Abstract
Entheses (insertion sites, osteotendinous junctions, osteoligamentous junctions) are sites of stress concentration at the region where tendons and ligaments attach to bone. Consequently, they are commonly subject to overuse injuries (enthesopathies) that are well documented in a number of sports. In this review, we focus on the structure–function correlations of entheses on both the hard and the soft tissue sides of the junction. Particular attention is paid to mechanical factors that influence form and function and thus to exploring the relationship between entheses and exercise. The molecular parameters indicative of adaptation to mechanical stress are evaluated, and the basis on which entheses are classified is explained. The application of the ‘enthesis organ’ concept (a collection of tissues adjacent to the enthesis itself, which jointly serve the common function of stress dissipation) to understanding enthesopathies is considered and novel roles of adipose tissue at entheses are reviewed. A distinction is made between different locations of fat at entheses, and possible functions include space-filling and proprioception. The basic anchorage role of entheses is considered in detail and comparisons are explored between entheses and other biological ‘anchorage’ sites. The ability of entheses for self-repair is emphasized and a range of enthesopathies common in sport are reviewed (e.g. tennis elbow, golfer's elbow, jumper's knee, plantar fasciitis and Achilles insertional tendinopathies). Attention is drawn to the degenerative, rather than inflammatory, nature of most enthesopathies in sport. The biomechanical factors contributing to the development of enthesopathies are reviewed and the importance of considering the muscle–tendon–bone unit as a whole is recognized. Bony spur formation is assessed in relation to other changes at entheses which parallel those in osteoarthritic synovial joints.
Keywords: biomechanical, enthesopathy, fibrocartilage, insertion sites, osteotendinous junctions
Introductory remarks
In engineering terms, tendons and ligaments can be regarded as machines with multiple moving parts (fibrils, fibres and fascicles) that perform the basic function of force transfer to and from the skeleton. They distribute the loads applied to them dynamically in order to execute movement patterns. Their complex response to loading allows for multi-axis bending, and this adds to the stress concentration in the region where they attach to bone. This attachment site will be referred to in this review as an ‘enthesis’, but it is also known as an ‘insertion site’, or an ‘osteotendinous’ or ‘osteoligamentous’ junction. It is stress concentration at the hard–soft tissue interface which makes entheses vulnerable to acute or overuse injuries in sport. Conditions such as tennis and golfer's elbow, jumper's knee and various Achilles insertional tendinopathies are well known to primary-care physicians, orthopaedic surgeons, rheumatologists and sports medicine specialists alike. However, the importance of entheses extends beyond this, as they are also the primary target organ in a collection of rheumatic conditions known as the seronegative spondyloarthropathies, the best known of which is ankylosing spondylitis (Benjamin & McGonagle, 2001). Intriguingly, not all entheses are equally targeted in these patients and one of the theories accounting for the differential effect is that the inflammatory enthesopathies that afflict these patients are most typical of entheses that are subject to mechanical trauma (Benjamin & McGonagle, 2001). Entheses are also of clinical significance to orthopaedic surgeons who may be faced with the challenge of reattaching a tendon or ligament to bone, particularly when creating an autograft for a ruptured anterior cruciate ligament (ACL). In the present review, we focus on the structure–function correlations of entheses and pay particular attention to mechanical factors that influence form and function and thus to the relationship between entheses and exercise. The concept of mechanical load influencing the structure of a musculoskeletal tissue is well recognized and is part of the growing subject of mechanobiology (Benjamin, 2002; Benjamin & Hillen, 2002). It is the basis of the ‘form follows function’ principle which underpins Wolff's law (see Milz et al. 2005 for further details). However, far less attention has been paid to tendons and ligaments than to bone and much of the current interest has its roots in the pioneering work of Ploetz (1938), Kummer (1959, 1962, 1978) and Pauwels (1980).
The classification of entheses and the ‘enthesis organ’ concept
Classification of entheses
Over the past two decades in which our laboratory has focused on entheses, we have distinguished two broad categories of attachment sites according to their structure. We have termed them fibrous and fibrocartilaginous according to the type of tissue present at the attachment site (Fig. 1a–d; Benjamin & Ralphs, 1995; Benjamin & McGonagle, 2001). They equate to the indirect and direct attachments, respectively, of Woo et al. (1988) – a terminology which highlights the absence of a periosteum at fibrocartilaginous entheses and hence a ‘direct’ attachment of the tendon/ligament to the bone. Both classifications have a different basis from that of Biermann (1957) and Knese & Biermann (1958). These authors based their classification system on the location of entheses associated with long bones, i.e. chondral–apophyseal entheses which are found at the ends of the long bones and periosteal–diaphyseal attachments which occur on the shafts. The former are fibrocartilaginous and the latter are fibrous.
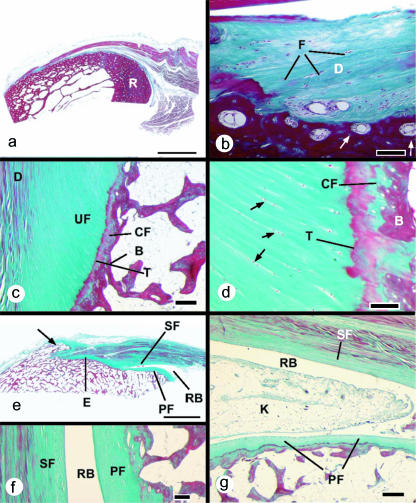
Fig. 1.
Histological sections (stained with Masson's trichrome) of entheses from dissecting room cadavers. (a) A macroscopic view of the fibrous enthesis at the insertion of pronator teres on the mid-shaft of the radius (R). Note the thick layer of cortical bone. Scale bar = 5 mm. (b) Higher magnification view of the pronator teres enthesis, showing the presence of dense fibrous connective tissue (D) at the bone–tendon junction. Fibroblasts are evident (F), but no fibrocartilage cells. Note the presence of osteons at the attachment site (arrows). Scale bar = 100 µm. (c) A typical fibrocartilaginous enthesis (Achilles tendon) showing the four zones of tissue at the bone–tendon interface: dense fibrous connective tissue (D), uncalcified fibrocartilage (UF), calcified fibrocartilage (CF) and bone (B). The two fibrocartilage zones are separated from each other by a tidemark (T). Note that the tidemark is straight, but the tendon–bone interface (i.e. the junction between calcified and non-calcified fibrocartilage) is highly irregular (arrows). This is critical for the integrity of the junction. Scale bar = 300 µm. (d) A higher magnification view of the fibrocartilage zones at the Achilles tendon enthesis. The zone of uncalcified fibrocartilage is characterized by rows of fibrocartilage cells (arrows) that are separated from each other by parallel bundles of collagen fibres. T, tidemark; B, bone. Scale bar = 100 µm. (e) A macroscopic view of the Achilles tendon enthesis organ. This consists of the enthesis itself (E), a thick periosteal fibrocartilage (PF) on the superior tuberosity (ST) of the calcaneus, a sesamoid fibrocartilage (SF) in the deep surface of the tendon, an intervening retrocalcaneal bursa (RB) and the tip of Kager's fat pad (not visible in this section). Note the enthesophyte (arrow) in the most inferior part of the enthesis. Scale bar = 5 mm. (f) A higher magnification view of the sesamoid (SF) and periosteal (PF) fibrocartilages that lie either side of the retrocalcaneal bursa (RB). Scale bar = 100 µm. (g) The wedge-shaped tip of Kager's fat pad (K) protrudes into the retrocalcaneal bursa (RB) in a plantarflexed foot. PF, periosteal fibrocartilage; SF, sesamoid fibrocartilage. Scale bar = 500 µm.
At fibrous entheses, the tendon or ligament attaches either directly to the bone or indirectly to it via the periosteum. In both cases, dense fibrous connective tissue connects the tendon/ligament to the periosteum and there is no evidence of (fibro)cartilage differentiation (Fig. 1a,b). However, as their name suggests, fibrocartilaginous entheses are sites where chondrogenesis has occurred and thus four zones of tissue are commonly present: pure dense fibrous connective tissue, uncalcified fibrocartilage, calcified fibrocartilage and bone (Fig. 1c,d). The inclusion of a zone of ‘pure dense fibrous connective tissue’ and a zone of ‘bone’ at a fibrocartilaginous enthesis highlight the difficulty of defining with any degree of precision where such an enthesis begins and ends. The dense fibrous connective tissue is of course continuous with (and indistinguishable from) that of the rest of the tendon/ligament and equally the bone at an enthesis blends imperceptibly with that in the remainder of the skeleton. This absence of sharp boundaries together with the minute size of the critical zones which define a fibrocartilaginous enthesis (i.e. the two fibrocartilage zones) are perhaps a major reason why entheses have attracted relatively little attention from biochemists and molecular biologists.
The subdivision of entheses into fibrous and fibrocartilaginous types has been regarded by Hems & Tillmann (2000) as being too simple and not reflecting the range of morphological variations which they have observed. They advocate a distinction between periosteal, bony and fibrocartilaginous attachments – though the first two are clearly subdivisions of fibrous entheses. Their comments stem in particular from a study of the attachments of the masticatory muscles in humans. Here, there are striking variations in structure between one part of an enthesis and another (Hems & Tillmann, 2000). Although these regional variations are certainly more striking than they are in most entheses in the limbs, it is not the case that limb tendons have a uniform enthesis structure. By contrast, most so-called fibrocartilaginous entheses consist of one part which is fibrocartilaginous and another which is largely fibrous (see Benjamin et al. 2002 for further discussion). Typically, the fibrous area is in the most superficial or distal part of the enthesis.
The enthesis organ concept
Many tendons and ligaments approach their attachment sites obliquely, and as a result they often make contact with the bone just before their attachment in certain positions of the joint on which they act. This contact between tendon/ligament and bone influences stress dissipation at the enthesis itself, and has led Benjamin & McGonagle (2001) to propose the concept of an ‘enthesis organ’. They define an ‘enthesis organ’ as a collection of related tissues at and near the enthesis, which serve a common function of stress dissipation. The enthesis organ concept is widely applicable, but is most clearly understood by considering the attachment of the Achilles tendon to the calcaneus (Fig. 1e–g). Thus, the earlier work of Rufai et al. (1995) was pivotal in developing the enthesis organ concept. The Achilles enthesis organ comprises the osteotendinous junction itself (characterized by a prominent enthesis fibrocartilage), a sesamoid fibrocartilage near the deep surface of the tendon, immediately adjacent to the osteotendinous junction, a complementary periosteal fibrocartilage covering the superior tuberosity of the calcaneus, and the wedge-shaped tip of the retromalleolar (preAchilles) fat pad which protrudes into the retrocalcaneal bursa (Fig. 1e–g). During dorsiflexion of the foot, the sesamoid and periosteal fibrocartilages are pressed against each other with a compressive force which varies according to the size of the tuberosity. The point of contact between tendon and bone creates a fulcrum which provides the Achilles tendon with a long lever arm and thus a slight mechanical advantage in its action on the calcaneus. The bursa promotes free movement between tendon and bone and the fat pad is thought to serve a variety of functions. It protrudes into the bursa in a plantarflexed foot, but is retracted during dorsiflexion (Canoso et al. 1988; Theobald et al. 2006). The suggested mechanical functions of the fat pad include reducing friction between the tendon and the bone, preventing the tendon from kinking under load (by cushioning its deep surface), and acting as a variable space filler (i.e. a plunger) so that negative pressure does not develop in the bursa during plantarflexion (Theobald et al. 2006). However, as it contains a variety of sensory nerve endings, it may also have a proprioceptive function in monitoring insertional angle changes between tendon and bone during foot movements (Shaw et al. 2005). Ultrastructural studies on the comparable enthesis organ of the rat Achilles tendon have shown that its sesamoid and periosteal fibrocartilages are covered with a layer of acellular, electron-dense material that forms an articular surface lamina preventing direct cellular contact with the bursal cavity (Rufai et al. 1996). Proximally, the bursa is lined with a vascular synovium, but as in other synovial joints, this is absent over the articular surfaces themselves (Benjamin & McGonagle, 2001).
Enthesis organs can also be recognized at many other insertion sites in the body and have been described in detail by Benjamin et al. (2004a). What most have in common is either that the tendon/ligament attaches to a pit or that there is a small bony protuberance next to the enthesis. In both cases, contact is made between tendon/ligament and bone immediately next to the enthesis and this dissipates stress concentration away from the enthesis itself and often leads to the formation of distinctive sesamoid and periosteal fibrocartilages. Some enthesis organs have a particularly complex structure, for there are ‘multi-enthesis organs’ where two or more neighbouring tendons or ligaments share an enthesis. They include the sites of the entheses of tibialis posterior and the plantarcalcaneonavicular ligament (Moriggl et al. 2003) and the shared enthesis of popliteus and the lateral collateral ligament of the knee joint (Benjamin et al. 2004a).
The concept of an enthesis organ is of particular significance to clinicians, as it can help to explain patterns of injury or why the symptoms associated with a particular enthesopathy are diffuse. A few examples will suffice. (1) It is well recognized that patients with lateral epicondylosis (‘tennis elbow’) often present to their practitioners with pain that radiates into the neighbouring collateral or annular ligaments (Bredella et al. 1999). Our anatomical studies have shown that the enthesis of the extensor carpi radialis brevis muscle merges imperceptibly with that of the lateral collateral ligament of the elbow joint and that this in turn fuses with the annular ligament of the superior radioulnar joint (Milz et al. 2004). Consequently, considerable load-sharing takes place between all of these structures. (2) The Achilles tendon is one of the commonest sites of enthesopathy in patients with ankylosing spondylitis (Braun et al. 2000). Magnetic resonance imaging of such patients frequently reveals signal changes adjacent to rather than at the enthesis itself (Olivieri et al. 1998). This could relate to the contact made between the tendon and the bone immediately proximal to the enthesis at the sites characterized by sesamoid and periosteal fibrocartilages (Benjamin & McGonagle, 2001). (3) The anterior talofibular ligament (ATFL) of the ankle joint is the most common site of ankle sprains and the ligament can either be damaged in mid-substance or suffer an avulsion fracture (Kumai & Benjamin, 2002). Damage to the ATFL is particularly common in basketball and soccer players (Garrick, 1977). If the ligament avulses, it almost universally does so at its fibular rather than its talar end, and Kumai & Benjamin (2002) have suggested that this is because only the distal end of the ligament has an enthesis organ. This has a stress-shielding influence on the talar attachment – but makes the fibular one more vulnerable to injury.
The structure of fibrocartilaginous entheses
Tissue zonation
The classic description of a fibrocartilaginous enthesis is that the fibrocartilage cells in the zone of uncalcified fibrocartilage are arranged in longitudinal rows between parallel bundles of collagen fibres (Fig. 1d; Cooper & Misol, 1970). However, this regular alignment is frequently not evident. The developmental study of Gao et al. (1996) suggests that where rows of fibrocartilage cells are present at an enthesis, they reflect the prior alignment of the fibroblasts from which they differentiate. By definition, fibrocartilage is a transitional tissue (Benjamin & Evans, 1990) and the question of whether fibrocartilage cells nearer the hard tissue boundary differ from those nearer the ‘pure’ tendon/ligament end of the boundary has not been properly addressed. Neither has the exact nature of the tidemark, i.e. the basophilic line separating the zones of uncalcified and calcified fibrocartilage (Fig. 1c,d). The zone of calcified fibrocartilage is always small and may occasionally be locally absent (Suzuki et al. 2005). However, the proportion of the enthesis subchondral bone plate which consists of calcified fibrocartilage increases with age, because of a thinning of the cortical bone (Bloebaum & Kopp, 2004). This is believed to contribute to the increased brittleness of the cortical shell and thus its vulnerability to fracture (Shea et al. 2002). Tetracycline labelling studies clearly demonstrate that the uptake is significantly less in the calcified fibrocartilage than the bone, illustrating that turnover is minimal in the latter (Bloebaum & Kopp, 2004). Thus, with endosteal resorption, the cortex increasingly comes to be represented by a brittle tissue with little capacity for repair. Calcified fibrocartilage is typically less cellular than its uncalcified equivalent, probably because the deposition of calcium salts in the extracellular matrix (ECM) leads to cell death.
Fibrocartilage differentiation from tendon/ligament cells at the enthesis involves changes in cell shape and cell–cell interactions. The cells lose contact with their neighbours, round-up and enlarge (Ralphs et al. 1998), and express cartilage markers, especially type II collagen and aggrecan (Waggett et al. 1998). Nothing is known specifically about how these changes are regulated, and little for fibrocartilages in general, although members of the TGF beta superfamily are involved. The fibrocartilage present in tendons which change direction around bony pulleys en route to their enthesis (‘wrap-around tendons’, Vogel & Koob, 1989) develops in association with compressive load under the influence of TGF beta (Robbins et al. 1997), and patellar tendon cells are known to form cartilaginous tissue on treatment with bone morphogenetic protein (BMP; Sato et al. 1988). BMPs2, 4 and GDF5 (growth and differentiation factor 5, also known as BMP12) are associated with normal and pathological fibrocartilage differentiation in the intervertebral disc and during fracture healing (Bostrom et al. 1995; Takae et al. 1999; Nakase et al. 2001). GDF5, along with GDF6 and 7, is also involved in control of early tendon differentiation (Wolfman et al. 1997). The extent of the similarities between enthesial fibrocartilage differentiation and early chondrogenesis are unclear. Both tissues start as type I collagen producers and switch to producing cartilage markers. In cartilage, the factors outlined above, notably BMP2 and 4, are associated with up-regulating the expression of the cartilage transcription factor SOX9, which along with SOX5 and SOX6 regulate chondrogenesis from the early cell condensation (e.g. Lefebvre et al. 2001). TGF beta also promotes chondrogenesis (Zhou et al. 2004). Other transcription factors are also likely to be important, e.g. cKrox which down-regulates type I collagen and up-regulates type II collagen (Galera et al. 1996; Ghayor et al. 2000; Widom et al. 2001), and Cbfa1 which suppresses type I collagen synthesis under the control of BMP4 and 7 (Tsuji et al. 1998). Thus, a key question is to what extent is fibrocartilage development at entheses controlled by the same factors as normal cartilage development and the differentiation of fibrocartilage in wrap-around tendons.
Avascularity
All fibrocartilages associated with normal entheses or enthesis organs are avascular and this contributes to a poor healing response at and near attachment sites. The avascularity reflects the mechanical conditions to which the tissues are subject – notably compression. No attention has yet been directed to the factors which control angiogenesis during enthesis development and we currently rely on information derived from studying fibrocartilages elsewhere. Here, the development of blood vessels is controlled by a variety of angiogenic and anti-angiogenic factors. The former include vascular endothelial growth factor (VEGF) and the latter endostatin, a small (20 kDa) molecule that has been identified as the C terminal fragment of the NC1 domain of type XVIII collagen (O’Reilly et al. 1997). Endostatin has recently been detected with a pericellular distribution around the fibrocartilage cells that are found in adult human tendons in the avascular wrap-around regions and its expression is probably mechanically induced (Pufe et al. 2004). However, VEGF is absent at such sites (Petersen et al. 2002). The mechanism of action of endostatin is unknown, but Pufe et al. (2004) have considered several possibilities – an action via endostatin or VEGF receptors on endothelial cells (Kim et al. 2000; Karumanchi et al. 2001), or an influence on endothelial cell function by binding to ECM proteins (Furumatsu et al. 2002).
Adipose tissue
Fat is a common feature of many entheses, but its significance is rarely addressed. It is often regarded simply as a sign of degeneration or aging in tendons or ligaments and is sometimes called tendolipomatosis (Jozsa & Kannus, 1997). Although not contesting that fat can certainly be evidence of degeneration, Benjamin et al. (2004b) have highlighted several important normal functions that adipose tissue could serve at entheses and have distinguished between different locations of fat at insertion sites. They describe endotenon, epitenon, meniscoid and insertional angle fat. Endotenon fat lies within the films of loose connective tissue that separate adjacent fascicles in a tendon or ligament. Although endotenon is not an obvious feature of many entheses (being most typical of tendon/ligament mid-substance), a fatty endotenon is a conspicuous feature of tendons/ligaments that flare out considerably at their attachment site, e.g. the insertion of peroneus longus on the first metatarsal bone and the medial cuneiform. At such locations, the fat acts as a packing tissue filling up space between bundles of collagen fibres. Epitenon fat lies in the loose connective tissue on the surface of the tendon/ligament, meniscoid fat is a feature of meniscal folds of synovium that project into subtendinous bursae, and insertional angle fat occupies the space between the tendon/ligament and the bone as the tendon/ligament approaches its enthesis obliquely. All of these adipose tissue depots, but particularly insertional angle fat, contain blood vessels and nerves and could play an important role in proprioception. One could envisage a function for insertional angle fat in monitoring the changes in the angle at which a tendon/ligament meets the bone with joint movement. The tip of the retromalleolar fat pad in humans contains prominent lamellated corpuscles (Benjamin et al. 2005) and Shaw et al. (2005) have demonstrated that the equivalent fat pad in the rat is richly supplied with autonomic nerves, including those containing substance P and calcitonin gene-related peptide (CGRP). Intriguingly, it also contains numerous mast cells. Significantly, the fat is the only region of the Achilles tendon enthesis organ that is innervated (Shaw et al. 2005).
Molecular composition of enthesis ECM
The composition of the ECM of entheses may be directly related to the mechanical demands at the soft/hard tissue interface. Here, local compressive stresses occur, especially at sites with an oblique insertional angle of the tendon or ligament fibres. The compressive stress triggers functional adaptation of the molecular composition of the ECM, which leads to the presence of cartilage-related molecules (Benjamin et al. 2002). At such entheses, these molecules occur in addition to the typical components of dense connective tissue and at sites with relatively high compression, the former may even replace the latter completely.
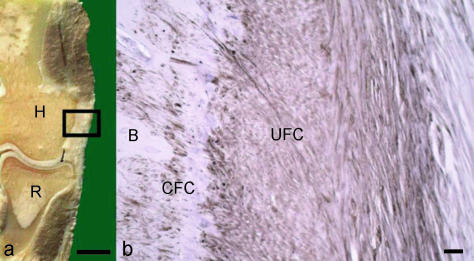
Glycosaminoglycans (GAGs) are typical components of the ECM at entheses subject to compression. They include dermatan and keratan sulphate, which can also be found in the tensile regions of tendons and ligaments (Milz et al. 2005). Chondroitin 4 sulfate, and with increasing compression, chondroitin 6 sulfate, are found when the tissue can be recognized histologically as fibrocartilage (Milz et al. 1998). Proteoglycans (which contain GAGs) such as versican and tenascin, which are typical components of dense connective tissue, become less evident, and eventually disappear locally, when the compressive stress increases above a certain threshold. By contrast, immunohistochemical labelling of aggrecan and link protein (Fig. 2) becomes more and more prominent (Milz et al. 2004, 2005).
Fig. 2.
(a) Frontal saw cut through the lateral part of a human elbow joint. The rectangle represents the part of the common extensor origin that is shown in fig. b. H, humerus; R, radius. Scale bar = 1 cm. (b) Immunohistochemical labelling for aggrecan at the enthesis of the common extensor origin. B, bone; UFC, uncalcified fibrocartilage; CFC, calcified fibrocartilage. Scale bar = 100 µm.
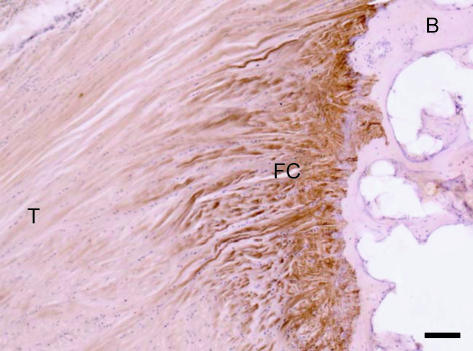
In entheses of particularly cartilaginous, almost hyaline-like phenotype, type I collagen (the prevailing collagen of tendons, ligaments and bone) disappears and is replaced by type II collagen (Fig. 3). Such a region appears like a gap between two collagen I-containing tissues (i.e. tendon/ligament and bone) and therefore the appearance has been called the ‘gap phenomenon’ (Boszczyk et al. 2003a; Milz et al. 2004). It can also be observed for versican and occasionally tenascin (Boszczyk et al. 2003a; Milz et al. 2004). The region positive for type II collagen includes the mineralized and unmineralized fibrocartilage and in many cases these tissues also label for aggrecan, link protein and chondroitin 6 sulfate (Boszczyk et al. 2003a; Milz et al. 2004). Type III and VI collagens may equally occur in the fibrous and fibrocartilaginous zones of chondral entheses, but with different distribution patterns in the ECM (Waggett et al. 1998; Milz et al. 2001).
Fig. 3.
Immunohistochemical labelling for type II collagen at the insertion of the central slip of the extensor tendon to the base of the middle phalanx of a second toe. The enthesis fibrocartilage (FC) has labelled strongly for type II collagen, but the bone (B) and tendon (T) are not labelled. Scale bar = 100 µm.
In patients with rheumatoid arthritis, the presence of type II collagen, aggrecan and link protein is of clinical relevance, because these molecules have been identified as autoantigens during the autoimmune response (Glant et al. 1998; Guerassimov et al. 1998; Myers et al. 2001). Fibrocartilaginous regions of the enthesis presenting these molecules constitute potential targets for the extra-articular manifestation of rheumatoid arthritis (Boszczyk et al. 2003a). A comparable mechanism has also been discussed for other diseases with an autoimmune-related aetiology (e.g. spondylarthopathies), as they have comparable predilection sites for the occurrence of clinical symptoms (Benjamin & McGonagle, 2001).
Magnetic resonance (MR) imaging appearance of entheses
Two particular technical developments have recently made entheses a focus of interest in MR imaging. The first of these is ultrashort echo time (TE), or UTE imaging (Bergin et al. 1991; Robson et al. 2003), and the second is magic angle imaging (Oatridge et al. 2001; Gatehouse & Bydder, 2003). In MR terms, fibrocartilage is one of about 20 tissues including tendons, ligaments, menisci and cortical bone which have short, or very short transverse relaxation times (T2). This means that the magnetization induced in them by the MR imaging process decays away very quickly. This decay is described by the exponential time constant T2 and these tissues have short T2. As a result of the rapid decay of the signal, it is not detectable, or only just detectable by the time that clinical MR systems can be switched on in receive mode. Even if some signal is detectable initially after enabling the receive mode of the MR system, the process of spatially encoding the signal takes further time and this cannot be accomplished if the signal decays further and disappears. All of the tissues at a fibrocartilaginous enthesis (i.e. the zones of dense fibrous connective tissue, uncalcified fibrocartilage, calcified fibrocartilage and bone) have short T2. They thus produce little or no signal with all conventional clinical MR pulse sequences and consequently appear as signal voids. By shortening the time to the beginning of the data reception (TE) by a factor of 20–200, and by using techniques which can spatially encode data as soon as the gradient system is enabled, it is possible to detect signal from the tissues of interest at entheses, and to encode it. Once this has been done, it is then possible to use radiofrequency pulses to manipulate this signal and distinguish the tissues (Robson et al. 2003, 2004).
In magic angle imaging, the MR signal received from highly ordered, collagen-rich tissues such as tendons and ligaments depends on the orientation of their fibres to the static magnetic field of the system (B0). In particular, if the fibres are at 55° to B0, the interactions between spins (which are modulated by the term [3cos2θ − 1] is nearly zero. Interactions between nuclei are minimized and the tissue T2 is prolonged (Fullerton et al. 1985; Henkelman et al. 1994). As a consequence, the signal from these highly ordered tissues does not rapidly decay to zero and is detectable even with conventional imaging system pulse sequences (Oatridge et al. 2001; Gatehouse & Bydder, 2003). Differences in fibre orientation result in differences in signal and this can be used as a source of image content. Whereas fibrous connective tissue in a tensional mid-tendon/ligament region has an essentially linear arrangement of fibres, sesamoid fibrocartilage has a basket-weave type of pattern. Enthesis fibrocartilage tends to have a linear pattern more or less perpendicular to that of the underlying cortical bone. Orientation of entheses tissues at or around 0°, 55° and 90° to B0 can result in very different signal intensities from these tissues and permit them to be distinguished.
It should be noted that although the normal features of entheses cannot be recognized with conventional sequences, in enthesitis, tissue T2 are prolonged (this is a common reaction to a variety of pathological processes and is attributed to increased water, loss of order, cellular infiltration, microcyst or cyst formation, and other causes). This prolongation enables abnormalities to be seen with conventional sequences in advanced disease, although their precise localization may be difficult without concurrent, detailed demonstration of the normal anatomy which has a short T2. Thus, osteitis associated with seronegative disease can be recognized as a late feature of enthesitis (McGonagle et al. 2002; McGonagle, 2003). The capacity to image the normal anatomy of entheses and to use this as a basis for recognizing abnormalities in early disease are potential technical advantages which are only now being applied to clinical disease.
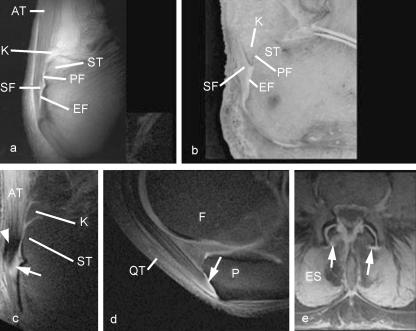
The close correspondence between anatomical studies and MR imaging with UTE sequences (Benjamin et al. 2004b; Robson et al. 2004) is apparent in Fig. 4(a,b), sagittal views of the insertion of the Achilles tendon. Enthesis, sesamoid and periosteal fibrocartilage can be separately identified. A similar result can be achieved with magic angle imaging. By placing the bulk of the tendon fibres a little away from 55°, low signal is seen in the tendon next to the sesamoid fibrocartilage, but high signal is seen in the sesamoid fibrocartilage itself, because its basket-weave pattern means that some of its fibres are at 55° to B0 (Fig. 4c). Using either or both of these techniques, it is possible to identify enthesis fibrocartilage at the quadriceps tendon insertion (Fig. 4d) as well as the patellar tendon origin and insertion. Entheses have also been visualized at many different sites around the body, including the shoulder, elbow, fingers, spine and pelvis. One particular area of interest has been the demonstration of fibrocartilage in the dorsal capsule of the facet joints. These have been observed on histological studies (Boszczyk et al. 2001) and the presence of fibrocartilage has been confirmed with immunocytochemistry (Boszczyk et al. 2003b). However, the fibrocartilage has not previously been demonstrable in life, as in this case of a patient with degenerative spine disease (Fig. 4e).
Fig. 4. MR images of entheses.
(a) A UTE image and (b) a corresponding methacrylate section of the Achilles tendon (AT) enthesis, cut in the mid-sagittal plane to show the periosteal (PF), sesamoid (SF) and enthesis (EF) fibrocartilages, which can be identified in the UTE sequence. K, Kager's fat pad; ST, superior tuberosity. Panel b is reproduced with permission from Robson et al. Clin Radiol 2004, 59, 729. (c) A sagittal magic angle image of the Achilles tendon enthesis. Low signal is seen in the tendon posterior to the calcaneus (arrowhead), but high signal is seen in the sesamoid fibrocartilage (arrow). (d) Sagittal magic angle image of the quadriceps tendon (QT) insertion on the patella (P). The enthesis fibrocartilage (arrow) has a high signal. F, femur.
(e) Tranverse UTE image of the lumbar spine in a patient with degenerative spine disease. High signal is seen in the region of the dorsal capsule of the lumbar facet joints (arrows). ES, erector spinae.
Finally, it should be noted that conventional and UTE sequences are useful for demonstrating fat associated with entheses and enthesis organs. This tissue normally provides a high signal and is easy both to visualize and to suppress with MR imaging (Benjamin et al. 2004b).
The functions of entheses
Anchorage and stress dissipation
The attachment of a tendon/ligament to the skeleton is clearly the basic function of any enthesis and central to force transmission. Thus, tendons and ligaments often flare out at their attachment sites as an adaptation to securing skeletal anchorage and resisting the effects of insertional angle change. Flaring of entheses is particularly striking in the limbs, e.g. at the Achilles tendon, the insertion of peroneus brevis and the tibial attachment of the ACL. It is also an obvious but understated fact that most tendons and ligaments do not attach to the skeleton in an isolated manner. The enthesis of one often blends with that of another, so that many bony attachment sites overlap and this adds to the stability of the anchorage (Benjamin et al. 2004a). There are also many unnamed fibrous connections linking tendons and ligaments directly together near their attachment sites. Both these statements are in line with the concept of myofascial continuity explained in detail by Myers (2001). He proposes that what may appear to be discrete muscles are mechanically linked to each other by fascia that establish important lines of force transmission.
There are repeated misconceptions in the literature about how this anchorage is achieved. It is erroneous to think that this always hinges on ‘Sharpey's fibres’, yet the concept is deeply entrenched in the literature. Yes, some tendons/ligaments certainly do have Sharpey's fibres – notably fibrous entheses in regions where there is substantial cortical bone, at the sites where the periodontal ligament is attached to cementum and alveolar bone (Raspanti et al. 2000) and at the sites of surgical reattachment of tendons and ligaments to the skeleton (Johnson, 2005). Yet, a great many tendons and ligaments attach to areas of bone where there is virtually no cortex (short bones and the epiphyses and apophyses of long bones). At such sites, there seems little chance of deeply penetrating collagen fibres crossing the tissue boundary, although it does not completely negate the possibility that some fibres cross the divide. Such entheses are invariably fibrocartilaginous and collagen fibre continuity across the hard/soft tissue boundary occurs predominantly at the level of the tidemark which separates calcified and non-calcified fibrocartilage. It is perhaps these fibres which should be regarded as the functional equivalent of Sharpey's fibres in a fibrous enthesis. Milz et al. (2002) have suggested that it is the complex interdigitation of the layer of calcified fibrocartilage with the adjacent bone that secures attachment. Thus, there are parallels with the mechanism by which enamel and dentine are joined together in a tooth (Marshall et al. 2001). The dentinoenamel junction is markedly scalloped to increase the bonding between the tissues, and similar ‘scalloping’ occurs at fibrocartilaginous entheses. As there may be gradients of mineral content across the dentinoenamel junction that have a role in reducing stress concentration (Marshall et al. 2001), it would be interesting to know if the same applies to entheses.
There are intriguing parallels between entheses and numerous other biological interfaces, and these may add to our understanding of mechanical adaptations at insertion sites. As Waite et al. (2004) have eloquently stated, the widespread evolution of stiff scaffolds in the animal kingdom for ‘frame, integument, and appendages’ has created challenges in linking hard and soft tissues which nature has had to overcome with parallel adaptations. Thus, other biological interface regions presenting mechanical challenges which parallel those faced at entheses include the hard jaws of marine polychaete worms that anchor to soft tissue at their base, and the byssus threads which anchor mussels to rocky shores (Waite et al. 2004). It is worth noting that byssus threads are viewed as ‘extracorporeal tendons’ (they are collagenous structures) and like long human tendons, they show regional differentiation along their length (Bell & Gosline, 1996).
It is also worth considering parallels between the mechanical anchorage of trees via their root systems and entheses. Like tendons and ligaments, plants are subject to mechanical forces created by their static loading, the influence of the wind, and the slope of the ground (Ennos et al. 1993). A remarkably small proportion of the plant actually participates in securing firm skeletal anchorage and it is clearly the same with tendons and ligaments. Thus, in both cases the necessary anchorage is created with a minimum investment in structural material. This maximizes the proportion of the tendon/ligament that can remain compliant and flexible and thus serve other functions (e.g. energy storage and changing the direction of muscle pull – Benjamin & Ralphs, 1998). Just as the lateral roots of a tree fan out from the point where the trunk meets the ground, equally the bony spicules beneath many entheses can radiate in all directions (Suzuki et al. 2005). There are other entheses (e.g. Achilles and patellar tendons) where there is considerable anisotropy of superficial trabeculae (Suzuki et al. 2005). Collectively, these may be likened to the tap root of a tree. Although the trabecular network is generally ignored when considering entheses, it obviously plays an important part in tendon/ligament anchorage and stress dissipation.
There are also useful comparisons to explore with non-biological composites. Humans are constantly seeking to join together materials of different physical properties (e.g. ceramic to metal) or to create surfaces that are resistant to contact damage (e.g. for magnetic storage media). What material scientists try to do is to create materials with graded mechanical properties that can then resist damage more effectively than their homogeneous counterparts (Suresh, 2001). This exactly parallels what fibrocartilage is believed to do at entheses. The presence of the two zones of fibrocartilage between the tendon/ligament ‘proper’ and the bone contributes to stress dissipation at entheses by ensuring that there is a gradual change in mechanical properties between hard and soft tissues (Woo et al. 1988). As Hems & Tillmann (2000) have emphasized, tendon and bone have similar tensile strength, but the elastic modulus of bone is approximately 10 times larger than that of tendon. Hence, a primary function of entheses must be to balance such widely different elastic moduli.
According to Suresh (2001), gradients at interface regions smooth stress distribution, eliminate singularities in stress, reduce stress concentration, improve the strength of the bonding and decrease the risk of fracture (i.e. failure). He also points out that the major mechanical difficulty which arises from joining a stiff scaffold (bone in the context of entheses) to a softer material (tendon or ligament) is ‘contact deformation and damage’. Here, it may be useful to note Waite et al.'s (2004) metaphor of a wicker basket filled with blackberries. The fruit in contact with the basket walls is always the first to be damaged. It is the requirement for smooth stress distribution which accounts for why discrete (well circumscribed) entheses, at which the tendon/ligament attaches to a small, precisely localized region of bone, are fibrocartilaginous, and why this tissue is not a typical feature of entheses where the surface area of the junctional region is large (Thomopoulos et al. 2003).
The paucity of attempts to study biomechanical aspects of entheses in relation to junctional properties is largely because of practical difficulties of recording strain levels within such a small volume of tissue and the transitional nature of the region with no clear boundaries that define it. Maganaris et al. (2004) argue that loading on entheses is non-uniform across the attachment site and they cite several studies which all show that the pathology occurs in the regions where strain levels are lowest. They make the interesting suggestion that the regions most vulnerable to damage at entheses are initially stress-shielded and that tensile failure may not be a key feature of enthesopathy. They have rightly drawn attention to the fact that clinically recognizable enthesopathy occurs more frequently in the deep than the superficial part of an enthesis. This corresponds with a regional difference in the prominence of enthesis fibrocartilage – which is generally more conspicuous in the deepest parts of entheses (Benjamin et al. 1986; Woo et al. 1988). Fibrocartilage is an adaptation to compression and/or shear (Benjamin & Ralphs, 2004) and the deep part of an attachment site is compressed by the superficial part. It is these compressive forces that may be pertinent to understanding enthesopathies.
In the early 1990s, Benjamin and colleagues published a series of papers which all suggested that there was a correlation between the quantity of uncalcified fibrocartilage at an enthesis and the degree of ‘insertional angle change’ that occurred during joint movement (Evans et al. 1990; Benjamin et al. 1991, 1992). An ‘insertional angle change’ is the change in the angle at which the tendon/ligament meets the bone as the joint(s) is moved. It is suggested that the stiffened ECM that typifies the fibrocartilaginous region of a tendon/ligament promotes the gradual bending of the collagen fibres as they approach the hard tissue interface. This function of enthesis fibrocartilage has often been compared with that of a grommet on an electrical plug – an analogy first used by Schneider (1956).
Although tendons and ligaments are often viewed as non-distensible, they do have the ability to stretch and recoil by approximately 6% of their original length without any obvious signs of damage. It is in recognition of this that Knese & Biermann (1958) proposed their ‘stretching-brake theory’ of enthesis fibrocartilage function. These authors pointed out that the stiffened ECM at a fibrocartilaginous enthesis should limit the narrowing of an elongated tendon/ligament at this region. The theory is an attractive one, but largely ignored by subsequent authors and thus one which remains unsubstantiated. Nevertheless, Milz et al. (2005) have suggested that a stretching brake function could well operate at the entheses of the human acetabular ligament. This is a very short ligament, which has conspicuous fibrocartilaginous entheses, yet exhibits little insertional angle change with joint loading. They argue that the rapid increase of tensile stress on this ligament during load bearing is likely to produce a biologically relevant shear stress that acts as the mechanical stimulus for fibrocartilage formation.
Entheses as mini growth plates
Knese & Biermann (1958) have suggested that entheses can act as growth plates for apophyses at tendon and ligament attachment sites. This is supported by the developmental study of Gao et al. (1996) on the femoral attachment of the medial collateral ligament of the rat knee joint. Gao et al. (1996) exploited age-related changes of labelling in types I and II collagen to show that the cartilage at the enthesis is initially derived from that of the embryonic bone rudiment. Equally, however, they showed that this hyaline cartilage is eroded during endochondral ossification and replaced by enthesis fibrocartilage that develops within the adjacent ligament by fibroblast metaplasia. Despite this, little is known about the molecular control of cell maturation at entheses. Given the similarities with the growth plate, the terminal differentiation of enthesis fibrocartilage cells invites comparison with the control of chondrocyte differentiation in epiphysial growth plates. This is a multistep process regulated by a complex network of signalling systems. Early stages involve control of chondrocyte proliferation, followed by hypertrophy and apoptosis, angiogenesis and osteogenesis. Proliferation, which in the growth plate is regulated by IGF (e.g. Olney & Mougey, 1999), appears not to be a major issue at the enthesis, as there is no evidence for enhanced tendon or fibrocartilage cell proliferation (Woo et al. 1988). However, control of the later stages is significant. In the epiphysial growth plate, this involves a complex interaction of a variety of signalling molecules, some of which are also involved in early tendon, cartilage and fibrocartilage differentiation as indicated above. Cessation of proliferation and onset of hypertrophy is stimulated by FGFs and BMPs (Volk et al. 1998; de Crombrugghe et al. 2000), with a negative feedback regulation of hypertrophy and enhancement of proliferation provided by Ihh produced by hypertrophic chondrocytes acting on the perichondrium to produce PTHrP (de Crombrugghe et al. 2001; Vortkamp, 2001). At the transcriptional level, hypertrophy is regulated by Cbfa1 (Leboy et al. 2001; Takeda et al. 2001). Although there are differences in the organization of tissues and cells, the similarities are sufficient to make it important to discover the extent to which controls operating in the growth plate also occur in the enthesis.
Following fibrocartilage ‘hypertrophy’, angiogenesis and osteogenesis occur following the erosion of the terminal ‘hypertrophic’ fibrocartilage cells of the enthesis (Benjamin et al. 2000). Nothing is known about how this happens at entheses, but again the analogy is with growth plates. Chondrocyte hypertrophy is followed by apoptosis and vascular invasion, fibrocartilage cells express hypertrophic markers and then undergo regulated cell death (Yamada, 1976) before the space they occupy is invaded by blood vessels. In cartilage, hypertrophic chondrocytes express VEGF (Gerber et al. 1999; Colnot & Helms, 2001), the major stimulator of angiogenesis, and expression is modulated by cartilage-promoting growth factors FGFa FGFb, TGF beta and IGF-1 (Garcia-Ramirez et al. 2000; Gerber & Ferrara, 2000). GDF5, which is associated with tendon differentiation and has been linked to fibrocartilage formation (Bostrom et al. 1995; Takae et al. 1999; Nakase et al. 2001), also has angiogenic effects (Yamashita et al. 1997). VEGF is bound to cartilage ECM, and can be released by matrix metalloproteinases (MMPs) (Vu et al. 1998; Gerber et al. 1999), so the expression of these is clearly important in growth plate angiogenesis. Mt1-MMP and MMP9 are expressed by the perichondrium in developing cartilage rudiments, whereas MMP13 occurs in late hypertrophic cells, and is important in the differentiation process – inhibition of collagenase arrests hypertrophy (Kim et al. 1999; Colnot & Helms, 2001; Wu et al. 2002). VEGF itself can also have direct effects on osteoblast differentiation (Deckers et al. 2000), thus linking angiogenesis to osteogenesis. Thus, it is clear that determining the control of angiogenesis in the enthesis is important in understanding its development and growth.
Enthesis turnover
Whether entheses are capable of repair and whether a ‘normal’ fibrocartilaginous enthesis can reform after the surgical reattachment of a tendon or ligament are questions of considerable clinical importance. Although a few authors report failure to re-establish a normal attachment site (e.g. Dovan et al. 2005), there are numerous reports claiming the restoration of enthesis structure at anchorage sites after surgery. Some of the more recent studies include those of Martinek et al. (2002), Uhthoff et al. (2002) and Wong et al. (2003). However, authors often comment on a poor quality of repair in their reconstructions (Thomopoulos et al. 2002), the slow nature of the healing process (Silva et al. 2002) or the greater time it takes for an enthesis to be remodelled structurally, than for its mechanical properties to be restored (Walsh et al. 2004). It is thus important to note that the restoration of a normal enthesis can be significantly enhanced by treating tendon–bone grafts with a variety of biologically active modulators. Martinek et al. (2002) have shown the adenovirus gene transfer of BMP 2 enhances the integration of a semitendinosus graft in rabbits as an ACL replacement. Lattermann et al. (2004) have demonstrated that tendon transplant healing is improved when adenoviral gene delivery is applied to the bone side of the interface. According to Mihelic et al. (2004), the integration of graft and bone tissue during the reconstruction of the sheep ACL can be promoted by a coating of BMP-7 (osteogenic protein-1). BMP-7 encouraged the formation of a denser network of trabecular bone at the reattachment site than was evident in controls. It is also worth noting that BMP-13 induces neotendon formation with fibrocartilage cell differentiation in skeletal muscle of athymic nude rats (Helm et al. 2001). Suturing periosteum onto the surface of reattached tendons can also promote healing, perhaps because of the availability of stem cells that this creates (Chen et al. 2003). It should also be noted that the type of surface to which a graft is attached has a bearing on the healing outcome. According to Soda et al. (2003), ligament reconstructions are more successful when ligaments are reattached to compact rather than cancellous bone, although St Pierre et al. (1995) had earlier reported no difference. Aoki et al. (2001) have considered the effect of attaching grafts to cancellous bone rather than calcified fibrocartilage. They attributed the poor attachment to the calcified fibrocartilage layer of the dog infraspinatus enthesis to a barrier effect of this tissue, inhibiting angiogenesis.
Despite the considerable recent interest in the biology of mesenchymal stem cells (MSCs) as it relates to tissue repair in the musculoskeletal system, few authors have considered them in relation to entheses. Yet a greater understanding of this topic clearly has an important bearing on methods for enhancing enthesis repair and securing a stable tendon/ligament–bone graft in the surgical reconstruction of an enthesis. When one considers the number of ACL replacements that are performed world-wide by orthopaedic surgeons each year, it is obvious that this is a priority area that needs to be addressed in future research. It is encouraging therefore to report that Lim et al. (2004) have recently investigated the potential of applying MSCs at a tendon–bone junction for enhancing the surgical reconstruction of an enthesis. They found that coating hamstring tendon autografts in rabbits with MSCs resulted in the formation of a substitute ACL enthesis that was more biologically normal than that formed in control animals where the autograft was reattached without any stem-cell coating. The application of MSCs encouraged fibrocartilage formation at the interface that was a characteristic feature of the normal insertion site. Unfortunately, the authors did not use labelled MSCs, so they could not be certain whether the cartilage was formed from cells introduced experimentally or cells recruited locally. There would seem to be two potential sources of local stem cells: undifferentiated cells in the endotenon or MSCs in the enthesis bone marrow. Fibrocartilage is known to form within the endotenon of tendons in ‘wrap-around’ regions (Benjamin et al. 1995) and the thin character of the subchondral bone plate at entheses, combined with the common microscopic local absence of bone at these sites (Suzuki et al. 2005), offers the potential for bone marrow stem cells to make direct contact with the soft tissue side of an enthesis and thus contribute to enthesis fibrocartilage repair. Evidence for fibrocartilage formation at areas of local bone absence has been seen at the insertion site of iliopsoas, and cartilage formation within the bone marrow of entheses has also been observed (M. Benjamin, unpublished observations).
Enthesopathies in sport
An enthesopathy is usually defined as a pathological change at an enthesis. Rheumatological conditions including rheumatoid arthritis, spondylarthropathy, CPPD (calcium pyrophosphate deposition disease) and DISH (diffuse idiopathic skeletal hyperostosis) account for a major proportion of insertional tendinopathies or enthesopathies seen in clinical practice. In certain studies, however, up to 50% of the injuries suffered by athletes who exercise daily involve tendons, tendon sheaths and tendon insertions Orava & Leppilahti (1999). The most common anatomical sites for sport-associated enthesopathies are the rotator cuff, the lateral epicondyle of the humerus, the lower pole of the patella, the Achilles tendon insertion and the plantar fascia of the heel. Enthesopathies of the groin and extensor mechanism of the knee are relatively infrequent but are often more difficult for clinicians to manage (Renstrom, 1992). Because fibrous tendon insertions are not very common, we shall focus on fibrocartilaginous tendon insertional problems where the pathological changes are localized at or near the osteotendinous junction.
Abundant histological evidence of the most common enthesopathies rarely, if ever, demonstrates evidence of inflammation within the affected enthesis or enthesis organ. Although misnomers such as chronic lateral epicondylitis and patellar tendonitis persist, inflammatory processes are rarely involved in these conditions. Classic inflammatory changes are not frequently seen in chronic athletic tendon conditions and histopathological features in tendinopathic tendons are clearly different from normal tendons, showing an exaggerated dysfunctional repair response. Microscopically, these tendons show thinning, disruption of collagen fibres, increased vascularity and cellularity, granulation tissue, increased proteoglycan content in the ECM, and microtears (Khan et al. 1999). Similarly, age-related microscopic and biochemical pathological changes including tenocyte degeneration, accumulation of lipids, amorphous ECM and calcium deposits can be seen as early as age 15 years in the Achilles, biceps brachii, tibialis anterior, quadriceps and patellar tendons (Adams et al. 1974).
Biomechanical factors contributing to the development of enthesopathies have become a topic of debate. Tendons demonstrate viscoelastic, time-dependent behaviour and exhibit adaptive responses to altered loading patterns. It has been argued that insertional tendinopathies may not be purely a tensile injury, but rather that altered mechanics such as compression or stress-shielding may be important. Both tendon compression and a decrease in tendon load (stress-shielding) will induce changes similar to those seen in an insertional tendinopathy. Biomechanical studies show that the strains within a tendon near its insertion site are in fact non-uniform (Maganaris et al. 2004). If the material properties are similar throughout the tendon, forces transferred through the insertion site may preferentially load the side of the tendon that is usually not principally affected. These areas of compressive loading correspond to the sites where tendinopathic characteristics are typically seen. Thus, the presence of differential strains opens the possibility of alternative biomechanical explanations for the pathology found in enthesopathies. High-resolution, real-time ultrasound scanning confirms the applicability of these findings in human tendons in vivo (Fukunaga et al. 2001). An application of this concept is worthy of discussion. The clinical success observed following operative release of the adductor longus lends some support to the theory of stress-shielding. In this surgery, the superficial section of the normal adductor longus tendon is released at a point distal to its insertion. This may have the effect of transferring stress from the superficial section of the tendon to the stress-shielded deeper fibres, and the induction of normal loads in both the deeper and the superficial fibres of the tendon may assist in tendon recovery.
Because muscle is more compliant than tendon or bone, the transfer of mechanical stress from muscle to bone (rather than vice versa) facilitates the physiological transmission of forces and stress dissipation across the ‘muscle–tendon–bone’ unit. This offers a mechanical advantage during loading (compared with stress transfer in the opposite direction) by minimizing the effects of stress concentration. However, it is possible that a change in stiffness of the ‘muscle–tendon’ unit following exercise, injury and/or fatigue alters force transfer and that some enthesopathies may be a secondary consequence. Thus, it is well known that muscle–tendon stiffness is altered in exercises inducing muscle fatigue (Horita et al. 2003) and this may account for some enthesopathies. Certainly, it is likely that enthesopathies can result from ground reaction forces, as these are often considerably greater than the force produced by the muscle. Because bone is stiffer than a muscle–tendon unit, the stress dissipation from bone to tendon must be considerable. It is well known that training on hard surfaces, e.g. concrete, can increase the risk of Achilles insertional problems (Nichols, 1989). Landing heavily or repeatedly on such surfaces can send shock waves through the body which are partly absorbed by the Achilles tendon. Soft surfaces (e.g. grass) absorb some of the force generated by landing heavily on the ground during jumping or running. In exercises involving high ground impact forces, the transfer of the reaction force from ground to bone, from bone to tendon and from tendon to muscle may cause injury, particularly if the movement is not controlled. For example, insufficient strength of the gastrocnemius, soleus and tibialis anterior muscles will result in poor control of foot pronation during landing (Marigold et al. 2004). This in turn will change the alignment of the calcaneus and thus the insertional angle of the Achilles tendon, perhaps making it vulnerable to injury. It should be noted that in exercises involving successive landing cycles, there is a repeated two-way transfer of mechanical stress between muscle and bone. This may injure the vulnerable junctional zones (i.e. the myotendinous junction and the enthesis). It should also be noted that muscles function as ‘shock absorbers’ and, in this respect, they must counterbalance a shock-absorbing function of their antagonists. Well-balanced agonist and antagonist sets of muscles preserve joint alignment and reduce the risk of injuries that result when one muscle group is weaker than its opposing muscles. For example, jogging places more stress on the hamstring and calf muscles than it does on quadriceps femoris (Andriacchi & Birac, 1993). This creates a muscle imbalance that can lead to knee injuries such as jumper's knee. The logical extension of this argument is that muscle balance training may reduce the risk of enthesopathy.
Thus, although it would seem that there are many ways in which enthesopathies could occur, the source of pain is unknown. Clinically, pain at entheses has been attributed to inflammatory processes, but as it has become evident that tendinopathies in general are degenerative rather than inflammatory conditions (Khan et al. 2002), a combination of mechanical and biochemical causes for enthesopathies is more attractive. It is thus interesting to note that Shaw et al. (2005) have shown that the normal rat Achilles tendon enthesis is aneural, although it is well known that other parts of tendons are innervated (Jozsa & Kannus, 1997). Four types of nerve endings have been identified: free nerve endings, Ruffini corpuscles, Pacinian corpuscles and Golgi tendon organs (Jozsa & Kannus, 1997). Although much of the innervation of tendons parallels their microvasculature, some of the neural elements terminate in the tissues in close proximity to tissue mast cells. Given the known activity of mast cells, recent thinking is that the neural–mast cell interaction may lead to release of mast cell components that could modulate pain (Hart et al. 1995). It is intriguing that mast cells are conspicuous in conjunction with nerve fibres in Kager's fat pad of the rat, an integral part of the Achilles tendon enthesis organ (Shaw et al. 2005).
The diagnosis of enthesopathy is primarily a clinical one, but Doppler ultrasonography and MR imaging have recently been used to confirm emerging ideas about the pathophysiology of enthesopathies. Both techniques provide excellent anatomical representation of tendons and show changes not previously recognized in patients with enthesis disorders, although these changes do not necessarily predict prognosis or outcome. In the patellar and Achilles tendons for example, increased signal on MR imaging (Khan et al. 1996; Movin et al. 1998a,b) and hypoechoic regions on ultrasonography (Cook et al. 1998) reflect collagen degeneration rather than ongoing inflammation. However, identical findings have been noted in tendons of totally asymptomatic jumping athletes (Yu et al. 1995). Indeed, in clinical practice the source of pain in patients with enthesopathies is open to debate. Furthermore, a prospective study with longitudinal follow-up showed that the presence of an ultrasonographic abnormality in patellar tendons did not predict poor prognoses in elite basketball players (Cook et al. 1998). Taken together, these findings support the hypothesis that sport-related enthesopathies represent a non-inflammatory condition with pathological changes that favour tendon degeneration rather than acute inflammation.
The optimal treatment approach for an athlete with an enthesopathy is still evolving. Although outcomes of both non-operative and operative management are improving, these strategies are still mostly empirically based and often lack robust scientific evidence. Current therapeutic protocols are characterized by wide variability ensuing from anecdotal experience rather than evidence. Moreover, numerous reports in recent years have shattered previous doctrines and dogmatic belief on tendon overuse. Histopathological and biochemical evidence has indicated that the underlying pathology of tendinopathy is not an inflammatory tendonitis, but a degenerative tendinosis. Consequently, enthesopathy-related pain is probably not due to inflammation, but its exact origin remains unexplained. Accordingly, in pursuit of pathology- and evidence-based management, conservative therapy has shifted from anti-inflammatory strategies towards a comprehensive rehabilitation programme emphasizing eccentric strengthening of the affected limb and muscle–tendon unit (Young et al. 2005). The traditional concept of tensile failure may not be the essential feature of the pathomechanics of insertional tendinopathies. Incorporating different joint-position exercises may exert more controlled stresses on these affected areas of the tendon, possibly allowing better maintenance of the mechanical strength of that tendon region and therefore preventing injury. Such exercises could stress a healing area of the tendon in a controlled manner and thus stimulate healing once an injury has occurred. Investigations are underway to prove whether such principles should be incorporated into rehabilitation programmes. Another recent novel approach to the treatment of insertional tendinopathies involves the use of extracorporeal shock wave lithotripsy (ESWT). Emerging evidence suggests that short-term use of this modality results in tissue neovascularization through increased levels of VGEF and endothelial nitric oxide synthase (eNOS) (Wang et al. 2003). Others have shown that ESWT produces short-term pain relief through decreased levels of substance P along with reduced levels of CGRP immunoreactivity in the dorsal root ganglion (Maier et al. 2003). Despite these postulated mechanisms of action, the results of ESWT for insertional tendinopathies (lateral epicondyle of the elbow, patellar tendon, plantar fascia) remain somewhat mixed. Patient selection parameters and exact methods remain for future investigation.
Enthesophytes (bony spurs)
Bony spurs (enthesophytes) are well documented at numerous entheses as bony outgrowths that extend from the skeleton into the soft tissue of a tendon or ligament at its enthesis (Fig. 1e). They can occur in association with high levels of physical activity (Smillie, 1970), in patients with seronegative spondyloarthropathy and in those suffering from DISH. They become more common with increasing age and are more frequently found in males than females (Rogers et al. 1997). Enthesophytes are comparable with the osteophytes which form around the articular surfaces of synovial joints in patients with osteoarthritis. Indeed, Rogers et al. (1997) have made the interesting suggestion that osteophyte and enthesophyte formation are linked and that both represent a skeletal response to stress. On the basis of a comprehensive study of a large number of skeletons from archaeological sites, they have proposed that some individuals have a greater tendency to form bone than others, both at the margins of joints and at entheses. This argues for a genetic basis to spur formation at both locations and suggests that some individuals can be regarded as ‘bone formers’. Such individuals form bone at levels of mechanical stress that do not trigger comparable osteogenesis in others. It is significant that in individuals in whom enthesophytes are most common (those with DISH – Mazieres & Rovensky, 2000), there is also a high incidence of osteophytes (Rogers et al. 1997). It has been suggested that osteophytes develop to modify the loading on synovial joints whose stability has been compromised by injury or disease (Bullough & Vigorta, 1984). They may represent an adaptation to injury where they serve to limit abnormal movement and help to restore a functional joint surface. Whether enthesophytes represent a comparable adaptation at entheses is unclear. Whether their presence reduces the risk of failure at the hard/soft tissue interface or whether they are ever a feature of bone at sites of tendon or ligament avulsion are important questions that remain unanswered.
In view of the association between osteophytes and osteoarthritis, it is of interest to note that at entheses where bony spurs are well documented, e.g. the Achilles tendon and the plantar fascia, osteoarthritic-like degenerative changes (fissures and cell clusters) have also been noted in the enthesis fibrocartilage of the tendon or ligament (Rufai et al. 1995; Kumai & Benjamin, 2002). We know relatively little about bony spur formation, although enthesophytes are widely assumed to be ‘traction spurs’, i.e. to develop in response to high tensile forces within a tendon or ligament (Rogers et al. 1997). However, there is little evidence to support this assumption, which has been challenged by Kumai & Benjamin (2002) on the basis of their study of spur formation in the plantar fascia. They have pointed out that the majority of plantar fascial spurs develop on the deep surface of the ligament rather than within it and it is thus difficult to see how the spurs could be subject to high tensile load within the ligament. The later radiological study of Abreu et al. (2003) confirms that the spurs rarely occur in the plantar fascia, but more often in the entheses of abductor digiti minimi and flexor digitorum brevis.
The presence of fibrocartilage at the tip of many enthesophytes suggests that endochondral ossification could play a role in their formation. This is supported by the work of Benjamin et al. (2000) on the rat Achilles tendon, which demonstrated the development of bony spurs by vascular invasion along the rows of fibrocartilage cells at the enthesis. They have argued that bony spur formation at this site is essentially an extension of normal enthesis growth and that the longitudinal orientation of the bony spurs, along the long axis of the tendon or ligament, reflects the orientation of the rows of fibrocartilage cells.
Chambler et al. (2004) have shown that osteocytes at the tip of the human acromion (where bony spurs commonly grow into the coracoacromial ligament) express elevated levels of alkaline phosphatase (indicative of osteoblastic activity) and tartrate-resistant acid phosphatase (TRAP, indicative of osteoclastic activity). Furthermore, glucose-6-phosphate dehydrogenase (G6PD) and alkaline phosphatase activity are greater on the inferior surface of the acromial tip than on the superior surface. This corresponds with the area of most intense mineral apposition in bony spur formation (Chambler et al. 2003).
References
- Abreu MR, Chung CB, Mendes L, Mohana-Borges A, Trudell D, Resnick D. Plantar calcaneal enthesophytes: new observations regarding sites of origin based on radiographic, MR imaging, anatomic, and paleopathologic analysis. Skeletal Radiol. 2003;32:13–21. doi: 10.1007/s00256-002-0585-x. [DOI] [PubMed] [Google Scholar]
- Adams CMW, Bayliss OB, Baker RWR, Abdulla YH, Huntercraig CJ. Lipid deposits in aging human arteries, tendons, and fascia. Atherosclerosis. 1974;19:429–440. doi: 10.1016/s0021-9150(74)80007-9. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Birac D. Functional testing in the anterior cruciate ligament-deficient knee. Clin Orthop Relat Res. 1993;288:40–47. [PubMed] [Google Scholar]
- Aoki M, Oguma H, Fukushima S, Ishii S, Ohtani S, Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment. J Shoulder Elbow Surg. 2001;10:123–128. doi: 10.1067/mse.2001.111963. [DOI] [PubMed] [Google Scholar]
- Bell E, Gosline J. Mechanical design of mussel byssus: material yield enhances attachment strength. J Exp Biol. 1996;199:1005–1017. doi: 10.1242/jeb.199.4.1005. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ. Fibrocartilage. A Review. J Anat. 1990;171:1–15. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Donthineni Rao R, Findlay JA, Pemberton DJ. Quantitative differences in the histology of the attachment zones of the meniscal horns in the knee joint of man. J Anat. 1991;177:127–134. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Newell RLM, Evans EJ, Ralphs JR, Pemberton DJ. The structure of the insertions of the tendons of biceps brachii, triceps and brachialis in elderly dissecting room cadavers. J Anat. 1992;180:327–332. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Qin S, Ralphs JR. Fibrocartilage associated with human tendons and their pulleys. J Anat. 1995;187:625–633. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Functional and developmental anatomy of tendons and ligaments. In: Gordon SL, Blair SJ, Fine LJ, editors. Repetitive Motion Disorders of the Upper Extremity. Rosemont: American Academy of Orthopaedic Surgeons; 1995. pp. 185–203. [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arth Rheum. 2000;43:576–583. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M. Tendons are dynamic structures that respond to changes in exercise levels. Scand J Med Sci Sports. 2002;12:1–2. doi: 10.1046/j.0905-7188.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Hillen B. Mechanical influences on cells, tissues and organs –‘mechanical morphogenesis’. Eur J Morph. 2002;40:69–73. doi: 10.1076/ejom.40.2.69.15449. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons – tendon entheses. Comp Biochem Phys A Mol Integr Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The ‘enthesis organ’ concept – why enthesopathies may not present as focal insertional disorders. Arth Rheum. 2004a;50:3306–3313. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Redman S, Milz S, et al. Fat at entheses – the rheumatological implications of its distribution: a potential site of pain and stress dissipation? Ann Rheum Dis. 2004b;63:1549–1555. doi: 10.1136/ard.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. The biology of fibrocartilage cells. Int Rev Cytol. 2004;233:1–45. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Theobald P, Suzuki D, Toumi H. The anatomy of the Achilles tendon. In: Maffulli N, Almekinders L, editors. The Achilles Tendon. Berlin: Springer; 2005. in press. [Google Scholar]
- Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction imaging. Radiology. 1991;179:777–781. doi: 10.1148/radiology.179.3.2027991. [DOI] [PubMed] [Google Scholar]
- Biermann. Die Knochenbildung im Bereich Periostaler-Diaphysär Sehnen- und Bandansätze. Z Zellforsch. 1957;46:635–671. [PubMed] [Google Scholar]
- Bloebaum RD, Kopp DV. Remodeling capacity of calcified fibrocartilage of the hip. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:736–739. doi: 10.1002/ar.a.20066. [DOI] [PubMed] [Google Scholar]
- Bostrom MP, Lane JM, Berberian WS, et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13:357–367. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- Boszczyk BM, Boszczyk AA, Putz R, Buttner A, Benjamin M, Milz S. An immunohistochemical study of the dorsal capsule of the lumbar and thoracic facet joints. Spine. 2001;26:E338–E343. doi: 10.1097/00007632-200108010-00006. [DOI] [PubMed] [Google Scholar]
- Boszczyk AA, Boszczyk BM, Putz R, Benjamin M, Milz S. Expression of a wide range of fibrocartilage molecules at the entheses of the alar ligaments possible antigenic targets for rheumatoid arthritis? J Rheumatol. 2003a;30:1420–1425. [PubMed] [Google Scholar]
- Boszczyk BM, Boszczyk AA, Korge A, et al. Immunohistochemical analysis of the extracellular matrix in the posterior capsule of the zygapophysical joints in patients with degenerative L4–5 motion segment instability. J Neurosurg. 2003b;99(Suppl. 1):27–33. doi: 10.3171/spi.2003.99.1.0027. [DOI] [PubMed] [Google Scholar]
- Braun J, Khan MA, Sieper J. Enthesitis and ankylosis in spondyloarthropathy: What is the target of the immune response? Ann Rheum Dis. 2000;59:985–994e. doi: 10.1136/ard.59.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella MA, Tirman PF, Fritz RC, Feller JF, Wischer TK, Genant HK. MR imaging findings of lateral ulnar collateral ligament abnormalities in patients with lateral epicondylitis. Am J Roentgenol. 1999;173:1379–1382. doi: 10.2214/ajr.173.5.10541124. [DOI] [PubMed] [Google Scholar]
- Bullough P, Vigorta V. Atlas of Orthopaedic Pathology. London: Gower; 1984. [Google Scholar]
- Canoso JJ, Liu N, Traill MR, Runge VM. Physiology of the retrocalcaneal bursa. Ann Rheum Dis. 1988;47:910–912. doi: 10.1136/ard.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambler AF, Pitsillides AA, Emery RJ. Acromial spur formation in patients with rotator cuff tears. J Shoulder Elbow Surg. 2003;12:314–321. doi: 10.1016/s1058-2746(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Chambler A, Rawlinson S, Emery R, Pitsillides A. Quantitative cytochemical evidence for local increases in bone turnover at the acromial enthesis of the human coracoacromial ligament. J Rheumatol. 2004;31:2216–2225. [PubMed] [Google Scholar]
- Chen CH, Chen WJ, Shih CH, Yang CY, Liu SJ, Lin PY. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Arthroscopy. 2003;19:290–296. doi: 10.1053/jars.2003.50014. [DOI] [PubMed] [Google Scholar]
- Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100:245–250. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- Cook JL, Khan KM, Harcourt PR, Kiss ZS, Fehrmann MW, Griffiths L, Wark JD. Patellar tendon ultrasonography in asymptomatic active athletes reveals hypoechoic regions: a study of 320 tendons: Victorian Institute of Sport Tendon Study Group. Clin J Sport Med. 1998;8:73–77. doi: 10.1097/00042752-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg. 1970;52A:1–20. [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol. 2001;13:721–727. doi: 10.1016/s0955-0674(00)00276-3. [DOI] [PubMed] [Google Scholar]
- Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- Dovan TT, Ritty T, Ditsios K, Silva MJ, Kusano N, Gelberman RH. Flexor digitorum profundus tendon to bone tunnel repair: a vascularization and histologic study in canines. J Hand Surg. 2005;30A:246–257. doi: 10.1016/j.jhsa.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ennos AR, Crook MJ, Grimshaw C. A comparative study of the anchorage systems of Himalayan balsam Impatiens glandulifera and mature sunflower Helianthus annuus. J Exp Bot. 1993;44:133–146. [Google Scholar]
- Evans EJ, Benjamin M, Pemberton DJ. Fibrocartilage in the attachment zones of the quadriceps tendon and patellar ligament of man. J Anat. 1990;171:155–162. [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc R Soc Lond B Biol Sci. 2001;268:229–233. doi: 10.1098/rspb.2000.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton GD, Cameron IL, Ord VA. Orientation of tendons in the magnetic field and its effect on T2 relaxation times. Radiology. 1985;155:433–435. doi: 10.1148/radiology.155.2.3983395. [DOI] [PubMed] [Google Scholar]
- Furumatsu T, Yamaguchi N, Nishida K, et al. Endostatin inhibits adhesion of endothelial cells to collagen I via alpha (2) beta (1) integrin, a possible cause of prevention of chondrosarcoma growth. J Biochem (Tokyo) 2002;131:619–626. doi: 10.1093/oxfordjournals.jbchem.a003142. [DOI] [PubMed] [Google Scholar]
- Galera P, Park RW, Ducy P, Mattei MG, Karsenty G. c-Krox binds to several sites in the promoter of mouse type I collagen genes. Structure/function study and developmental expression analysis. J Biol Chem. 1996;271:21331–21339. doi: 10.1074/jbc.271.35.21331. [DOI] [PubMed] [Google Scholar]
- Gao J, Messner K, Ralphs JR, Benjamin M. An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat Embryol. 1996;194:399–406. doi: 10.1007/BF00198542. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramirez M, Toran N, Andaluz P, Carrascosa A, Audi L. Vascular endothelial growth factor is expressed in human fetal growth cartilage. J Bone Miner Res. 2000;15:534–540. doi: 10.1359/jbmr.2000.15.3.534. [DOI] [PubMed] [Google Scholar]
- Garrick JM. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977;5:241–242. doi: 10.1177/036354657700500606. [DOI] [PubMed] [Google Scholar]
- Gatehouse PD, Bydder GM. Magnetic resonance imaging of short T2 components in tissue. Clin Radiol. 2003;58:1–19. doi: 10.1053/crad.2003.1157. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med. 2000;10:223–228. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- Ghayor C, Herrouin JF, Chadjichristos C, Ala-Kokko L, Takigawa M, Pujol JP, Galera P. Regulation of human COL2A1 gene expression in chondrocytes. Identification of C-Krox-responsive elements and modulation by phenotype alteration. J Biol Chem. 2000;275:27421–27438. doi: 10.1074/jbc.M002139200. [DOI] [PubMed] [Google Scholar]
- Glant TT, Buzas EI, Finnegan A, Negroiu G, Cs-Szabo G, Mikecz K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812–3819. [PubMed] [Google Scholar]
- Guerassimov A, Zhang Y, Banerjee S, et al. Autoimmunity to cartilage link protein in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 1998;25:1480–1484. [PubMed] [Google Scholar]
- Hart DA, Frank CB, Bray RC. Inflammatory processes in repetitive motion and overuse syndromes: potential role of neurogenic mechanisms in tendons and ligaments. In: Gordon SL, Blair SJ, Fine LJ, editors. Repetitive Motion Disorders of the Upper Extremity. Rosemont: American Academy of Orthopaedic Surgeons; 1995. pp. 247–262. [Google Scholar]
- Helm GA, Li JZ, Alden TD, et al. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001;95:298–307. doi: 10.3171/jns.2001.95.2.0298. [DOI] [PubMed] [Google Scholar]
- Hems T, Tillmann B. Tendon entheses of the human masticatory muscles. Anat Embryol (Berl) 2000;202:201–208. doi: 10.1007/s004290000107. [DOI] [PubMed] [Google Scholar]
- Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ. Anisotropy of NMR properties of tissues. Magn Reson Med. 1994;32:592–601. doi: 10.1002/mrm.1910320508. [DOI] [PubMed] [Google Scholar]
- Horita T, Komi PV, Hamalainen I, Avela J. Exhausting stretch-shortening cycle (SSC) exercise causes greater impairment in SSC performance than in pure concentric performance. Eur J Appl Physiol. 2003;88:527–534. doi: 10.1007/s00421-002-0716-z. [DOI] [PubMed] [Google Scholar]
- Johnson RB. Synthesis of alveolar bone Sharpey's fibers during experimental tooth movement in the rat. Anat Rec a Discov Mol Cell Evol Biol. 2005;284A:485–490. doi: 10.1002/ar.a.20179. [DOI] [PubMed] [Google Scholar]
- Jozsa LG, Kannus P. Human Tendons: Anatomy, Physiology, and Pathology. Champaign, IL: Human Kinetics; 1997. [Google Scholar]
- Karumanchi SA, Jha V, Ramchandran R, et al. Cell surface glypicans are low-affinity endostatin receptors. Mol Cell. 2001;7:811–822. doi: 10.1016/s1097-2765(01)00225-8. [DOI] [PubMed] [Google Scholar]
- Khan KM, Bonar F, Desmond PM, et al. Patellar tendinosis (jumper's knee): findings at histopathologic examination, US and MR imaging: Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200:821–827. doi: 10.1148/radiology.200.3.8756939. [DOI] [PubMed] [Google Scholar]
- Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- Khan KM, Cook JL, Kannus P, Maffulli N, Bonar SF. Time to abandon the ‘tendinitis’ myth. BMJ. 2002;324:626–627. doi: 10.1136/bmj.324.7338.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80:159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- Kim YM, Jang JW, Lee OH, et al. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- Knese K-H, Biermann H. Die Knochenbildung an Sehnen- und Bandsätzen im Bereich ursprünglich chondraler Apophysen. Z Zellforsch Mikrosk Anat. 1958;49:142–187. [PubMed] [Google Scholar]
- Kumai T, Benjamin M. Heel spur formation and the subcalcaneal enthesis of the plantar fascia in man. J Rheumatol. 2002;29:1957–1964. [PubMed] [Google Scholar]
- Kummer B. Bauprinzipien Des Säugerskelettes. Stuttgart: Thieme; 1959. [Google Scholar]
- Kummer B. Funktioneller Bau und funktionelle Anpassung des Knochens. Anat Anz. 1962;110:261–293. [Google Scholar]
- Kummer B. Mechanische Beanspruchung und funktionelle Anpassung des Knochens. Verh Anat Ges. 1978;72:21–46. [PubMed] [Google Scholar]
- Lattermann C, Zelle BA, Whalen JD, et al. Gene transfer to the tendon-bone insertion site. Knee Surg Sports Traumatol Arthrosc. 2004;12:510–515. doi: 10.1007/s00167-003-0482-4. [DOI] [PubMed] [Google Scholar]
- Leboy P, Grasso-Knight G, D’Angelo M, et al. Smad–Runx interactions during chondrocyte maturation. J Bone Joint Surg. 2001;83(Suppl. 1):S15–S22. [PubMed] [Google Scholar]
- Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9(Suppl. A):S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med. 2004;34:1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- Maier M, Averbeck B, Milz S, Refior HJ, Schmitz C, Maier M. Substance P and prostaglandin E2 release after shock wave application to the rabbit femur. Clin Orthop. 2003;406:237–245. doi: 10.1097/01.blo.0000030173.56585.8f. [DOI] [PubMed] [Google Scholar]
- Marigold DS, Eng JJ, Tokuno CD, Donnelly CA. Contribution of muscle strength and integration of afferent input to postural instability in persons with stroke. Neurorehabil Neural Repair. 2004;18:222–229. doi: 10.1177/1545968304271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GW, Jr, Balooch M, Gallagher RR, Gansky SA, Marshall SJ. Mechanical properties of the dentinoenamel junction: AFM studies of nanohardness, elastic modulus, and fracture. J Biomed Mater Res. 2001;54:87–95. doi: 10.1002/1097-4636(200101)54:1<87::aid-jbm10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg. 2002;84A:1123–1131. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Mazieres B, Rovensky J. Non-inflammatory enthesopathies of the spine: a diagnostic approach. Baillieres Best Pract Res Clin Rheumatol. 2000;14:201–217. doi: 10.1053/berh.1999.0062. [DOI] [PubMed] [Google Scholar]
- McGonagle D, Benjamin M, Marzo-Ortega H, Emery P. Advances in the understanding of entheseal inflammation. Curr Rheumatol Rep. 2002;6:500–506. doi: 10.1007/s11926-002-0057-2. [DOI] [PubMed] [Google Scholar]
- McGonagle D. Diagnosis and treatment of enthesitis. Rheum Dis Clin North Am. 2003;3:549–560. doi: 10.1016/s0889-857x(03)00044-9. [DOI] [PubMed] [Google Scholar]
- Mihelic R, Pecina M, Jelic M, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- Milz S, McNeilly C, Putz R, Ralphs JR, Benjamin M. Fibrocartilages in the extensor tendons of the interphalangeal joints of human toes. Anat Rec. 1998;252:264–270. doi: 10.1002/(SICI)1097-0185(199810)252:2<264::AID-AR11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Milz S, Schluter T, Putz R, Moriggl B, Ralphs JR, Benjamin M. Fibrocartilage in the transverse ligament of the human atlas. Spine. 2001;15:1765–1771. doi: 10.1097/00007632-200108150-00007. [DOI] [PubMed] [Google Scholar]
- Milz S, Rufai A, Buettner A, Putz R, Ralphs JR, Benjamin M. Three dimensional reconstructions of the Achilles tendon enthesis in man. J Anat. 2002;200:145–152. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz S, Tischer T, Buettner A, et al. The molecular composition and pathology of entheses on the medial and lateral epicondyles of the humerus – a structural basis for epicondylitis? Ann Rheum Dis. 2004;63:1015–1021. doi: 10.1136/ard.2003.016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz S, Benjamin M, Putz R. Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol. 2005 in press. [PubMed] [Google Scholar]
- Moriggl B, Kumai T, Milz S, Benjamin M. The structure and histopathology of the ‘enthesis organ’ at the navicular insertion of the tendon of tibialis posterior in man. J Rheumatol. 2003;30:508–517. [PubMed] [Google Scholar]
- Movin T, Kristoffersen-Wiberg M, Rolf C, Aspelin P. MR imaging in chronic Achilles tendon disorder. Acta Radiol. 1998a;39:126–132. doi: 10.1080/02841859809172165. [DOI] [PubMed] [Google Scholar]
- Movin T, Kristoffersen-Wiberg M, Shalabi A, Gad A, Aspelin P, Rolf C. Intratendinous alterations as imaged by ultrasound and contrast medium enhanced magnetic resonance in chronic achillodynia. Foot Ankle Int. 1998b;19:311–317. doi: 10.1177/107110079801900508. [DOI] [PubMed] [Google Scholar]
- Myers TW. Anatomy Trains. Myofascial Meridians for Manual and Movement Therapists. Edinburgh: Churchill Livinstone; 2001. [Google Scholar]
- Myers LK, Higgins GC, Finkel TH, et al. Juvenile arthritis and autoimmunity to type II collagen. Arthritis Rheum. 2001;44:1775–1781. doi: 10.1002/1529-0131(200108)44:8<1775::AID-ART313>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Nakase T, Ariga K, Miyamoto S, et al. Distribution of genes for bone morphogenetic protein-4-6, growth differentiation factor-5, and bone morphogenetic protein receptors in the process of experimental spondylosis in mice. J Neurosurg. 2001;94:68–75. doi: 10.3171/spi.2001.94.1.0068. [DOI] [PubMed] [Google Scholar]
- Nichols AW. Achilles tendinitis in running athletes. J Am Board Fam Pract. 1989;2:196–203. [PubMed] [Google Scholar]
- O’Reilly MS, Behm T, Sing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Oatridge A, Herlihy AH, Thomas RW, et al. Magnetic resonance: magic angle imaging of the Achilles tendon. Lancet. 2001;358:1610–1611. doi: 10.1016/S0140-6736(01)06661-2. [DOI] [PubMed] [Google Scholar]
- Olivieri I, Barozzi L, Padula A, et al. Retrocalcaneal bursitis in spondyloarthropathy: assessment by ultrasonography and magnetic resonance imaging. J Rheumatol. 1998;25:1352–1357. [PubMed] [Google Scholar]
- Olney RC, Mougey EB. Expression of the components of the insulin-like growth factor axis across the growth-plate. Mol Cell Endocrinol. 1999;156:63–71. doi: 10.1016/s0303-7207(99)00144-6. [DOI] [PubMed] [Google Scholar]
- Orava S, Leppilahti J. In: Overuse Injuries of Tendons in Athletes. Jakob RP, Fulford P, Horan F, editors. London: The British Editorial Society of Bone and Joint Surgery; 1999. [Google Scholar]
- Pauwels F. Biomechanics of the Locomotor Apparatus. Berlin: Springer-Verlag; 1980. [Google Scholar]
- Petersen W, Pufe T, Kurz B, Mentlein R, Tillmann B. Angiogenesis in fetal tendon development: Spatial and temporal expression of the angiogenetic peptide vascular endothelial growth factor (VEGF) Anat Embryol. 2002;205:263–270. doi: 10.1007/s00429-002-0241-1. [DOI] [PubMed] [Google Scholar]
- Ploetz E. Funktioneller Bau und funktionelle Anpassung der Gleitsehnen. Z Orthop. 1938;67:212–234. [Google Scholar]
- Pufe T, Petersen WJ, Miosge N, et al. Endostatin/collagen XVIII – an inhibitor of angiogenesis – is expressed in cartilage and fibrocartilage. Matrix Biol. 2004;23:267–276. doi: 10.1016/j.matbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Benjamin M, Waggett AD, Russell DC, Messner K, Gao J. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat. 1998;193:215–222. doi: 10.1046/j.1469-7580.1998.19320215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspanti M, Cesari C, De Pasquale V, et al. A histological and electron-microscopic study of the architecture and ultrastructure of human periodontal tissues. Arch Oral Biol. 2000;45:185–192. doi: 10.1016/s0003-9969(99)00145-4. [DOI] [PubMed] [Google Scholar]
- Renstrom P. Tendon and muscle injuries in the groin area. Clin Sports Med. 1992;11:815–8312. [PubMed] [Google Scholar]
- Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–211. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27:825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- Robson MD, Benjamin M, Gishen P, Bydder GM. Magnetic resonance imaging of the Achilles tendon using ultrashort (UTE) pulse sequences. Clin Radiol. 2004;59:727–735. doi: 10.1016/j.crad.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shepstone L, Dieppe P. Bone formers: osteophyte and enthesophyte formation are positively associated. Ann Rheum Dis. 1997;56:85–90. doi: 10.1136/ard.56.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufai A, Ralphs JR, Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res. 1995;13:585–593. doi: 10.1002/jor.1100130414. [DOI] [PubMed] [Google Scholar]
- Rufai A, Ralphs JR, Benjamin M. Ultrastructure of fibrocartilages at the insertion of the rat Achilles tendon. J Anat. 1996;189:185–191. [PMC free article] [PubMed] [Google Scholar]
- Sato K, Miura T, Iwata H. Cartilaginous transdifferentiation of rat tenosynovial cells under the influence of bone morphogenetic protein in tissue culture. Clin Orthop. 1988;236:233–239. [PubMed] [Google Scholar]
- Schneider H. Zur Struktur der Sehnenansatzzonen. Z Anat. 1956;119:431–456. [PubMed] [Google Scholar]
- Shaw HM, Santer RM, Watson A, Benjamin M. The innervation of the enthesis organ of the rat Achilles tendon. J Anat. 2005 in press. [Google Scholar]
- Shea JE, Hallows RK, Bloebaum RD. Experimental confirmation of the sheep model for studying the role of calcified fibrocartilage in hip fractures and tendon attachments. Anat Rec. 2002;266:177–183. doi: 10.1002/ar.10051. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Boyer MI, Ditsios K, et al. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. J Orthop Res. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Smillie IS. Injuries of the Knee Joint. Edinburgh: Churchill Livingstone; 1970. [Google Scholar]
- Soda Y, Sumen Y, Murakami Y, Ikuta Y, Ochi M. Attachment of autogenous tendon graft to cortical bone is better than to cancellous bone: a mechanical and histological study of MCL reconstruction in rabbits. Acta Orthop Scand. 2003;74:322–326. doi: 10.1080/00016470310014256. [DOI] [PubMed] [Google Scholar]
- St Pierre P, Olson EJ, Elliott JJ, O’Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Joint Surg. 1995;77A:1856–1866. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- Suresh S. Graded materials for resistance to contact deformation and damage. Science. 2001;292:2447–2451. doi: 10.1126/science.1059716. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Toumi H, McGonagle D, Emery P, Benjamin M. The structure of bone at tendon and ligament attachment sites (entheses) J Anat. 2005 in press. [Google Scholar]
- Takae R, Matsunaga S, Origuchi N, et al. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine. 1999;24:1397–1401. doi: 10.1097/00007632-199907150-00002. [DOI] [PubMed] [Google Scholar]
- Takeda S, Bonnamy JP, Owen MJ, Ducy P, Karsenty G. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 2001;15:467–481. doi: 10.1101/gad.845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald PS, Bydder G, Nokes L, Dent C, Pugh N, Benjamin M. The functional significance of Kager's fat pad in relation to the Achilles tendon. J Anat. 2006;208:91–97. doi: 10.1111/j.1469-7580.2006.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Hattersley G, Rosen V, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Ito Y, Noda M. Expression of the PEBP2alphaA/AML3/CBFA1 gene is regulated by BMP4/7 heterodimer and its overexpression suppresses type I collagen and osteocalcin gene expression in osteoblastic and nonosteoblastic mesenchymal cells. Bone. 1998;22:87–92. doi: 10.1016/s8756-3282(97)00267-6. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK, Seki M, Backman DS, Trudel G, Himori K, Sano H. Tensile strength of the supraspinatus after reimplantation into a bony trough: an experimental study in rabbits. J Shoulder Elbow Surg. 2002;11:504–509. doi: 10.1067/mse.2002.126760. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Volk SW, Luvalle P, Leask T, Leboy PS. A BMP responsive transcriptional region in the chicken type X collagen gene. Bone Miner Res. 1998;13:1521–1529. doi: 10.1359/jbmr.1998.13.10.1521. [DOI] [PubMed] [Google Scholar]
- Vortkamp A. Interaction of growth factors regulating chondrocyte differentiation in the developing embryo. Osteoarthritis Cartilage. 2001;9(Suppl. A):S109–S117. [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggett AD, Ralphs JR, Kwan AP, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998;16:457–470. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- Waite JH, Lichtenegger HC, Stucky GD, Hansma P. Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry. 2004;43:7653–7662. doi: 10.1021/bi049380h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh WR, Harrison JA, Van Sickle D, Alvis M, Gillies RM. Patellar tendon-to-bone healing using high-density collagen bone anchor at 4 years in a sheep model. Am J Sports Med. 2004;32:91–95. doi: 10.1177/0363546503260709. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Kuender Y, Wang FS, Huang CC, Yang LJ. Shock wave induces neovascularization at the tendon-bone junction. J Orthop Res. 2003;21:984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- Widom RL, Lee JY, Joseph C, Gordon-Froome I, Korn JH. The hcKrox gene family regulates multiple extracellular matrix genes. Matrix Biol. 2001;20:451–462. doi: 10.1016/s0945-053x(01)00167-6. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MW, Qin L, Lee KM, et al. Healing of bone-tendon junction in a bone trough: a goat partial patellectomy model. Clin Orthop. 2003;413:291–302. doi: 10.1097/01.blo.0000076802.53006.5b. [DOI] [PubMed] [Google Scholar]
- Woo S, Maynard J, Butler D, et al. Ligament, tendon, and joint capsule insertions to bone. In: Woo SL-Y, Buckwalter JA, editors. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge: Am Acad Orthop Surg; 1988. pp. 133–166. [Google Scholar]
- Wu CW, Tchetina EV, Mwale F, et al. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- Yamada M. Ultrastructural and cytochemical studies on the calcification of the tendon-bone joint. Arch Histol Jpn. 1976;39:347–378. doi: 10.1679/aohc1950.39.347. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Shimizu A, Kato M, et al. Growth/differentiation factor-5 induces angiogenesis in vivo. Exp Cell Res. 1997;235:218–226. doi: 10.1006/excr.1997.3664. [DOI] [PubMed] [Google Scholar]
- Young MA, Cook JL, Purdam CR, Kiss ZS, Alfredson H. Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med. 2005;39:102–105. doi: 10.1136/bjsm.2003.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JS, Popp JE, Kaeding CC, Lucas J. Correlation of MR imaging and pathologic findings in athletes undergoing surgery for chronic patellar tendinitis. Am J Roentgenol. 1995;165:115–118. doi: 10.2214/ajr.165.1.7785569. [DOI] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]