Abstract
Mandibular condylar cartilage is the principal secondary cartilage, differing from primary cartilage in its rapid differentiation from progenitor cells (preosteoblasts/skeletoblasts) to hypertrophic chondrocytes. The expression of three transcription factors related to bone and cartilage formation, namely Runx2, Osterix and Sox9, was investigated at the onset of mouse mandibular condylar cartilage formation by in situ hybridization. Messenger RNAs for these three molecules were expressed in the condylar anlage, consisting of preosteoblasts/skeletoblasts, at embryonic day (E)14. Hypertrophic chondrocytes appeared at E15 as soon as cartilage tissue appeared. Runx2 mRNA was expressed in the embryonic zone at the posterior position of the newly formed cartilage, in the bone collar and in the newly formed cartilage, but expression intensity in the newly formed cartilage was slightly weaker. Osterix mRNA was also expressed in the embryonic zone and in the bone collar, but was at markedly lower levels in the newly formed cartilage. Sox9 mRNA was continuously expressed from the embryonic zone to the newly formed cartilage. At this stage, Sox5 mRNA was expressed only in the newly formed cartilage. These results suggest that reduced expression of Osterix in combination with Sox9–Sox5 expression is important for the onset of condylar (secondary) cartilage formation.
Keywords: Indian hedgehog, preosteoblast, secondary cartilage, transcription factor
Introduction
Mandibular condylar cartilage is the principal secondary cartilage and differs somewhat from primary cartilage skeleton (Beresford, 1981). Silbermann et al. (1987) maintained that condylar cartilage develops from already differentiated progenitor cells called ‘skeletoblasts’, which are differentiated from undifferentiated mesenchymal cells, and function as osteochondro progenitor cells that can differentiate into either chondrocytes of secondary cartilage or mature osteoblasts. Several histological and histochemical studies indicate that condylar cartilage is derived from alkaline phosphatase (ALP)-positive, type I collagen mRNA-expressing progenitor cells continuous to the ossifying mandible (Shibata et al. 1996, 1997a; Miyake et al. 1997; Fukada et al. 1999), indicating that skeletoblasts have preosteoblast characteristics. Hence, we fundamentally support Silbermann's hypothesis. In addition, newly formed chondrocytes simultaneously express mRNA for collagen types I, II, X and aggrecan (Shibata et al. 1997a; Fukada et al. 1999), indicating that the progenitor cells (preosteoblasts/skeletoblasts) for this cartilage rapidly differentiate into hypertrophic chondrocytes. This is also characteristic of avian secondary cartilage (Buxton et al. 2003). We further demonstrated that the hypertrophic cell zone rapidly extends over the following few days (Shibata et al. 1996, 1997a; Fukada et al. 1999), and that bone sialoprotein mRNA expression is more extensively expressed than that in the tibial cartilage at the corresponding stage (Shibata et al. 2002). These features of differentiation differ from those of primary cartilage, including limb bud cartilage, although many common structural features also exist (Beresford, 1981).
Several transcription factors essentially involved in bone and/or cartilage formation have recently been established. Sox9 (SRY-box containing gene 9) is an essential factor for chondrocyte differentiation, and is expressed in the chondrogenic region (Wright et al. 1995; Ng et al. 1997; Zhao et al. 1997; Bi et al. 1999), directly regulates cartilage-specific genes (Bell et al. 1997; Lefebvre et al. 1997; Xie et al. 1999; Sekiya et al. 2000) and induces ectopic cartilage formation when misexpressed (Bell et al. 1997; Healy et al. 1999). In addition, Sox9 interacts with Sox5 (L-Sox5) and Sox6 (Lefebvre et al. 1998; Smits et al. 2001). Akiyama et al. (2002) demonstrated that Sox9 plays essential roles in the successive steps from undifferentiated mesenchymal cells to proliferating chondrocytes, and is required for Sox5 and Sox6 expression. Further, Mori-Akiyama et al. (2003) demonstrated that Sox9 is required for determination of the chondrogenic lineage in cranial neural crest cells.
Runx2 (runt-related transcription factor 2) is an essential transcription factor for bone formation, and Runx2 gene knockout mice completely lack bone tissue (Komori et al. 1997; Otto et al. 1997; Hoshi et al. 1999; Inada et al. 1999). Runx2 also regulates hypertrophic chondrocyte differentiation (Inada et al. 1999; Kim et al. 1999; Enomoto et al. 2000; Takeda et al. 2001; Ueta et al. 2001). Recently, Yoshida et al. (2004) demonstrated that Runx2 and Runx3 are essential for chondrocyte maturation, and that Runx2 regulates limb growth through the induction of Indian hedgehog (Ihh).
In avian secondary cartilage formation, Buxton et al. (2003) reported that Runx2-expressing preosteoblasts exit from the cell cycle and rapidly differentiate into hypertrophic chondrocytes, which is correlated with the up-regulation of Sox9. To date, there have been no studies related to the function of transcription factors for mouse secondary cartilage formation, although Runx2-deficient mice lack mandibular condylar cartilage (Shibata et al. 2004).
Furthermore, Nakashima et al. (2002) reported that the gene knockout of a novel zinc-finger-containing transcription factor called Osterix causes a complete lack of bone formation, demonstrating that Osterix is another essential factor for osteoblast differentiation. They further suggested an inhibitory role of Osterix in the chondrocyte differentiation pathway, as ectopic cartilage is formed in various regions of Osterix-deficient mice. However, the function of Osterix in secondary cartilage formation is unknown.
Based on these studies, we hypothesized that these three transcription factors, Sox9, Runx2 and Osterix, are involved in the formation of mouse secondary cartilage. The present study examined whether these transcription factors have similar functions to those in primary cartilage, using in situ hybridization in mandibular condylar cartilage as a model system.
As described above, chondrocytes of condylar cartilage rapidly differentiate into hypertrophic chondrocytes and the classification of zones is not established until embryonic day (E)16, and thus we propose that ‘the onset of condylar cartilage formation’ and ‘the subsequent differentiation process’ should be examined separately. Therefore, we focused mainly on onset during E14–16 in the present study.
Materials and methods
All animals were housed in facilities approved by the Tokyo Medical and Dental University. The animal-use protocol conformed to the NIH guidelines as stated in the Principles of Laboratory Animal Care (NIH publication no. 86-23, revised 1985), and was reviewed and approved by the Screening Committee for Animal Research at the Tokyo Medical and Dental University.
Tissue preparation
A total of ten pregnant ICR mice, of E14–16 (08:00 am on the day of the vaginal plug was designated as E0), were used for this study. At each time point, the pregnant mice were killed by cervical dislocation under ether anaesthesia, after which each fetal mouse was killed by cervical dislocation. The heads were then removed and immersed in 4% paraformaldehyde (0.1 m phosphate buffer, pH 7.4) for 1 day at 4 °C. The specimens were decalcified with 10% EDTA for 7 days at 4 °C and then embedded in paraffin using standard procedures. Sections (5 µm) were cut in the coronal plane, perpendicular to the sagittal plane, and parallel to the long axis of the condylar process of the mandible. Sections were stained with 0.1% toluidine blue (0.1 m phosphate buffer, pH 7.4) for histological examination.
RNA probes for in situ hybridization
Probes for cartilage matrix proteins, including aggrecan, and collagen types II and X were as used in previous studies (Fukada et al. 1999; Shibata et al. 2003a). Probes for the transcription factors, including Runx2, Osterix and Sox9, were kindly donated by Dr Kazuhisa Nakashima (Molecular Pharmacology, Department of Regulation of Internal Environment and Reproduction, Graduate School, Tokyo Medical and Dental University). These probes were used in a previous in situ hybridization study (Nakashima et al. 2002). Total RNA was extracted from the rib cartilage of newborn mice, and cDNAs for Ihh and Sox5 were then synthesized by reverse transcription-polymerase chain reaction (PCR) using a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech, Tokyo, Japan). The primers used were as follows: Ihh, forward, 5′(897)-ACCACCTVAGACCGTGACCGAAA-3′ (919), reverse, 5′(1674)-TTCAGCTTCCTGCCCCAGACACG-3′ (1651) amplified length, 778 bp (NCBI No. NM 010544); Sox5, forward, 5′(1769)-GAGCCCCACATAAAGCGTCCAAT-3′ (1791), reverse, 5′(2425)-ACCACAGTCTGTTGGCCCTTATGA-3′ (2402) amplified length, 657 bp (NCBI No. NM 01144). The Sox5 gene has two isoforms including a short form and a long form (L-Sox5) (Lefebvre et al. 1998). Although the probe used in this study recognized both types of Sox5, the short form of Sox5 is exclusively expressed in testis, and therefore it is reasonable to assume that our probe recognized L-Sox5 in the cartilage tissue. After identification of homology by sequencing, the PCR products were subcloned into pCRII vectors (Stratagene, La Jolla, CA, USA), and antisense and sense RNA probes were synthesized. Some probes were labelled with 35S-UTP using a riboprobe in vitro transcription system (Promega, Madison, WI, USA) while the others were labelled with digoxigenin using a DIG-labeling kit (Roche Diagnostics, Mannheim, Germany).
In situ hybridization using the digoxigenin-labelled probes and nucleic acid detection kit (Roche Diagnostics) was performed as previously described (Fukada et al. 1999; Shibata et al. 2002). When using 35S-UTP-labelled probes, sections were dipped with emulsion (NTB2, Kodak, Rochester, NY, USA) after hybridization and RNAase treatment, then exposed for 1 week at 4 °C for autoradiography. Sections were observed after counterstaining with nuclear fast red or haematoxylin. Sense probes were used as negative controls.
Results
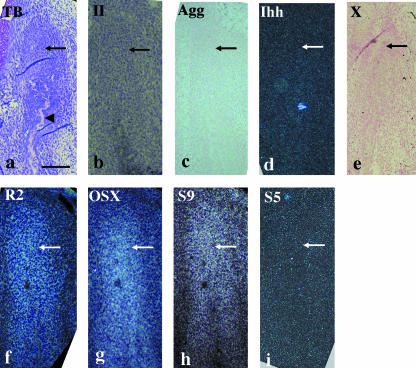
At E14, the anlage of the future condylar process (termed the condylar anlage), consisting of a mesenchymal cell condensation, was first observed in the posterior position of the ossifying mandible, as described previously (Shibata et al. 1996, 1997a, 2002; Fukada et al. 1999). Matrix metachromasia, indicative of cartilage formation, was not observed in the anlage at this stage (Fig. 1a). Type II collagen, aggrecan, Ihh and type X collagen mRNA were not expressed in the condylar anlage (Fig. 1b–e). Runx2, Osterix and Sox9 mRNA were expressed in the condylar anlage (Fig. 1f–h), whereas Sox5 mRNA was not (Fig. 1i).
Fig. 1.
Condylar anlage in coronal plane at E14. Toluidine blue staining (a) and in situ hybridization using digoxigenin-labelled probes (c,e) and 35S-UTP-labelled probes (b,d,f–i) of a bright field (b) or dark fields (d,f–i). (a) Although bone tissue (arrowhead) has formed, metachromasia is never seen in the anlage consisting of condensed mesenchymal cells corresponding to preosteoblasts/skeletoblasts (arrow). (b–e) Type II collagen (b), aggrecan (c), Ihh (d) and type X collagen (e) mRNA are not expressed in the condylar anlage (arrows). (f–i) Runx2 (f), Osterix (g) and Sox9 (h) mRNA are expressed in the condylar anlage, but Sox5 (i) mRNA is not (arrows). Width of view = 100 µm.
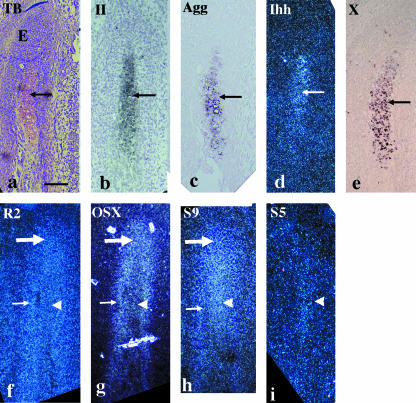
At E15, a metachromatically stained matrix was first detected in the condylar anlage, indicating the first formation of condylar cartilage. The bone collar formed around the newly formed cartilage. The region posterior to the newly formed cartilage, consisting of mesenchymal cell condensation, was termed ‘the embryonic zone’, and appears equivalent to ‘the germinal region’ in avian secondary cartilage (Buxton et al. 2003) (Fig. 2a). Type II collagen, aggrecan, Ihh and type X collagen mRNA were simultaneously expressed in the newly formed cartilage, but not in the embryonic zone or the bone collar (Fig. 2b–e). Runx2 mRNA was expressed in the embryonic zone, in the bone collar and in the newly formed cartilage, but expression intensity in the newly formed cartilage was slightly weaker compared with other positive regions within the same section (Fig. 2f). Osterix mRNA was expressed in the embryonic zone and in the bone collar, as seen in the condylar anlage at E14, but expression was markedly weaker in the newly formed cartilage compared with other positive regions within the same section (Fig. 2g). Sox9 mRNA was expressed in the embryonic zone and in the newly formed cartilage, as seen in the condylar anlage at E14, but expression was slightly weaker in the bone collar compared with other positive regions within the same section (Fig. 2h). Sox5 mRNA was only expressed in the newly formed cartilage (Fig. 2i).
Fig. 2.
Condylar anlage in coronal plane at E15. Toluidine blue staining (a) and in situ hybridization using digoxigenin-labelled probes (c,e) and 35S-UTP-labelled probes (b,d,f–i) of a bright field (b) or dark fields (d, f–i). (a) A metachromatically stained matrix is first recognized in the anlage (arrow). The embryonic zone (E) posteriorly to the metachromatically stained matrix consists of condensed mesenchymal cells. (b–e) Type II collagen (b), aggrecan (c), Ihh (d) and type X collagen (e) mRNA are simultaneously expressed in the newly formed condylar cartilage (arrows). (f) Runx2 mRNA is expressed in the embryonic zone (large arrow), in the bone collar (small arrow) and in the newly formed cartilage (arrowhead) with slightly weaker expression intensity. (g) Osterix mRNA is expressed in the embryonic zone (large arrow) and in the bone collar (small arrow), but remarkably reduced in the newly formed cartilage (arrowhead). (h) Sox9 mRNA is expressed in the embryonic zone (large arrow) and in the newly formed cartilage (arrowhead), but slightly reduced in the bone collar (small arrow). (i) Sox5 mRNA is only expressed in the newly formed cartilage (arrowhead). Width of view = 100 µm.
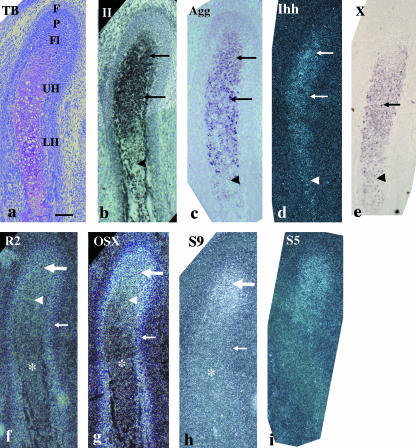
At E16, the condylar cartilage had increased in length, particularly in the hypertrophic cell zone. The zones usually present in the growing condyle (Luder et al. 1988) had become distinct: the fibrous cell (articulation) zone, polymorphic cell zone, flattened cell zone, upper hypertrophic cell zone and lower hypertrophic cell zone were all evident (Fig. 3a). Type II collagen and aggrecan mRNA had similar expression patterns and were expressed from the flattened cell zone to the upper hypertrophic cell zone, but were reduced in the lower hypertrophic cell zone (Fig. 3b,c). Ihh mRNA was expressed from the flattened cell zone to the upper hypertrophic cell zone, and was slightly reduced in the lower hypertrophic cell zone (Fig. 3d). Type X collagen mRNA was expressed throughout the entire hypertrophic cell zone, but was weaker in the lower hypertrophic cell zone (Fig. 3e). Runx2 mRNA was strongly expressed in the bone collar, from the fibrous cell zone to the polymorphic cell zone, as seen in the embryonic zone at E15, was moderately expressed in the flattened cell zone and was weakly expressed throughout the entire hypertrophic cell zone (Fig. 3f). Osterix mRNA was strongly expressed in the bone collar, from the fibrous cell zone to the polymorphic cell zone, as seen in the embryonic zone at E15, and was weakly expressed in the flattened cell zone, while markedly reduced expression was evident throughout the entire hypertrophic cell zone (Fig. 3g). Sox9 mRNA was strongly expressed from the polymorphic cell zone to the flattened cell zone, as seen in the embryonic zone and newly formed cartilage at E15; however, reduced expression was evident throughout the entire hypertrophic cell zone and in the bone collar (Fig. 3h). The pattern of Sox5 mRNA expression was similar to that of Sox9 (Fig. 3i).
Fig. 3.
Condylar cartilage in coronal plane at E16. Toluidine blue staining (a) and in situ hybridization using digoxigenin-labelled probes (c,e) and 35S-UTP-labelled probes (b,d,f–i) of a bright field (b) or dark fields (d, f–i). (a) The condylar cartilage has increased in length, especially the hypertrophic cell zone. The classification of zones becomes distinct: the fibrous cell (articulation) zone (F), polymorphic cell zone (P), flattened cell zone (Fl), upper hypertrophic cell zone (UH) and lower hypertrophic cell zone (LH) are evident. (b,c) Type II collagen (b) and aggrecan (c) mRNA are expressed from the flattened cell zone to the upper hypertrophic cell zone (arrows), but reduced in the lower hypertrophic cell zone (arrowheads). (d) Ihh mRNA is expressed from the flattened cell zone to upper hypertrophic cell zone (arrows), but slightly reduced in the lower hypertrophic cell zone (arrowhead). (e) Type X collagen mRNA is expressed throughout the entire hypertrophic cell zone (arrow), but more waekly expressed in the lower hypertrophic cell zone (arrowhead). (f) Runx2 mRNA is strongly expressed in the bone collar (small arrow), from the fibrous cell zone to the polymorphic cell zone (large arrow), moderately in the flattened cell zone (arrowhead) and weakly in the entire hypertrophic cell zone (*). (g) Osterix mRNA is strongly expressed in the bone collar (small arrow), from the fibrous cell zone to the polymorphic cell zone (large arrow), weakly in the flattened cell zone (arrowhead) but remarkably reduced in the entire hypertrophic cell zone (*). (h) Sox9 mRNA is expressed from the polymorphic cell zone to the flattened cell zone (large arrows), but reduced in the entire hypertrophic cell zone (arrowhead) and in the bone collar (small arrow). (i) Sox5 mRNA shows similar expression pattern to that of Sox9. Width of view = 100 µm.
Discussion
In the present study, the mRNA of three transcription factors, Runx2, Osterix and Sox9, were simultaneously expressed in the mesenchymal cells of the condylar anlage at E14, indicating that these three transcription factors are, more or less, involved in condylar cartilage formation. As these cells express ALP activity and type I collagen mRNA (Miyake et al. 1997; Shibata et al. 1997a; Fukada et al. 1999), they differentiated into preosteoblasts, corresponding to ‘skeletoblasts’, which can differentiate into either chondrocytes of secondary cartilage or mature osteoblasts (Silbermann et al. 1987).
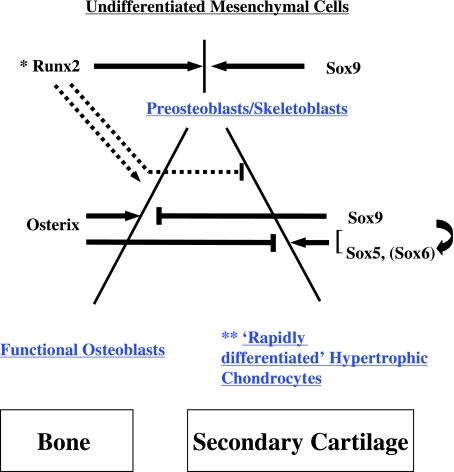
Akiyama et al. (2002) investigated Sox9 expression during the differentiation of primary cartilage, and reported that Sox9 plays essential roles in successive steps from undifferentiated mesenchymal cells to proliferating chondrocytes. Here, we assumed that ALP activity is an important marker of the differentiation of secondary cartilage, because ALP activity is not expressed until hypertrophic chondrocytes develop during the differentiation of primary cartilage, whereas secondary cartilage is derived from ALP-positive progenitor cells corresponding to preosteoblasts/skeletoblasts (Miyake et al. 1997; Shibata et al. 1997a). Therefore, preosteoblasts/skeletoblasts in the present study are apparently located downstream of undifferentiated mesenchymal cells in terms of ALP activity, and express Sox9 mRNA. Furthermore, condensed mesenchymal cells in Osterix-deficient mice and Runx2-deficient mice have preosteoblast characteristics (Nakashima et al. 2002; Shibata et al. 2004) and still express Sox9 mRNA (Nakashima et al. 2002; Yoshida et al. 2004), indicating that Sox9 is possibly involved in the differentiation of the undifferentiated mesenchymal cells to preosteoblasts/skeletoblasts during the differentiation of secondary cartilage (Fig. 4).
Fig. 4.
Involvement of transcription factors at the onset of mouse condylar (secondary) cartilage formation. Differentiation of osteoblasts is entirely according to Nakashima et al. (2002). Differentiation of the secondary cartilage is according to Silbermann et al. (1987) and Buxton et al. (2003), with modifications. Blue letters indicate ALP-positive cells. Inhibitory function of Osterix co-operating with stimulating function of Sox9–Sox5 (Sox6) may be important for the onset of condylar (secondary) cartilage formation. At this stage, ALP-positive preosteoblasts/skeletoblasts differentiate into functional osteoblasts or rapidly differentiate into hypertrophic chondrocytes of the secondary cartilage. *Lack of Runx2 does not necessary inhibit the formation of preosteoblasts/skeletoblasts, but does extensively inhibit their maturation (Komori et al. 1997; Shibata et al. 2004). **‘Rapidly differentiated’ hypertrophic chondrocytes can simultaneously express collagen types I, II and X, aggrecan, Ihh, bone sialoprotein and osteopontin (Shibata et al. 1997a, 2002; Fukada et al. 1999).
Runx2 and Osterix mRNA were also expressed in preosteoblasts/skeletoblasts in the present study. Although mesenchymal cells with preosteoblast characteristics did not form a sufficient amount of condensation in the calvaria and perichondrial regions of the limbs in the Runx2-deficient mice (Komori et al. 1997), a sufficient amount of mesenchymal condensation, which expresses Runx2, was formed in the membranous skeletal elements in Osterix-deficient mice, suggesting that Runx2 is required for the differentiation of undifferentiated mesenchymal cells into preosteoblasts. At the same time, Osterix acts downstream of Runx2 and is required for the differentiation of preosteoblasts into functional osteoblasts (Nakashima et al. 2002). Furthermore, Runx2 also plays an important role in the maturation of the mesenchymal condensation in membranous skeletal elements (Shibata et al. 2004). It is possible therefore that Runx2 is also required in the transition from undifferentiated mesenchymal cells to preosteoblasts/skeletoblasts during the differentiation of secondary cartilage, whereas Osterix might work downstream of this step (see Fig. 4).
Condylar cartilage was first detected at E15, and chondrocytes in the newly formed cartilage rapidly differentiated into hypertrophic chondrocytes expressing mRNA for collagen types II and X, aggrecan, and Ihh. Furthermore, these ‘rapidly differentiated’ hypertrophic chondrocytes differ from those in the primary cartilage in expression of the mRNA for type I collagen (Fukada et al. 1999), bone sialoprotein and secreted phosphoprotein 1 (osteopontin) (Shibata et al. 2002). In primary cartilage, Sox9 accelerates chondrocyte differentiation in proliferating chondrocytes, but regulates inhibition in the hypertrophy step (Akiyama et al. 2002). Sox9 mRNA was strongly expressed in newly formed chondrocytes of the condylar cartilage at E15, suggesting that Sox9 stimulates chondrocyte differentiation at the onset of condylar cartilage formation, although chondrocytes in the newly formed condylar cartilage rapidly differentiate into hypertrophic chondrocytes.
Interactions between Sox9 and Sox5 (L-Sox5) or Sox6 are related to cartilage formation (Lefebvre et al. 1998; Smits et al. 2001; Akiyama et al. 2002). Sox5 was first expressed in the newly formed cartilage in the present study, indicating that the Sox9–Sox5 system can also be applied to the onset of condylar cartilage formation. In addition, Runx2, in co-operation with Runx3, accelerates chondrocyte hypertrophy by inducing Ihh expression in the primary cartilage (Yoshida et al. 2004). In the present study, mRNA for Runx2 and Ihh was expressed in the newly formed condylar cartilage, but the expression intensity for Runx2 mRNA was slightly weaker compared with that of preosteoblasts/skeletoblasts in the embryonic zone and cells in the bone collar within the same section at E15. Therefore, Runx2 appears to be involved primarily in bone collar formation at this stage, and might, unlike primary cartilage, inhibit the onset of condylar cartilage formation.
Osterix mRNA expression was markedly reduced in the newly formed cartilage compared with the condylar anlage at E14 and the embryonic zone at E15. Nakashima et al. (2002) suggested that Osterix has an inhibitory action in cartilage formation. Thus, the reduced expression of Osterix expression in combination with Sox9–Sox5 expression might be important for the onset of condylar cartilage formation.
To examine the function of transcription factors for secondary cartilage formation, Buxton et al. (2003) investigated the expression of Runx2 and Sox9 in avian secondary cartilage, and described two routes to chondrocyte hypertrophy. Whereas chondrocytes regulated by Sox9 differentiate into prehypertrophic/hypertrophic chondrocytes mediated by the up-regulation of Runx2 in primary cartilage formation, preosteoblasts regulated by Runx2 differentiate into prehypertrophic/hypertrophic chondrocytes mediated by the up-regulation of Sox9 in secondary cartilage formation. We agree with their proposed concept in principle, but note some differences related to the onset of mouse condylar (secondary) cartilage formation. Sox9 is first expressed in newly formed avian secondary cartilage, not in preosteoblasts, which are precursors of secondary chondrocytes (Buxton et al. 2003), whereas Sox9 is already expressed in preosteoblasts/skeletoblasts in mice. Thus, we suggest that, instead of Sox9 as in avian cartilage, up-regulation of Sox 5 (also presumably Sox6) is involved in the onset of mouse secondary cartilage formation. Second, we would like to emphasize the importance of Osterix functioning at this site. Furthermore, we propose that hypertrophic chondrocytes of the primary cartilage and ‘rapidly differentiated’ hypertrophic chondrocytes of the secondary cartilage should be discriminated. Our model of the onset of mouse condylar (secondary) cartilage formation is summarized in Fig. 4.
In the present study, the hypertrophic cell zone markedly increased in length from E15 to 16, indicating that the mode of differentiation (rapid differentiation) observed at the onset of condylar cartilage formation is still predominant at this stage. The findings in the upper part of the condylar cartilage (from the fibrous cell zone to the upper hypertrophic cell zone) and in the bone collar at E16 confirm the role of these transcription factors at the onset of condylar cartilage formation described above.
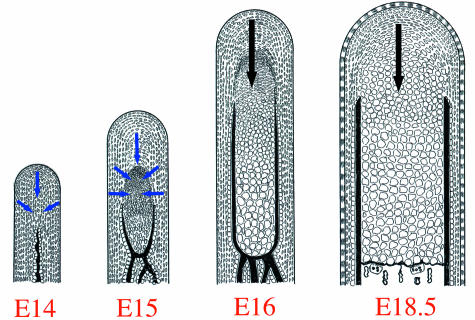
In this case, hypertrophic chondrocytes are most likely supplied from the preosteoblasts/skeletoblasts around the newly formed cartilage, suggesting that the direction of differentiation is convergent. The classification of zones observed in growing cartilage was first established at E16, although areas expressing each marker gene largely overlapped, indicating that subsequent differentiation progresses gradually (gradual differentiation) along the posterior–anterior direction. As described above, we believe that ‘the onset of condylar cartilage formation’ and ‘the subsequent differentiation process’ should be discussed separately. Furthermore, endochondral bone formation starts with resorption of the bone collar by osteoclasts at E16 (Shibata et al. 1996, 1997b) and is progressing at E18.5 (Shibata et al. 2003b). These previous studies and present results lead to the model of differentiation mode of the condylar cartilage from E14 to 18.5, as described in Fig. 5.
Fig. 5.
Schematic representations of histological features of mouse mandibular condylar cartilage from E14 to 16 and E18.5. Blue arrows indicate the direction of ‘rapid differentiation’ seen at the onset of condylar cartilage formation (from E14 to 16). Black arrows indicate the direction of ‘gradual differentiation’ seen in the subsequent differentiation process (E16~). At the onset of condylar cartilage formation, preosteoblasts/skeletoblasts rapidly differentiated into hypertrophic chondrocytes, and consequently the hypertrophic cell zone extensively increases in length. The direction of differentiation is convergent. At E16, the mode of differentiation is changed to the ‘gradual differentiation’ and its direction is restricted to posterior–anterior. Simultaneously, the endochondral bone formation starts with the resorption of bone collar by osteoclasts at this age, and is progressing at E18.5.
E16 may also be regarded as the turning point between the two types of differentiation, and Sox9 and Sox5 mRNA were strongly expressed from the polymorphic cell zone to the flattened cell zone, but were reduced throughout the entire hypertrophic cell zone at E16. Furthermore, in postnatal rat mandibular condylar cartilage, Runx2 is restrictively expressed in the prehypertrophic and hypertrophic cell zone (Rabie et al. 2004). These expression patterns are similar to those of the primary cartilage (Akiyama et al. 2002; Yoshida et al. 2004). Thus, we hypothesize that the regulation of transcription factors in the ‘subsequent differentiation process’ might have a similar pattern to that of primary cartilage. Further analysis of transcription factors during postnatal periods is required to confirm this hypothesis.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (No. 17591901) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Akiyama H, Chaboissier M-C, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DM, Leung KKH, Wheatley SC, et al. Sox9 directly regulates the type-II collagen gene. Nature Genet. 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- Beresford WA. Chondroid Bone, Secondary Cartilage and Metaplasia. Baltimore: Urban and Schwarzenberg; 1981. [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nature Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Buxton PG, Hall B, Archer CW, Francis-West P. Secondary chondrocytes-derived Ihh stimulates proliferation of periosteal cells during chick development. Development. 2003;130:4729–4739. doi: 10.1242/dev.00610. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Enomoto-Iwamoto M, Iwamoto M, et al. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T. In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J Anat. 1999;195:321–329. doi: 10.1046/j.1469-7580.1999.19530321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy C, Uwanogho D, Sharpe PT. Regulation and role of Sox9 in cartilage formation. Dev Dyn. 1999;215:69–78. doi: 10.1002/(SICI)1097-0177(199905)215:1<69::AID-DVDY8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hoshi K, Komori T, Ozawa H. Morphological characterization of skeletal cells in cbfa1-deficient mice. Bone. 1999;25:639–651. doi: 10.1016/s8756-3282(99)00223-9. [DOI] [PubMed] [Google Scholar]
- Inada M, Yasui T, Nomura S, et al. Maturational disturbance of chondrocytes in cbfa1-deficient mice. Dev Dyn. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80:159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, et al. Targeted disruption of cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. Sox9 is a potent activator of the chondrocyte-specific enhancer of the proa1 (II) collagen gene. Mol Cell Biol. 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luder HU, Leblond CP, Von Der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and Type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- Miyake T, Cameron AM, Hall BK. Stage-specific expression patterns of alkaline phosphatase during development of the first arch skeleton in inbred C57BL/6 mouse embryos. J Anat. 1997;190:239–260. doi: 10.1046/j.1469-7580.1997.19020239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of chondrogenic cell linege in the cranial neural crest. Proc Natl Acad Sci USA. 2003;100:9360–9365. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, et al. The novel zink finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Ng Ling-Jim, Wheatley S, Muscat GEO, et al. Sox9 binds DNA, activates transcription, and coexpresses with Type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Rabie ABM, Tang GH, Hägg U. Cbfa1 couples chondrocytes maturation and endochondral ossification in rat mandibular condylar cartilage. Archs Oral Biol. 2004;49:109–118. doi: 10.1016/j.archoralbio.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, et al. Sox9 enhances aggrecan gene promotor/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275:10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- Shibata S, Suzuki S, Tengan T, Ishii M, Kuroda T. A histological study of the developing condylar cartilage of the fetal mouse mandible using coronal sections. Arch Oral Biol. 1996;41:47–54. doi: 10.1016/0003-9969(95)00105-0. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Yamashita Y. Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. J Anat. 1997a;191:561–570. doi: 10.1046/j.1469-7580.1997.19140561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Suzuki S, Yamashita Y. An ultrastructural study of cartilage resorption at the site of endochondral bone formation in the fetal mouse mandibular condyle. J Anat. 1997b;191:65–76. doi: 10.1046/j.1469-7580.1997.19110065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y. In situ hybridization and immunohistochemistry of bone sialoprotein and secreted phosphoprotein 1 (osteopontin) in the developing mouse mandibular condylar cartilage compared with limb bud cartilage. J Anat. 2002;200:309–320. doi: 10.1046/j.1469-7580.2002.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Imai H, Abe T, Yamashita Y. In situ hybridization and immunohistochemistry of versican, aggrecan, and link protein and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003a;203:425–432. doi: 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Suda N, Fukada K, Ohyama K, Yamashita Y, Hammond VE. Mandibular coronoid process in parathyroid hormone-related protein-deficient mice shows ectopic cartilage formation accompanied by abnormal bone modeling. Anat Embryol (Berl) 2003b;207:35–44. doi: 10.1007/s00429-003-0325-6. [DOI] [PubMed] [Google Scholar]
- Shibata S, Suda N, Yoda S, et al. Runx2-deficient mice lack mandibular condylar cartilage and have deformed Meckel's cartilage. Anat Embryol (Berl) 2004;208:273–280. doi: 10.1007/s00429-004-0393-2. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Reddi AH, Hand AR, Leapman RD, von der Mark K, Franzen A. Further characterization of the extracellular matrix in the mandibular condyle in neonatal mice. J Anat. 1987;151:169–188. [PMC free article] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, et al. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta C, Iwamoto M, Kanatani N, et al. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol. 2001;153:87–99. doi: 10.1083/jcb.153.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nature Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Xie WF, Zhang X, Sakano S, Lefebvre V, Sandell LJ. Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J Bone Miner Res. 1999;14:757–763. doi: 10.1359/jbmr.1999.14.5.757. [DOI] [PubMed] [Google Scholar]
- Yoshida CA, Yamamoto H, Fujita T, et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Gene Dev. 2004;18:952–963. doi: 10.1101/gad.1174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]