Abstract
Vital labelling of hard tissues was used to examine the periodicity of features of dental enamel microstructure. Fluorescent labels were administered pre- and postnatally to developing macaques (Macaca nemestrina), which were identified histologically in dentine and related to accentuated lines in enamel, allowing for counts of features within known-period intervals. This study demonstrates that cross-striations represent a daily rhythm in enamel secretion, and suggests that intradian lines are the result of a similar 12-h rhythm. Retzius lines were found to have a regular periodicity within individual dentitions, and laminations appear to represent a daily rhythm that also shows 12-h subdivisions. The inclusion of intradian lines and laminations represents the first empirical evidence for their periodicities in primates; these features frequently complicate precise measurements of secretion rate and Retzius line periodicity, which are necessary for determination of crown formation time. The biological basis of incremental feature formation is not completely understood; long-period features may result from interactions between short-period rhythms, although this does not explain the known range of Retzius line periodicities within humans or among primates. Studies of the genetic, neurological and hormonal basis of incremental feature formation are needed to provide more insight into their physiological and structural basis.
Keywords: biological clock, cross-striation, intradian line, lamination, Retzius line
Introduction
Biological rhythms have been identified in both plants and animals, including single-cell organisms and fungi, and they appear to be ubiquitous in eukaryotes (reviewed in Hastings, 1997). Rhythm periodicities may range from a few hours to a yearly interval. The control of rhythm is related to intrinsic (endogenous) factors (formally referred to as ‘biological clocks’), as well as extrinsic (exogenous) factors, which are discussed further below. Scrutton (1978) suggested that any organism with ‘preservable hard parts’ formed by a ‘continually additive mode of growth’ may provide evidence of rhythms. Common examples include annual rings in the trunks of trees, or the daily or annual bands in hard tissues of marine organisms (reviewed in Smith, 2004). This type of evidence has proved useful for assessing chronological age or reconstructing past environments, as well as for documenting changes in day length millions of years ago. Structural evidence of biological rhythms in dental hard tissues has also been recognized for over a century, and recent anthropological studies have used features in enamel, dentine and cementum to make inferences about the rate and duration of dental development, stress experienced during development, the age or season at death of developing individuals, and even the pace of life history (reviewed in Dean, 2000; Smith, 2004).
Identification of known-period features is the most fundamental aspect of analyses of hard tissue development (Scrutton, 1978). Despite a number of illustrative experimental studies carried out in the 1930s and 1940s by Japanese and US researchers, certain fundamental aspects of incremental dental development have recently been called into question (reviewed in Boyde, 1989; Risnes, 1990; FitzGerald, 1998; Smith, 2004). Several studies have attempted to refute criticisms by experimentally or deductively establishing the periodicity of incremental dental features, and these include a number of PhD dissertations (e.g. FitzGerald, 1996; Antoine, 2000; Smith, 2004). The aim of this study is to demonstrate experimentally the periodicity of several types of incremental features found in enamel, which are defined and illustrated below. This is accomplished by using dental tissues that have been labelled in vivo, which provide known-period intervals that may be identified in histological sections and used to determine the repeat intervals of features. This study represents the most comprehensive examination of biological rhythms recorded in dental enamel of a single species, and provides experimental confirmation of the validity of results based on analyses of incremental features.
Background
Enamel development and incremental features
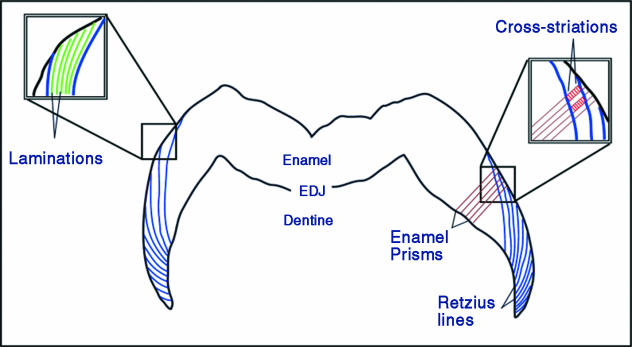
Enamel formation begins at the tip of the dentine horn, and proceeds by apposition towards the future surface of the tooth and by extension towards the future cervix of the tooth. Initially, a highly organic enamel matrix is secreted on top of a dentine framework. This matrix is eventually replaced almost entirely by minerals, which are arranged in crystallized bundles that make up fundamental units known as enamel prisms. During this process, changes in the enamel secretory rhythm, chemical composition and/or the position of the developing enamel front are recorded as incremental features (reviewed in Boyde, 1989; Smith, 2004). Four classes of these features are defined and examined below: cross-striations, intradian lines, Retzius lines and laminations (Fig. 1).
Fig. 1.
Illustration of three of the four classes of incremental enamel features examined in this study. Long-period Retzius lines run from the enamel dentine junction (EDJ, inner black border) to the surface of the enamel (outer black border). In the magnified box on the right, a series of cross-striations (in red) perpendicular to enamel prisms may be counted between a pair of Retzius lines, which yields the Retzius line periodicity. On the left, an enlarged box shows lamination (in green), running parallel to Retzius lines but with a much closer spacing. Due to the scale of these structures, it was not possible to illustrate intradian lines, which appear as small subdivisions of cross-striations (modified from Smith et al. 2003).
Cross-striations are defined as light and dark bands that cross enamel prisms perpendicularly with a common interval of about 3–6 µm, and have been described in numerous studies. The most comprehensive model of their formation was illustrated by Boyde (1989), who specifically posited that they are formed every 24 h with an orientation that is parallel to the secretory face (Tomes' process) of the enamel-forming cell (ameloblast) (e.g. Boyde, 1989, fig. 7, p. 338). A second class of features is known as intradian lines, fine bands that divide cross-striations into two or three segments. Assuming that cross-striations are daily features, each intradian line must represent less than 24 h of enamel secretion. However, their exact rhythmicity is unknown (reviewed in Smith et al. 2003).
Fig. 7.
Transmitted light micrograph of Retzius lines formed between minocycline injections (labelled M) (individual CF 326). Two to three cross-striations are seen between the first label and the Retzius line above it (white line labelled l), followed by six Retzius lines (numbered 2–7). Given that the labels were administered 28 days apart, this indicates that each interval (1–2, 2–3, 3–4, etc.) represents 4 days. The 4-day Retzius line periodicity in this tooth can also be seen in the intervals between Retzius lines 1–2 and 2–3.
Retzius lines are a prominent type of internal long-period features found in primate enamel, which may also be seen encircling the surface of the tooth as concentric rings or troughs (termed perikymata). These structures are generally formed at an oblique angle to enamel prisms (as well as cross-striations and intradian lines), presumably due to their origin from a lateral (shoulder) portion of the ameloblast (e.g. Risnes, 1990, figs 12 and 13, pp. 142–143). They represent the successive positions of the enamel-forming front. The periodic nature of this feature, which may be assessed by the number of cross-striations between successive lines and is known to vary among primates (reviewed in Smith et al. 2003), has been one of the more contentious topics in the study of enamel microstructure (reviewed in FitzGerald, 1998). The final structure examined here is a poorly known class of incremental features termed laminations. Laminations appear parallel to Retzius lines, and have a similar spacing to cross-striations, but they do not tend to cross prisms perpendicularly, and may be found in aprismatic enamel. It has been unclear how these features relate to cross-striations or intradian lines (Smith et al. 2003, 2004).
Materials and methods
Histological material
The dental material examined included 98 histological sections of maxillary and mandibular teeth (dc, dp3, dp4, M1) from 17 immature pigtailed macaques (Macaca nemestrina), representing various developmental stages from birth to 458 days old. The majority of the sections were generated in the late 1970s/early 1980s as part of study on bone growth (Newell-Morris & Sirianni, 1982; Sirianni, 1985). Ten new sections were generated from a left upper dp4 and M1 and a lower M1 according to standard histological procedures (Reid et al. 1998a,b). The lower M1 was obtained from an individual that did not undergo fluorescent labelling (described below).
The sections were examined under transmitted, tandem scanning reflected, laser confocal and fluorescent light microscopy. Areas within sections that were determined to be oblique to a vertical plane through the long axis of the tooth were not used to assess the periodicity of incremental features, owing to the likelihood of interference artefacts. Sections that showed clear growth disturbances in the enamel were identified, and disturbances were matched to labels in the dentine using fluorescent light microscopy. Regions showing clear incremental features in association with confirmed labels were selected and imaged under high magnification. The number of each specific feature (cross-striations, intradian lines, Retzius lines, laminations) was determined between markers, and then divided by the injection interval (in days) to yield a periodicity (number of features per day). Features were also related to one another to provide additional confirmation.
Treatment
An ideal study of the periodicity of incremental features in enamel requires a labelling protocol that leaves distinct markers over known-period intervals (e.g. Mimura, 1939; Schour & Hoffman, 1939; Okada, 1943). Several studies have examined experimentally labelled macaque dentitions in the past few decades (Molnar et al. 1981; Newell-Morris & Sirianni, 1982; Bromage, 1991; Dean, 1993). The material originally used by Newell-Morris & Sirianni (1982) was considered to be most suitable for a comprehensive study of incremental formation, as the individuals were completing deciduous tooth formation and beginning permanent tooth development while the labels were administered; thus, it was used as the basis of the current study.
During the original study on bone growth rates, individuals were injected 3–5 times with 1–3 fluorescent labels: minocycline hydrochloride, xylenol orange and DCAF (2,4-Bis [N,N′ Di (carbomethyl) aminomethyl] fluorescein), during the final 2 months of life (Newell-Morris & Sirianni, 1982; Sirianni, 1985). Intravenous injections were given to pregnant females at 20–50 mg kg−1 pregnant weight, and postnatal injection doses were 35 mg kg−1. Dates of conception, injections, birth and death are given in Table 1. Although the three labels were chosen because of their minimal effect on bone growth, they were all apparent in the dentine under fluorescent light microscopy (Fig. 2), although the colours of each varied slightly depending on the type of illumination used. Minocycline appeared to cause the most evident growth disturbance in both the enamel and the dentine (Fig. 3), although at these doses, these labels did not appear to disrupt the production of enamel increments for long; daily increments within the injection intervals were present as expected (discussed below). Xylenol orange and DCAF did not appear to cause growth disturbances as frequently as did minocycline.
Table 1.
Material and treatment record of Macaca nemestrina used in this study
| Prenatal | Postnatal | ||||||
|---|---|---|---|---|---|---|---|
| CF | Event | Age* | Interval | CF | Event | Age† | Interval |
| 285 | XO | 129 | – | 317 | M | 2 | – |
| DCAF | 136 | 7 | XO | 4 | 2 | ||
| M | 143 | 7 | DCAF | 6 | 2 | ||
| M | 153 | 10 | M | 8 | 2 | ||
| Birth | 170 | 17 | Sacrifice | 8.7 | 0.7 | ||
| XO | 181 | 11 | |||||
| M | 193 | 12 | 319 | M | 6 | – | |
| Sacrifice | 199 | 6 | DCAF | 8 | 2 | ||
| XO | 10 | 2 | |||||
| 296 | DCAF | 146 | – | M | 12 | 2 | |
| XO | 157 | 11 | Sacrifice | 13 | 1 | ||
| M | 167 | 10 | |||||
| Stillborn | 170 | 3 | 324 | DCAF | 14 | – | |
| M | 16 | 2 | |||||
| 300 | DCAF | 142 | – | XO | 18 | 2 | |
| XO | 151 | 9 | M | 20 | 2 | ||
| M | 159 | 8 | Sacrifice | 20.9 | 0.9 | ||
| Birth | 178 | 19 | |||||
| Sacrifice | 186 | 8 | 325 | XO | 10 | – | |
| M | 12 | 2 | |||||
| 302 | DCAF | 127 | – | DCAF | 14 | 2 | |
| XO | 136 | 9 | M | 16 | 2 | ||
| M | 144 | 8 | Sacrifice | 16.4 | 0.4 | ||
| Stillborn | 145 | 1 | |||||
| 326 | XO | 18 | – | ||||
| 303 | DCAF | 122 | – | M | 20 | 2 | |
| XO | 131 | 9 | DCAF | 22 | 2 | ||
| M | 139 | 8 | M | 24 | 2 | ||
| Birth | 160 | 21 | Sacrifice | 24.7 | 0.7 | ||
| Sacrifice | 166 | 6 | |||||
| 330 | DCAF | 22 | – | ||||
| 320 | M | 146 | – | M | 24 | 2 | |
| M | 153 | 7 | XO | 26 | 2 | ||
| M | 159 | 6 | M | 28 | 2 | ||
| M | 167 | 8 | Sacrifice | 28.7 | 0.7 | ||
| Birth | 172 | 5 | |||||
| M | 174 | 2 | 336 | M | 57 | – | |
| Sacrifice | 177 | 3 | XO | 59 | 2 | ||
| DCAF | 61 | 2 | |||||
| 327 | M | 146 | – | Sacrifice | 62.6 | 1.6 | |
| M | 153 | 7 | |||||
| M | 160 | 7 | 337 | M | 57 | – | |
| Birth | 166 | 6 | XO | 59 | 2 | ||
| M | 167 | 1 | DCAF | 61 | 2 | ||
| M | 171 | 4 | Sacrifice | 65.4 | 4.2 | ||
| Sacrifice | 174 | 3 | |||||
| M6898 | no treatment | ||||||
| 333 | M | 159 | – | ||||
| M | 166 | 7 | |||||
| Birth | not recorded? | ||||||
| M | 172 | 6 | |||||
| XO | 183 | 11 | |||||
| DCAF | 197 | 14 | |||||
| Sacrifice | 200 | 3 | |||||
CF indicates the code of each individual in the original study. Event indicates administration of one of the three labels: XO = xylenol orange, M = minocycline hydrochloride, DCAF = 2,4-Bis [N,N′ Di (carbomethyl) aminomethyl] fluorescein, or birth, stillbirth or sacrifice.
Age is postconception days.
Age is weeks after birth. Interval is interval between events, in days for prenatal individuals and in weeks for postnatal individuals.
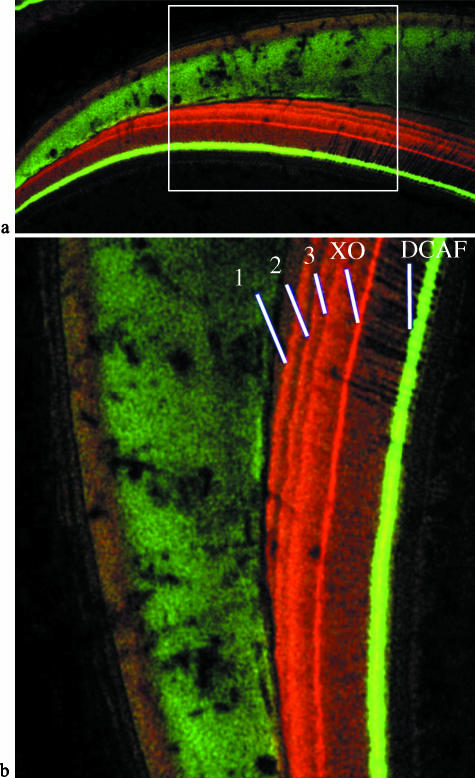
Fig. 2.
(a,b) Laser confocal scanning microscope images of a deciduous third premolar. (a) Overviews of the lateral and cervical enamel (cervix to the left), showing the area enlarged and rotated in (b) (white box). (b) Enamel is the faintly illuminated green tissue on the left, and the dentine (on the right) shows five fluorescent lines that correspond to the series of five injections given to this individual (CF 333). Line 1 (red/orange) represents the first minocycline injection, followed 7 days later by a second minocycline injection (Line 2), 6 days later by a third minocycline injection (Line 3), 11 days later by a xylenol orange injection (Line XO, red) and 14 days later by a DCAF injection (Line DCAF, bright green). This animal was killed 3 days later.
Fig. 3.
Fluorescence and transmitted light microscope images showing a series of accentuated lines (growth disturbances) related to minocycline injections (individual CF 327). Fluorescence microscopy (top image) and light microscopy (bottom image) of the same area showing five labels (numbered) in the dentine (above EDJ), which correspond with the same five labels in the enamel (below EDJ). The enamel dentine junction (EDJ) is labeled on the left, and the cervix is to the right of the images.
Incremental features
Cross-striations
This study provides additional support for the hypothesis that features identified as cross-striations, light and dark bands that cross prisms perpendicularly, are the result of a 24-h secretion rhythm. Lines in enamel matched to labels in dentine showed the same number of cross-striations between them as days between respective injections (Fig. 4), which was observed in several sections from multiple individuals. This is consistent with experimental studies by Mimura (1939), Okada (1943) and Bromage (1991), and the deductive study by Antoine (2000). Given the consistent appearance and periodicity of cross-striations, counts and measurements may be used to establish the periodicity of other incremental features, as well as the rate and duration of enamel formation.
Fig. 4.
Light microscope image of cross-striations in cervical enamel. In this individual (CF 300), one xylenol orange injection (XO) was followed 8 days later by a minocycline injection (M). Eight cross-striations (indicated by red lines) can be clearly distinguished between these two labels, confirming the daily nature of this feature. The cervix is to the lower left.
Intradian lines
This study suggests that intradian lines, defined here as thin bands that subdivide daily lines, are the result of a 12-h rhythm in enamel production. Intradian lines are generally difficult to resolve clearly, as they may be very closely spaced and require high magnification, which results in poorer definition of features (Fig. 5). In some cases, it appeared that there were either two or three intradian lines between cross-striations. However, information from underlying layers may give the impression of additional structures in the focal plane. While manually focusing through sections, features tended to shift position and number. When viewing these regions under lower magnification with more distinct resolution, it appeared that a single subdivision is located in the centre of the interval between cross-striations (implying two evenly spaced 12-h increments). It was not possible to count intradian lines between pairs of labels in any section, as they were generally very localized, or not clear in long successive runs. However, it was possible to show an inclusive series of eight intradian lines between a pair of Retzius lines in two teeth with 4-day Retzius line periodicities, representing additional evidence that these features have a 12-h periodicity (Fig. 6). Daily laminations (discussed below) also infrequently showed single subdivisions that appeared to be equivalent to 12-h subdivisions.
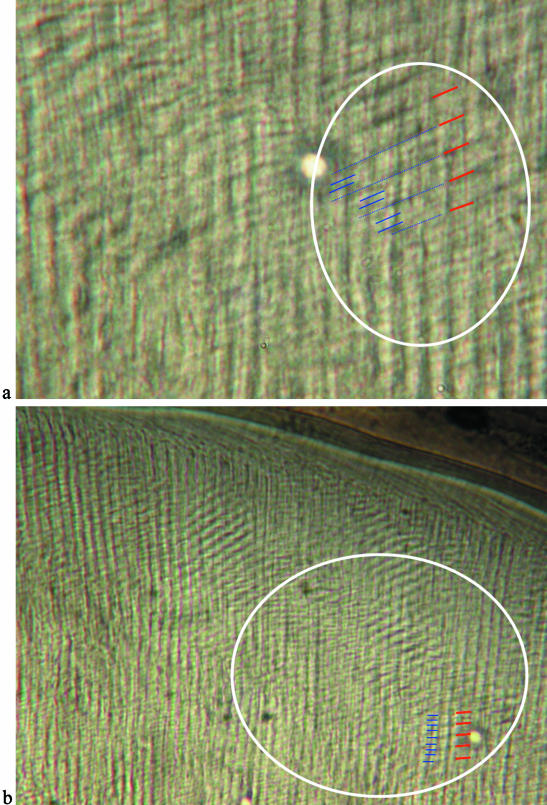
Fig. 5.
(a,b) Polarized light micrographs showing the relationship between intradian lines and cross-striations (individual CF 336). (a) High-magnification polarized light image of an area where 2–3 intradian lines (blue) are visible between cross-striations (red), and (b) the same area at lower magnification showing pairs of intradian lines between cross-striations. The enamel surface is at the top of these images.
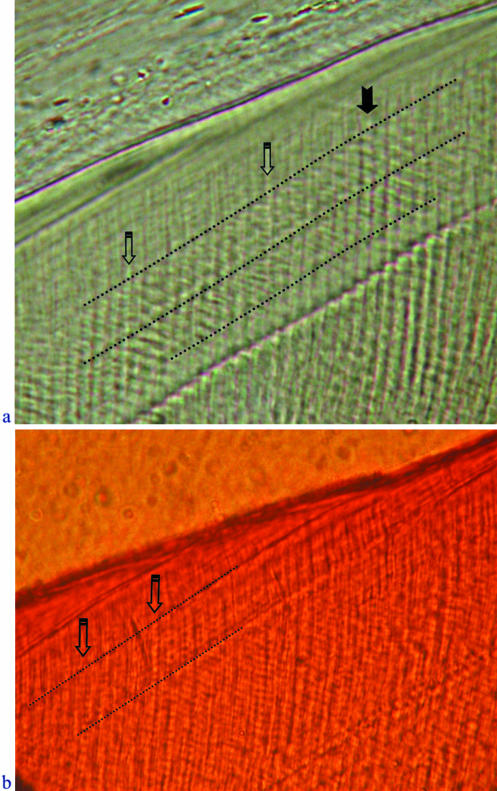
Fig. 6.
(a,b) Transmitted light micrographs of intradian lines between Retzius lines. (a) Four cross-striations are evident along a vertical prism (solid arrow) between a pair of Retzius lines (dotted lines), and eight intradian lines are present within the same interval (open arrows, downward-pointing prisms) (individual CF 326). (b) Eight intradian lines (open arrows) between a pair of Retzius lines (dotted lines) (individual M6898). In both images the enamel surface is the dark diagonal line near the top, and the cervix is to the lower left.
Experimental work in dentine has previously confirmed the presence of subdaily features (Kawasaki et al. 1977; Rosenberg & Simmons, 1980; Ohtsuka & Shinoda, 1995; Ohtsuka-Isoya et al. 2001), which will also be referred to here as intradian lines. Work by Rosenberg & Simmons (1980) and Ohtsuka & Shinoda (1995) suggested that 2–3 intradian lines are formed in dentine per day. However, in the former study, daily and intradian lines were typically identified in different preparations, and were not illustrated together in the same section. These authors contrasted the ratio of the average subdaily repeat interval (∼10 µm) with the average daily repeat interval (∼20 µm, sometimes up to 30 µm) to argue for the presence of an 8-h and/or 12-h rhythm, but they did not illustrate subdaily lines between daily lines. Ohtsuka & Shinoda (1995) presented more convincing evidence for intradian lines using rats injected with lead salt markers. They showed several images where more than two and fewer than three intradian lines were formed per day (14 lines/5 days, 7 lines/3 days). They also contrasted the average 16–24-µm daily line spacing with the subdaily 6–8-µm spacing, which they regarded as additional evidence for an 8-h rhythm. Most recently, Ohtsuka-Isoya et al. (2001) illustrated intradian lines between labels that showed a 12-h periodicity, but did not report lines with an 8-h periodicity.
After their initial description in enamel by Gustafson (1959), it appeared that intradian lines were not generally recognized and accepted until the 1990s (e.g. Dean & Scandrett, 1996; FitzGerald, 1996). Boyde (1989) suggested that they were the result of artefacts from other layers (also noted by Antoine et al. 1999). Smith et al. (2003, 2004) first demonstrated conclusively that they are genuine structural phenomena by documenting them with scanning electron microscopy (SEM) and tandem scanning reflected light microscopy (TSRLM), methods that are not influenced by optical artefacts that may be present in conventional transmitted or polarized light microscopy. However, given the variable appearance of intradian lines, and the potential for optical interference from light microscopy, it is suggested that cross-striations be used preferentially for determination of rate and time instead of intradian lines.
Retzius lines
It was difficult to determine experimentally the periodicity of Retzius lines. Few sections showed Retzius lines between labels; Retzius lines were often best defined near the enamel surface, while the labels were often clearest near the enamel dentine junction (EDJ), and the two generally did not extend far enough into the mid-thickness enamel to be related to one another. It was possible to relate Retzius lines to a known-period interval in one section (Fig. 7). In this case, a pair of minocycline lines was identified in the cervical enamel and traced to the surface, representing 28 days of enamel formation. Six complete Retzius line intervals (seven lines in total) plus four cross-striations were seen between these two labels. This suggests that each Retzius line interval represents 4 days, which is consistent with observations made on cross-striations between individual pairs of Retzius lines in this individual. Where it was possible to make counts, the number of cross-striations between pairs of Retzius lines was equal within a section, tooth and/or individual. This is consistent with several large studies on human dentitions (e.g. Beynon, 1992; FitzGerald, 1996; Reid et al. 2002).
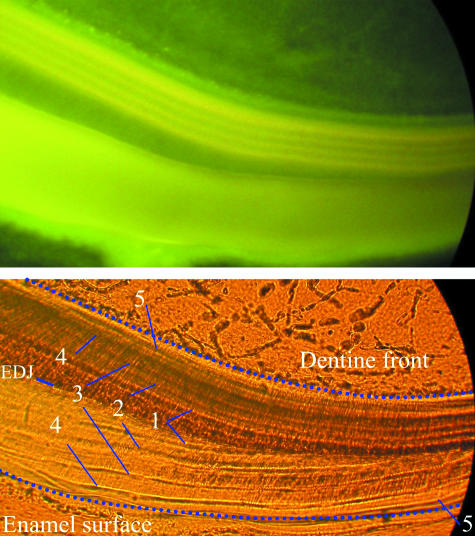
Laminations
This study demonstrates that laminations, closely spaced bands that follow the course of the developing enamel front, show a daily periodicity (Fig. 8). Laminations were observed to be most common in the enamel over the dentine horn, along the EDJ in the cervical region, and in the outer enamel in association with aprismatic enamel and/or Retzius lines. As noted in Smith et al. (2003), 2004), the development of laminations in specific regions may be related to aprismatic enamel production or to a certain type of prism packing pattern (i.e. Pattern 1), but this requires further study. Laminations were also occasionally observed to show a subdivision (Fig. 9), seen in several sections in the cervical and lateral enamel at the EDJ. When it was possible to image these subdivisions, they consistently appeared mid-way between laminations, representing additional evidence that a class of subdaily features is produced every 12 h.
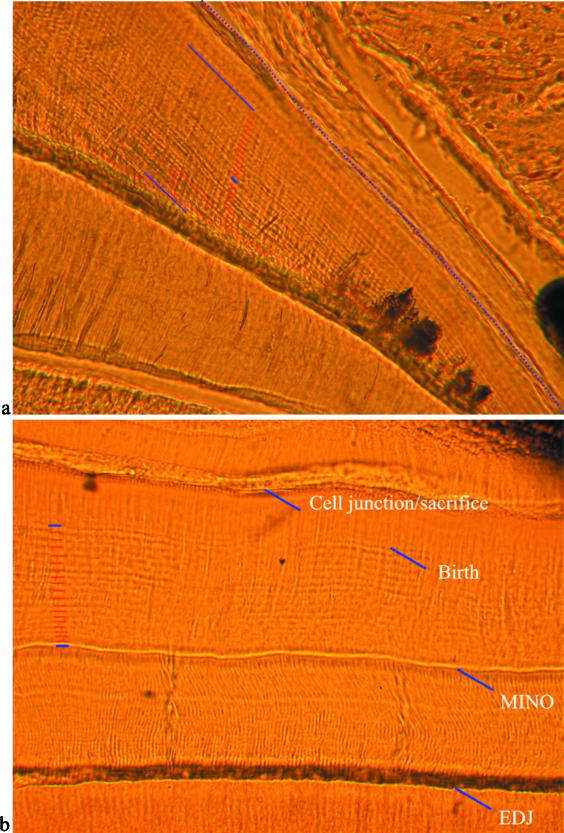
Fig. 8.
(a,b) Transmitted light micrographs showing the daily nature of laminations. (a) Laminations (labelled with red lines) between injections (labelled in blue) near the cervix (to the right). This individual (CF 326) received a minocycline injection, followed 2 weeks later with a DCAF injection, 2 weeks after that with a minocycline injection, and was killed shortly after the final injection. Twenty-eight laminations can be seen between the first and third labels, which are equal to 28 days of formation. (b) Laminations in the occlusal enamel of a mandibular dp4 cut transversely. In this individual (CF 300), a prenatal minocycline injection was given, which was followed by birth 19 days later, and the animal was killed 8 days after that. This image shows the enamel dentine junction (EDJ) at the bottom, a growth disturbance caused by minocycline (MINO), 19 laminations (red lines on the left) between this disturbance and the neonatal line (Birth), and eight laminations (not labelled) between this disturbance and the end of enamel formation (sacrifice).
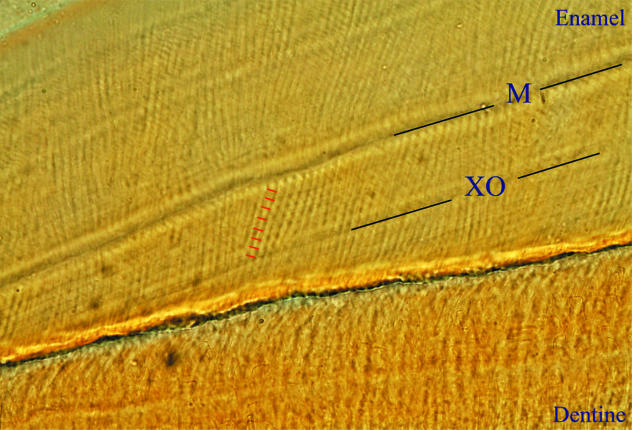
Fig. 9.
(a,b) Transmitted light micrograph showing subdaily lines (blue lines) between laminations (red lines) in (a) lateral enamel at the enamel dentine junction (EDJ), and (b) cervical enamel of a transverse section (individual CF 336).
Bromage (1991) also inadvertently provided similar evidence of the daily nature of laminations by figuring and counting a series between labels that corresponded to the number of days of formation (Bromage, 1991, figs 4 and 6, pp. 208 and 210). He did not distinguish cross-striations from laminations, although both can be distinguished from one another in his figures. Additional evidence for the daily nature of laminations comes from observations in the current study that cross-striations and laminations may be seen in register with one another (Fig. 10), as also implied by Whittaker (1982), Risnes (1998) and Li & Risnes (2004). Despite this finding, laminations should not be used to determine the periodicity of Retzius lines. It is suggested that the common appearance of Retzius lines as broad dark bands is often enhanced by the optical superimposition of laminations. When images are optically through-focused, the ‘Retzius line’ may shift position and split into two parallel lines (illustrated in Smith, 2004, fig. 3.16a–f, pp. 165–167). This may result in underestimation of Retzius line periodicity when laminations are counted as a proxy for cross-striations (such as in Fig. 7 in the interval between Retzius lines 2 and 3; also see Smith et al. 2003, 2004).
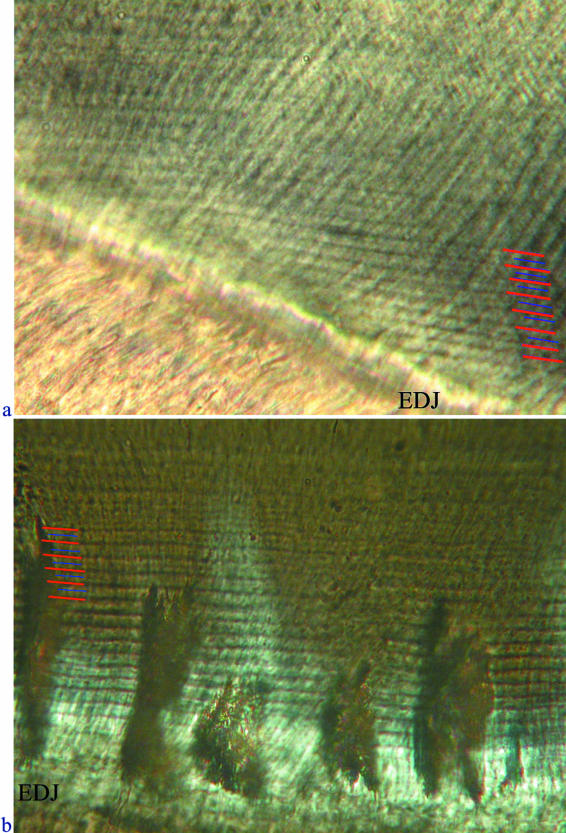
Fig. 10.
Transmitted light micrograph showing nine laminations in register with nine cross-striations (between the two dotted isochronous lines), confirming that these features are of equal periodicity (individual M6898).
One possible explanation for laminations is that they are structurally equivalent to cross-striations (Tafforeau, 2004). However, observations of focal-plane-specific information (SEM and TSRLM) show that laminations do not simply result from an optical illusion of cross-striations in register (‘stairstep configuration’ seen at times under light microscopy; e.g. Smith, 2004, fig. 1.13a–d, pp. 53–54). Laminations generally bisect prisms obliquely, and cannot be structurally distinguished from Retzius lines, except for the shorter intervals between successive laminations and the external manifestation of Retzius lines as perikymata. It is possible that, during cross-striation production (from the Tomes' process of the ameloblasts), laminations result from the circadian rhythm manifesting across the entire enamel-forming front (secretory ameloblasts), yet any developmental model of lamination formation must account for their unique orientation (relative to cross-striations), which is suggested to be due to their separate and discrete origin.
The geometric relationship between laminations and Retzius lines in enamel is similar to the relationship between von Ebner's (daily) lines and Andresen's (long-period) lines in dentine. In both instances, daily features run parallel to long-period features. Given the difficulty of accurately resolving successive daily lines in dentine, cross-striations are generally used to determine the periodicity of long-period lines in both tissues (Dean et al. 1993; Dean & Scandrett, 1996). This study has demonstrated that the geometric relationship between cross-striations and Retzius lines is more ideal for assessing rate or time, as cross-striations are generally orientated at an angle to Retzius lines, and may be less prone to optical superimposition with Retzius lines than are laminations.
Biological clocks
The physiological basis of biological rhythms has been extensively studied; both the eye and two specific regions of the forebrain are considered to be important for the production and maintenance of these rhythms: the pineal gland and the suprachiasmatic nucleus (SCN). Hastings (1997) reviewed evidence that suggests that there is a photoreceptive clock that is based in the eye, which produces melatonin in a circadian rhythm. Numerous studies have shown that this is not the only source of rhythm maintenance, as animals that have been experimentally blinded continue to demonstrate circadian rhythms. The pineal gland is located near the centre of the brain, posterior to the hypothalamus (which houses the SCN), and has been frequently noted for its potential role in maintaining circadian rhythms (reviewed in Hastings, 1997; Haus & Touitou, 1997). The main secretory product of the pineal gland is melatonin, which shows a circadian (and possibly circaseptan) production rhythm during the night, and is known to affect the production of a number of hormones as well as the maintenance of several physiological cycles (Vollrath et al. 1975; Haus & Touitou, 1997).
Experimental studies have demonstrated that the SCN is the mammalian control centre of biological rhythms (e.g. Reppert, 1995; Jin et al. 1999; Yamazaki et al. 2000; Ohtsuka-Isoya et al. 2001). Experimental evidence suggests that changing light–dark cycles may trigger the production of specific proteins that induce a chemical response in the hypothalamus, which releases hormones via the pituitary that affect a number of physiological activities. Ohtsuka-Isoya et al. (2001) have presented convincing evidence that the SCN is related to the production of daily lines in dentine, as rats in which the SCN is fully or partially lesioned experience respective permanent or temporary loss of circadian dentine increments. These authors noted that, given that there is no direct neurological connection between the SCN and the odontoblasts (dentine-forming cells), it is likely that there is a hormonal cue that triggers or maintains the production of daily increments. Several hormones that target odontoblasts include corticosterone, growth hormone, thyroxines and parathyroid hormone, which may play a role in the network from the SCN to the odontoblasts (reviewed in Ohtsuka-Isoya et al. 2001). Future work should examine specific hormonal influences on incremental development in dental hard tissues.
It has been suggested that the structure of circadian increments in dental hard tissues relates to variations in the level of carbonate and calcium (e.g. Okada, 1943; Boyde, 1989). Boyde (1989) hypothesized that circadian rhythms in ameloblast metabolism may be responsible for changes in p CO2, which lead to regular differences in the mineral composition of enamel that manifest as cross-striations. These differences are apparent when enamel is viewed under backscattered SEM. The majority of studies on circadian rhythms in secretory cells and hard tissue development have been conducted on dentine. Okada (1943) demonstrated that during the day, calcium levels in the blood plasma remained fairly constant, but at night there was a steady decrease. He suggested that this evidence provided a physiological explanation for the production of light bands in dentine during the day and dark bands at night; when blood plasma became more acidic (during the day), calcium levels in the blood increased, which decreased the precipitation of calcium in hard tissues, and produced a white, calcium-poor line. The opposite scenario at night produced a dark, calcium-rich line. Miani & Miani (1971) and Shinoda (1984) also demonstrated that feeding cycles and/or activity patterns may influence the production of circadian features.
Relatively little is known about the relationships between or among the biological clocks that control the production of incremental features in dental hard tissues. Given the diversity and complexity of cellular-level rhythms (reviewed in Roenneberg & Merrow, 2001), it is entirely possible that multiple independent rhythms are responsible for the production of different incremental features within a developmental system. Ohtsuka & Shinoda (1995) reported the presence of two types of short-period features (daily and subdaily) in rodent dentine that develop at different times after birth. These authors suggested that the coexistence of these two lines may result from two independent oscillatory mechanisms. This was further supported by the recent study of Ohtsuka-Isoya et al. (2001), who found that SCN obliteration correlated with cessation of daily lines, but not intradian lines in rodent dentine. Newman & Poole (1974), 1993) postulated that the existence of two circadian rhythms in enamel production could account for the relationship between Retzius lines and cross-striations. They suggested that a precise 24-h rhythm and a free-running circadian rhythm may run in tandem, regularly producing a Retzius line when the two cycles were most offset from one another. This theory of multiple physiological circadian cycles is supported by experimental work of Roenneberg & Morse (1993) on rhythms in a unicellular organism. They noted ‘phase jumps’ roughly every 7 days, which occurred when a faster circadian rhythm corrected itself relative to a slower rhythm, and suggested that separate but coupled (approximately circadian) oscillators may produce a rhythm that appears to be controlled by a 7-day clock. However, it is not clear how the range of known Retzius line periodicities (2–12 days among primates; reviewed in Smith et al. 2003) may be produced through interactions between known daily and subdaily short-period features. Additional work is needed to determine if and how interactions among incremental features relate to their periodic and structural manifestations.
In conclusion, this study has documented all of the known classes of incremental enamel features in experimentally labelled primate material. The results of this study are in agreement with several recent experimental studies that have examined the periodicity, secretion rate and development of incremental features in enamel and dentine. It is clear that two classes of daily features are formed in enamel (cross-striations and laminations), both of which may show 12-h subdivisions (intradian lines), and long-period features (Retzius lines) are also formed with a consistent periodicity. Collectively, these studies represent convincing evidence of incremental periodicities, as well as the correspondence of short- and long-period features between enamel and dentine (e.g. Dean et al. 1993; Dean & Scandrett, 1995, 1996), which may yield highly accurate estimates of secretion rate, formation time and age at death (Smith et al. in press).
Acknowledgments
Don Reid, Pam Walton, David Coleflesh and Milan Hadravsky provided invaluable technical assistance. In addition, Lawrence Martin, Anthony Olejniczak, Allison Cleveland, Don Reid, Bill Jungers, Dan Lieberman, Gary Schwartz and two anonymous reviewers provided helpful comments on earlier versions of the manuscript. Joyce Sirianni, Laura Newell-Morris and Daris Swindler kindly provided the original material. Chris Dean, Lawrence Martin, Don Reid, Daniel Antoine, Paul Tafforeau, Alexander Fabing and Bela Vigh also contributed to helpful discussions of incremental periodicities. This study was funded by Stony Brook University IDPAS Travel and Research Awards, NSF Dissertation Improvement Grant award 0213994 and support from the Max Planck Society.
References
- Antoine D, Dean C, Hillson S. The periodicity of incremental structures in dental enamel based on the developing dentition of post-medieval known-age children. In: Mayhall JT, Heikkinen T, editors. Dental Morphology '98: Proceedings of the 11th International Symposium on Dental Morphology. Oulu: Oulu University Press; 1999. pp. 48–55. [Google Scholar]
- Antoine D. University College London: 2000. Evaluating the periodicity of incremental structures in dental enamel as a means of studying growth in children from past human populations. PhD dissertation. [Google Scholar]
- Beynon AD. Circaseptan rhythms in enamel development in modern humans and Plio-Pleistocene hominids. In: Smith P, Tchernov E, editors. Structure, Function and Evolution of Teeth. London: Freund; 1992. pp. 295–309. [Google Scholar]
- Boyde A. Enamel. In: Oksche A, Vollrath L, editors. Handbook of Microscopic Anatomy Vol. V/6: Teeth. Berlin: Springer; 1989. pp. 309–473. [Google Scholar]
- Bromage TG. Enamel incremental periodicity in the pig-tailed macaque: a polychrome fluorescent labeling study of dental hard tissues. Am J Phys Anthropol. 1991;86:205–214. [Google Scholar]
- Dean MC. Daily rates of dentine formation in macaque tooth roots. Int J Osteoarch. 1993;3:199–206. [Google Scholar]
- Dean MC, Beynon AD, Reid DJ, Whittaker DK. A longitudinal study of tooth growth in a single individual based on long- and short-period incremental markings in dentine and enamel. Int J Osteoarch. 1993;3:249–264. [Google Scholar]
- Dean MC, Scandrett AE. Rates of dentine mineralization in permanent human teeth. Int J Osteoarch. 1995;5:349–358. [Google Scholar]
- Dean MC, Scandrett AE. The relation between long-period incremental markings in dentine and daily cross-striations in enamel in human teeth. Arch Oral Biol. 1996;41:233–241. doi: 10.1016/0003-9969(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Dean MC. Progress in understanding hominoid dental development. J Anat. 2000;197:77–101. doi: 10.1046/j.1469-7580.2000.19710077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald CM. University of Cambridge: Tooth crown formation and the variation of enamel microstructural growth markers in modern humans. PhD dissertation. [Google Scholar]
- FitzGerald CM. Do enamel microstructures have regular time dependency? Conclusions from the literature and a large-scale study. J Hum Evol. 1998;35:371–386. doi: 10.1006/jhev.1998.0232. [DOI] [PubMed] [Google Scholar]
- Gustafson A-G. A morphologic investigation of certain variations in the structure and mineralization of human dental enamel. Odontol Tidskr. 1959;67:366–472. [Google Scholar]
- Hastings MH. The vertebrate clock: localisation, connection, and entrainment. In: Redfern PH, Lemmer B, editors. Physiology and Pharmacology of Biological Rhythms. Berlin: Springer; 1997. pp. 1–28. [Google Scholar]
- Haus E, Touitou Y. Chronobiology and development of aging. In: Redfern PH, Lemmer B, editors. Physiology and Pharmacology of Biological Rhythms. Berlin: Springer; 1997. pp. 95–134. [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, De Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Tanaka S, Ishikawa T. On the incremental lines in human dentine as revealed by tetracycline labelling. J Anat. 1977;123:427–436. [PMC free article] [PubMed] [Google Scholar]
- Li C, Risnes S. SEM observations of Retzius lines and prism cross-striations in human dental enamel after different acid etching regimes. Arch Oral Biol. 2004;49:45–52. doi: 10.1016/s0003-9969(03)00195-x. [DOI] [PubMed] [Google Scholar]
- Miani A, Miani C. Circadian advancement rhythm of the calcification front in dog dentin. Minerva Stomatol. 1971;20:169–178. [PubMed] [Google Scholar]
- Mimura F. The periodicity of growth lines seen in enamel. Kobyo-Shi. 1939;13:454–455. (in Japanese) [Google Scholar]
- Molnar S, Przybeck TR, Gantt DG, Elizondo RS, Wilkerson JE. Dentin apposition rates as markers of primate growth. Am J Phys Anthropol. 1981;55:443–453. doi: 10.1002/ajpa.1330550405. [DOI] [PubMed] [Google Scholar]
- Newell-Morris L, Sirianni JE. Factors and Mechanisms Influencing Bone Growth. New York: Alan R. Liss; 1982. Parameters of bone growth in the fetal and infant macaque (Macaca nemestrina) humerus as documented by trichromatic bone labels; pp. 243–258. [PubMed] [Google Scholar]
- Newman HN, Poole DFG. Observations with scanning and transmission electron microscopy on the structure of human surface enamel. Arch Oral Biol. 1974;19:1135–1143. doi: 10.1016/0003-9969(74)90242-8. [DOI] [PubMed] [Google Scholar]
- Newman HN, Poole DFG. Dental enamel growth. J R Soc Med. 1993;86:61. [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M, Shinoda H. Ontogeny of circadian dentinogenesis in the rat incisor. Arch Oral Biol. 1995;40:481–485. doi: 10.1016/0003-9969(95)00002-7. [DOI] [PubMed] [Google Scholar]
- Ohtsuka-Isoya M, Hayashi H, Shinoda H. Effect of suprachiasmatic nucleus lesion on circadian dentin increment in rats. Am J Physiol Reg Comp Physiol. 2001;280:R1364–R1370. doi: 10.1152/ajpregu.2001.280.5.R1364. [DOI] [PubMed] [Google Scholar]
- Okada M. Hard tissues of animal body: highly interesting details of Nippon studies in periodic patterns of hard tissues are described. The Shanghai Evening Post Special Edition, Health, Recreation and Medical Progress. 1943. pp. 15–31.
- Reid DJ, Schwartz GT, Dean C, Chandrasekera MS. A histological reconstruction of dental development in the common chimpanzee, Pan troglodytes. J Hum Evol. 1998a;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Beynon AD, Ramirez Rozzi FV. Histological reconstruction of dental development in four individuals from a medieval site in Picardie, France. J Hum Evol. 1998b;35:463–477. doi: 10.1006/jhev.1998.0233. [DOI] [PubMed] [Google Scholar]
- Reid D, Ferrell R, Walton P. Histologically derived canine crown formation times from a medieval Danish sample. Am J Phys Anthropol Suppl. 2002;34:129. [Google Scholar]
- Reppert SM. Interaction between the circadian clocks of mother and fetus. In: Chadwick DJ, Ackrill K, editors. Circadian Clocks and Their Adjustment. Chichester: John Wiley & Sons; 1995. pp. 198–211. [DOI] [PubMed] [Google Scholar]
- Risnes S. Structural characteristics of staircase-type Retzius lines in human dental enamel analyzed by scanning electron microscopy. Anat Rec. 1990;226:135–146. doi: 10.1002/ar.1092260203. [DOI] [PubMed] [Google Scholar]
- Risnes S. Growth tracks in dental enamel. J Hum Evol. 1998;35:331–350. doi: 10.1006/jhev.1998.0229. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Morse D. Two circadian oscillators in one cell. Nature. 1993;362:362–364. doi: 10.1038/362362a0. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Circadian systems: different levels of complexity. Phil Trans R Soc Lond. 2001;356:1687–1696. doi: 10.1098/rstb.2001.0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GD, Simmons DJ. Rhythmic dentinogenesis in the rabbit incisor: circadian, ultradian, and infradian periods. Calcif Tissue Int. 1980;32:29–44. doi: 10.1007/BF02408519. [DOI] [PubMed] [Google Scholar]
- Schour I, Hoffman MM. Studies in tooth development. II. The rate of apposition of enamel and dentin in man and other mammals. J Dent Res. 1939;18:161–175. [Google Scholar]
- Scrutton CT. Periodic growth features in fossil organisms and the length of the day and month. In: Broche P, Sundermann J, editors. Tidal Friction and the Earth's Rotation. Berlin: Springer-Verlag; 1978. pp. 154–196. [Google Scholar]
- Shinoda H. Biological rhythms recorded in teeth. Chem Today. 1984;9:34–40. [Google Scholar]
- Sirianni JE. Nonhuman primates as models for human craniofacial growth. In: Watts E, editor. Nonhuman Primate Models for Human Growth and Development. New York: Alan R. Liss, Inc; 1985. pp. 95–124. [Google Scholar]
- Smith TM, Martin LB, Leakey MG. Enamel thickness, microstructure and development in Afropithecus turkanensis. J Hum Evol. 2003;44:283–306. doi: 10.1016/s0047-2484(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Smith TM. Stony Brook University: 2004. Incremental development of primate dental enamel. (available online at: http://www.paleoanthro.org/dissertation_list.htm) PhD dissertation. [Google Scholar]
- Smith TM, Martin LB, Reid DJ, de Bouis L, Koufos GD. An examination of dental development in Graecopithecus freybergi (=Ouranopithecus macedoniensis) J Hum Evol. 2004;46:551–577. doi: 10.1016/j.jhevol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Smith TM, Reid DJ, Sirianni JE. J Anat. Vol. 208. 2006. The accuracy of histological assessments of dental development and age at death; pp. 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafforeau P. Université de Montpellier II: Aspects Phylogénétiques et Fonctionnels de la Microstructure de l'Email Dentaire et de la Structure Tridimensionnelle des Molaires Chez les Primates Fossiles et Actuels: Apports de la Microtomographie à Rayonnement X Synchrotron. PhD dissertation. [Google Scholar]
- Vollrath L, Kantarjian A, Howe C. Mammalian pineal gland: 7-day rhythmic activity? Experientia. 1975;31:458–460. doi: 10.1007/BF02026378. [DOI] [PubMed] [Google Scholar]
- Whittaker DK. Structural variations in the surface zone of human tooth enamel observed by scanning electron microscopy. Arch Oral Biol. 1982;27:383–392. doi: 10.1016/0003-9969(82)90147-9. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]