Abstract
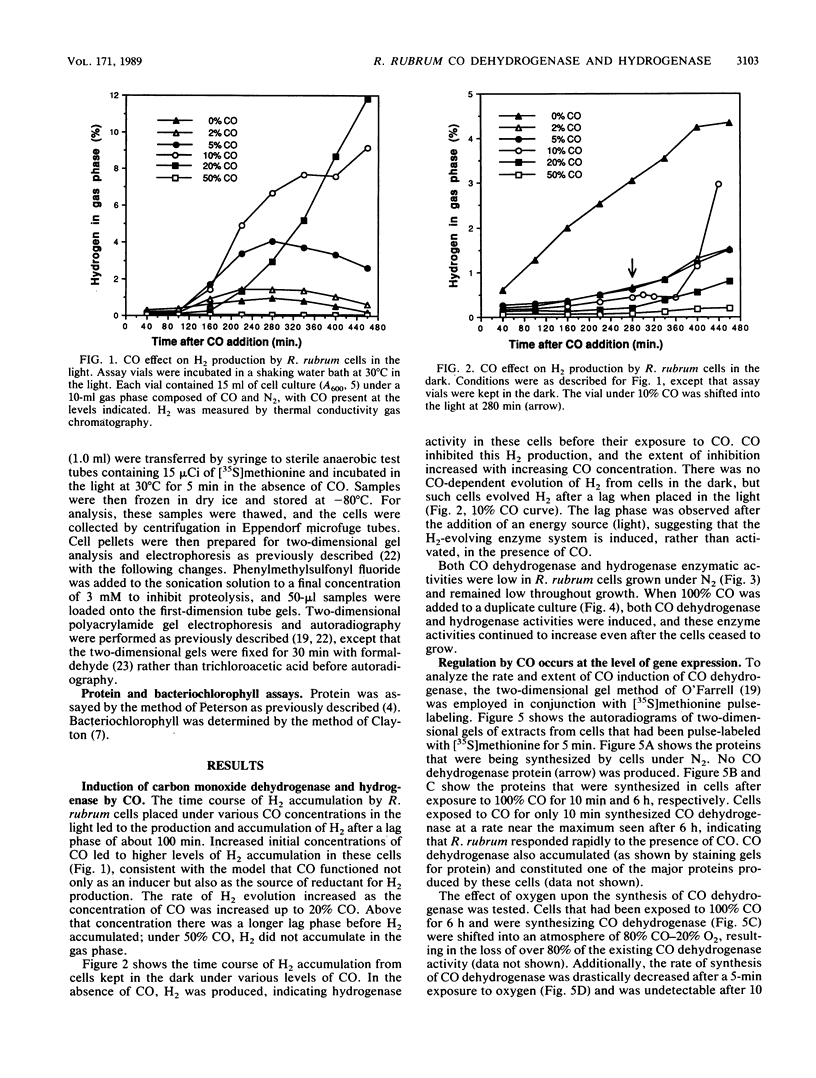
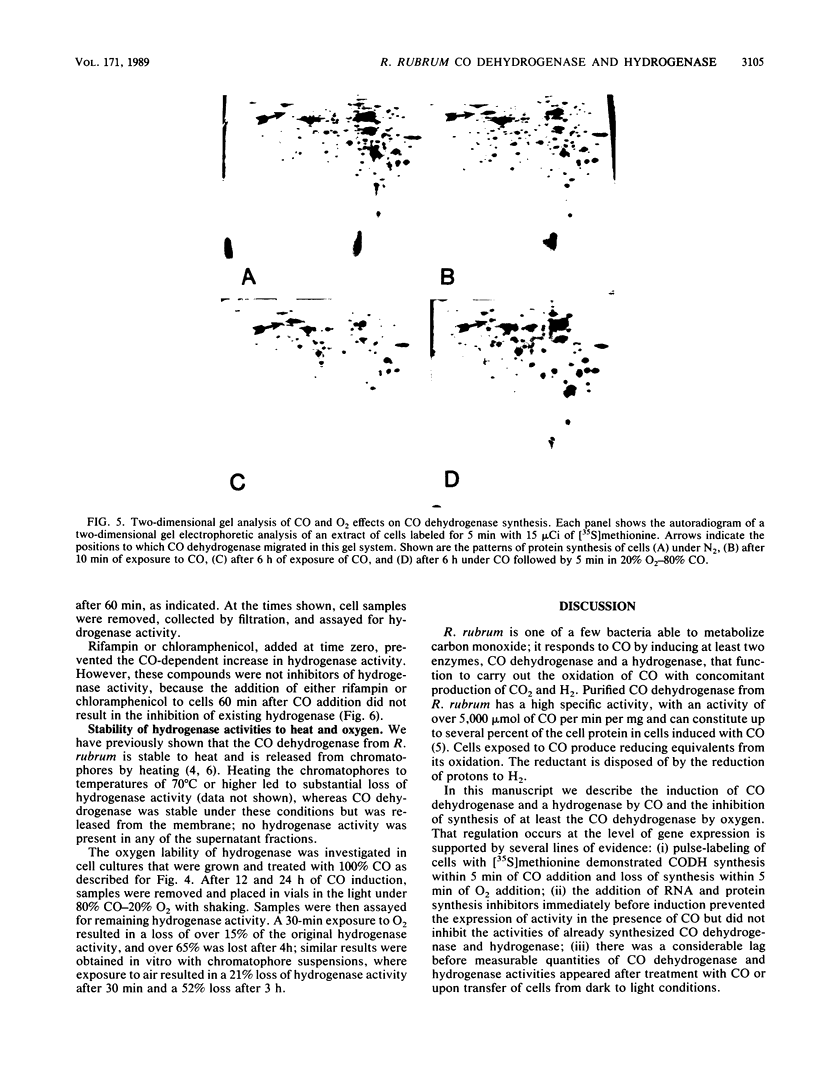
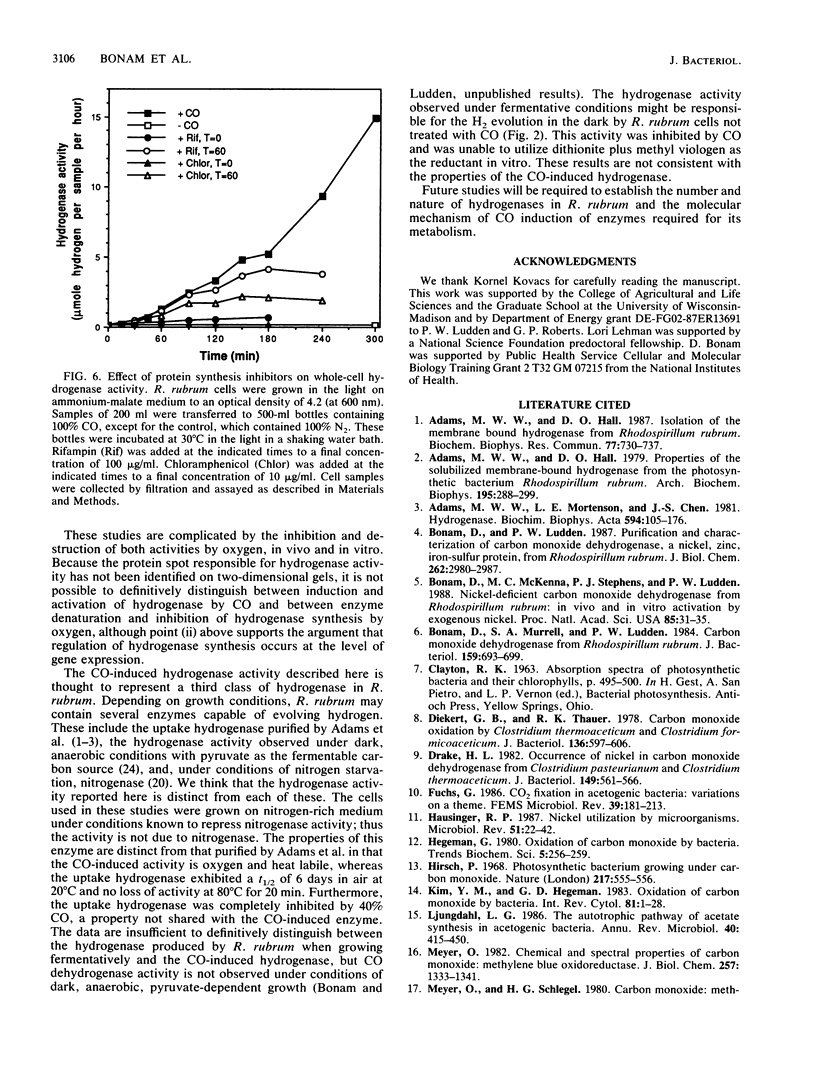
Exposure of the photosynthetic bacterium Rhodospirillum rubrum to carbon monoxide led to increased carbon monoxide dehydrogenase and hydrogenase activities due to de novo protein synthesis of both enzymes. Two-dimensional gels of [35S]methionine-pulse-labeled cells showed that induction of CO dehydrogenase synthesis was rapidly initiated (less than 5 min upon exposure to CO) and was inhibited by oxygen. Both CO dehydrogenase and the CO-induced hydrogenase were inactivated by oxygen in vivo and in vitro. In contrast to CO dehydrogenase, the CO-induced hydrogenase was 95% inactivated by heating at 70 degrees C for 5 min. Unlike other hydrogenases, this CO-induced hydrogenase was inhibited only 60% by a 100% CO gas phase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Isolation of the membrane-bound hydrogenase from Rhodospirillum rubrum. Biochem Biophys Res Commun. 1977 Jul 25;77(2):730–737. doi: 10.1016/s0006-291x(77)80039-9. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Properties of the solubilized membrane-bound hydrogenase from the photosynthetic bacterium Rhodospirillum rubrum. Arch Biochem Biophys. 1979 Jul;195(2):288–299. doi: 10.1016/0003-9861(79)90355-2. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Bonam D., Ludden P. W. Purification and characterization of carbon monoxide dehydrogenase, a nickel, zinc, iron-sulfur protein, from Rhodospirillum rubrum. J Biol Chem. 1987 Mar 5;262(7):2980–2987. [PubMed] [Google Scholar]
- Bonam D., McKenna M. C., Stephens P. J., Ludden P. W. Nickel-deficient carbon monoxide dehydrogenase from Rhodospirillum rubrum: in vivo and in vitro activation by exogenous nickel. Proc Natl Acad Sci U S A. 1988 Jan;85(1):31–35. doi: 10.1073/pnas.85.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam D., Murrell S. A., Ludden P. W. Carbon monoxide dehydrogenase from Rhodospirillum rubrum. J Bacteriol. 1984 Aug;159(2):693–699. doi: 10.1128/jb.159.2.693-699.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L. Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):561–566. doi: 10.1128/jb.149.2.561-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. Photosynthetic bacterium growing under carbon monoxide. Nature. 1968 Feb 10;217(5128):555–556. doi: 10.1038/217555a0. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Oxidation of carbon monoxide by bacteria. Int Rev Cytol. 1983;81:1–32. doi: 10.1016/s0074-7696(08)62333-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Meyer O. Chemical and spectral properties of carbon monoxide: methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982 Feb 10;257(3):1333–1341. [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Gene-product relationships of the nif regulon of Klebsiella pneumoniae. J Bacteriol. 1980 Oct;144(1):210–216. doi: 10.1128/jb.144.1.210-216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Schön G., Voelskow H. Pyruvate fermentation in Rhodospirillum rubrum and after transfer from aerobic to anaerobic conditions in the dark. Arch Microbiol. 1976 Feb;107(1):87–92. doi: 10.1007/BF00427872. [DOI] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Metabolism of carbon monoxide by Rhodopseudomonas gelatinosa: cell growth and properties of the oxidation system. J Bacteriol. 1983 Sep;155(3):956–965. doi: 10.1128/jb.155.3.956-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim B. T., Uffen R. L. Membrane association of the carbon monoxide oxidation system in Rhodopseudomonas gelatinosa. J Bacteriol. 1983 Jan;153(1):571–573. doi: 10.1128/jb.153.1.571-573.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Kerby R., Krzycki J. A. Single-carbon chemistry of acetogenic and methanogenic bacteria. Science. 1985 Mar 8;227(4691):1167–1173. doi: 10.1126/science.3919443. [DOI] [PubMed] [Google Scholar]



