypoconid, respectively. In the entoconid, a neonatal line could not be found, and thus it was not possible to determine the age at initiation (indicated by ‘?’) or age at death. Note that the scales are different on the three graphs. Data are from Table 1.
The presence of accentuated lines in Specimens 3–5 permitted a secondary chronology of development to be determined through comparisons of simultaneously forming regions of enamel. For example, a pair of accentuated lines was identified in the enamel of Specimen 5; initial calculations suggested that the first line was formed at approximately 139 days of age in the metaconid, 138 days in the protoconid and 128 days in the hypoconid, showing differences of 1–11 days between estimates. The most marked accentuated line (E1) was then matched to a marked line in the dentine in each cusp, which permitted an iterative chronology to be determined from the cessation of dentine formation (at death) to the formation of the accentuated line (Fig. 5). Based on counts of Andresen lines, the marked line was determined to have occurred 53 increments, or 212 days, before death. When the time of formation was adjusted from the known age at death, the iterative chronology suggested very little prenatal enamel formation in the metaconid and hypoconid, and none in the entoconid, which initiated 36 days after birth. Given the 23- to 32-day average underestimates of the metaconid and hypoconid when compared with the known age at death, it is likely that one or all of the following are true: the neonatal lines were not correctly identified, the cuspal formation time was underestimated and/or the number of earliest-formed Retzius lines was underestimated. Considering the difficulty of imaging Retzius lines near the cusp tips in this tooth, it is likely that this last reason was the primary cause of the underestimates, particularly in the hypoconid.
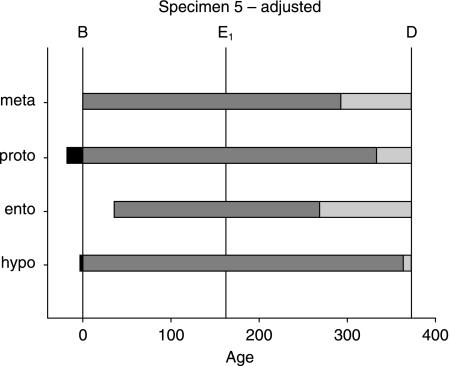
Fig. 5.
Adjusted developmental chronology in Specimen 5. Information derived from long-period lines formed after a marked accentuated line (E1) is used to adjust developmental time by iteratively working from death to birth (see text for details). Prenatal enamel formation is shown in black, followed by postnatal crown formation (grouped cuspal and imbricational) in dark grey, and root formation in light grey. Birth (B), event (E1) and age at death (D) are approximated by vertical lines. Cusp codes are given in Fig. 4(c).
Enamel extension and crown formation time
The adjusted crown formation times derived from knowledge of the actual age at death were compared with times derived from the application of Shellis' formula (Table 2). Shellis' formula yielded estimates of crown formation times that are significantly greater than the actual times (Wilcoxon signed ranks test: Z = −3.297, n = 14, P < 0.01). These ranged from 5 to 86% more than the actual time, and were 43% greater on average. This was due to underestimation of extension rate, as revealed by comparison with the actual average extension rate. This is consistent with comparisons of calculated and measured local extension rates over short intervals (Table 3). The extension rates predicted from Shellis' formula generally yielded a lower value than the actual local extension rates, which resulted in significant overestimates of formation time (Wilcoxon signed ranks test: Z = −2.778, n = 19, P < 0.01). Absolute differences between the calculated and known formation times ranged from 4 to 171%, with an average difference of 39%. Average time differences were: 70% (SD = 70, n = 4 areas) for the dc areas, 35% (SD = 20, n = 13 areas) for dp4s and 4% (n = 2) for M1s. In each of the two molars, the angle of intersection of the developing enamel front (D) was greater than in most of the deciduous teeth, which was easier to measure and may have resulted in more accurate determination of the local extension rate.
Table 2.
Adjusted crown formation time and extension in Macaca nemestrina
| Shellis' | Known | |||||||
|---|---|---|---|---|---|---|---|---|
| Spec. | Cusp | EDJ | Time | Rate | Time | Rate | Diff. | % Error |
| 1 | Metaconid | 4210 | 228 | 18.46 | 1841 | 22.88 | 44 | 23.91 |
| 2 | Protoconid | 4689 | 326 | 14.38 | 2201 | 21.31 | 106 | 48.18 |
| 3 | Paracone | 5470 | 427 | 12.81 | 295 | 18.54 | 132 | 44.75 |
| Protocone | 5433 | 415 | 13.09 | 367 | 14.80 | 48 | 13.08 | |
| 4a | Metaconid | 4280 | 366 | 11.69 | 285 | 15.02 | 81 | 28.42 |
| Protoconid | 6380 | 476 | 13.40 | 374 | 17.06 | 102 | 27.27 | |
| 4b | Paracone | 5445 | 502 | 10.85 | 287 | 18.97 | 215 | 74.91 |
| Protocone | 6257 | 788 | 7.94 | 424 | 14.76 | 364 | 85.85 | |
| Metacone | 5042 | 526 | 9.58 | 286 | 17.63 | 240 | 82.47 | |
| Hypocone | 6268 | 609 | 10.29 | 426 | 14.71 | 183 | 42.96 | |
| 5 | Metaconid | 4596 | 335 | 13.72 | 318 | 14.45 | 17 | 5.34 |
| Protoconid | 5603 | 466 | 12.02 | 378 | 14.82 | 88 | 23.28 | |
| Entoconid | 3660 | 429 | 8.53 | 2332 | 15.71 | 196 | 84.12 | |
| Hypoconid | 5091 | 447 | 11.39 | 399 | 12.76 | 48 | 12.03 | |
Spec., specimen number as described in the text. Cusp, derived from first molars. EDJ, enamel–dentine junction length (µm), Shellis' values derived from the application of his formula using measurements from a minimum of four areas along the EDJ (see text for formula); Time, predicted formation time (days); Rate, average extension rate (µm day−1), determined by dividing EDJ by time. Known values derived from actual age at death, from which root formation was subtracted and prenatal time was added. Time, prenatal plus postnatal enamel formation (days); Rate, extension rate (µm day−1), determined by dividing EDJ by time. Diff., difference score, calculated by subtracting the actual crown formation time from the calculated formation time, % Error, difference score divided by the actual time × 100.
Incomplete crown.
Original estimate not adjusted due to lack of neonatal line.
Table 3.
Measured and calculated extension rate in Macaca nemestrina
| Known | Shellis | Difference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF | Tooth | Area | Event | Dist | Time1 | Rate | Min. d | I | D | c | Int. c | Time2 | Diff | % Error |
| 300 | dp4 m | lat/cer | XO | 4.6 | 66.3 | 11.4 | 19.04 | |||||||
| Mino | 260 | 8 | 32.50 | 4.8 | 76.8 | 13.0 | 19.15 | 19.09 | 14 | 6 | 75.00 | |||
| Birth | 633 | 19 | 33.32 | 3.9 | 74.1 | 6.0 | 34.62 | 26.88 | 24 | 5 | 26.32 | |||
| 303 | dp4 m | lat/cer | DCAF | 5.5 | 61.2 | 7.1 | 36.05 | |||||||
| XO | 260 | 9 | 28.89 | 5.4 | 59.4 | 7.8 | 31.18 | 33.61 | 8 | −1 | −11.11 | |||
| Mino | 284 | 8 | 35.50 | 5.0 | 70.6 | 9.1 | 27.78 | 29.48 | 10 | 2 | 25.00 | |||
| Birth | 521 | 21 | 24.81 | 5.2 | 66.4 | 7.0 | 36.73 | 32.25 | 16 | −5 | −23.81 | |||
| dp4 d | lat/cer | DCAF | 4.5 | 59.1 | 6.2 | 33.23 | ||||||||
| XO | 287 | 9 | 31.89 | 5.1 | 58.1 | 8.3 | 26.98 | 30.11 | 10 | 1 | 11.11 | |||
| Mino | 274 | 8 | 34.25 | 4.9 | 59.8 | 8.7 | 25.21 | 26.10 | 10 | 2 | 25.00 | |||
| 320 | dc lab | lat/cer | Mino | 5.6 | 42.5 | 10.3 | 16.69 | |||||||
| Mino | 417 | 7 | 59.57 | 5.1* | 46.0 | 6.9 | 26.77 | 21.73 | 19 | 12 | 171.43 | |||
| Mino | 267 | 6 | 44.50 | 4.6 | 48.7 | 4.8 | 38.12 | 32.45 | 8 | 2 | 33.33 | |||
| Mino | 421 | 8 | 52.63 | 4.3 | 39.4 | 5.6 | 24.51 | 31.32 | 13 | 5 | 62.50 | |||
| Mino | 263 | 7 | 37.57 | 4.3 | 43.5 | 4.0 | 39.21 | 31.86 | 8 | 1 | 14.29 | |||
| dp4 m | lat/cer | Mino | 4.3 | 54.9 | 9.2 | 19.25 | ||||||||
| Mino | 244 | 7 | 34.86 | 4.7 | 56.4 | 7.0 | 29.28 | 24.27 | 10 | 3 | 42.86 | |||
| Mino | 194 | 6 | 32.33 | 3.9 | 49.1 | 6.3 | 24.15 | 26.71 | 7 | 1 | 16.67 | |||
| Mino | 299 | 8 | 37.38 | 4.4 | 52.6 | 7.4 | 24.24 | 24.19 | 12 | 4 | 50.00 | |||
| Mino | 208 | 7 | 29.71 | 3.7 | 52.3 | 9.1 | 16.01 | 20.13 | 10 | 3 | 42.86 | |||
| 325 | M1 m | lat/cer | Mino | 3.7 | 77.2 | 16.8 | 11.17 | |||||||
| Mino | 409 | 28 | 14.61 | 4.0* | 74.0* | 11.0 | 18.68 | 14.92 | 27 | −1 | −3.57 | |||
| 326 | M1 m | lat/cer | Mino | 3.5 | 83.4 | 16.8 | 11.11 | |||||||
| Mino | 396 | 28 | 14.14 | 3.6 | 82.8 | 10.7 | 18.45 | 14.78 | 27 | −1 | −3.57 | |||
| 333 | dp4 d | lat | Mino | 5.2 | 57.9 | 10.5 | 21.00 | |||||||
| Mino | 175 | 7 | 25.00 | 4.5 | 49.1 | 11.2 | 14.23 | 17.62 | 10 | 3 | 42.86 | |||
| Mino | 148 | 6 | 24.67 | 4.5 | 54.9 | 11.6 | 15.35 | 14.79 | 10 | 4 | 66.67 | |||
CF, individual macaque; Tooth, tooth type, m, mesial section, d, distal section, lab, labial aspect; Area, region of the enamel where the measurements were made, either the approximate border of the lateral and cervical thirds or the lateral enamel third; Event, birth or injection, XO, xylenol orange, Mino, minocycline, DCAF, (2,4Bis) N,N′ Di aminomethyl fluorescein; Dist., length of the interval along the EDJ (µm); Time1, known time between labels (days); Rate, actual extension rate (µm day−1), determined by division of the distance by the known time; Min. d, minimum (measured) average local cross-striation spacing in (µm day−1); I, angle of intersection of the enamel prisms with the enamel–dentine junction (EDJ); D, angle of the developing enamel front/labels with the EDJ; c, calculated extension rate using the formula proposed by Shellis (1984a, b): extension rate (c) = d[(sin I/tan D) − cos I] (µm day−1); Int. c, average extension rate of the upper and lower interval boundaries (µm day−1); Time2, predicted time of formation (days), determined by division of the distance by the average calculated extension rate; Diff., difference score, calculated by subtracting known time (Time1) from Shellis' calculated time (Time2); % Error, difference score divided by known time × 100.
Estimated value.
Discussion
Accuracy of crown formation time/age at death
Radiographic information indicates that pig-tailed macaques initiate first molar crown formation prior to birth, completing mandibular first molar formation at 341 days of age and maxillary first molar formation at 372 days on average (both sexes averaged from Sirianni & Swindler, 1985). This is in general agreement with the results of this study. Crown formation time may be estimated histologically with a high degree of accuracy, particularly in sections that preserve well-defined incremental features, and are not cut oblique to the ideal plane of section (discussed further below).
Few studies have examined material of known age at death (Stringer et al. 1990; Huda & Bowman, 1995; Antoine, 2000; Schwartz et al. in press), or made comparisons with an equivalent amount of dentine (Dean et al. 1993a; Beynon et al. 1998; Dean, 1998; Smith et al. 2004). Early studies suggested that counts of incremental features are accurate to about ±10%, but did not test this empirically (Boyde, 1963; Dean & Beynon, 1991). Antoine (2000) estimated the age at death in several individuals from the Spitalfields collection and compared his estimates with known ages at death, yielding an accuracy of 94% or greater. His study utilized material of exceptional quality, which permitted counts of cross-striations throughout the crown. Antoine did not test the accuracy of more common methods that involve counts of both short- and long-period increments, which are most often applied to material of ‘average quality’. Regardless, the similarity between the accuracy of Antoine's study and that or ours suggests that histological methods in general (direct counts of increments and/or estimation of appositional growth) may yield accurate estimations of crown formation time and/or age at death.
Sources of error
It is not surprising that poorer quality sections yielded less accurate estimations of crown formation time, as both short-period and long-period features were difficult to image. It was difficult to assess DSR where there was a lack of clarity of cross-striations, which complicated cuspal enamel estimation. In addition, if Retzius line periodicity is not clear, an additional source of error may be introduced into imbricational enamel formation time. For example, had the periodicity of any of the (crown complete) molars been 5 instead of 4 days, 30–71 additional days of imbricational formation time would have been added (as well as up to 54 additional days of root formation). A difference of a single day in periodicity may affect molar crown formation time estimation by approximately 2–6 months in longer-forming hominoid dentitions (depending on tooth and cusp type).
Material of suboptimal quality may also show poorly defined long-period lines. In this study, Retzius lines were often unclear in the first-formed enamel near the cusp tips, or in the last-formed cervical enamel. Errors in counting these structures are related to the periodicity; teeth with low periodicity, such as the macaques in this study, show a smaller number of days per line than hominoids. The exclusion of a few Retzius lines would represent less time than in hominoids with higher periodicities. Identification of the neonatal line represents an additional complication. If this is not identified when present, underestimations of prenatal enamel formation may result, as well as overestimations of the postnatal cuspal enamel formation. Alternatively, incorrect identification may cause either over- or underestimation of the prenatal and postnatal cuspal enamel formation times. Unfortunately, it is difficult to validate estimations of prenatal enamel.
The issue of section obliquity and its potential confounding affects on histological analyses has been considered only recently (Risnes, 1999; Antoine, 2000; Dean & Schrenk, 2003; Smith et al. 2003, 2004). There are several potential problems with section planes that do not represent the true axial thickness of cuspal enamel and the profile of the dentine horn: exaggerated cuspal thickness, exaggerated prism path length, exaggerated daily secretion rate and/or number of cross-striations, and poor definition of Retzius lines (Smith, 2004). Obliquely sectioned cusps with inflated enamel thicknesses may yield overestimated cuspal formation time when determined from enamel, and underestimated time when determined from dentine (Smith et al. 2004). Because the ideal plane of section preserves the thinnest cuspal enamel, which may show the slowest secretion rates (Risnes, 1999, fig. 7, p. 319), deviations from this plane will necessarily increase both. It was difficult to confirm this in the present study, as the quality of cross-striations in the cuspal enamel was generally poor. However, cusps with thicker enamel often yielded higher DSR than other sections from the same individuals. Some evidence from two sections of Graecopithecus freybergi also supports this (Smith et al. 2004). In addition, the current study suggests that obliquity generally results in underestimated EDJ length, as sections that showed the thinnest cuspal enamel and the most well-defined dentine horns generally had the longest cusp-specific EDJ lengths (see also Martin, 1983; Smith, 2004). This implies that obliquity will also affect the accuracy of crown formation time determined from the application of Shellis' extension rate formula (discussed further below).
A final area that may influence the accuracy of crown formation time estimation is the three-dimensional course of prisms, which may lead to underestimation of cuspal formation time when linear thickness is used (Risnes, 1986). However, given the degree of overestimation found in this study without correction, it may not be necessary to apply a correction factor for accurate determination of cuspal formation time. Although many early studies relied on Risnes's (1986) correction factor for cuspal enamel formation time in humans, others have suggested that this correction factor may not be an appropriate factor for fossil hominids or other primates (e.g. Beynon & Wood, 1987; Dean et al. 1993b; Dean, 1998; Dirks, 1998; Reid et al. 1998a; Macho et al. 2003; Smith et al. 2003, 2004). Antoine (2000) also showed that the complication imposed by decussation in human teeth could be minimized when cross-striations were counted along prisms that did not show marked decussation. The degree of accuracy of this method showed that, contraRisnes (1986) and Macho et al. (2003), decussation does not prohibit accurate determination of formation time from cross-striations. There appears to be substantial variation in the course of prisms through cuspal enamel among primates, and appropriate corrections appear to be specific to taxa, or even cusps within taxa, but these corrections may not always be necessary for accurate assessment.
Extension rate and crown formation time
The present study has demonstrated that the application of Shellis' extension rate formula generally overestimates enamel formation time due to underestimation of extension rate. Shellis (1998) noted that estimates of extension rate are more likely to be prone to error in rapidly formed teeth, such as those examined in this study, but he did not test this. In taxa that show a progressive reduction in extension rate from crown initiation to completion, the earliest-formed enamel (which is sometimes prenatal) will have the lowest angles of intersection between the forming front and the EDJ, due to relatively fast extension. These acute angles often prove to be difficult to measure accurately (Shellis, 1984a; Grine & Martin, 1988), and fewer ‘intersections’ are available to measure due to the typical lack of clarity of incremental features in the earliest-formed enamel. In this study, it was not possible to sample the initial 500–2000 µm of EDJ length in any cusp. In addition, it was frequently more difficult to find clearly defined daily lines in deciduous teeth, which may have introduced additional error. Unless numerous areas are sampled, particularly during the initial period of formation (as in Dean, 1998), artificially low extension rates result in an overestimation of time (Shellis, 1984a).
It is possible that Shellis' formula may alternatively result in an underestimation of crown formation time in certain situations, although this did not occur in the present study. It is well established that the DSR in hominoids varies based on the position within the crown (e.g. Beynon et al. 1991; Reid et al. 1998a,b). In his early reports, Shellis (1984a,b) suggested the average DSR is 4.8 µm day−1 in the inner enamel among primates, which he revised and re-reported as 3.9 µm day−1 in 1998. However, Shellis (1998) failed to note that inner enamel secretion rate decreases from cuspal to cervical enamel. Thus, the use of a single average value for inner enamel DSR is not appropriate for calculating extension rate along the entire length of the EDJ. If the secretion rate determined from cuspal inner enamel was used for lateral and cervical enamel extension calculations, this would yield an inflated extension rate and a lower crown formation time. It is possible that the low values for human crown formation times reported in Shellis (1984a) (relative to recent data on human crown formation time from Reid et al. 1998b) were due to the use of an overestimated inner DSR.
Additionally, Shellis (1998) reported an average daily secretion rate of 3.8 µm day−1 for Pan inner enamel, which is greater than most published reports for inner enamel secretion rates in this genus (Beynon et al. 1991; Dean, 1998; Reid et al. 1998a; Smith, 2004). By his calculation, Shellis (1998) reported that a single Pan M1 formed in 1.46 years, which is lower than the values for this tooth type reported by Reid et al. (1998a) and Smith (2004). However, if extension rate is recalculated using average inner enamel secretion rates of 3.0 or 2.5 µm day−1, Pan crown formation time increases from 1.46 years to 1.85 or 2.22 years, respectively. These times are closer to the average values for a large sample of mesial cusps of lower first molars of Pan (Smith, 2004). Smith (2004) also demonstrated that Shellis' tooth specimen of Pan was cut distal to the ideal plane of section, and did not preserve the entire EDJ. As noted above, oblique sections (including those cut mesial or distal to the ideal plane of section) do not preserve the complete profile of the EDJ, which may yield underestimates of crown formation time using Shellis' method, as the entire length (or period) of extension is not represented.
In conclusion, this study has demonstrated that assessments of crown formation time and age at death based on counts and measurements of short- and long-period incremental features may yield accurate results. The degree of accuracy is related to the clarity of incremental features and the plane of section; oblique planes of section generally led to overestimated cuspal developmental time. Poorly defined incremental features may lead to either overestimated or underestimated formation times. The study also tested the accuracy of a method for estimation of extension rate and crown formation time proposed by Shellis (1984a,b, 1998). When compared with results derived from known periods of formation, this formula underestimated extension rate and significantly overestimated formation time over short periods and for overall crown development. This was particularly apparent in deciduous teeth, which may be due to the lack of clarity of incremental features and the relatively fast rate of extension. It is suggested that results derived from this method should be regarded with caution, and additional work is needed to demonstrate the accuracy of this method. This also suggests that studies that have used crown formation time data reported in Shellis (1998) may be problematic (e.g. Macho, 2001).
Acknowledgments
This study benefited from the expert technical assistance of Pam Walton. In addition, Lawrence Martin, Anthony Olejniczak, Bill Jungers, Allison Cleveland, Dan Lieberman, Gary Schwartz and two anonymous reviewers provided helpful comments on the manuscript. Laura Newell-Morris and Daris Swindler kindly assisted with the material. This study was funded by Stony Brook University IDPAS Travel and Research Awards (T.M.S.), NSF Dissertation Improvement Grant award 0213994 (T.M.S.), and by the Max Planck Institute for Evolutionary Anthropology.
References
- Antoine D. University College London, London: Evaluating the periodicity of incremental structures in dental enamel as a means of studying growth in children from past human populations. PhD dissertation. [Google Scholar]
- Beynon AD, Wood BA. Patterns and rates of enamel growth in the molar teeth of early hominids. Nature. 1987;326:493–496. doi: 10.1038/326493a0. [DOI] [PubMed] [Google Scholar]
- Beynon AD, Dean MC, Reid DJ. On thick and thin enamel in hominoids. Am J Phys Anthropol. 1991;86:295–309. [Google Scholar]
- Beynon AD, Dean MC, Leakey MG, Reid DJ, Walker A. Comparative dental development and microstructure of Proconsul teeth from Rusinga Island, Kenya. J Hum Evol. 1998;35:163–209. doi: 10.1006/jhev.1998.0230. [DOI] [PubMed] [Google Scholar]
- Boyde A. Estimation of age at death of young human skeletal remains from incremental lines in the dental enamel. Excerpta Med Int Congr Series. 1963;80:36–46. [Google Scholar]
- Bromage TG. Enamel incremental periodicity in the pig-tailed macaque: a polychrome fluorescent labeling study of dental hard tissues. Am J Phys Anthropol. 1991;86:205–214. [Google Scholar]
- Dean MC, Beynon AD. Histological reconstruction of crown formation times and initial root formation times in a modern human child. Am J Phys Anthropol. 1991;86:215–228. [Google Scholar]
- Dean MC, Beynon AD, Reid DJ, Whittaker DK. A longitudinal study of tooth growth in a single individual based on long- and short-period incremental markings in dentine and enamel. Int J Osteoarch. 1993a;3:249–264. [Google Scholar]
- Dean MC, Beynon AD, Thackeray JF, Macho GA. Histological reconstruction of dental development and age at death of a juvenile Paranthropus robustus specimen, SK 63, from Swartkrans, South Africa. Am J Phys Anthropol. 1993b;91:401–419. doi: 10.1002/ajpa.1330910402. [DOI] [PubMed] [Google Scholar]
- Dean MC. The nature and periodicity of incremental lines in primate dentine and their relationship to periradicular bands in OH 16 (Homo habilis) In: Moggi-Cecchi J, editor. Aspects of Dental Biology: Paleontology, Anthropology and Evolution. Florence: International Institute for the Study of Man; 1995. pp. 239–265. [Google Scholar]
- Dean MC, Scandrett AE. The relation between long-period incremental markings in dentine and daily cross-striations in enamel in human teeth. Arch Oral Biol. 1996;41:233–241. doi: 10.1016/0003-9969(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Dean MC. A comparative study of cross striation spacings in cuspal enamel and of four methods of estimating the time taken to grow molar cuspal enamel in Pan, Pongo and Homo. J Hum Evol. 1998;35:449–462. doi: 10.1006/jhev.1998.0208. [DOI] [PubMed] [Google Scholar]
- Dean MC, Schrenk F. Enamel thickness and development in a third permanent molar of Gigantopithecus blacki. J Hum Evol. 2003;45:381–387. doi: 10.1016/j.jhevol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Dirks W. Histological reconstruction of dental development and age of death in a juvenile gibbon (Hylobates lar) J Hum Evol. 1998;35:411–425. doi: 10.1006/jhev.1997.0185. [DOI] [PubMed] [Google Scholar]
- Dirks W, Reid DJ, Jolly CJ, Phillips-Conroy JE, Brett FL. Out of the mouths of baboons: stress, life history, and dental development in the Awash National Park hybrid zone, Ethiopia. Am J Phys Anthropol. 2002;118:239–252. doi: 10.1002/ajpa.10089. [DOI] [PubMed] [Google Scholar]
- FitzGerald CM. Do enamel microstructures have regular time dependency? Conclusions from the literature and a large-scale study. J Hum Evol. 1998;35:371–386. doi: 10.1006/jhev.1998.0232. [DOI] [PubMed] [Google Scholar]
- Grine FE, Martin LB. Enamel thickness and development in Australopithecus and Paranthropus. In: Grine FE, editor. Evolutionary History of the ‘Robust’ Australopithecines. New York: Aldine de Gruyter; 1988. pp. 3–42. [Google Scholar]
- Huda TFJ, Bowman JE. Age determination from dental microstructure in juveniles. Am J Phys Anthropol. 1995;97:135–150. doi: 10.1002/ajpa.1330970206. [DOI] [PubMed] [Google Scholar]
- Kelley J, Smith TM. Age at first molar emergence in early Miocene Afropithecus turkanensis and life-history evolution in the Hominoidea. J Hum Evol. 2003;44:307–329. doi: 10.1016/s0047-2484(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Macho GA. Primate molar crown formation times and life history evolution revisited. Am J Primatol. 2001;55:189–201. doi: 10.1002/ajp.1054. [DOI] [PubMed] [Google Scholar]
- Macho GA, Jiang Y, Spears IR. Enamel microstructure – a truly three-dimensional structure. J Hum Evol. 2003;45:81–90. doi: 10.1016/s0047-2484(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Martin LB. University College London, London: The relationships of the later Miocene Hominoidea. PhD dissertation. [Google Scholar]
- Newell-Morris L, Sirianni JE. Factors and Mechanisms Influencing Bone Growth. New York: Alan R. Liss; 1982. Parameters of bone growth in the fetal and infant macaque (Macaca nemestrina) humerus as documented by trichromatic bone labels; pp. 243–258. [PubMed] [Google Scholar]
- Reid DJ, Schwartz GT, Dean C, Chandrasekera MS. A histological reconstruction of dental development in the common chimpanzee, Pan troglodytes. J Hum Evol. 1998a;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Beynon AD, Ramirez Rozzi FV. Histological reconstruction of dental development in four individuals from a medieval site in Picardie, France. J Hum Evol. 1998b;35:463–477. doi: 10.1006/jhev.1998.0233. [DOI] [PubMed] [Google Scholar]
- Risnes S. Enamel apposition rate and the prism periodicity in human teeth. Scand J Dent Res. 1986;94:394–404. doi: 10.1111/j.1600-0722.1986.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Risnes S. Mature enamel morphology and forming enamel dynamics: crystal orientation, crystal continuity, prism diameter, Retzius lines, and prism cross-striations. In: Mayhall JT, Heikkinen T, editors. Dental Morphology '98: Proceedings of the 11th International Symposium on Dental Morphology. Oulu, Finland: Oulu University Press; 1999. pp. 312–322. [Google Scholar]
- Schwartz GT, Reid DJ, Dean MC, Zihlman AL. A faithful record of stressful life events preserved in the dental development of a juvenile gorilla. Int J Primatol. in press. [Google Scholar]
- Shellis RP. Inter-relationships between growth and structure of enamel. In: Fearnhead RW, Suga S, editors. Tooth Enamel. Amsterdam: Elsevier Science; 1984a. pp. 467–471. [Google Scholar]
- Shellis RP. Variations in growth of the enamel crown in human teeth and a possible relationship between growth and enamel structure. Arch Oral Biol. 1984b;29:697–705. doi: 10.1016/0003-9969(84)90175-4. [DOI] [PubMed] [Google Scholar]
- Shellis RP. Utilization of periodic markings in enamel to obtain information on tooth growth. J Hum Evol. 1998;35:387–400. doi: 10.1006/jhev.1998.0260. [DOI] [PubMed] [Google Scholar]
- Sirianni JE. Nonhuman primates as models for human craniofacial growth. In: Watts E, editor. Nonhuman Primate Models for Human Growth and Development. New York: Alan R. Liss, Inc; 1985. pp. 95–124. [Google Scholar]
- Sirianni JE, Swindler DR. Growth and Development of the Pigtailed Macaque. Boca Raton, FL: CRC Press, Inc; 1985. [Google Scholar]
- Smith TM, Martin LB, Leakey MG. Enamel thickness, microstructure and development in Afropithecus turkanensis. J Hum Evol. 2003;44:283–306. doi: 10.1016/s0047-2484(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Smith TM. Stony Brook University, Stony Brook: Incremental development of primate dental enamel. (Available on-line at http://www.paleoanthro.org/dissertation_list.htm) PhD dissertation. [Google Scholar]
- Smith TM, Martin LB, Reid DJ, de Bouis L, Koufos GD. An examination of dental development in Graecopithecus freybergi (=Ouranopithecus macedoniensis) J Hum Evol. 2004;46:551–577. doi: 10.1016/j.jhevol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Smith TM. Experimental determination of the periodicity of incremental features in enamel. J Anat. 2006;208:99–113. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer CB, Dean MC, Martin RD. A comparative study of cranial and dental development within a recent British sample and among Neandertals. In: De Rousseau CJ, editor. Primate Life History and Evolution. New York: Wiley-Liss.; 1990. pp. 115–152. [Google Scholar]