Abstract
It has long been appreciated that studying the embryonic chick in ovo provides a variety of advantages, including the potential to control the embryo's environment and its movement independently of maternal influences. This allowed early workers to identify movement as a pivotal factor in the development of the locomotor apparatus. With an increasing focus on the earliest detectable movements, we have exploited this system by developing novel models and schemes to examine the influence of defined periods of movement during musculoskeletal development. Utilizing drugs with known neuromuscular actions to provoke hyperactivity (4-aminopyridine, AP) and either rigid (decamethonium bromide, DMB) or flaccid (pancuronium bromide, PB) paralysis, we have examined the role of movement in joint, osteochondral and muscle development. Our initial studies focusing on the joint showed that AP-induced hyperactivity had little, if any, effect on the timing or scope of joint cavity elaboration, suggesting that endogenous activity levels provide sufficient stimulus, and additional mobilization is without effect. By contrast, imposition of either rigid or flaccid paralysis prior to cavity formation completely blocked this process and, with time, produced fusion of cartilaginous elements and formation of continuous single cartilaginous rods across locations where joints would ordinarily form. The effect of these distinct forms of paralysis differed, however, when treatment was initiated after formation of an overt cavity; rigid, but not flaccid, paralysis partly conserved precavitated joints. This observation suggests that ‘static’ loading derived from ‘spastic’ rigidity can act to preserve joint cavities. Another facet of these studies was the observation that DMB-induced rigid paralysis produces a uniform and specific pattern of limb deformity whereas PB generated a diverse range of fixed positional deformities. Both also reduced limb growth, with different developmental periods preferentially modifying specific osteochondral components. Changes in cartilage and bone growth induced by 3-day periods of flaccid immobilization, imposed at distinct developmental phases, provides support for a diminution in cartilage elaboration at an early phase and for a relatively delayed influence of movement on osteogenesis, invoking critical periods during which the developing skeleton becomes receptive to the impact of movement. Immobilization also exerts differential impact along the proximo-distal axis of the limb. Finally, our preliminary results support the possibility that embryonic hyperactivity influences the potential for postnatal muscle growth.
Introduction
It is generally acknowledged that a dynamic adaptive relationship exists between the structural characteristics of connective tissues and their prevailing mechanical environment. Indeed, this relationship is examined in detail in several of the other papers in the current issue of this journal. Furthermore, it is commonly asserted that these structural characteristics, which provide functional competence, are maintained and also established, at least partly, by these adaptive mechanisms. There is indeed much evidence in a range of tissues supporting the contribution of these adaptive mechanisms to the maintenance of adult musculoskeletal competence. A role for such adaptation in developmental attainment of appropriate structure is, however, largely unsubstantiated. The extent to which movement controls acquisition of appropriate connective tissue structure during embryonic development remains therefore largely undefined. This review focuses on the role of movement or the mechanical sequelae it engenders in the embryonic development of joint cavities, and briefly examines the role of movement in cartilage and bone formation in the hind limb skeleton.
Joint formation
The processes responsible for synovial joint development are usually divided into two phases. The first involves the formation of cartilaginous anlagen and the intervening interzones in which the joints will develop, i.e. limb ‘patterning’. The second involves the formation of the articular cartilage, synovium and other related structures within the joint. This depends on elaboration of the joint cavity, containing synovial fluid, a process referred to as ‘cavitation’. This subdivision has meant, however, that each of these continuous phases is often examined without reference to the other (Lamb et al. 2003a). It is possible that this subdivision has led to confusion about the mechanisms involved. Thus, fundamental issues remain unresolved, such as (1) is the site at which a joint will form defined before or after the cartilaginous anlagen are produced? and (2) is cavitation achieved by controlled cell death? These issues are beyond the scope of this review but are examined critically elsewhere (Pacifici et al. 2005; A. Pitsillides and D. Ashhurst, personal observations).
Any review of joint formation would be incomplete without a brief overview of limb patterning. Limb patterning involves dynamic relationships between a thickened region of ectoderm, the apical ectodermal ridge (AER) and the underlying distal limb mesenchyme, or progress zone, which is retained in an undifferentiated, proliferative state during outgrowth. The ‘progress zone model’ predicts that the timing and location of the departure of each cell from the influence of the AER dictates its commitment to a specific fate (Wolpert et al. 1975; Storm et al. 1994; Tickle, 1995; Duprez et al. 1996; Capdevila & Johnson, 1998; Sanz-Ezquerro & Tickle, 2001; Tickle & Wolpert, 2002). Two recent studies suggest an alternative view: that progenitor cells are assigned segmentally to the stylopod, zeugopod and autopod during outgrowth, without later mixing of cells between adjacent segments (Dudley et al. 2002; Sun et al. 2002). This infers that different limb segments are therefore ‘specified’ as distinct domains. These studies provide insights into the mechanism(s) that determine the precise location of specific skeletal structures, and are very likely to impact on our understanding of joint development; it is clear that their relationships are beginning to be defined (de la Fuente & Helms, 2005; see Lamb et al. 2003a).
Regardless of the above, it is apparent that synovial joint cavity formation must successfully generate ‘new’, non-adherent surfaces by a process involving the assembly of a cell-free, fluid-filled separation, which will facilitate painless and almost frictionless articulation of a joint. As limb condensations are indeed discrete by this stage, it is apparent that cavitation occurs between the ends of predetermined cartilaginous elements to create surfaces that are continuous with the synovial lining and associated structures, including menisci (see Pitsillides, 1999). With physical support from an arrangement of musculature and ligaments, these structures provide diarthrodial joints with a range of movement required for locomotion. It is clear that the cavitation process also requires precise regulation. Diarthrodial joints have a range of finely adjusted anatomical arrangements and their development needs to be meticulously controlled. Such control ensures joint shape, dictates articular surface convexity/concavity, and controls congruity. Different joints may conceivably utilize diverse strategies for specifying their position, but a major supposition is that the cavitation process itself is achieved by a conserved mechanism in all joints.
The first explicit evidence that the location for joint formation has been specified involves the elaboration of an ‘interzone’ of mesenchymal cells defining a boundary between opposed skeletal elements. Thus, joints form at sites between discrete chondrogenic regions, the length and location of which are created by prior limb patterning events. These expand appositionally to form the opposing cartilage anlagen. A fundamental role for these intervening mesenchymal interzones is evident when one considers that their specification interrupts what might otherwise develop into a single, continuous cartilaginous anlage (or rod). The morphology of interzones can vary from a simple thin layer of closely compacted cells between the developing articular surfaces in human joints, to a more obvious three-layered structure containing a central laminar layer bordered on either side by chondrogenous layers that interface with the developing epiphyses in chick interphalangeal joints (Archer et al. 1994; Edwards et al. 1994; Pitsillides, 1999). Neither the basis of this divergence in interzone structure nor the potential implications for joint formation are understood. It is evident, however, that interzones remain isolated from, or indeed actively antagonize, any stimuli that promote neighbouring chondrogenesis (Lizarraga et al. 2002). Recent studies have emphasized the importance of several factors, including stanniocalcin, parathyroid hormone-related protein, α5β1 integrin, Wnt9A and Wnt/β-catenin signalling in the early stages of synovial joint formation (Vortkamp et al. 1996; Hartmann & Tabin, 2001; Stasko & Wagner, 2001; Garciadiego-Cazares et al. 2004; Guo et al. 2004). Later, the interzones become flattened and attenuated by continued expansion of the neighbouring elements. The presumptive capsule, initially continuous with the interzones, and the developing synovium both become vascularized. Ligament and tendon insertions develop from additional lateral condensations surrounding the presumptive joint (Benjamin & Ralphs, 1997) and tissue separation begins within the avascular centre of this interzone where the precise differentiation that is essential for joint cavitation takes place (Osborne et al. 2002a).
Joint formation was originally thought to depend upon surrounding tissues (Fell & Canti, 1934). However, the early stages of joint formation are now known to be relatively unaffected by the removal of surrounding cartilaginous tissues (Holder, 1977). It is also known that removal of interzones results in the moderately unrestricted expansion and eventual union of cartilage segments to induce joint fusion. This knowledge strengthens the notion that interzones must act to restrict local cartilage differentiation. It appears that the cellular origins of skeletal elements are initially homologous, only losing their capacity to change once they have responded to exclusive differential stimuli (Edwards & Francis-West, 2001). Thus, the removal of a specific cell population will result in the loss of the tissues for which they represent a progenitor pool.
The joint cavitation process within the interzone requires extremely precise spatial control over the position at which separation between the elements will occur: the ‘plane of cleavage’. A specific characteristic that unambiguously identifies cells within these early presumptive joint regions as those that will create a plane of cleavage has yet to be described. Several reviews conclude that this process is regulated by localized cell death (Spitz & Duboule, 2001; Mariani & Martin, 2003), but critical evaluation indicates that there is little firm evidence that cell death actively participates in cavitation (A. Pitsillides and D. Ashhurst, unpublished data). It appears that the formation of a joint space involves changes in composition of the extracellular matrix (ECM) and our studies indicate that selective increases in local synthesis, export and cellular binding of hyaluronan (HA), as well as other specific modifications in ECM quality, may contribute to joint cavitation (Edwards et al. 1994; Pitsillides et al. 1995a; Dowthwaite et al. 1998; Pitsillides, 1999; Kavanagh et al. 2002a). It is noteworthy, however, that Pacifici et al. (2005) recently postulated that a dual mechanism, initially dependent on cell death and reliant on local HA production only later, could provide the basis for a unifying mechanism. This possibility is currently being examined.
Role of movement in joint formation
Many studies have concluded that a lack of muscular activity results in the fusion of opposing joint elements and absence of articular cavities, indicating that embryonic limb movement serves an essential role in joint cavity formation (Fell & Canti, 1934; Hamburger & Waugh, 1940; Lelkes, 1958; Drachman & Sokoloff, 1966; Murray & Drachman, 1969; Yasuda, 1973; Ruano-Gil et al. 1978, 1980; Mitrovic, 1982; Osborne et al. 2002a,b). Thus, numerous modes of eradicating movement including the immobilization of limbs in ovo by administration of botulinum toxin, decamethonium bromide (DMB) or succinylcholine, neurectomy or simply the maintenance of limbs in organ culture have been shown to influence joint formation. These studies support the cumulative view that restricting normal embryonic limb movement prevents cavity formation in previously uncavitated joints, and results in new cartilaginous ‘fusions’ by promoting the regression of previously cavitated joints. One simple interpretation of these findings is that both the acquisition and the maintenance of joint cavities rely upon movement-induced mechanical stimuli. Although these studies endorse a role for movement in joint cavitation, they do not necessarily provide mechanistic understanding. Movement may, for example, contribute by creating differential patterns of growth, by physically disrupting tissue at the joint line, or by altering the local ECM composition.
Indeed, it has been shown using 3H-thymidine labelling of grafted limbs that while division and growth took place in cartilaginous areas, there was very little evidence for growth in interzonal areas, suggesting that cavitation may depend upon differential patterns of growth (see Wolpert et al. 1975). Our labelling studies for Ki67, a proliferating cell nuclear antigen, in developing human limbs also support low proliferation of interzone cells compared with chondrogenic regions, as do similar studies in rabbits (Edwards et al. 1994; Kavanagh et al. 2002b). The possibility that movement controls joint cavitation by modulating local growth remains, however, largely unaddressed. It has nevertheless been shown that the prevention of cavitation, the stunting of morphogenesis and the induction of skeletal distortions are achieved with only mild effects on the growth of chick limbs paralysed for 3 days (Drachmann & Sokoloff, 1966). Similar studies using long-term (days 7–19) chick immobilization to induce cavitation failure in almost all joints was also shown to cause only a slight delay in development (Murray & Drachmann, 1969). Therefore, differential growth would appear insufficient as a sole mediator of the influence of movement in joint cavity formation.
At this point it may be worthwhile to consider the postnatal changes that occur in cartilage; in a basic modification, its chondrocytes acquire a vertical columnar organization with distinct horizontal strata associated with endochondral growth (Stevens et al. 1999). In Monodelphis domestica, articular cartilage growth is thought to depend on a balance between endochondral ossification at the tissue base (the subchondral plate) and the rate or duration of appositional cell production from stem cells resident at the articular surface (Archer et al. 2003; Dowthwaite et al. 2004). The surface articular chondrocytes may thus be derived either from epiphyseal growth cartilage or from a non-chondrogenic source in the joint interzone. Our recent description of a non-invasive model for joint loading through natural points of articulation will allow us to determine whether mechanical cues regulate the recruitment and behaviour of this surface zone stem cell population of chondrocytes (De Souza et al. 2005a).
Returning to the mechanism by which cavitation is achieved, it is also possible that mechanical forces generated by muscle contraction directly disrupt cell–cell cohesion along the plane of cleavage, leading to a mere liquefaction of tissue substance. This is unlikely, as the morphogenetic events leading up to cavitation are intrinsically determined and it would appear pointless to predefine this population if mechanical disruption alone is responsible for creating a joint in their midst, thereafter. Moreover, the diverse joint shapes have incredible, inherent precision and it seems unlikely that they would rely on a seemingly crude, imprecise disruptive force generated by movement alone for their elaboration. Rather, cavitation appears to be a process of selective differentiation (Hamerman et al. 1970).
Another possibility is that movement regulates the localized ECM (re)modelling or synthesis that is required for normal cavitation, and that this also underpins the blockade of joint cavitation in immobilized limbs. Fell & Canti's (1934) description of joint tissue chondrification in cultured chick limbs is consistent with this view. Many authors have since described fibrous, cartilaginous or even bony ‘fusions’ across presumptive joint sites in immobilized limbs. This suggests that movement does indeed contribute to the alterations in ECM synthesis that normally accompany tissue separation at these sites. It has been known for some time that early cavities contain an anionic polysaccharide (Andersen, 1961; Anderson, 1965). For this and other reasons (Craig et al. 1990) we hypothesized that increases in HA synthesis and its localized retention is a principal event in joint cavity formation. Our studies reveal that enzymatic metalloproteinase-mediated degradation of fibrous and adherent ECM constituents is unlikely (Edwards et al. 1996) and that the participation of phagocytosing macrophages in cavitation is also doubtful (Takabatake & Yamamoto, 1991; Edwards et al. 1994). We have, however, confirmed that selective increases in local synthesis (UDPGD activity), export and cell binding of HA may contribute to cavitation by a mechanism reliant on changing ECM composition (Pitsillides et al. 1995a,b; Dowthwaite et al. 1998; Kavanagh et al. 2002a; see Pitsillides, 1999).
The significance of these events has been endorsed by our findings in DMB-immobilized limbs, where a failure to form joint cavities was associated with merging of the interzone with articular surfaces to form a homogeneous rounded cell zone uniting opposed cartilages, culminating ultimately in their cartilaginous fusion (see Ruano-Gil et al. 1978; Kavanagh et al. 2006). This was supported by studies showing that administration of HA-oligosaccharides, capable of disrupting the association between HA and its known cell surface-associated binding partners, such as CD44, could block joint cavitation in ovo (Dowthwaite et al. 1998). In both circumstances we found that cells in ‘new’ fusion tissues exhibited decreased UDPGD expression and activity levels, decreased CD44 and moesin expression and changes in Alcian blue staining at presumptive joint lines that were consistent with local decreases in HA synthesis, accumulation and binding (Dowthwaite et al. 1998; Bastow et al. 2005). It is therefore tempting to speculate that movement promotes cavitation by stimulating HA synthesis by cells at the joint line, and that its withdrawal results in an ECM unsuitable for friction-free movement. This is supported by analyses of cellular phenotypes that indicate that this specialized differentiation requires local shear forces (Wilkinson et al. 1993; Pitsillides et al. 1999a; Pitsillides, 1999).
Further mechanistic deciphering of this mechanodependent process is also provided by in vitro studies. These established that cells derived from embryonic chick articular surfaces: (i) retain their differentiation status in vitro and (ii) exhibit increases in UDPGD, CD44, cellular HA-binding capacity and HA-synthase3 mRNA in response to short periods of dynamic mechanical strain stimulation (Dowthwaite et al. 1999, 2003). These results suggest that movement-related mechanical stimuli contribute to the acquisition of this articular surface cell phenotype. Attempts to define an upstream regulator of this phenotype recently culminated in the identification of the selective activation of the MEK–ERK pathway in cells at sites of presumptive cavitation, and loss of this activation in immobilized limbs. Activation of this specific mitogen-activated protein kinase (MAPK) cascade was also induced in articular surface cells by mechanical strain stimuli in vitro. We also demonstrated the relevance of such MEK–ERK pathway activation to the joint cavitation process by using selective inhibitors and specific transfection strategies to show that ERK activation status directly regulates cellular HA synthesis and binding (Bastow et al. 2004, 2005; Lewthwaite et al. 2006). Together with studies showing that MEK–ERK pathway activation negatively regulates chondrogenesis, this shows a pivotal role for this MAPK in the joint formation process.
It may be pertinent to re-emphasize at this point that immobilization inhibits cavitation without affecting earlier joint specification, outgrowth or patterning of the limb. These earlier events are intrinsically regulated and independent of muscular activity (Fell & Canti, 1934; Drachmann & Sokoloff, 1966; Mitrovic, 1982; see Lamb et al. 2003a). This provides the basis for identifying genes linked unequivocally to the cavitation process (or to patterning). We have used in ovo immobilization to distinguish between genes that impact upon different phases of joint formation. We have found differential regulation of growth/differentiation factor-5 and FGF-2/4 by immobilization in ovo (Kavanagh et al. 2006). Our findings support a direct mechano-dependent role for FGF-2 but not FGF-4 in cavitation and the likely influence of GDF-5 predominantly in chondrogenesis. Future, gain/loss-of-function studies coupled with immobilization will allow factors operating independently of movement, but which directly promote cavitation, to be unambiguously defined.
Targeting specific developmental ‘windows’ using selective neuromuscular strategies
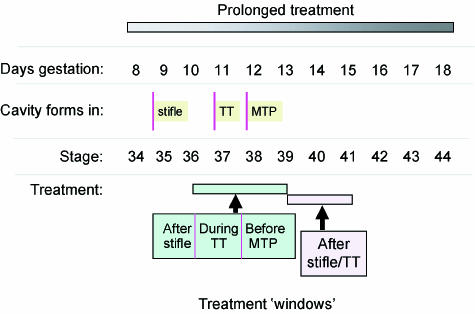
Chick embryos are often used to study long bone and joint development (Hamilton, 1952; Farquharson et al. 1996). Avian joints exhibit an anatomy, akin to reptiles, that is adapted for flight and they initially contain fibro-cartilaginous articular surfaces (Archer, 1994). Nevertheless, chick joint morphogenesis closely resembles that in humans (O’Rahilly & Gardner, 1978). As birds are oviparous, their development is not complicated by maternal influences, and manipulation in ovo is therefore relatively easy and non-invasive. More importantly, the temporo-spatial sequence of chick developmental events and specifically the formation of limbs are extremely well documented (Hamburger & Hamilton, 1992; Nalin et al. 1995). This documentation allows distinct phases in the development of selected joints to be examined at specific, defined times. ‘Targeting’ interference to those events taking place before, during or after cavitation within individual joints is therefore feasible (Fig. 1). Through use of neuromuscular agents, this is exploited in the next section to explore how particular components of the mechanical milieu contribute to the initial elaboration or subsequent maintenance of joint cavities. This strategy is later modified to define how movement contributes to cartilage and bone formation during skeletal development.
Fig. 1.
Diagram to demonstrate the scope to influence distinct phases of cavitation in particular joints. This provides details on the days of gestation, the relative stages of chick development as well as the time at which cavitation commences in the knee (stifle), tibiotarsal (TT) and metatarsophalangeal (MTP) joints. Also shown are the ‘treatment windows’ (stages 36–39 and 39–41) that allow distinct phases of cavitation (before, during or after) of these joints to be selectively targeted. In most studies, treatment with drugs used to evoke immobilization has started relatively early during development and only the relatively long-term effects of treatment have been examined.
Differential effects of ‘rigid’ and ‘relaxed’ paralysis
It is established that long periods of in ovo DMB treatment achieve ‘rigid’ limb paralysis and result in chick joint fusion (Drachman & Sokoloff, 1966). DMB blocks depolarization irreversibly by binding to post-junctional membranes of motor endplates and resists acetylcholinesterase degradation (Bowman & Rand, 1980). Administration initially causes repetitive muscle firing, but ultimately ‘block by depolarization’ prevents action potential propagation. Tension of such tetanus does not wane; muscles respond with a spasticity causing legs to extend rigidly and the head to thrust back. Muscular activity engenders many mechanical stimuli, including a static loading of joints by virtue of sustained muscle contractions, but also in dynamic stimuli resulting from their intermittent and discontinuous quality. Accordingly, DMB removes the dynamic component of skeletal movement, but is likely to retain the static loading it exerts across diarthrodial joints by virtue of muscle contraction.
Pancuronium bromide (PB), by contrast, produces flaccid or ‘relaxed’ paralysis. Acting as a non-depolarizer it competitively antagonizes acetylcholine receptors and acetylcholinesterase makes its actions reversible (Bowman & Rand, 1980; Crossland, 1980; Taylor, 1990; Rang et al. 1995). PB is therefore likely to remove both the dynamic component of skeletal movement and any joint loading achieved by muscle contraction. PB administration to premature infants produces a reduced manually applied hip and knee joint flexion and ankle joint dorsiflexion, suggesting that flaccid paralysis reduces neonatal joint mobility and that spontaneous activity prevents such contracture (Fanconi et al. 1995). We used PB and DMB to manipulate skeletal movement in ovo, and compared their effects at early as well as late phases of joint formation to define the contribution of movement and joint loading to initial cavitation and later maintenance.
Having confirmed efficacy by direct observation of embryonic motility in ovo, we showed that three days of paralysis before cavitation with either PB or DMB resulted in a failure in joint cavitation (Osborne et al. 2002a,b). Even partially developed joints exhibited severely retarded cavities, with fused anlagen and an apparent absence of menisci and cruciate ligaments. Thus, skeletal movement appears to be a necessity during initial formation of the overt cavity. Immobilization with PB after joint cavity formation also promoted significant joint fusion. At the same stage, however, paralysis with DMB failed to exert such a significant influence, with all joints remaining cavitated, albeit with abnormal articular surfaces (Osborne et al. 2002a,b). Thus, once a cavity is established, muscularly induced static loading contributes to its preservation whilst flaccid paralysis promotes a more rapid decline in these precavitated joints. These observations suggest that the ‘dynamic’ aspects of movement are important in forming a joint cavity and that ‘static’ compressive force contributes to the preservation of joint cavities once formed.
Embryonic movement and positional deformity
In the context of this series of reviews, it would be pertinent to comment on the positional deformities induced by these two forms of paralysis. This may familiarize colleagues in related fields with the many roles of early embryo movement (Beckham et al. 1977). Three days of immobilization (stages 36–39 or 39–41) with DMB induced a reproducible well-recognized, contracted-flexed phenotype, while PB initially produced relaxed body posture, lacking muscular tone (Osborne et al. 2002a,b). Mid-gestation d-tubocurarine also induces many contractures resembling arthrogryposis multiplex congenita, reduced weight and multiple positional deformities of the extremities, ‘fetal akinesia deformation sequence’ (Drachman & Coulombre, 1962; Moessinger, 1983). Although use of PB has already been associated with neonatal deformity, joints appear to develop normally in mice lacking skeletal muscle, suggesting that movement may not, however, be required for mammalian joint development (Hasty et al. 1993). It is possible that embryo movements instigated by maternal activity are sufficient in mammals.
We found that PB induces a significant range of fixed, surprisingly diverse positional deformities, heterogeneity of phenotype producing roughly equal numbers of feet in extreme flexed and extreme extended positions (Lamb et al. 2003b). In addition to limb, spinal and craniofacial deformities, PB also restricted the normal geometric pattern of weight gain to an arithmetic accretion. Because the observed patterns of limb growth disturbance that are induced by PB treatment are relatively homogeneous, we consider it unlikely that this variety of ‘positional’ phenotypes can be explained on the basis of indirect effects via growth regulation. Prolonged but not acute PB treatment promoted jaw prognathism and torticollis that became more pronounced and severe with increasing duration. This can be contrasted with the lack of any marked effect on kyphosis. Increased duration of PB treatment also promoted greater knee flexion and hyperextension of tibiotarsal joints. Speculatively, this may reflect the long-term consequences of a complete isolation from the earliest developmental movements. Thus, DMB appears to drive particular deformities tonically to produce a single pattern of ‘stereotypic’ contractures (Hosseini & Hogg, 1991a,b; Osborne et al. 2002a,b). By contrast, PB treatment results in a phenotypic ‘plasticity’ that appears to be the result of ‘muscularly unlimited’ contractures (Lamb et al. 2003b). Not surprisingly, neuromuscular agents also have actions beyond the skeletal system, e.g. PB and DMB both result in a reduced heart rate. In egg-bound embryos this may reduce blood flow, oxygen and nutrient delivery, and so decrease growth. As many authors have described the effects of rigid paralysis, the remainder of this review will focus on the effects of flaccid paralysis, imposed using various strategies, to address the role of movement in cartilage and bone formation during development.
Flaccid immobilization discloses discrete periods in cartilage and bone development on the basis of their ‘mechanosensitivity’
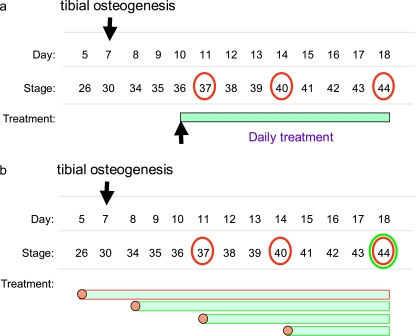
It is established that spontaneous movement is detectable in embryo chick limbs from as early as 5 days of gestation (Hamburger & Balaban, 1963). Evidently, movement starts well before those periods that we have ‘targeted’ (from 8 to 12 days, see above). We have monitored embryo movement at this later time and found that it consists of variable periods of spontaneous activity (∼10–40 s min−1). Cartilaginous differentiation is also known to be a very early event (above) whereas osteogenesis, of chick tibiae for example, is not observed until day 7 (Scott-Savage & Hall, 1979; Hall, 1987; see Hall, 2005). It is apparent that the influence of movement on skeletal growth can be addressed by examining the effects of PB-induced immobility from day 10; we therefore deployed an alternative strategy (Fig. 2a).
Fig. 2.
Diagrammatic representation of distinct targeting strategies used to treat embryonic chicks to establish the effects of flaccid immobilization on hind-limb development. (a) Dosing strategy used to establish the short- and long-term effects of flaccid, relaxed paralysis (induced by daily treatment with pancuronium bromide) on skeletal development. Treatment commenced at stage 36 (day 10) and effects examined in chicks at stages 37, 40 and 44 (days 11, 14 and 18, respectively). (b) Dosing strategy used to establish the stage at which the effects of pancuronium bromide-induced flaccid paralysis exert their impact on skeletal development. Treatment commenced at stages 26, 43, 37 and 40 (days 5, 8, 11 and 14, respectively) and the effects on development examined at stage 44 (day 18). Also shown for reference is the stage at which tibial osteogenesis commences. Together, these ‘treatment windows’ (a and b) will allow the phase at which flaccid immobilization exerts influence on particular facets of skeletal development to be determined.
We have chosen to initiate PB treatment at a fixed time during development (day 10) and to evaluate its effects along the proximal–distal axis of the limb, after various times thereafter (days 11, 14 and 18). Our investigations disclosed several findings, including: (i) that the length of all skeletal elements was reduced (femur-P2) by PB treatment; (ii) that this was more dramatic in the most distal elements; (iii) that tibial cartilage/bone ratios were relatively unaffected between days 10 and 14, but showed a decline in bone accretion between days 14 and 18; and (iv) that epiphyseal but not diaphyseal widths were reduced (day 14, Table 1) to a greater extent in the relatively ‘less mature’ distalmost elements (Lamb et al. 2003b). These findings agree with those using alternative forms of paralysis showing that such restraint diminishes long bone growth predominantly of the phalanges. Our studies provide some rationale for previous observations indicating that paralysed elements exhibit modified growth trajectories, which make them stouter (Bertram et al. 1997). These analyses suggest a ‘switch’ from a PB-induced restriction of cartilage expansion at earlier stages to a selective restraint upon ossification only at later stages. In addition, it also indicates that there is a developmental phase during which the ossification of long bones is relatively insensitive to mechanical stimuli engendered by movement. This is an intriguing possibility that has been addressed in detail by modifying our ‘targeted’ immobilization strategy still further (Fig. 2b).
Table 1.
Effect of decamethonium bromide (DMB), pancuronium bromide (PB) and 4-aminopyridine (AP) on knee (K), tibiotarsal (TT) and metatarso-phalangeal (MTP) joint epiphyseal breadth. Treatment from stages 36 to 39 (* P < 0.05; ** < 0.01 vs. TS)
| Proximal epiphysis (cm ± SEM) | Distal epiphysis (cm ± SEM) | |||||
|---|---|---|---|---|---|---|
| Treatment | K | TT | MTP | K | TT | MTP |
| Control (TS) | 0.20 (0.000) | 0.22 (0.008) | 0.11 (0.007) | 0.28 (0.014) | 0.19 (0.008) | 0.11 (0.007) |
| DMB | 0.15* (0.014) | 0.14** (0.008) | 0.05** (0.004) | 0.21* (0.008) | 0.11** (0.008) | 0.05** (0.000) |
| PB | 0.16** (0.008) | 0.16** (0.008) | 0.06** (0.008) | 0.21* (0.008) | 0.13** (0.008) | 0.06** (0.008) |
| AP | 0.19 (0.007) | 0.23 (0.008) | 0.13 (0.000) | 0.30 (0.023) | 0.20 (0.000) | 0.12 (0.004) |
To address the notion that endochondral long bone ossification may consist of phases that can be distinguished on the basis of their sensitivity to mechanical stimulation, we initiated flaccid paralysis from days 8, 11 and 14 and examined impact at day 18. Our preliminary studies have demonstrated that bone growth of individual skeletal elements was as severely affected by PB-evoked immobility that commenced on day 14 as it was by the more prolonged immobility from day 8. This is despite the fact that these elements will have been undergoing ossification for at least the previous 6 days (days 8–14). This supports a relatively late acquisition of the ‘mechano-sensitivity’ of bone and that embryo bone growth includes an early phase during which it is more or less insensitive to mechanical cues.
These findings can be interpreted in many ways. One possibility is that intrinsic, genetic factors are responsible for initiating bone formation and that extrinsic, epigenetic factors, including mechanical sequelae brought about by movement, predominantly impact upon this process only at later stages of development. Taken together with the induction of positional deformity by PB (see above), it is tempting to suggest that PB isolates embryos from extrinsic stimuli and allows intrinsic mechanisms to dominate development. If so, it is apparent that epigenetic mechanical influences may not act only to condition future mobility but may also prolong any immobility experienced during earlier development. It is questionable therefore whether these PB-induced changes in skeletal architecture should be considered as an apt ‘active process’ of functional adaptation or whether these changes represent the convergence toward a musculoskeletal ‘blueprint’ upon which the mechanical consequences of movement might act.
Another interpretation relies on the concept that there are ‘critical periods’ during development of the skeleton. Studies by Hall (1977) examining the effect of administering single doses of thallium on particular days of development and examining their specific impact upon tibial growth and organization at various times thereafter have indeed defined critical periods in tibial development. Studies showing that identical critical periods were retained in vitro have confirmed that these responses to thallium represent an intrinsic property of the developing tibia (Hall, 1985). Thus, the tibia was found to show sensitivity to thallium only between days 6 and 8 and that the dramatic reduction in thallium-induced tibial deformation evident on day 9 coincided with a decline in chick growth rate during this phase of development (Hall, 1977, 1985). However, the relationship between this ‘critical period’ in tibial growth and development, associated with changes in overall growth rate and defined by its sensitivity to exogenous thallium, and the response of the tibia to PB-induced immobilization shown by us to be acquired at later times (day 14 or so) has not yet been defined. It remains possible therefore that our findings point to a ‘critical period’ during which the tibia, in this instance, becomes receptive to mechanically derived cues and exhibits a mechanoadaptive capacity. Further work using targeted developmental ‘windows’ for administration of neuromuscular agents (described herein, see also Hall, 1985) will allow this to be addressed.
We have also shown that although PB limits long bone length, it does not significantly affect diaphyseal width, indicating that these latter growth processes are less reliant upon movement (Lamb et al. 2003b). This notion is consistent with the studies of Hosseini & Hogg (1991a), which showed that DMB-induced paralysis from day 6 to 11 failed to modify histological features of tibial chondrification, initial perichondrial ossification and vascular invasion, but that more prolonged paralysis results in reduced bone formation. The importance of differentiating between the processes of growth in bone length and width has already been stressed by Bertram et al. (1997), and our studies suggest that growth plate-associated cellular populations are more sensitive to immobility than periosteal osteoblasts. This is consistent with diminished rates of chondrocyte proliferation and recruitment in chick growth plates and the significant reductions in clavicle, mandible and long bone growth induced by DMB treatment (Hall & Herring, 1990; Germiller & Goldstein, 1997). These considerations stress the importance of muscular activity for normal skeletal growth and development (Rauch & Schoenau, 2001) and can be related to the implementation of daily physical exercise regimes for very low-birth-weight infants (Moyer-Mileur et al. 2000). A need to revisit use of PB-like drugs in premature infants is highlighted (see Lamb et al. 2003b).
Determining the effects of embryonic hyperactivity
The concept that components of the adult musculoskeletal system are in a dynamic adaptive relationship with their mechanical environment is supported by many studies that have examined the effects of disuse/’unloading’ and exercise/‘overloading’, including our own (De Souza et al. 2005a,b). We therefore sought evidence that embryo ‘exercise’, through induction of hyperactivity, also impacts on the developing musculoskeletal system. To achieve hyperactivity we have used 4-aminopyridine (AP), a non-selective voltage-sensitive K+ channel blocker that augments impulse-evoked acetylcholine release and post-junctional excitability to maintain depolarization (Osborne, 2000; Lewthwaite et al. 2003). AP may re-excite axons by prolonging the presynapse action potential to cause repetitive firing and may augment muscle contractility via an unknown direct action on muscle (Bowman & Rand, 1980; Rang et al. 1995).
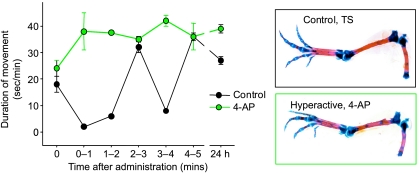
Having confirmed that AP indeed induced a longer duration of movement (Fig. 3), we found that AP did not dramatically affect temporal or morphological development of chick hind limb articular joints. This suggests that the duration and magnitude of mechanical forces imparted by normal movement are sufficient to regulate the histotypical proliferation and differentiation involved in joint cavity formation, and that excess stimuli are devoid of significant influence on this process. This compares favourably with results from chick quadratojugal joints maintained in vitro, which showed that only two daily flexures of the presumptive joint were sufficient for differentiation and maintenance of tissues of the functional joint (Hall, 1968; see Buxton et al. 2003). Our preliminary observations suggest, nevertheless, that AP-treated chicks have larger patellae, and articular surfaces with a ‘mushroom-like’ shape. These are consistent with studies indicating that reserpine-induced hypermotility results in the formation of larger than normal joint cavities (Ruano-Gil et al. 1985). Our findings also provided evidence of an increase in body weight and that AP treatment produced larger hind limb joint areas and increases in the length of some distal elements in these otherwise apparently normal chicks (Osborne, 2000; Lewthwaite et al. 2003). Measurement of cartilage/bone area ratios did not disclose any pronounced effects of AP-induced hyperactivity on endochondral ossification between stages 36 and 39. More recent studies have established that tibial and femur length were increased in chicks that were treated with AP at an identical developmental stage, but examined later (Heywood et al. 2005). These studies indicate that embryonic hyperactivity may promote growth of the skeletal elements.
Fig. 3.
AP induces rapid and sustained embryonic hyperactivity. Changes in the duration of limb movement (s min−1) at various times after treatment of stage 36 chick embryos with a single in ovo dose of 4-aminopyridine (AP). Control (Tyrode's solution, black circles) and AP-induced hyperactivity (green circles). Data are expressed as mean ± SEM (n = 3 for each treatment).
Skeletal muscle is likely to exhibit pronounced mechanically induced increases in mass in adults. Thus, it is possible that AP-induced hyperactivity and its mechanical sequelae impact on muscle as well as cartilage and bone development. The formation of muscle fibres occurs in two stages. In the first early embryonic stage, primary fibres form through the fusion of newly developed myoblasts. In the second stage, secondary fibres form using the primary fibres already formed as a scaffold (see Van Horn & Crow, 1989; Wigmore & Dunglison, 1998). Thus, increases in muscle fibre number acquired during the developmental stages could enhance the potential for postnatal muscle growth. We have found that AP-induced hyperactivity from days 10 to 13 induces significant increases in nuclear number per cross-sectional area in the semitendinosus muscle, without any effect on the total muscle area (Lewthwaite et al. 2003). This result has been supported by the observations of Heywood et al. (2005), who also disclosed similar AP-induced increases in the potential for postnatal muscle growth, as well as increases in mean body mass. These data suggest that embryonic hyperactivity promotes a phenotype with greater post-hatch muscle growth potential, and that this may extend to promoting long bone length when appropriately targeted during development.
The above highlights the possibility that a relationship exists between musculoskeletal adaptation to extrinsic mechanical stimuli and growth potential. Indeed, it is broadly acknowledged that greater scope for increases in bone mass and architecture in adaptation to load-bearing are associated with those periods of fast adult growth (see Parfitt, 1994). However, It remains to be determined whether this relationship extends to embryonic periods of geometric growth (weight gain) during which it might be hypothesized that the mechanical sequelae of movement will exert their most profound influence. This is obviously a difficult hypothesis to test directly.
Our previous studies have therefore used an indirect approach to examine whether the scope for such mechano-adaptive responses is indeed related to growth rate (Dallas et al. 1993; Pitsillides et al. 1995b). We utilized embryonic tibiotarsi from distinct breeds of chicken that are genetically selected for inherently different growth rates and compared increases in autocoid release and osteoblast glucose 6-phosphate dehydrogenase (G6PD) activity as quantitative markers of the mechanical strain-induced response. Somewhat surprisingly, this showed that the greatest capacity for strain-induced increases in nitric oxide release and osteoblast G6PD activity were apparent in the slower growing breeds and that the lowest capacities were apparent in the fastest growing chickens (Pitsillides et al. 1999a). These observations are consistent with those that have shown that slower-growing chickens demonstrate significant increases in adult bone strength when maintained in conditions that promote greater load-bearing movement (Gregory et al. 1991; Fleming et al. 1994), whereas faster-growing chickens fail to exhibit appropriate increases in bone strength when subjected to regimens that are designed to increase load-bearing through exercise (Patterson et al. 1986). This observation suggests that intrinsically high rates of growth may effectively desensitize bones to extrinsic adaptive stimuli. Moreover, it suggests that the adaptability of bone to load-bearing is a feature that can be selected for genetically. It will be intriguing to compare the mechanisms and changes in gene expression that might underpin the apparent acquisition of mechanosensitivty of embryonic bone in these chickens with divergent growth rates. It is also possible that such growth-related adaptability may extend to other components of the musculoskelatal system, but this remains to be addressed.
Conclusions and considerations
There are many perspectives from which knowledge of the contribution of movement to embryonic development can be viewed. A capacity to regenerate joint tissues, such as articular surfaces, subchondral bone, menisci or ligaments, would transform modern therapeutic approaches to cartilage repair and regeneration. Many methods, including implantation of artificial matrices, perichondrium, periosteum, and transplanted chondrocytes and mesenchymal stem cells, have been utilized in attempts to repair osteochondral defects and there is an awareness that cartilage formation can be promoted by controlled loading and motion. However, these approaches have not yet been used successfully to stimulate formation of tissue that duplicates the composition and mechanical properties of articular cartilage. Nevertheless, regeneration often recapitulates embryonic processes of tissue formation (Erlacher et al. 1998) and so defining the mechanisms by which mechanical cues ‘engineer’ these architectural changes during development may indeed facilitate their therapeutic repair. Translation of avian studies to mammals is likely as it is known that chickens also develop osteoarthritis and joint pathology due to trauma and that certain breeds are prone to dyschondroplasia (Thorp, 1996; Anderson-MacKenzie et al. 1998).
There are a few differences that should be considered before these results in chick are extrapolated to other species. Chick epiphyses remain cartilaginous until maximum bone size is reached, and they contain a proliferation zone that is penetrated by vascular canals from the epiphysis (Randall & Reece, 1996). Our studies have therefore concentrated on the slow-growing White leghorn chicken breed, which undergoes even calcification of the cartilaginous matrix. It will be interesting to examine whether immobilization and hyperactivity evoke similar changes in the developing limbs of breeds that have been selected for their very high inherent rates of growth.
It is also possible that such studies will provide information on how embryonic movement contributes to developmental attainment of functional competence. Our findings and those of others raise an intriguing question: do individual early episodes of embryo movement orchestrate subsequent changes to facilitate an even greater scope for movement during later episodes? If this were indeed the case, it would question the notion that purely adaptive mechanisms are responsible for establishing the characteristics of connective tissues that reflect their prevailing mechanical environment. Some evidence points to the existence of ‘critical periods’ of skeletal development (Hall, 1977, 1985) and our studies evoke later periods of development during which movement begins to exert a dramatic and significant contribution. Clearly, both the mechanical sequelae of movement and the structural characteristics of these tissues are changing rapidly during development; it therefore seems unlikely that a purely adaptive capacity underpins this relationship.
Our studies have made major assumptions regarding the mechanical consequences of rigid and relaxed paralysis and the precise changes in stimuli they engendered. It is therefore vital that these issues are revisited wherever possible in a context in which external control of these mechanical factors can be exploited in vitro; this will facilitate the mechanistic deciphering of these events. In the context of the developing joint cavity, it is known that embryonic movement does not modify the earliest limb patterning events that control the location at which a joint will ultimately form. However, movement does contribute to later events that co-ordinate the cavity-forming process at such sites; movement and particularly the loading it engenders across the joint are also likely to contribute to maintaining joint cavities once formed. Indeed, immobilization-induced modifications in ECM composition at the site of the presumptive joint are consistent with the idea that mechano-adaptive changes contribute to the initial elaboration and later maintenance of the structures that are required in the joint to facilitate articulation. It is germane that many syndromes are associated with the development of prenatal contractures in humans (Swinyard & Bleck, 1985). There is a large body of evidence indicating that these phenotypes can be attained via effects on the central nervous system, motor endplates or by primary degeneration of muscle. These considerations provide clues to the factors that control musculoskeletal development and to the role of movement.
The normal response of bone to load-bearing in the growing and adult animal is known to rely on the ‘novelty’ of the stimulus. Thus, osteogenic/anti-resorptive changes in bone cell behaviour are stimulated by novel distributions, frequencies or magnitudes of mechanical strain application that are sufficiently different from the mechanical strains to which the bone is architecturally adapted (Rubin & Lanyon, 1984; Rubin et al. 2001; De Souza et al. 2005a). It may therefore be pertinent to consider our findings from this standpoint. Thus, a central question is whether similar arguments also apply during development. Is it likely that all ‘new’ mechanical experiences during development will have marked effects on bone modelling and that their novelty is ensured by the fact that the developing embryo will ‘always’ be experiencing these influences for the first time? Our recent findings suggest that this might not necessarily be the case, and that bone does not initially exhibit an inherent sensitivity, but appears to acquire its sensitivity to the mechanical milieu as part of its development. If so, then identification of the changes in gene expression that coincide with such shifts in the response of bone to the mechanical environment may be beneficial in controlling bone mass and architecture where they are compromised by disease. Finally, we have demonstrated that early developmental changes in movement may translate into longer-term changes in muscle's capacity for growth. This highlights the possibility that defined periods of embryonic movement may impact upon the future adaptability of individual components of the musculoskeletal system to mechanically derived stimuli.
Acknowledgments
I am extremely grateful to Drs Anne Osborne, Emma Kavanagh, Katherine Lamb, Jo Lewthwaite and Edward Bastow for their major contributions to our work presented herein. This work has been supported by grants from the Arthritis Research Campaign and the Wellcome Trust and by PhD studentship support from the BBSRC. More recent contributors to this work to which I am greatly indebted include Dr Caroline Wheeler-Jones for her contribution to identifying signalling events at the joint line, and Drs Chantal Chenu and Imelda McGonnell for their collaboration in recent experiments aimed at defining the critical periods during which embryonic motility modifies bone development.
References
- Andersen H. Histochemical studies on the histogenesis of the knee joint and superior tibio-fibular joint in human foetuses. Acta Anat. 1961;46:279–303. doi: 10.1159/000141791. [DOI] [PubMed] [Google Scholar]
- Anderson H. Development, morphology and histochemistry of the early synovial tissue in human fetuses. Acta Anat. 1965;58:90–115. doi: 10.1159/000142577. [DOI] [PubMed] [Google Scholar]
- Anderson-MacKenzie JM, Hulmes DJ, Thorp BH. Effects of body mass and genotype on avian degenerative joint disease pathology and articular cartilage proteoglycan distribution. Clin Exp Rheumatol. 1998;16:403–408. [PubMed] [Google Scholar]
- Archer CW. Skeletal development and osteoarthritis. Ann Rheum Dis. 1994;53:624–630. doi: 10.1136/ard.53.10.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer CW, Morrison H, Pitsillides AA. Cellular aspects of the development of diarthrodial joints and articular cartilage. J Anat. 1994;184:447–456. [PMC free article] [PubMed] [Google Scholar]
- Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Today. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- Bastow ER, Lewthwaite JC, Wheeler-Jones CPD, Pitsillides AA. Extracellullar regulated kinase (ERK) modulates hyaluronan dependent pericellular matrix formation by embryonic chick articular surface cells. In: Hascall VC, Balazs E, editors. Chapter 6: Hyaluronan and the Musculoskeletal System. New Jersey: Matrix Biology Institute; 2004. pp. 523–528. Hyaluronan: Structure, Metabolism, Biological Activities, Therapeutic Applications. [Google Scholar]
- Bastow E, Lamb K, Osborne A, et al. Selective activation of the MEK-ERK pathway is regulated by mechanical stimuli in forming joints and promotes pericellular matrix formation. J Biol Chem. 2005;280:1749–1758. doi: 10.1074/jbc.M414495200. [DOI] [PubMed] [Google Scholar]
- Beckham C, Dimond R, Greenlee TK., Jr The role of movement in the development of a digital flexor tendon. Am J Anat. 1977;150:443–459. doi: 10.1002/aja.1001500306. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Tendons and ligaments-an overview. Histol Histopathol. 1997;12:1135–1144. [PubMed] [Google Scholar]
- Bertram JE, Greenberg LS, Miyake T, Hall BK. Paralysis and long bone growth in the chick: growth shape trajectories of the pelvic limb. Growth Dev Aging. 1997;61:51–60. [PubMed] [Google Scholar]
- Bowman WC, Rand MJ. Textbook of Pharmacology. Cambridge: Blackwell Scientific Publications; 1980. Striated muscle and neuromuscular transmission; pp. 17.1–17.56. [Google Scholar]
- Buxton PG, Hall B, Archer CW, Francis-West P. Secondary chondrocyte-derived Ihh stimulates proliferation of periosteum during chick development. Development. 2003;130:4729–4739. doi: 10.1242/dev.00610. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998;197:205–217. doi: 10.1006/dbio.1997.8824. [DOI] [PubMed] [Google Scholar]
- Craig FM, Bayliss MT, Bentley G, Archer CW. A role for hyaluronan in joint development. J Anat. 1990;171:17–23. [PMC free article] [PubMed] [Google Scholar]
- Crossland J. Drugs That Block Neuromuscular or Ganglionic Transmission. New York: Churchill Livingstone; 1980. [Google Scholar]
- Dallas SL, Zaman G, Pead MJ, Lanyon LE. Early strain-related changes in cultured embryonic chick tibiotarsi parallel those associated with adaptive modeling in vivo. J Bone Miner Res. 1993;8:251–259. doi: 10.1002/jbmr.5650080302. [DOI] [PubMed] [Google Scholar]
- De Souza RL, Matsuura M, Eckstein F, Rawlinson SC, Lanyon LE, Pitsillides AA. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone. 2005a;37:810–818. doi: 10.1016/j.bone.2005.07.022. [DOI] [PubMed] [Google Scholar]
- De Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J Bone Miner Res. 2005b;20:2159–2168. doi: 10.1359/JBMR.050812. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Edwards JCW, Pitsillides AA. An essential role for the interaction between hyaluronan and hyaluronan-binding proteins during joint development. J Histochem Cytochem. 1998;46:641–651. doi: 10.1177/002215549804600509. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Ward AC, Suswillo RFL, Flanelly J, Archer CW, Pitsillides AA. Effect of mechanical strain on the metabolism of hyaluronan and hyaluronan-binding protein expression in embryonic chick fibrocartilage cells. Matrix Biol. 1999;18:523–532. doi: 10.1016/s0945-053x(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Flannery CR, Flannelly J, Lewthwaite JC, Archer CW, Pitsillides AA. A mechanism underlying the movement requirement for synovial joint cavitation. Matrix Biol. 2003;22:311–322. doi: 10.1016/s0945-053x(03)00037-4. [DOI] [PubMed] [Google Scholar]
- Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- Drachman D, Coulombre A. Experimental clubfoot and arthrogryposis multiplex congenita. Lancet. 1962;11:523–526. doi: 10.1016/s0140-6736(62)90399-9. [DOI] [PubMed] [Google Scholar]
- Drachman DB, Sokoloff L. The role of movement inembryonic joint development. Dev Biol. 1966;14:401–420. [Google Scholar]
- Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell L. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeltal elements in the chick limb. Mech Dev. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Francis-West PH. Bone morphogenetic proteins in the development and healing of synovial joints. Semin Arthritis Rheum. 2001;31:33–42. doi: 10.1053/sarh.2001.24875. [DOI] [PubMed] [Google Scholar]
- Edwards J, Wilkinson L, Jones M, et al. The formation of human synovial joint cavities: a possible role for hyaluronan and CD44 in altered joint cohesion. J Anat. 1994;185:355–367. [PMC free article] [PubMed] [Google Scholar]
- Edwards JC, Wilkinson LS, Soothill P, Hembry RM, Murphy G, Reynolds JJ. Matrix metalloproteinases in the formation of human synovial joint cavities. J Anat. 1996;188:355–360. [PMC free article] [PubMed] [Google Scholar]
- Erlacher L, Ng CK, Ullrich R, Krieger S, Luyten FP. Presence of cartilage-derived morphogenetic proteins in articular cartilage and enhancement of matrix replacement in vitro. Arthritis Rheum. 1998;41:263–273. doi: 10.1002/1529-0131(199802)41:2<263::AID-ART10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Fanconi S, Ensner S, Knecht B. Effects of paralysis with pancuronium bromide on joint mobility in premature infants. J Pediatr. 1995;127:134–136. doi: 10.1016/s0022-3476(95)70274-1. [DOI] [PubMed] [Google Scholar]
- Farquharson C, Rennie JS, Loveridge N, Whitehead CC. In vivo and in vitro effect of 1,25-dihydroxyvitamin D3 and 1,25-dihydroxy-16-ene-23-yne-vitamin D3 on the proliferation and differentiation of avian chondrocytes: their role in tibial dyschondroplasia. J Endocrinol. 1996;148:465–474. doi: 10.1677/joe.0.1480465. [DOI] [PubMed] [Google Scholar]
- Fell HB, Canti RG. Experiments on the development in vitro of the avian knee joint. Proc R Soc. 1934;B1176:316–351. [Google Scholar]
- Fleming RH, Whitehead CC, Alvey D, Gregory NG, Wilkins LJ. Bone structure and strength in laying hens housed in different husbandry systems. Br Poult Sci. 1994;35:651–662. doi: 10.1080/00071669408417731. [DOI] [PubMed] [Google Scholar]
- de la Fuente L, Helms JA. The fickle finger of fate. J Clin Invest. 2005;115:833–836. doi: 10.1172/JCI24840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadiego-Cazares D, Rosales C, Katoh M, Chimal-Monroy J. Coordination of chondrocyte differentiation and joint formation by alpha5beta1 integrin in the developing appendicular skeleton. Development. 2004;131:4735–4742. doi: 10.1242/dev.01345. [DOI] [PubMed] [Google Scholar]
- Germiller JA, Goldstein SA. Structure and function of embryonic growth plate in the absence of functioning skeletal muscle. J Orthop Res. 1997;15:362–370. doi: 10.1002/jor.1100150308. [DOI] [PubMed] [Google Scholar]
- Gregory NG, Wilkins LJ, Kestin SC, Belyavin CG, Alvey DM. Effect of husbandry system on broken bones and bone strength in hens. Vet Rec. 1991;128:397–399. doi: 10.1136/vr.128.17.397. [DOI] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18:2404–2417. doi: 10.1101/gad.1230704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. In vitro studies on the mechanical evocation of adventitious cartilage in the chick. J Exp Zool. 1968;168:283–306. doi: 10.1002/jez.1401680302. [DOI] [PubMed] [Google Scholar]
- Hall BK. Thallium-induced achondroplasia in chicken embryos and the concept of critical periods during development. Teratology. 1977;15:1–16. doi: 10.1002/tera.1420150102. [DOI] [PubMed] [Google Scholar]
- Hall BK. Critical periods during development as assessed by thallium-induced inhibition of growth of embryonic chick tibiae in vitro. Teratology. 1985;31:353–361. doi: 10.1002/tera.1420310306. [DOI] [PubMed] [Google Scholar]
- Hall BK. Earliest evidence of cartilage and bone development in embryonic life. Clin Orthop Relat Res. 1987;225:255–272. [PubMed] [Google Scholar]
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morph. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Amsterdam: Elsevier/Academic Press; 2005. [Google Scholar]
- Hamburger V, Waugh M. The primary development of the skeleton in nerveless and poorly innervated limb transplants of chick embryos. Physiol Zoo. 1940;13:367–380. [Google Scholar]
- Hamburger V, Balaban M. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev Biol. 1963;7:533–545. doi: 10.1016/0012-1606(63)90140-4. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hamerman D, Rosenberg LC, Schubert M. Diarthrodial joints revisited. J Bone Joint Surg Am. 1970;52:725–774. [PubMed] [Google Scholar]
- Hamilton HL. Sensitive periods during development. Ann NY Acad Sci. 1952;55:177–187. doi: 10.1111/j.1749-6632.1952.tb26533.x. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104:341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Heywood JL, McEntee GM, Stickland NC. In ovo neuromuscular stimulation alters the skeletal muscle phenotype of the chick. J Muscle Res Cell Motil. 2005;26:49–56. doi: 10.1007/s10974-005-9007-8. [DOI] [PubMed] [Google Scholar]
- Holder N. An experimental investigation into the early development of the chick elbow joint. J Embryol Exp Morph. 1977;39:115–127. [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. II. Effects on histogenesis of the tibia. J Anat. 1991a;177:169–178. [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Hogg DA. The effects of paralysis on skeletal development in the chick embryo. I. General effects. J Anat. 1991b;177:159–168. [PMC free article] [PubMed] [Google Scholar]
- Kavanagh E, Osborne AC, Ashhurst DE, Pitsillides AA. Keratan sulphate epitopes exhibit a conserved distribution during joint development that remains undisclosed on the basis of GAG charge density. J Histochem Cytochem. 2002a;50:1039–1047. doi: 10.1177/002215540205000806. [DOI] [PubMed] [Google Scholar]
- Kavanagh E, Abiri M, Bland YS, Ashhurst DE. Division and death of cells in developing synovial joints and long bones. Cell Biol Int. 2002b;26:679–688. doi: 10.1006/cbir.2002.0918. [DOI] [PubMed] [Google Scholar]
- Kavanagh E, Church VL, Osborne AC, et al. Differential regulation of GDF-5 and FGF-2/4 by immobilisation exposes distinct roles in joint formation. Dev Dynamics. 2006;235:826–834. doi: 10.1002/dvdy.20679. [DOI] [PubMed] [Google Scholar]
- Lamb KJ, Lewthwaite JC, Bastow ER, Pitsillides AA. Defining boundaries during joint cavity formation: going out on a limb. Int J Exp Pathol. 2003a;84:55–67. doi: 10.1046/j.1365-2613.2003.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb KJ, Lewthwaite JC, Lin J-P, et al. Diverse fixed positional deformities and bone growth restraint provoked by flaccid paralysis in embryonic chicks. Int J Exp Path. 2003b;84:191–199. doi: 10.1046/j.1365-2613.2003.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelks G. Experiments in vitro on the role of movement in the development of joints. J Embryol Exp Morph. 1958;6:183–186. [PubMed] [Google Scholar]
- Lewthwaite JC, Lamb KJ, Somaiya A, et al. Pharmacological induction of hyperactivity in embryonic chicks by the administration of 4-aminopyridine fails to accelerate joint formation but results in an increased capacity for skeletal muscle growth. J Physiol. 2003;552P:89. [Google Scholar]
- Lewthwaite JC, Bastow ER, Lamb KJ, Blenis J, Wheeler-Jones CPD, Pitsillides AA. A mechano-modulatory role for p38MAPK in regulating MEK-ERK pathway activation in embryonic joint articular surface cells. J Biol Chem. 2006 doi: 10.1074/jbc.M510680200. in press. [DOI] [PubMed] [Google Scholar]
- Lizarraga G, Lichtler A, Upholt WB, Kosher RA. Studies on the role of Cux1 in regulation of the onset of joint formation in the developing limb. Dev Biol. 2002;243:44–54. doi: 10.1006/dbio.2001.0559. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423:319–325. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- Mitrovic D. Development of the articular cavity in paralyzed chick embryos and in chick embryo limb buds cultured on chorioallantoic membranes. Acta Anat. 1982;113:313–324. doi: 10.1159/000145566. [DOI] [PubMed] [Google Scholar]
- Moessinger AC. Fetal akinesia deformation sequence: an animal model. Pediatrics. 1983;72:857–863. [PubMed] [Google Scholar]
- Moyer-Mileur LJ, Brunstetter V, McNaught TP, Gill G, Chan GM. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics. 2000;106:1088–1092. doi: 10.1542/peds.106.5.1088. [DOI] [PubMed] [Google Scholar]
- Murray PD, Drachman DB. The role of movement in the development of joints and related structures: the head and neck in the chick embryo. J Embryol Exp Morph. 1969;22:349–371. [PubMed] [Google Scholar]
- Nalin AM, Greenlee TK, Jr, Sandell LJ. Collagen gene expression during development of avian synovial joints: transient expression of types II and XI collagen genes in the joint capsule. Dev Dyn. 1995;203:352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Gardner E. The Embryology of Movable Joints. New York: Academic Press; 1978. [Google Scholar]
- Osborne AC. University of London: 2000. Mechanisms by which movement exerts its essential role on diarthrodial joint cavity development. PhD thesis. [Google Scholar]
- Osborne AC, Kavanagh E, Lamb KJ, Dowthwaite G, Archer CW, Pitsillides AA. Mechanomodulatory influences upon HA production in joint development and maintenance. In: Kennedy JF, Phillips GO, Williams PA, editors. Hyaluronan. Vol. 2. Cambridge: Woodhead Publishing; 2002a. pp. 303–310. [Google Scholar]
- Osborne AC, Lamb KJ, Lewthwaite JC, Dowthwaite GP, Wheeler-Jones CP, Pitsillides AA. Short-term rigid and flaccid paralysis diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J Musculoskeletal Neuronal Interact. 2002b;2:448–456. [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75:237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- Patterson PH, Cook ME, Crenshaw TD, Sunde ML. Mechanical properties of the tibiotarsus of broilers and poults loaded with artificial weight and fed various dietary protein levels. Poult Sci. 1986;65:1357–1364. doi: 10.3382/ps.0651357. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA. The role of hyaluronan in joint cavitation. In: Archer CW, Caterson B, Benjamin M, Ralphs JR, editors. Biology of the Synovial Joint. Singapore: Harwood Academic Publishers; 1999. pp. 41–62. [Google Scholar]
- Pitsillides AA, Archer CW, Prehm P, Bayliss MT, Edwards JCW. Alterations in hyaluronan synthesis during developing joints cavitation. J Histochem Cytochem. 1995a;43:263–273. doi: 10.1177/43.3.7868856. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Rawlinson SCF, Suswillo RFL, Bourrin S, Zaman G, Lanyon LE. Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re) modeling? FASEB J. 1995b;9:1614–1622. doi: 10.1096/fasebj.9.15.8529841. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Rawlinson SCF, Mosley JR, Lanyon LE. Genetic selection for enhanced growth at the expense of skeletal adaptability to loading. J Bone Miner Res. 1999a;14:980–987. doi: 10.1359/jbmr.1999.14.6.980. [DOI] [PubMed] [Google Scholar]
- Pitsillides AA, Skerry TM, Edwards JCW. The effect of immobilisation on the maintenance of hyaluronan concentrations in diarthrodial joints: alterations in synovial lining cellular phenotype. Rheumatology. 1999b;38:1108–1112. doi: 10.1093/rheumatology/38.11.1108. [DOI] [PubMed] [Google Scholar]
- Randall CJ, Reece RL. Color Atlas of Avian Histophaology. London: Times Mirror International Publisher Ltd; 1996. Chapter 9; pp. 171–186. [Google Scholar]
- Rang H, Dale M, Ritler J. Local Anaesthetics and Other Drugs That Affect Excitable Membranes. New York: Churchill Livingstone; 1995. [Google Scholar]
- Rauch F, Schoenau E. The developing bone: slave or master of its cells and molecules? Pediatr Res. 2001;50:309–314. doi: 10.1203/00006450-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Ruano-Gil D, Nardi-Vilardaga J, Tejedo-Mateu A. Influence of extrinsic factors on the development of the articular system. Acta Anat (Basel) 1978;101:36–44. doi: 10.1159/000144947. [DOI] [PubMed] [Google Scholar]
- Ruano-Gil D, Nardi-Vilardaga J, Teixidor-Johe A. Embryonic mobility and joint development. Folia Morph (Praha) 1980;28:1–3. [PubMed] [Google Scholar]
- Ruano-Gil D, Nardi-Vilardaga J, Teixidor-Johe A. Embryonal hypermobility and articular development. Acta Anat (Basel) 1985;123:90–92. doi: 10.1159/000146045. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- Rubin CT, Sommerfeldt DW, Judex S, Qin Y. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov Today. 2001;6:848–858. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Tickle C. Fingering the vertebrate limb. Differentiation. 2001;69:91–99. doi: 10.1046/j.1432-0436.2001.690203.x. [DOI] [PubMed] [Google Scholar]
- Scott-Savage P, Hall BK. The timing of the onset of osteogenesis in the tibia of the embryonic chick. J Morph. 1979;162:453–463. doi: 10.1002/jmor.1051620310. [DOI] [PubMed] [Google Scholar]
- Spitz F, Duboule D. The art of making a joint. Science. 2001;291:1713–1714. doi: 10.1126/science.1059665. [DOI] [PubMed] [Google Scholar]
- Stasko SE, Wagner GF. Possible roles for stanniocalcin during early skeletal patterning and joint formation in the mouse. J Endocrinol. 2001;171:237–248. doi: 10.1677/joe.0.1710237. [DOI] [PubMed] [Google Scholar]
- Stevens SS, Beaupre GS, Carter DR. Model of endochondral growth and ossification in long bones: biological and mechanobiological influence. J Orthop Res. 1999;17:646–653. doi: 10.1002/jor.1100170505. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418:501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- Swinyard CA, Bleck EE. The etiology of arthrogryposis (multiple congenital contracture) Clin Orthop Relat Res. 1985;194:15–29. [PubMed] [Google Scholar]
- Takabatake K, Yamamoto T. Morphology of the synovium during its differentiation and development in the mouse knee joint. A histochemical, SEM and TEM study. Anat Embryol (Berl) 1991;183:537–544. doi: 10.1007/BF00187902. [DOI] [PubMed] [Google Scholar]
- Taylor P. Agents acting at the neuromuscular junction and autonomic ganglia. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. pp. 166–186. [Google Scholar]
- Thorp BH. Diseases of the musculoskelatal system. In: Jordan FTW, Pattison M, editors. Poultry Diseases. London: W.B. Saunders; 1996. pp. 290–305. [Google Scholar]
- Tickle C. Vertebrate limb development. Curr Opin Genet Dev. 1995;4:478–484. doi: 10.1016/0959-437x(95)90052-i. [DOI] [PubMed] [Google Scholar]
- Tickle C, Wolpert L. The progress zone – alive or dead? Nature Cell Biol. 2002;4:E216–E217. doi: 10.1038/ncb0902-e216. [DOI] [PubMed] [Google Scholar]
- Van Horn R, Crow MT. Fast myosin heavy chain expression during early and late embryonic stages chicken skeletal muscle development. Dev Biol. 1989;134:279–288. doi: 10.1016/0012-1606(89)90100-0. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Wigmore PM, Dunglison GF. The generation of fiber diversity during myogenesis. Int J Dev Biol. 1998;42:117–125. [PubMed] [Google Scholar]
- Wilkinson LS, Moore AR, Pitsillides AA, Willoughby DA, Edwards JCW. Comparison of surface fibroblastic cells in subcutaneous air pouch and synovial lining: differences in uridine diphosphoglucose dehydrogenase activity. J Exp Path. 1993;74:113–115. [PMC free article] [PubMed] [Google Scholar]
- Wolpert L, Lewis JH, Summerbell D. Morphogenesis of the vertebrate limb. In: Porter R, Rivers J, editors. Cell Patterning. Elsevier: Ciba Found. Symp 29 Amersterdam; 1975. pp. 95–130. [DOI] [PubMed] [Google Scholar]
- Yasuda Y. Differentiation of human limb buds in vitro. Anat Rec. 1973;175:561–579. doi: 10.1002/ar.1091750305. [DOI] [PubMed] [Google Scholar]