Abstract
Senile sarcopenia, the loss of muscle mass associated with aging, is one of the main causes of muscle weakness and reduced locomotor ability in old age. Although this condition is mainly driven by neuropathic processes, nutritional, hormonal and immunological factors, as well as a reduction in physical activity, contribute to this phenomenon. Sarcopenia alone, however, does not fully account for the observed muscle weakness, as the loss of force is greater than that accounted for by the decrease in muscle size. As a consequence, a reduction in the force per unit area, both at single fibre and at whole muscle level, is observed. We recently suggested that at whole muscle level, this reduction in intrinsic force is the result of the combined effect of changes in (1) muscle architecture, (2) tendon mechanical properties, (3) neural drive (reduced agonist and increased antagonist muscle activity) and (4) single fibre-specific tension. Whereas several studies support the role of the last two factors in the loss of intrinsic muscle force with aging, alterations in muscle architecture and in tendon mechanical properties have also been shown to contribute to the above phenomenon. Indeed, sarcopenia of the human plantarflexors, represented by a 25% reduction in muscle volume, was found to be associated with a 10% reduction in fibre fascicle length and 13% reduction in pennation angle. These architectural alterations were accompanied by a 10% decrease in tendon stiffness, attributable to alterations in tendon material properties, as suggested by a 14% decrease in Young's modulus. Most of these changes may be reversed by 14 weeks of resistive training; both fibre fascicle length and tendon stiffness were found to be increased by 10 and 64%, respectively. Surprisingly, however, training had no effect on the estimated relative length–tension properties of the muscle, indicating that the effects of greater tendon stiffness and increased fascicle length cancelled out each other. It seems that natural strategies may be in place to ensure that the relative operating range of muscle remains unaltered by changes in physical activity, in old age.
Keywords: aging, muscle wasting, sarcopenia, tendon alterations
Introductory historical remarks
The muscle wasting and weakness that occur with aging have been a preoccupation of mankind since early Greek and Roman history (e.g. Diogenes 3rd century bc; Cicero 1st century bc). Classic Greeks notoriously abhorred aging as it represented corruption of their highly prized youthful vigour, as narrated by Socrates in book one of The Republic. The desire of maintaining bodily vigour is indeed epitomized by a statement of Diogenes (3rd century bc) who, speaking against the common predicament that one should slow down when reaching old age, compares himself to the last runner in a relay race and argues ‘would you have me slow down as I near the finish line?’. The widespread expectation among most members of the society that older men, because of their physically frailty, should refrain from any activities, including those not requiring bodily strength, is clearly despised by the Roman philosopher Cicero (44 bc) in his treatise ‘Cato Maior de senectute’ as he states: ‘… Grant that old age is devoid of strength; none is even expected of it. By law and by custom men of my age are exempt from those public services which cannot be rendered without body strength. Therefore, we are not only not required to do what we cannot perform but we are not required to do even as much as we can’, but a few passages later argues ‘it is our duty, my young friends, to resist old age; to compensate for its defects, to fight against it as we would fight a disease; to adopt a regimen of health; to practice moderate exercise; and to take just enough food and drink to restore our strength’. This concern of combating physical frailty in old age while maintaining an active role in life is indeed a fact as we have entered the second millennium with the proportion of elderly citizen exceeding that of young people.
If the problem of physical frailty in old age is to be effectively mitigated with the final goal of maintaining mobility and independence until the individual limit of chronological age, a full understanding of the aetiology of the mechanisms leading to muscle weakness must be achieved first. Hence, the aim of this paper is to review the mechanisms leading to muscle wasting in old age, the anatomical alterations and functional consequences of muscle wasting, the interactions of these changes with those of tendinous tissue and their effects, and the reversibility of these changes in response to increased loading.
Sarcopenia
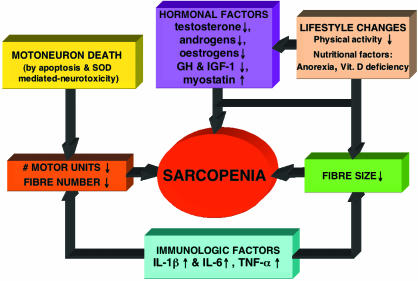
Senile sarcopenia, the loss of muscle mass associated with aging, is a main cause of muscle weakness in old age. This process has an onset at around the 6th decade, and by the 8th decade muscle mass attains a value approximating to 60% that of the 2nd decade (Lexell et al. 1988). The aetiology of sarcopenia is rather complex and involves multiple factors (Fig. 1), but there is general consensus that it is mainly driven by neuropathic changes leading to motoneuron death following alterations in superoxide dismutase activity and cell apoptosis. This process may also affect directly the muscle cell itself independently of the neuropathic processes as there is increasing evidence that apoptosis of skeletal myocytes contribues to sarcopenia (Dirks & Leeuwenburgh, 2005). Muscle cell apoptosis seems to be caused through the activation of specific signalling pathways, initiated by ligand binding of tumour necrosis factor alpha (TNF-α) to a cell membrane receptor, involving a cascade of caspases (endoproteases), which lead to the activation of an effector caspase responsible for the proteolytic events resulting in cell breakdown and death. In addition, the mitochondrion, commonly regarded as the ‘main regulator’ of apoptosis (Dirks & Leeuwenburgh, 2005), may provoke apoptosis via several different pathways, by releasing cytochrome c into the cytosol leading to the activation of effector caspases, but also through the release of pro-apoptotic proteins leading to DNA fragmentation. Stress to the endoplasmic reticulum may also lead to apoptosis, following the release of calcium into the cytosol, eventually leading to the activation of effector caspases.
Fig. 1.
Scheme summarizing the present concepts on the aetiology of sarcopenia.
As a result of the loss of motoneurons and of muscle cell apoptosis, the number of muscle fibres considerably decreases with aging (Lexell et al. 1988). Fibre size also decreases with age (atrophy) and this is probably due both to a decrease satellite cell proliferation due to an age-related decrease in the level of growth factors such as IGFs (Barton-Davis et al. 1998), as well as to a reduction in physical activity. In fact, recent evidence suggests that even ‘physically active’ septuagenarians (individuals aged 70–79 years) are about 20% less active than their vicenarian (individuals aged 20–29 years) counterparts (Morse et al. 2004). Besides, reduced physical activity may also lead to muscle fibre apoptosis as it has been found after hindlimb unweighting in rats (Allen et al. 1997). In addition to these neuropathic and physical activity-related changes, nutritional, hormonal and immunological factors are also known to contribute to sarcopenia. Malnutrition in aging is quite common, and this is due to a progressive a loss of appetite, a reduction in food intake and also to vitamin D deficiency, largely due to skin atrophy (Tawa & Goldberg, 1994). Furthermore, low vitamin D, in association with high parathyroid hormone levels, has been found to increase the risk of muscle wasting in old age (Visser et al. 2003). In addition, sarcopenia has recently been shown to be associated with vitamin D receptor genotype (Roth et al. 2004).
The hormonal and immunological alterations contributing to sarcopenia are represented by the withdrawal, or resistance, to those factors responsible for anabolism (decreased levels of GH, IGF-1, testosterone) and by an increased catabolic activity (increased levels of IL-1, IL-6, TNF-α, myostatin) (Doherty, 2003), and may also be due to vitamin D deficiency as vitamin D is known to protect muscle and bone from inflammatory cytokines (Schach, 1999). Despite the fact that sarcopenia is a major determinant of muscle weakness in old age, the loss of muscle strength exceeds that of muscle size and, as a consequence, there is a decline in force per unit of muscle cross-sectional area (Young et al. 1985; Klitgaard et al. 1990; Phillips et al. 1993; Jubrias et al. 1997; Macaluso et al. 2002; Morse et al. 2004). Several factors contribute to this phenomenon, frequently referred to as a deterioration in ‘muscle quality’. These factors can be grouped under two main categories: neuromuscular and tendinous. Each of these factors is discussed separately in the following sections.
Neuromuscular alterations in old age
Among the muscular changes, a reduction in single fibre-specific tension is one of the major factors contributing to the decline in intrinsic muscle force, and recent evidence suggests that this is tightly associated with a decrease in the number of actomyosin cross-bridges rather than in the force exerted by each cross-bridge (D’Antona et al. 2003). A reduction in excitation–contraction coupling may also contribute to the decrease in specific tension in old age (Delbono et al. 1997; Payne & Delbono, 2004). Other contributors include a reduction in neural drive to the agonist muscles and an increase in neural drive to the antagonist muscles. Several investigators have found a reduced activation capacity in older individuals (Winegard et al. 1996; Harridge et al. 1999; Yue et al. 1999; Scaglioni et al. 2002), whereas others reported no differences (Vandervoort & McComas, 1986; Phillips et al. 1992; De Serres & Enoka, 1998; Kent-Braun & Ng, 1999), although it is generally agreed that a considerable heterogeneity in activation capacity exists among muscles (Dowling et al. 1994). Both motor unit recruitment and firing frequency have been found to be reduced in older adults (Kamen et al. 1995; Yue et al. 1999), although a lower firing frequency may not necessarily lead to a decrease in activation capacity as motor unit fusion frequency is reduced with aging because of prolongation of twitch contraction time (Narici et al. 1991; Connelly et al. 1999). An increased co-activation of antagonist muscles, probably necessary for joint stabilization, has also been suggested as a possible mechanism for the loss of force with aging (Klein et al. 2001; Macaluso et al. 2002). However, no differences in co-activation were recently observed in the plantarflexor and dorsiflexor muscles when septuagenarian and vicenarian males where compared (Morse et al. 2004).
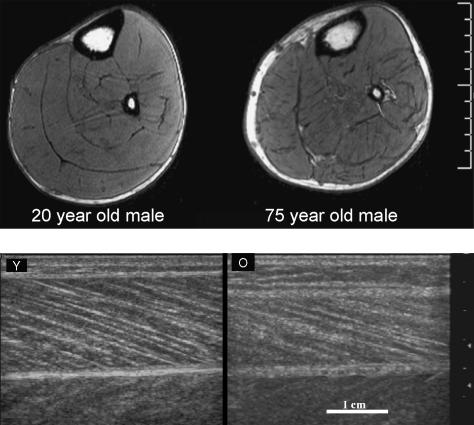
In addition to the above changes, aging also leads to marked alterations in muscle architecture that potentially contribute to the force loss (Klein et al. 2001; Narici et al. 2003). Recently, we were able to demonstrate not only that the gross size of muscle decreases with aging (findings based on MRI scanning, Fig. 2 top; Morse et al. 2004), but also that muscle fascicle lengths and pennation angles in older individuals (aged 70–81 years) were significantly smaller, by 10 and 13%, respectively, than those in younger adults (findings based on ultrasound scanning, Fig. 2 bottom; Narici et al. 2003; Morse et al. 2004). These later findings were based on ultrasound scanning, a method that has been validated by comparisons with direct anatomical measurements on cadaveric muscles (Narici et al. 1996; Kawakami et al. 1993). The above modifications in muscle architecture are unlikely to be associated with disuse, given that in one study (Narici et al. 2003) we purposely matched the level of physical activity of young and elderly individuals. Thus, we believe that the differences between age groups were caused by the process of aging per se. The smaller pennation angles and fascicle lengths found in older individuals suggests that, with aging, sarcomeres both in parallel and in series are lost. A reduction in fibre length due to a decrease in serial sarcomere number, not of sarcomere length, has also been observed in older mice (Hooper, 1981). These observations raise the issue on the mechanisms responsible for regulating sarcomere number in skeletal muscle. Prolonged muscle stretch is known to promote the addition of sarcomeres in series and in parallel, whereas a decrease in tension, as caused by immobilization in a shortened position, is known to result in a loss of sarcomeres (Williams & Goldspink, 1971, 1973). In vivo, however, a decrease in the passive tension necessary for sarcomere removal is likely to be induced not only by external mechanical constraints, e.g. an aging-associated reduction in functional joint range of movement (Grimston et al. 1993), but also by a reduction in the internal load, which is known to modulate myosin heavy chain and actin accumulation in contracting cells (Simpson et al. 1996). For a given muscle–tendon complex length, a reduction in the passive force at the ends of the whole complex might be achieved by decreasing the resistive force that the cytoskeleton and tendon develop on tension. For tendons at least, this indeed seems to be the case, as aging increases their tensile compliance (Vogel, 1980, 1983; Maganaris, 2001) as we will discuss in detail below. Nonetheless, whatever the exact mechanism responsible for the removal of muscle sarcomeres in parallel and in series, it is likely to involve post-transcriptional processes, as muscle growth, and hence atrophy, is not controlled by transcription (Russell et al. 2000). From a functional point of view, a loss of sarcomeres in parallel and in series is expected to alter both the length–tension as well as the force–velocity relationships (Lieber & Friden, 2000). Indeed, from a comparison of torque–velocity data from young and elderly men (Table 1), it emerges that the estimated maximum shortening velocity (Vmax) of the plantar flexor muscles of the elderly is 16% lower that that of the young (pers. unpublished data). However, this difference is reduced to 9% once Vmax values are normalized for fascicle length, i.e. after accounting for the different numbers of sarcomeres in series. Similarly, maximum isometric plantarflexion force was found to be 34% lower in the elderly, but only 18% lower once force values were normalized for the physiological cross-sectional area of this muscle group, thus accounting for the difference of sarcomeres both in series and in parallel. Hence, these findings suggest that differences in muscle architecture may account for about 50% of the loss in muscle function in the elderly. However, the actual functional outcome of these architectural alterations will also depend on tendon stiffness because, ceteris paribus, the force developed by the contractile component (CC) is not only affected by muscle architecture but also by the mechanical properties of the tendinous structures in series with the CC. These changes and their functional relevance are discussed in the following section.
Fig. 2.
Top: axial-plane MRI scans of the calf muscles of a young male aged 20 years and an older male aged 75 years matched for anthropometric characteristics and physical activity level. Bottom: sagittal-plane sonographs of the gastrocnemius medialis muscle in the same subjects. In the older individual (O) muscle fibre fascicle length and pennation angle are visibly smaller than in the younger adult (Y).
Table 1.
Absolute (radians s−1) and relative (lengths s−1) maximum velocity of shortening (Vmax) estimated from Hill's plots of isokinetic torque–velocity data in young adults and elderly individuals. The normalized Vmax values were obtained by dividing linear values of shortening velocity by fascicle length obtained by ultrasound
| Vmax absolute (rad s−1) | Vmax normalised (lengths s−1) | |
|---|---|---|
| Young | 5.73 | 1.98 |
| Elderly | 4.83 | 1.80 |
| % difference | −16.0 | −9.1 |
Tendon alterations in old age
Aging affects not only the CC, but also all collagenous structures, including tendons (for reviews see Butler et al. 1978; Viidik, 1982; Tuite et al. 1997; Kjaer, 2004). However, the results of experiments aimed to characterize the effect of age on tendon mechanical properties are inconsistent. Some cross-sectional studies (Shadwick, 1990) show that aging may result in stiffer and stronger tendons. However, others report opposite results (Vogel, 1980, 1983; Blevins et al. 1994; Nakagawa et al. 1996), or that aging has no effect on most tendon mechanical properties (Hubbard & Soutas-Little, 1984; Johnson et al. 1994; Flahiff et al. 1995). An explanation for the above inconsistency may relate to the population ages considered. Careful examination of the mammals’ age range investigated reveals a great variability, with some studies including very young animals (Shadwick, 1990; Nakagawa et al. 1996). Comparative data involving such populations may represent the effect of biological maturation and development, which may be different from that of the aging process, and should therefore be examined independently to avoid potentially confounding effects. In fact, when the effect of biological maturation has been controlled for, a consistency emerges across most comparative studies: excised tendons from mature animals are stiffer, stronger and more rebound resilient than tendons from older animals (Vogel, 1980, 1983; Blevins et al. 1994; Nakagawa et al. 1996). Reference, however, to in vitro quantitative results when interpreting in vivo function in relation to the aging process should be treated with caution. This is because: (1) The forces exerted on a tendon under in vivo conditions may be affected by age and differ from the force region over which measurements are taken under in vitro conditions. (2) To perform a tensile test in vitro, clamping of the specimen is necessary. Fixing a fibrous structure with clamps is inevitably associated with fibre slippage and/or stress concentration that may result in premature rupture. These measurement artefacts might be expected to be more profound in younger tendons because they are usually thicker than older tendons. (3) Many in vitro experiments have been performed using preserved tendons, which may have altered properties, affected to different degrees by aging. Moreover, in vitro methodologies require that the animal is then killed, which means that human experiments can only be performed if donor cadavers become available. Indeed, data on the effect of aging on the mechanical properties of human tendons are rather scarce (Blevins et al. 1994; Johnson et al. 1994; Flahiff et al. 1995). Recently, quick-release experiments have been used to estimate stiffness in elderly individuals (e.g. Valour & Pousson, 2003; Ochala et al. 2004). However, this technique provides a gross index of stiffness, which reflects the series elastic component of the entire group of the agonist–antagonist muscles acting on a particular joint – not the free tendon of a single muscle.
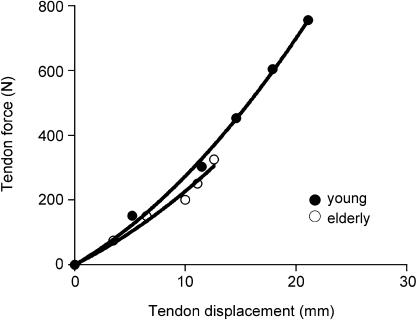
A method, however, has recently been developed that allows examination of mechanical properties in selected anatomically superficial single human tendons under in vivo conditions, thus circumventing the above problems (e.g. Maganaris & Paul, 1999; Kubo et al. 2001; Magnusson et al. 2001; Maganaris, 2002; Reeves et al. 2003). This in vivo method is based on real-time ultrasound scanning of a reference point along the muscle–tendon during an isometric contraction of the in-series muscle. The limb is fixed on the load cell of a dynamometer to record changes in joint torque during muscle activation and subsequent relaxation. The muscle forces generated by activation pull the tendon proximally and cause a longitudinal deformation, which is measured by the recorded displacement of the selected landmark (see Fig. 4). From the in vivo torque–deformation plots obtained, and additional measurements of the tendon's moment arm length and dimensions, and antagonist muscle electromyographic activity if the contraction is elicited voluntarily and not by isolated muscle stimulation, all relevant mechanical properties can be calculated. A number of versions of the above procedure have since been applied for the study of several human tendons and conditions, but the effect of aging has not been fully and systematically investigated. One recent pilot study examined differences in the mechanical properties of the gastrocnemius tendon between six healthy men aged 20–26 years and six healthy older men aged 69–80 years (Maganaris, 2001). Contractions were produced voluntarily and the gastrocnemius medialis myotendinous junction was used as a reference landmark (Fig. 3). The gastrocnemius tendon length, cross-sectional area and moment arm length were obtained using ultrasound and MRI scanning. The study showed that the older tendons were ∼15% more compliant than the younger tendons (Fig. 4), and that this difference was due to changes in the material of the tendon, as evidenced by the similarity in the tendon dimensions between the two age groups. A more recent in vivo study, however, showed that the tendon may change its cross-sectional area with age (Magnusson et al. 2003). In contrast to what might readily be assumed, the above authors showed that the older tendons were thicker (22% difference) than the younger tendons. In combination with the aging-induced reduction in contractile force potential in the in-series muscle, as evidenced by measurements of ankle plantarflexion torque, the above authors concluded that older tendons would be subjected to smaller tensile stresses than younger ones and they would therefore be less likely to rupture on tension. Others, however, have shown using in vitro animal preparations that aging decreases the collagen fibril diameter (Nakagawa et al. 1994; Gillis et al. 1997; Dressler et al. 2002). From the above studies it is apparent that collagen fibril atrophy alone cannot account for the deterioration of the mechanical properties in the entire tendon during senescence. The nature of the exact mechanisms involved cannot be revealed without analysing the miscrostructure and composition of tendons, tasks impossible to tackle with the current in vivo technology. Analysis of in vitro tendon material, however, indicates that aging is associated with (1) an increase in non-reducible collagen cross-linking, (2) a reduction in collagen fibril crimp angle, (3) an increase in elastin content, (4) a reduction in extracellular water and mucopolysacharide content, and (5) an increase in type V collagen (Viidik, 1982; Tuite et al. 1997; Kjaer, 2004). Interestingly, factor 1 would stiffen a tendon if operated alone, but factors 2, 3 and 4 might bring about the opposite result. Clearly, differences in tensile phenomenological response between younger and older whole tendon specimens would reflect the combined ‘net’ effect of all the above independent factors.
Fig. 4.
In vivo human gastrocnemius tendon force–elongation properties. Data show average values (n = 6 in each age group). At high forces, the ‘young’ curve is steeper than the ‘elderly’ curve.
Fig. 3.
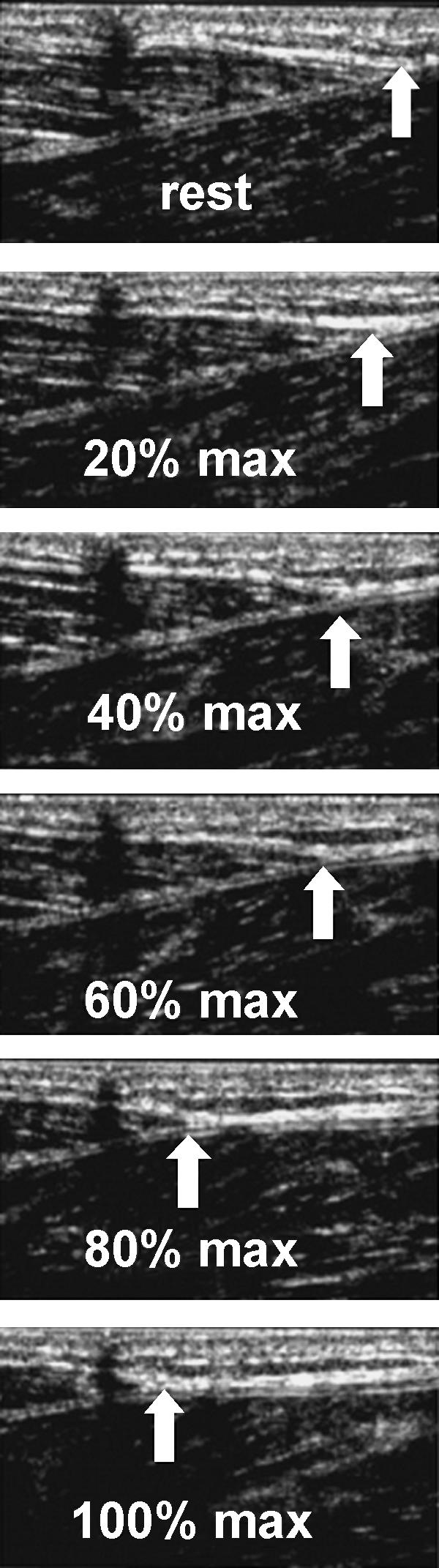
In vivo sagittal-plane sonographs of the human tibialis anterior (TA) tendon from rest to increasing intensity contraction (A to D) and back to rest by consequent relaxation (E to G). The white arrow points to the TA tendon origin, which was traced to obtain elongations. The shadow indicated by the black arrows is generated by a skin marker (from Maganaris & Paul, 2000).
The reduction in tendon stiffness by aging has at least two important functional implications. (1) For a given number of serial sarcomeres in a fibre, an older tendon would stretch more on muscle contraction, thus causing its sarcomere to shorten more than it would do if attached to a younger, less extensible tendon. The effect of this operating length change on the force-generating capability of the CC will depend on the portion of the length–tension relation over which the CC operates. If the ascending limb of the length–tension relation is used, as is the case in most human muscles (Cutts, 1988; Herzog et al. 1991; Ichinose et al. 1997), a reduction in contractile force would be expected owing to a change in sarcomere working length to a new length corresponding to less optimal myofilament overlap. This force reduction would be additional to any effect caused by muscle atrophy, reduced specific tension and neural activation. (2) Also, a more compliant tendon would require a longer time to be stretched than a stiffer tendon (Wilkie, 1949). This would mean that older tendons are less capable than younger tendons of transmitting fast forces from muscles to bones, which has implications for effectively reacting to avoid a slip or trip. Evidence for the association between tendon stiffness and speed of contractile force transmission is given by measurements of rate of force/torque development (Davies et al. 1986; Narici et al. 1996), showing that older individuals are ‘slower’ than younger ones in effectively contracting their muscles, a phenomenon which also encompasses the effect of aging-induced changes in myosin heavy chain composition and myosin molecule shortening speed.
Neuromuscular and tendon alterations with training in old age
In recent years, resistive exercise loading has been shown by numerous studies (for a review see Macaluso & De Vito, 2004) to be an effective method for attenuating, or even reversing to a certain degree, the detrimental effects of aging described in the above sections. Probably of most significance from these studies is the finding that older individuals can partly counteract muscle weakness with resistive loading, which most likely results from neuromuscular and tendinous adaptations.
Skeletal muscle still retains its capacity for adaptation into old age. Enlargement of muscle size measured using techniques such as computed tomography, ultrasound and more recently magnetic resonance imaging has been observed following resistive training programmes (e.g. Fiatarone et al. 1990; Ferri et al. 2003; Suetta et al. 2004). When expressed in terms of anatomical cross-sectional area, increases in muscle size following strength training programmes of ∼3 months duration can be expected to be in the region of 5–17% (Brown et al. 1990; Ferri et al. 2003), which is comparable with findings in young adults after similar periods of training (Jones & Rutherford, 1987; Garfinkel & Cafarelli, 1992). It has been suggested that muscle hypertrophy can largely explain the increase in strength with training. However, the relationship between gains in strength and muscle size depends greatly upon how these two variables have been measured and/or expressed. For example, strength gains may be reported as the repetition-maximum performed on the same devices that have been used for training. In this situation, the relative training-induced increase would usually be expected to exceed the gains in isometric strength, due to the specific nature of the strength test (e.g. Harridge et al. 1999). In order to assess the true maximum torque/force-producing capability in response to training programmes, the isometric strength may be the most appropriate choice. In terms of assessing muscle size, physiological cross-sectional area (PCSA, obtained by dividing muscle volume by optimal fascicle length) is the most appropriate parameter because skeletal muscle fibres usually lie at an angle to the whole muscle line of action. Furthermore, with aging there may be an increased infiltration of intramuscular fat and connective tissue (Kent-Braun et al. 2000; Macaluso et al. 2002), which means that the visible muscle area may be an overestimate of the actual contractile capability of the muscle. Both of the highlighted issues relating to muscle size may be modified by resistive loading and therefore should be considered when evaluating the relative contribution of muscle size changes to the increase in strength following such programmes. After 12 weeks of strength training in older men (Trappe et al. 2000) and older women (Trappe et al. 2001), it has been reported that the specific tension of single muscle fibres remains unchanged. By contrast, at the level of the whole muscle, it has recently been shown that 14 weeks of resistive loading increased the specific force of the vastus lateralis muscle (Reeves et al. 2004a). The observed increase in whole-muscle-specific force was independent of alterations in voluntary activation level due to the measurement of all relevant parameters during superimposed electrical stimulation, indicating that alternative factors must therefore be responsible. Thus, an increase in the force-producing capability of elderly muscle per unit area is one factor contributing to the observed strength gains following resistive training programmes.
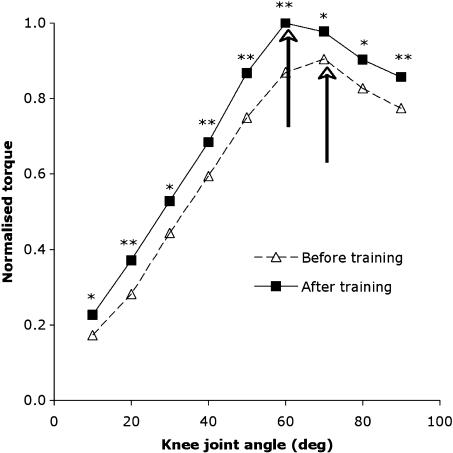
By using ultrasound imaging to study the internal muscle structure, architectural adaptations have been identified in elderly muscle following resistive loading. We have recently shown in the vastus lateralis muscle that following 14 weeks of strength training, the length of muscle fascicles and their pennation angle increase (Reeves et al. 2004a,b). These findings strongly suggest an increase of sarcomeres both in series and in parallel. A greater number of sarcomeres in parallel means that the muscle would be able to generate a higher maximum force. Although an increased number of sarcomeres in series suggests that a greater excursion range may be possible as compared with the pretraining situation with fewer sarcomeres in series, in vivo this would be limited by anatomical features that determine the range of motion about a joint, such as joint and relevant structure geometry. Relating to the production of force across the joint angular range, we have found that the relative increase in isometric knee extension torque following training is not constant across the joint range and that a training-induced shift in the optimal angle occurs (Reeves et al. 2004b; Fig. 5). The significance of this finding is that by testing isometric torque at just a single joint angle, the actual training-induced strength gains may be under- or overestimated. To avoid this, isometric torque should therefore be tested across a range of joint angles pre- and post-intervention.
Fig. 5.
Relative knee extensor angle–torque relation normalized. Dashed and solid arrows indicate the optimal angle before and after training, respectively. Asterisks denote increased torque after training (*P < 0.05, ** P < 0.01). Values are means (n = 9).
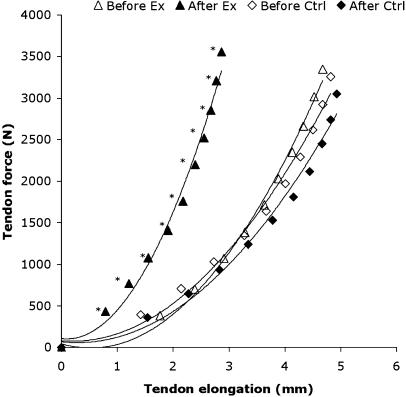
Although the muscular changes are recognized as the main adaptations to resistive training, tendons are also highly responsive to increased loading. Some animal models (Woo et al. 1980; Buchanan & Marsh, 2001) have shown that when tendons are subjected to loading levels above that normally experienced under physiological conditions, they respond by increasing their tensile stiffness. With the use of ultrasound imaging to scan the patellar tendon, it has been shown that resistive loading can increase the stiffness and Young's modulus of elderly human tendons (Reeves et al. 2003; Fig. 6). The increase in the tendon Young's modulus suggests that the stiffness increase occurred to due a change in the material properties of the tendon, and this has certain functional implications. More precisely (1) an increase in the rate of contractile force transmission, (2) a reduced likelihood of tendon strain injury and (3) changes in the force–length relation of the muscle would be expected.
Fig. 6.
The patellar tendon force–elongation curves for the resistance exercise group (Ex, n = 9) and a non-exercising control group (Ctrl, n = 9). Values are means; an asterisk denotes reduced elongation after resistive exercise training (P < 0.01). (From Reeves et al. 2003).
With respect to the first effect, it should be emphasized that the force-velocity characteristics of the muscle is influenced not only by muscle fibre composition but also by the stiffness of the tendon as this anatomical structure is placed between muscle and bone. In fact, Reeves et al. (2003) showed a training-induced increase in tendon stiffness was associated with a 25% faster development of joint torque. This finding implies that certain functional activities requiring a rapid generation of joint torque may benefit, such as an attempt to recover from a trip or slip. The second effect of a post-training reduced risk for tensile tendon rupture reflects the commonly reported finding that changes in collagenous tissue stiffness are accompanied by equivalent increases in tensile strength (e.g. Yamamoto et al. 1993; Majima et al. 1996; Matsumoto et al. 2003). The third effect relates again to the anatomical location of the tendon between muscle and bone. The extent of tendon elongation will directly affect the shortening of the in-series muscle. Thus, an increase in tendon stiffness with resistive training would be expected to cause a reduced shortening of muscle fibres and a change in the muscle's operating range. In the vastus lateralis muscle, however, the estimated operating range remained unchanged post-training (Reeves et al. 2004b). This finding was attributed to the increased fascicle length (and hence sarcomeric number) with training, an effect with the opposite result to that caused by the training-induced tendon stiffening. It seems that older muscle and tendon may interact and adapt in response to training in a way such that the muscle's operating range remains the same.
References
- Allen DL, Linderman JK, Roy RR, et al. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med. 1994;22:328–333. doi: 10.1177/036354659402200306. [DOI] [PubMed] [Google Scholar]
- Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol. 1990;69:1725–1733. doi: 10.1152/jappl.1990.69.5.1725. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol. 2001;90:164–171. doi: 10.1152/jappl.2001.90.1.164. [DOI] [PubMed] [Google Scholar]
- Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. Exerc Sport Sci Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- Cutts A. The range of sarcomere lengths in the muscles of the human lower limb. J Anat. 1988;160:79–88. [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino M, Adami R, et al. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CT, Thomas DO, White MJ. Mechanical properties of young and elderly human muscle. Acta Med Scand Suppl. 1986;711:219–226. doi: 10.1111/j.0954-6820.1986.tb08954.x. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998;84:284–291. doi: 10.1152/jappl.1998.84.1.284. [DOI] [PubMed] [Google Scholar]
- Delbono O, Renganathan M, Messi ML. Excitation-Ca2+ release-contraction coupling in single aged human skeletal muscle fiber. Muscle Nerve Suppl. 1997;5:S88–S92. doi: 10.1002/(sici)1097-4598(1997)5+<88::aid-mus21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 2005;35:473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Konert E, Ljucovic P, Andrews DM. Are humans able to voluntarily elicit maximum muscle force? Neurosci Lett. 1994;179:25–28. doi: 10.1016/0304-3940(94)90926-1. [DOI] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res. 2002;20:1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177:69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- Flahiff CM, Brooks AT, Hollis JM, van der Schilden JL, Nicholas RW. Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med. 1995;23:354–358. doi: 10.1177/036354659502300319. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- Garfinkel S, Cafarelli E. Relative changes in maximal force, EMG, and muscle cross-sectional area after isometric training. Med Sci Sports Exerc. 1992;24:1220–1227. [PubMed] [Google Scholar]
- Gillis C, Pool RR, Meagher DM, Stover SM, Reiser K, Willits N. Effect of maturation and aging on the histomorphometric and biochemical characteristics of equine superficial digital flexor tendon. Am J Vet Res. 1997;58:425–430. [PubMed] [Google Scholar]
- Grimston SK, Nigg BM, Hanley DA, Engsberg JR. Differences in ankle joint complex range of motion as a function of age. Foot Ankle. 1993;14:215–222. doi: 10.1177/107110079301400407. [DOI] [PubMed] [Google Scholar]
- Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Herzog W, Read LJ, Ter Keurs HEDJ. Experimental determination of force–length relations of intact human gastrocnemius muscles. Clin Biomech. 1991;6:230–238. doi: 10.1016/0268-0033(91)90051-Q. [DOI] [PubMed] [Google Scholar]
- Hooper ACB. Length, diameter and number of ageing skeletal muscle fibres. Gerontology. 1981;27:121–126. doi: 10.1159/000212459. [DOI] [PubMed] [Google Scholar]
- Hubbard RP, Soutas-Little RW. Mechanical properties of human tendon and their age dependence. J Biomech Eng. 1984;106:144–150. doi: 10.1115/1.3138471. [DOI] [PubMed] [Google Scholar]
- Ichinose Y, Kawakami Y, Ito M, Fukunaga T. Estimation of active force-length characteristics of human vastus lateralis muscle. Acta Anat. 1997;159:78–83. doi: 10.1159/000147969. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. The tensile and viscoelastic properties of human patellar tendon. J Ortop Res. 1994;12:796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- Jones DA, Rutherford OM. Human muscle strength training: the effects of three different regimens and the nature of the resultant changes. J Physiol. 1987;391:1–11. doi: 10.1113/jphysiol.1987.sp016721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubrias SA, Odderson IR, Esselman PC, Conley KE. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflüg Arch. 1997;434:246–253. doi: 10.1007/s004240050392. [DOI] [PubMed] [Google Scholar]
- Kamen G, Du Sison SVCC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–2744. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87:22–29. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol. 2000;88:662–668. doi: 10.1152/jappl.2000.88.2.662. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol. 2001;91:1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Mantoni M, Schiaffino S, et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Effects of different duration isometric contractions on tendon elasticity in human quadriceps muscles. J Physiol. 2001;536:649–655. doi: 10.1111/j.1469-7793.2001.0649c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of ageing atrophy? J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–472. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist–antagonist coactivation account for differences in torque between young and older women. Muscle Nerve. 2002;25:858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521:307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Hysteresis measurements in intact human tendon. J Biomech. 2000;33:1723–1727. doi: 10.1016/s0021-9290(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Maganaris CN. Proceedings of the Active Life Span Research Symposium, The Plasticity of the Motor System: Adaptations to Increased Use, Disuse and Ageing. UK: Manchester Metropolitan University; 2001. In vivo tendon mechanical properties in young adults and healthy elderly. [Google Scholar]
- Maganaris CN. In vivo force–length characteristics of human skeletal muscle. Acta Physiol Scand. 2001a;172:279–285. doi: 10.1046/j.1365-201x.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Maganaris CN. Proceedings of the Research Symposium Plasticity of the Motor System: Adaptations to Increased Use, Disuse and Ageing. UK: Manchester Metropolitan University; 2001b. In vivo tendon mechanical properties in young adults and healthy elderly. Available from http://www.mmu.ac.uk/c-a/exspsci/alSymposiumhtml. [Google Scholar]
- Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech. 2002;35:1019–1027. doi: 10.1016/s0021-9290(02)00047-7. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load–displacement propertries of the human triceps surae aponeurosis in vivo. J Physiol. 2001;531:277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci. 2003;58:123–127. doi: 10.1093/gerona/58.2.b123. [DOI] [PubMed] [Google Scholar]
- Majima T, Yasuda K, Fujii T, Yamamoto N, Hayashi K, Kaneda K. Biomechanical effects of stress shielding of the rabbit patellar tendon depend on the degree of stress reduction. J Orthop Res. 1996;14:377–383. doi: 10.1002/jor.1100140306. [DOI] [PubMed] [Google Scholar]
- Matsumoto F, Trudel G, Uhthoff HK, Backman DS. Mechanical effects of immobilization on the Achilles’ tendon. Arch Phys Med Rehabil. 2003;84:662–667. doi: 10.1016/s0003-9993(02)04834-7. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol. 2004;92:219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Majima T, Nagashima K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand. 1994;152:307–313. doi: 10.1111/j.1748-1716.1994.tb09810.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Hayashi K, Yamamoto N, Nagashima K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol. 1996;73:7–10. doi: 10.1007/BF00262803. [DOI] [PubMed] [Google Scholar]
- Narici MV, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol. 1996;71:1277–1281. doi: 10.1152/jappl.1991.71.4.1277. [DOI] [PubMed] [Google Scholar]
- Narici MV, Hoppeler H, Kayser B, et al. Human Quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand. 1996;157:175–186. doi: 10.1046/j.1365-201X.1996.483230000.x. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol. 2003;95:2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- Ochala J, Lambertz D, Pousson M, Goubel F, Hoecke JV. Changes in mechanical properties of human plantar flexor muscles in ageing. Exp Gerontol. 2004;39:349–358. doi: 10.1016/j.exger.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Payne AM, Delbono O. Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32:36–40. doi: 10.1097/00003677-200401000-00008. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Bruce SA, Newton D, Woledge RC. The weakness of old age is not due to failure of muscle activation. J Gerontol. 1992;47:M45–M49. doi: 10.1093/geronj/47.2.m45. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci. 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548:971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. Effect of resistance training on skeletal muscle-specific force in elderly humans. J Appl Physiol. 2004a;96:885–892. doi: 10.1152/japplphysiol.00688.2003. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV, Maganaris CN. In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol. 2004b;89:675–689. doi: 10.1113/expphysiol.2004.027797. [DOI] [PubMed] [Google Scholar]
- Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci. 2004;59:10–15. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- Russell B, Delara M, William WA. Form follows function: how muscle shape is regulated by work. J Appl Physiol. 2000;88:1127–1132. doi: 10.1152/jappl.2000.88.3.1127. [DOI] [PubMed] [Google Scholar]
- Scaglioni G, Ferri A, Minetti AE, et al. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol. 2002;92:2292–2302. doi: 10.1152/japplphysiol.00367.2001. [DOI] [PubMed] [Google Scholar]
- Schacht E. Rationale for treatment of involutional osteoporosis in women and for prevention and treatment of corticosteroid-induced osteoporosis with alfacalcidol. Calcif Tissue Int. 1999;65:317–327. doi: 10.1007/s002239900705. [DOI] [PubMed] [Google Scholar]
- Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol. 1990;68:1033–1040. doi: 10.1152/jappl.1990.68.3.1033. [DOI] [PubMed] [Google Scholar]
- Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM. Related mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Physiol. 1996;270:C1075–C1087. doi: 10.1152/ajpcell.1996.270.4.C1075. [DOI] [PubMed] [Google Scholar]
- Suetta C, Aagaard P, Rosted A, et al. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol. 2004;97:1954–1961. doi: 10.1152/japplphysiol.01307.2003. [DOI] [PubMed] [Google Scholar]
- Tawa NE, Goldberg AL. Protein and amino acid metabolism in muscle. In: Engel AG, Franzini-Amstrong G, editors. Myology. 2. Vol. 1. New York: McGraw-Hill; 1994. pp. 683–707. [Google Scholar]
- Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol. 2000;89:143–152. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol. 2001;281:C398–C406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- Tuite DJ, Renstrom PA, O’Brien M. The aging tendon. Scand J Med Sci Sports. 1997;7:72–77. doi: 10.1111/j.1600-0838.1997.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Valour D, Pousson M. Compliance changes of the series elastic component of elbow flexor muscles with age in humans. Pflugers Arch. 2003;445:721–727. doi: 10.1007/s00424-002-0871-4. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Viidik A. Age-related changes in connective tissues. In: Viidik A, editor. Lectures on Gerontology. London: Academic Press; 1982. pp. 173–211. [Google Scholar]
- Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Influence of maturation and ageing on mechanical and biochemical properties of connective tissue in rats. Mech Ageing Dev. 1980;14:283–292. doi: 10.1016/0047-6374(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Age dependence of mechanical properties of rat tail tendons (hysteresis experiments) Akt Gerontol. 1983;13:22–27. [PubMed] [Google Scholar]
- Wilkie DR. The relation between force and velocity in human muscle. J Physiol (Lond) 1949;110:249–280. doi: 10.1113/jphysiol.1949.sp004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971;9:751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116:45–56. [PMC free article] [PubMed] [Google Scholar]
- Winegard KJ, Hicks AL, Sale DG, Vandervoort AA. A 12-year follow-up study of ankle muscle function in older adults. J Gerontol A Biol Sci Med Sci. 1996;51:B202–B207. doi: 10.1093/gerona/51a.3.b202. [DOI] [PubMed] [Google Scholar]
- Woo SL, Ritter MA, Amiel D, et al. The biomechanical and biochemical properties of swine tendons – long term effects of exercise on the digital extensors. Connect Tissue Res. 1980;7:177–183. doi: 10.3109/03008208009152109. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K, Kaneda K. Effects of stress shielding on the mechanical properties of rabbit patellar tendon. J Biomech Eng. 1993;115:23–28. doi: 10.1115/1.2895466. [DOI] [PubMed] [Google Scholar]
- Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5:145–154. doi: 10.1111/j.1475-097x.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol A Biol Sci Med Sci. 1999;54:M249–M253. doi: 10.1093/gerona/54.5.m249. [DOI] [PubMed] [Google Scholar]