Abstract
A fine branch of the median nerve innervates the periosteum and medullary cavity of the cat humerus. After branching to innervate the periosteum on the medial surface of the humerus, the nerve enters and supplies the medullary cavity via a nutrient foramen, accompanied by a small artery and vein. The composition of the fibres in the nerve was examined using electron microscopy. Myelinated fibres with diameters of 0.8–6.6 µm and unmyelinated fibres with diameters of 0.1–1.4 µm were observed. These diameters indicate that afferent fibres of this nerve are confined within the Group III and IV categories, and may therefore be nociceptive or mechanoreceptive in function. In addition, autonomic efferent fibres may also be present in these fibre groups. As no fibre diameters greater than 7 µm were noted, it appears that Group I and II fibres are absent in this nerve. The fibre distribution suggests that the principal role of this nerve is to relay bone-related nociceptive or mechanoreceptive information to the central nervous system and to provide autonomic regulatory influences on the bone.
Keywords: bone nociception, bone-related afferents, nerve fibre diameter, sensory innervation of bone, sensory nerves
Introduction
In the sensory innervation of skin and muscle, there has been a long-standing and well-recognized dichotomy between large-diameter sensory nerve fibres associated with tactile or kinaesthetic sensation and smaller-diameter myelinated or unmyelinated fibres that mediate nociception (Erlanger & Gasser, 1937; Burgess & Perl, 1973). Whereas sensory nerve fibres, along with autonomic efferent fibres, are also known to end in association with mineralized bone, bone marrow and the periosteum, it appears that the bulk of the bone-associated fibres reported are in the small-diameter myelinated or unmyelinated fibre categories and terminate as free endings (Duncan & Shim, 1977; Grönblad et al. 1984; Hohmann et al. 1986; Bjurholm et al. 1988a,b; Hill & Elde, 1988, 1991; Mach et al. 2002). However, there are reports of some larger-diameter afferent fibres with specialized endings in the mandibular periosteum of the cat (Sakada & Maeda, 1967; Sakada & Aida, 1971) and suggestions of specialized endings that may or may not be associated with large fibres in human long-bone periosteum (Ralston et al. 1960), but the extent to which large sensory fibres innervate bone, and whether they have a role in innocuous mechanosensory perception are issues that require further investigation (Rowe et al. 2005).
The specific aim of this study was to determine whether large-diameter sensory fibres (Groups I and II) exist within a fine branch of the median nerve that supplies the periosteum and medullary cavity of the humerus in the cat. This is the first stage of a broader morpho-functional study of bone innervation and the role of individual bone afferents in bone pain or broader aspects of mechanoreception (Mahns et al. 2004, 2005; Rowe et al. 2005). A preliminary report of this work has been presented in abstract form (Ivanusic et al. 2004).
Materials and methods
Three adult cats (each ∼3 kg in weight) were anaesthetized with either α-chloralose (70 mg kg−1 i.v.) or sodium pentobarbitone (60 mg kg−1 i.p.) and a skin incision made longitudinally, over the entire medial aspect of the upper forelimb (left forelimb for animal 1, right forelimb for animals 2 and 3). Biceps and triceps muscles were reflected to expose the medial aspect of the humerus and a fine nerve, associated with small blood vessels, which supplies both the periosteum and the nutrient foramen of the humerus. This fine nerve does not innervate nearby tissues such as muscle (our observations), and can be followed proximally to the point where it joins the parent median nerve. The nerve was separated from the blood vessels by careful blunt dissection, and immersed (in vivo) in fixative consisting of 2% paraformaldehyde and 2.5% gluteraldehyde in phosphate-buffered saline (PBS; pH 7.2) for 20 min, before an ∼5-mm-long segment was removed and placed in the same fixative and stored at 4 °C overnight. In order to ensure that we sampled a part of the nerve that innervated both the medullary cavity and the periosteum of the humerus, the segment was cut proximal to the visible branches to these bony sites, at ∼5 mm from the nutrient foramen. The nerve was post-fixed in 2% osmium tetroxide (in PBS; pH 7.2) for 2 h, dehydrated in graded concentrations of ethanol and embedded in LR White resin. Transverse ultrathin sections were cut on a Reichardt Ultracut E ultramicrotome, picked up on Formvar-coated slot grids, treated with uranyl acetate and lead citrate, and examined using a Zeiss 10 CR transmission electron microscope (TEM) and inbuilt camera. Semithin sections were also cut and stained with 1% toluidine blue (in dH2O) on glass slides.
Ultrathin sections were photographed at various magnifications (1500–20 000×). A complete montage of each nerve was constructed from photomicrographs taken at 5000× or 10 000× and printed to A4 size (total magnification, 13 500× or 27 000×), and the diameter of every myelinated fibre in each of the three nerves was measured directly from these montages using a calibrated scale bar. In order to compare values directly with the classic descriptions of myelinated fibres (Gasser & Grundfest, 1939; Lloyd, 1943; Gasser, 1955), the diameter of each myelinated fibre was determined from its outside border (including the myelin sheath). The presence of small-diameter, unmyelinated fibres in bone has been acknowledged by many others (see Introduction), and was therefore not examined in detail in this study. We presented data regarding unmyelinated fibres in only one sample by way of confirmation of earlier published reports. The unmyelinated fibre diameters were determined from a montage of photomicrographs taken at 10 000× and printed to A4 size (total magnification, 27 000×).
All fibre diameters were reported as the average of the maximum and minimum diameter measured for each fibre. It has been reported that determining the diameters in this way accounts well for irregularities in the shape of fibres (Rydzewski & Evans, 1977), produces smaller variability and requires less digitization time than other methods, with little difference in the diameters calculated (Ewart et al. 1989). One of the most common alternative methods is based on measuring the cross-sectional area of a fibre and then calculating the diameter of a circle with an equivalent area (Karnes et al. 1977; Auer, 1994). We compared our method with this by digitizing some of the photomicrographs taken of one of our nerve samples, pseudo-randomly selecting 30 of the fibre profiles and applying both techniques using Image J software (NIH). There was a highly significant, positive correlation between the diameters reported using these two methods (Spearman's correlation; rs = 0.9742, P < 0.0001), indicating that our method is as accurate as others for the measurement of fibre size within peripheral nerves such as the nerve to the cat humerus. These data also confirm the accuracy of the diameter measurements made by a single observer because any significant ‘intra-observer’ variation would be reflected in a poorer correlation. Low-power photographs (100×) of semithin sections were taken on an Olympus microscope with a SPOT camera.
All experiments conformed to the Australian National Health and Medical Research Council code of practice for the use of animals in research, and were approved by the University of New South Wales Animal Care and Ethics Committee.
Results
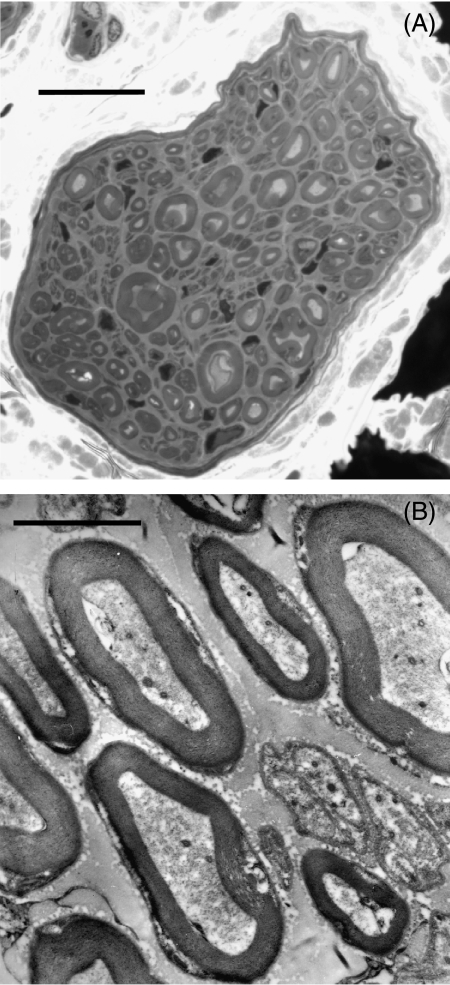
The low-power cross-sectional view (Fig. 1A) of the nerve to the cat humerus (diameter, 45 µm) reveals that the diameter of myelinated fibres varied, but did not exceed 7 µm. At higher magnification (Fig. 1B), both myelinated and unmyelinated fibres were observed, with the thickness of the myelin sheaths appearing to vary in proportion to myelinated fibre diameter. Unmyelinated fibres were often observed in groups in which a Schwann cell association was apparent. No fasciculation of the nerve was observed.
Fig. 1.
(A) Low-power light micrograph (100×) of a cross-section of the nerve to the humerus (scale bar = 10 µm). (B) Transmission electron micrograph (10 000×) of a portion of the nerve to the humerus, containing myelinated and unmyelinated fibres (scale bar = 2 µm).
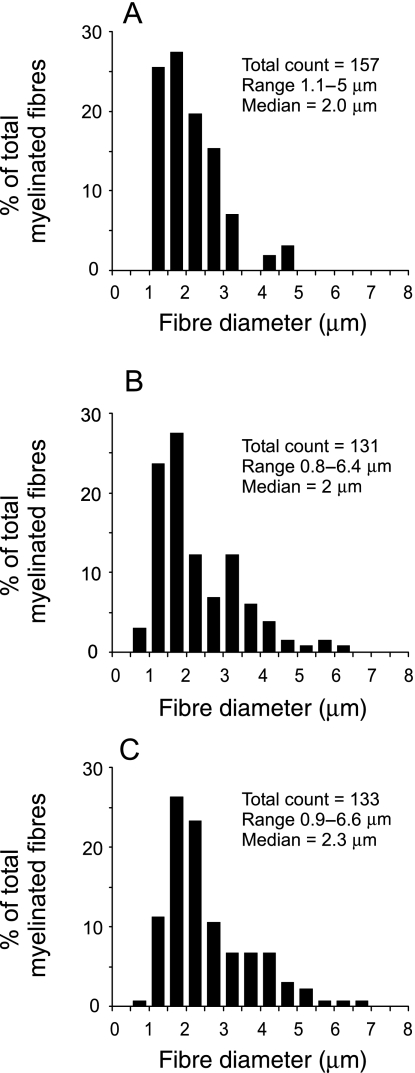
The distribution of myelinated fibre diameters within each of the three nerves (Fig. 2A–C) ranged from 0.9 to 6.6 µm, but almost all fibres (> 95% in each sample) were between 1 and 5 µm in diameter. All three distributions displayed a single peak, dominated by relatively small fibres, conferring on each distribution a median diameter value of ∼2 µm. Within these samples, myelin sheath thickness ranged from 0.1 to 1.8 µm.
Fig. 2.
The distribution of myelinated fibre diameters within three samples (A–C) of the median nerve branch that supplies the humerus bone. The y-axis reflects the proportion (%) of the total number of myelinated fibres counted in each nerve. The number of fibres counted, the range of diameters measured and the median diameter are presented for each nerve in the top right-hand side of each graph.
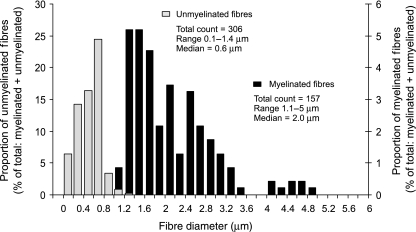
The detailed distribution of unmyelinated fibre diameters was measured in only one experiment (see Methods) and is plotted in Fig. 3, for the same nerve for which myelinated fibre diameter distributions are presented in Fig. 2(A). The unmyelinated fibres in this nerve numbered 306, almost double that of the myelinated fibres (157), and had a unimodal diameter distribution covering the range 0.1–1.4 µm.
Fig. 3.
The distribution of myelinated and unmyelinated fibre diameters within the nerve to the cat humerus. This sample was from the same nerve for which the data in Fig. 2(A) were derived. The y-axis reflects the proportion (%) of the total number of myelinated and unmyelinated fibres counted in the nerve. The number of fibres counted, the range of diameters measured and the median diameter are presented for the myelinated and unmyelinated fibre groups.
Discussion
Few studies have described the fibre composition of nerves innervating bone and, as none of these has been conducted for the humerus, this study is the first to report the diameter and distribution of fibres in the nerve to this long bone of the upper limb.
The analysis reveals that the nerve to the cat humerus contains only two groups based on fibre diameters, one a small myelinated (1–7 µm) fibre group, and the other an unmyelinated (0.1–1.3 µm) fibre group. This conclusion is based, first, on the finding that the distributions of myelinated fibre diameter and myelin sheath thickness had only a single peak, and second, on the absence of fibres of diameter > 7 µm, indicating an absence of large (Group I or II) fibre classes in this nerve. Based on comparisons with fibre diameters in the classical studies of Gasser & Grundfest (1939), Lloyd (1943) and Gasser (1955) on peripheral nerves such as the cutaneous saphenous nerve and the mixed nerve supplying the gastrocnemius muscle of the cat, we may conclude that the afferent nerves that supply the humerus consist of only Group III (small myelinated) and Group IV (unmyelinated) fibres. There is agreement that afferent fibres of these groups are associated predominantly with a nociceptive function (Burgess & Perl, 1973). However, there is evidence that suggests that some small-diameter, myelinated (Burgess & Perl, 1967; Burgess et al. 1968; Koltzenburg et al. 1997) and unmyelinated (Vallbo et al. 1993, 1999; Olausson et al. 2002) fibres in the hairy skin may also respond to light mechanical stimuli and produce percepts that have been described as non-painful. We cannot rule out the possibility that equivalent subgroups exist within the nerve to the cat humerus. Among the unmyelinated fibre group supplying the humerus, there may also be efferent fibres concerned with the autonomic regulation of the vasculature (Duncan & Shim, 1977) or those that effect the excitability of sensory afferents (Jänig et al. 1996).
There are two studies of the fibre composition of nerves to bone that allow a direct comparison with our study (Tokunaga, 1967; Seike, 1976). Tokunaga (1967) reported two populations of myelinated fibre diameters in the nerve supplying the canine tibia and suggested that they could be classified as Group II and III based on their diameters. However, it is not surprising that the larger afferents were observed in this study because it appears that the nerve may have been sampled proximal to the point at which branches leave this nerve to innervate muscle or aggregations of Pacininan corpuscles (PCs), which Tokunaga observed were in the vicinity of the periosteum. However, he also noted that the diameter of myelinated fibres in the branch of the nerve to the medullary cavity was confined to the Group III range with diameters between 1 and 4 µm. He did not deal with the unmyelinated fibre distribution as these could not be adequately sampled with the use of light microscopy. Seike (1976) also reported that both Group II and III myelinated fibres were present in this same nerve, but again, the level at which the nerve was sampled may have resulted in the inclusion of muscle or PC afferents in the sample.
Our finding of an absence of a large-fibre (Group I and II) sensory component in a nerve supplying a major long bone such as the humerus provides an excellent model for studies of the central processing of nociceptive inputs recruitable in the absence of concurrent large-fibre input. The use of the nerve to the cat humerus for this purpose would therefore be free of the potential ambiguities of interpretation consequent upon the concurrent activation of both nociceptive and large-diameter (Group I and II) mechanosensory inputs. The ease with which this nerve can be exposed and the distance over which it can be separated from surrounding vascular tissue further lends itself to electrophysiological analysis at the single fibre level, permitting characterization of response properties for individual afferent fibres in relation to the issue of bone pain and mechano-sensitivity (Mahns et al. 2004, 2005; Rowe et al. 2005).
Acknowledgments
We would like to thank C. Yeo and J. Norman for their technical assistance. This study was supported by the National Health and Medical Research Council of Australia.
References
- Auer RN. Automated nerve fibre size and myelin sheath measurement using microcomputer-based digital image analysis: theory, method and results. J Neurosci Meth. 1994;51:229–238. doi: 10.1016/0165-0270(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988a;9:165–171. doi: 10.1016/0196-9781(88)90023-x. [DOI] [PubMed] [Google Scholar]
- Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988b;25:119–125. doi: 10.1016/0165-1838(88)90016-1. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190:541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER. Cutaneous mechanoreceptors and nociceptors. In: Iggo A, editor. Handbook of Sensory Physiology. II. New York: Springer-Verlag; 1973. pp. 29–78. [Google Scholar]
- Duncan CP, Shim SS. J. Edouard Samson Address: the autonomic nerve supply of bone. An experimental study of the intraosseous adrenergic nervi vasorum in the rabbit. J Bone Joint Surg Br. 1977;59:323–330. doi: 10.1302/0301-620X.59B3.19482. [DOI] [PubMed] [Google Scholar]
- Erlanger J, Gasser HS. Electrical Signs of Nervous Activity. Philadelphia: University of Pennsylvania Press; 1937. [Google Scholar]
- Ewart DP, Kuzon WM, Jr, Fish JS, McKee NH. Nerve fibre morphometry: a comparison of techniques. J Neurosci Meth. 1989;29:143–150. doi: 10.1016/0165-0270(89)90026-5. [DOI] [PubMed] [Google Scholar]
- Gasser HS, Grundfest H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian fibers. Am J Physiol. 1939;127:393–414. [Google Scholar]
- Gasser HS. Properties of dorsal root unmedullated fibers on the two sides of the ganglion. J Gen Physiol. 1955;38:709–728. doi: 10.1085/jgp.38.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönblad M, Liesi P, Korkala O, Karaharju E, Polak J. Innervation of human bone periosteum by peptidergic nerves. Anat Rec. 1984;209:297–299. doi: 10.1002/ar.1092090306. [DOI] [PubMed] [Google Scholar]
- Hill EL, Elde R. Calcitonin gene-related peptide-immunoreactive nerve fibers in mandibular periosteum of rat: evidence for primary afferent origin. Neurosci Lett. 1988;85:172–178. doi: 10.1016/0304-3940(88)90347-3. [DOI] [PubMed] [Google Scholar]
- Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- Ivanusic J, Mahns D, Sahai V, Tracey D, Rowe M. The fibre composition of the median nerve branch that supplies the cat humerus. Proc Aust Health Med Res Congr. 2004;1424:305. (abstract) [Google Scholar]
- Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996;113:161–184. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- Karnes J, Robb R, O'Brien PC, Lambert EH, Dyck PJ. Computerized image recognition for morphometry of nerve attribute of shape of sampled transverse sections of myelinated fibers which best estimates their average diameter. J Neurol Sci. 1977;34:43–51. doi: 10.1016/0022-510x(77)90090-9. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC. Neuron patterns controlling transmission of ipsilateral hindlimb reflexes in cat. J Neurophysiol. 1943;6:293–315. [Google Scholar]
- Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Mahns D, Sahai V, Ivanusic J, Tracey D, Rowe M. A peripheral nerve preparation for studying the response characteristics of individual sensory nerve fibres of periosteal origin. Proc Aust Health Med Res Congr. 2004;1423:304. (abstract) [Google Scholar]
- Mahns D, Ivanusic J, Sahai V, Rowe M. Dual cortical representation of inputs arising from small diameter, bone-associated afferent fibres in the cat. Proc Aust Neurosci Soc. 2005;16:83. (abstract) [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Ralston HJ, 3rd, Miller MR, Kasahara M. Nerve endings in human fasciae, tendons, ligaments, periosteum, and joint synovial membrane. Anat Rec. 1960;136:137–147. doi: 10.1002/ar.1091360208. [DOI] [PubMed] [Google Scholar]
- Rowe MJ, Tracey DJ, Mahns DA, Sahai V, Ivanusic JJ. Mechanosensory perception: are there contributions from bone-associated receptors? Clin Exp Pharmacol Physiol. 2005;32:100–108. doi: 10.1111/j.1440-1681.2005.04136.x. [DOI] [PubMed] [Google Scholar]
- Rydzewski JJ, Evans MH. Diameter distribution spectral of myelinated axons in the median and ulnar nerves of the Soay sheep. J Anat. 1977;123:813–818. [PMC free article] [PubMed] [Google Scholar]
- Sakada S, Maeda K. Characteristics of innervation and nerve ending in cat's mandibular periosteum. Bull Tokyo Dent Coll. 1967;8:77–94. [PubMed] [Google Scholar]
- Sakada S, Aida H. Localization and shape of Golgi-Mazzoni corpuscles in the facial bones’ periosteum of the cat. Bull Tokyo Dent Coll. 1971;12:235–253. [Google Scholar]
- Seike W. Electrophysiological and histological studies on the sensibility of the bone marrow nerve terminal. Yonago Acta Med. 1976;20:192–211. [PubMed] [Google Scholar]
- Tokunaga J. The innervation of the diaphysis of the cat tibia. J Anat. 1967;101:125–136. [PMC free article] [PubMed] [Google Scholar]
- Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]