Abstract
The extracellular matrix (ECM) of connective tissues enables linking to other tissues, and plays a key role in force transmission and tissue structure maintenance in tendons, ligaments, bone and muscle. ECM turnover is influenced by physical activity, and both collagen synthesis and metalloprotease activity increase with mechanical loading. This can be shown by determining propeptide and proteinase activity by microdialysis, as well as by verifying the incorporation of infused stable isotope amino acids in biopsies. Local tissue expression and release of growth factors for ECM such as IGF-1, TGF-beta and IL-6 is enhanced following exercise. For tendons, metabolic activity (e.g. detected by positron emission tomography scanning), circulatory responses (e.g. as measured by near-infrared spectroscopy and dye dilution) and collagen turnover are markedly increased after exercise. Tendon blood flow is regulated by cyclooxygenase-2 (COX-2)-mediated pathways, and glucose uptake is regulated by specific pathways in tendons that differ from those in skeletal muscle. Chronic loading in the form of physical training leads both to increased collagen turnover as well as to some degree of net collagen synthesis. These changes modify the mechanical properties and the viscoelastic characteristics of the tissue, decrease its stress-susceptibility and probably make it more load-resistant. The mechanical properties of tendon fascicles vary within a given human tendon, and even show gender differences. The latter is supported by findings of gender-related differences in the activation of collagen synthesis with exercise. These findings may provide the basis for understanding tissue overloading and injury in both tendons and skeletal muscle.
Keywords: blood flow, collagen, growth factors, inflammation, protein synthesis
Introduction
For many centuries, the importance of tendons as force-transmitting elements that link skeletal muscle to bone and promote movement has been well appreciated. More recently, the key role of intramuscular connective tissue has also been recognized as enabling lateral force transmission between contracting muscle fibres (for references see Purslow, 2002; Kjær, 2004). As late as in the 1960s, tendons were considered to be relatively non-vascular, inert and inelastic structures, although now it is well accepted that tendons have the ability to store and recoil energy. It is only over the last decade that the dynamic nature of the extracellular matrix (ECM) of tendon and skeletal muscle has begun to be appreciated. In addition, the occurrence of overuse injuries in tendons such as the Achilles and the patellar tendon related to both occupational and leisure activity has underlined the general view that loading can result in pathological changes in the ECM that go beyond simple mechanical rupture. The phenomenon of overuse injuries suggests that there are metabolic processes that control the balance between physiological and pathological adaptation to mechanical loading (Kjær, 2004).
Tendon metabolism and flow
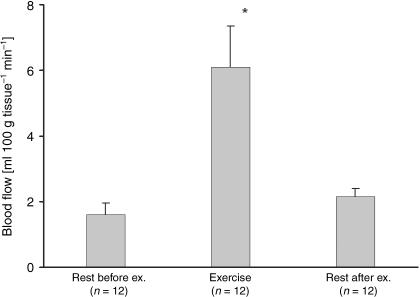
It is clear from routine light microscopy studies of tendons that they contain a relatively small number of fibroblasts in the tendon itself and in its sheath (McNeilly et al. 1996). At the same time, the use of confocal microscopy has revealed that there are elaborate processes extending between the intratendinous fibroblasts promoting cell–cell contact. This implies that tendons have a network of fibroblasts that provide the basis for a dynamic process between cells and the ECM (McNeilly et al. 1996). The blood flow within and around tendon has been shown to increase three- to seven-fold with exercise (Fig. 1; Langberg et al. 1998, 1999a). In addition, indirect estimation of tendon oxygen uptake has also shown that mechanical loading increases this by three- to six-fold (Boushel et al. 2000a,b, 2001). Together, these data imply that neither reduced blood flow nor ischaemia seems to occur in tendons with exercise (Boushel et al. 2001; Kjær, 2004). An important question to address is how blood flow is regulated. Several vasodilators (e.g. bradykinin and adenosine) have been shown to be elevated in peritendinous tissue with exercise (Langberg et al. 2002b). Furthermore, prostaglandins (PGs), and especially prostacyclin, have a vasodilatory effect, and together with nitric oxide (NO) and endothelial-derived hyperpolarization factor (EDHF), they are important in regulating the blood flow via skeletal muscle during exercise (Boushel et al. 2002, 2004). Whereas PG alone does not seem to have any vasodilatory effect in skeletal muscle during contraction, the release and thus increased tissue concentrations of PG has an important vasodilatory role in mechanically loaded tendon. During exercise, blood flow increase in both tendinous and peritendinous tissue is reduced by 40%, mainly through cyclooxygenase-2 (COX-2)-specific pathways (Langberg et al. 2003). Because PGs play a nociceptive role and act in concert with other substances during inflammatory processes, and play a role in regulating blood flow, the question arises as to whether it is beneficial to inhibit PG release with non-steroidal anti-inflammatory drugs (or even to block blood flow by sclerosing the peritendinous vessels) in situations where individuals suffer from tendinopathy or peritendinitis (Kjær, 2004).
Fig. 1.
Blood flow values during rest and exercise determined by 133Xe washout in the peritendinous space 5 cm proximal to the insertion of the human Achilles tendon. (From Langberg et al. 1999a.)
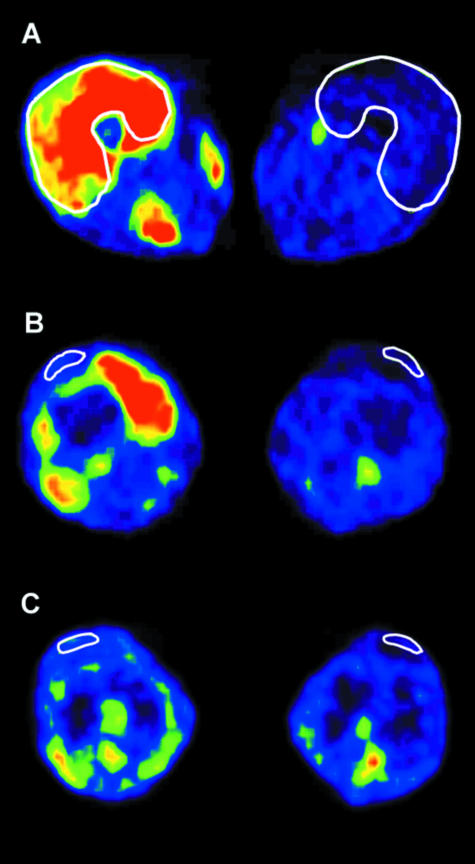
The use of near-infrared spectroscopy in combination with a dye-dilution technique (Boushel et al. 2000) shows that there is no sign of pronounced oxygen desaturation or hypoxia during mechanical loading (Boushel et al. 2001), and that there is a tight coupling between the moderate drop in tissue oxygen saturation and the increase in tissue blood flow seen with exercise. Furthermore, it can be demonstrated that glucose uptake is increased with exercise in both the human Achilles and the patellar tendon (Fig. 2; Kalliokoski et al. 2005; Hannukainen et al. 2005). Positron emission tomography (PET) shows that the uptake of 18-labelled fluor deoxyglucose (18F-FDG) into tendon is increased with moderate loading and that this change is not quantitatively correlated with the simultaneous uptake of glucose into the adjacent contracting muscle (Kalliokoski et al. 2005). This indicates that metabolic activity, the uptake of metabolic substrates and blood flow are regulated in a specialized way in tendon that is independent of that occurring in skeletal muscle.
Fig. 2.
Positron emission tomography (PET) images from the regions of quadriceps muscle (A), quadriceps tendon (B) and patellar tendon (C) in the exercising and resting leg of one subject. White lines show the regions of interest. (From Kalliokoski et al. 2005.)
Tendon and collagen synthesis
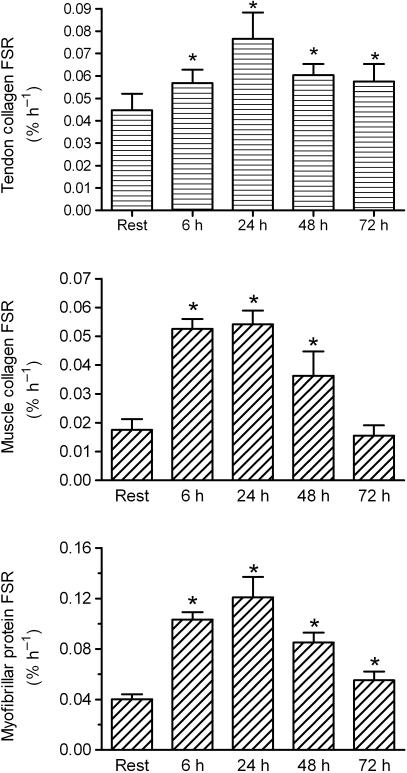
Determination of protein synthesis in the ECM of tendon and skeletal muscle has been studied in the resting state both in animals using radioactive isotopes and in humans using circulating pro-collagen propeptides as markers for collagen synthesis typical of those used to evaluate bone turnover (Langberg et al. 1999b, 2000). More recently, the introduction of stable (non-radioactive) isotopes of amino acids has allowed protein synthesis rates to be measured in human tissue. When using labelled proline (either 13C or 15N) infused at a high dose in a vein 2–4 h before measurements (‘flooding dose’), the collagen synthesis rate can be calculated from the amount of labelled proline incorporated into the tissue from a biopsy of tendon, ligament, skin, muscle or bone (Babraj et al. 2005). Interestingly, collagen synthesis in human tendon rises by around 100% with just one bout (60 min) of acute exercise, and the elevated collagen synthesis is still present 3 days after exercise (Fig. 3; Miller et al. 2005). In skeletal muscle, the rate of collagen synthesis also increases with exercise, in a time-dependent manner that follows the increase in myofibrillar protein synthesis with exercise (Miller et al. 2005). This suggests a more intimate interplay between adaptations in skeletal muscle fibres and the endo-, peri- and epimysium (Purslow, 2002). Interestingly, it has been shown that with intense exercise, intramuscular connective tissue shows considerable evidence of injury, which leads to an activation of satellite cell activity (Crameri et al. 2004a,b). This again hints at a possible link between intramuscular ECM and contractile muscle fibres. Furthermore, there is an increased expression of collagen in intramuscular ECM following intense exercise in animal studies. With in situ hybridization, it can be shown that the majority of the increased expression of collagen type I is in the perimysium (Koskinen et al. 2001, and Koskinen, personal communication). In addition to increased collagen synthesis in the ECM of human tendon and skeletal muscle, there is also increased protein degradation. The activity of matrix metalloproteinases (MMPs) increases immediately after acute exercise, when enzyme activity is determined in the interstitial peritendinous tissue by microdialysis (Koskinen et al. 2004). Thus, protein turnover in tendon seems to mimic that of myofibrillar protein, i.e. acute exercise is associated with an immediate increase in proteolytically driven degradation of collagen (and other proteins), and 1–3 days after exercise there is a marked increase in collagen synthesis (Kjær, 2004; Trappe et al. 2004; Miller et al. 2005). This contributes to our understanding of why overuse of tendon tissue can occur. If training sessions are too close to one another, an athlete may not gain maximum benefit from the stimulated collagen synthesis, but is instead likely to be in a net state of collagen catabolism. In line with this hypothesis, it is interesting to note that investigations of collagen turnover with the onset of regular training show that protein synthesis as well as degradation are chronically elevated 4 weeks into the training period, whereas protein synthesis remains high throughout a 12-week training cycle but that degradation is slowly reduced (Langberg et al. 2001). These results came from using microdialysis fibres placed peritendinously around the Achilles tendon to measure the local interstitial concentration of propeptides (PICP and PINP) and collagen degradation products (ICTP etc.) (Langberg et al. 1999a, 2001). This suggests that there may be a period early in training where turnover of collagen in tendon is increased in order to ‘restructure’ and adapt the tendon to the increased loading pattern. It is not until training is prolonged that there is a net collagen synthesis. We thus speculate that this provides the basis for a net enlargement of the tendon cross-section area (CSA) (and volume). Animal training studies have shown that tendon enlargement occurs with chronic training (Birch et al. 1999). Similarly, in humans cross-sectional studies comparing runners and sedentary individuals show that runners have larger Achilles tendon CSA than their age-, gender- and weight-matched sedentary counterparts (Rosager et al. 2002). However, short-term training studies have not been able to detect enlargements of human tendons (Hansen et al. 2003). This indicates a certain delay time – perhaps because of relative overloading initially – before tendon enlargement occurs. In line with this, earlier animal studies confirm that short-term training reduces the size of a tendon rather than increases it (Birch et al. 1999). Interestingly, tendons in which there is chronic pain and signs of tendinopathy can increase their collagen synthesis, and perhaps part of the effect that controlled training has on the tendon is simply to favour collagen synthesis, which in turn will ultimately strengthen it (Langberg et al. unpub. obs.). Conversely, it has been shown that both ageing (Reeves et al. 2003) and disuse (Maganaris et al. 2006) may alter the material properties of tendon, as evidenced by reduced stiffness, which can be meaningfully reversed with resistance exercises (Reeves et al. 2003).
Fig. 3.
The fractional synthesis rate of tendon collagen protein, muscle collagen protein and myofibrillar protein. (Adapted from Miller et al. 2005.)
Regulation of collagen synthesis
Growth factors and hormones are involved in the regulation of ECM synthesis in connective tissue, but little is known about their role in adjusting collagen synthesis to load. IGF-1 (and its binding proteins), TGF-beta and IL-6 have been shown to be present in human tendon tissue, and their concentration in the interstitial peritendinous tissue increases with exercise (Langberg et al. 2002a; Heinemeier et al. 2003). Furthermore, in an animal model in which the Achilles tendon is overloaded, expression of IGF-1 and TGF-beta is increased with loading (J. L. Olesen, personal communication). On this basis there is reason to believe that these hormones and growth factors are important for tendon collagen synthesis during exercise. As in vitro data have shown that oestrogen inhibits collagen synthesis and that some connective tissue injuries such as ligament ruptures (e.g. anterior cruciate ligament) are more frequent in women, it is interesting to find that collagen synthesis in tendons is lower in women than in men, and that it rises less with exercise (Miller et al. 2006). This fits with recent data on the mechanical properties of patellar tendon fascicles in both men and women that show that the stress-to-failure was less in women than in men (S. P. Magnusson, pers. comm.).
Muscle, tendon and bone interaction
In relation to tendon loading and overloading it is important to acknowledge that tendons are heterogeneous along both their length and their width. Furthermore, there is evidence of internal shear stress in the tendon (Bojsen-Møller et al. 2003; 2004). The heterogeneity of tendons has been demonstrated within the human Achilles tendon. Here, it seems that the CSA of the tendon differs along its length, and that the enlargement of the tendon to loading is also site specific (Magnusson & Kjær, 2003, Magnusson et al. 2003). It should also be noted that tendons have fibrocartilaginous entheses zones at their bony attachments that consist of dense fibrous connective tissue, uncalcified fibrocartilage, calcified fibrocartilage and bone (Benjamin & Ralphs, 2001). These are specialized zones that help dissipate bending, dissipate stress and compression, and withstand shear (Benjamin & Ralphs, 2001). When evaluating the mechanical properties of the human patellar tendon, it is now possible to study this in individual fascicles and to show that although fascicles from both the anterior and the posterior parts of the patellar tendon can be stretched around 8% before failure, the stress they can tolerate is only half as much on the posterior side (Haraldsson et al. 2005). The ‘weak’ location of the tendon coincides with the location of pathological changes in patellar tendinopathy (‘jumper's knee’). In the Achilles tendon, it is well known that the muscles which contribute to its formation (gastrocnemius and soleus) have different mechanical effects upon the tendon. The movement at the distal aponeurosis between the two muscles during isometric plantar flexion performed either with the knee straight or with it bent shows that the shear between gastrocnemius and soleus is around 4–5 mm (Bojsen-Møller et al. 2004). This could easily cause internal shear within the Achilles tendon and thus contribute to injury.
Conclusion
In conclusion, the ECM of both human tendon and skeletal muscle tissue reacts dynamically to mechanical loading and this increases collagen synthesis. The time pattern of this adaptation may limit athletic training, and help us to understand why overuse injuries occur in work, sport and recreational activities.
References
- Babraj JA, Cuthbertson DJR, Smith K, et al. Collagen synthesis in human musculoskeletal tissues: methodology, validation and physiological response. Am J Physiol. 2005;289:E864–E869. doi: 10.1152/ajpendo.00243.2005. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Entheses – the bony attachments of tendons and ligaments. Ital J Anat Embryol. 2001;106:151–157. [PubMed] [Google Scholar]
- Birch HL, McLaughlin L, Smith RK, Goodship AE. Treadmill exercise-induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Vet J. 1999;30:222–226. doi: 10.1111/j.2042-3306.1999.tb05222.x. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller J, Hansen P, Aagaard P, Kjær M, Magnusson SP. Measurement of in vivo vastus lateralis tendon-aponeurosis compliance. Scand J Med Sci Sports. 2003;13:259–265. doi: 10.1034/j.1600-0838.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller J, Hansen P, Aagaard P, Svantesson U, Kjær M, Magnusson SP. Differential displacement between the aponeurosis of the soleus and medial gastrocnemius during voluntary plantarflexion contraction observed in vivo. J Appl Physiol. 2004;97:1908–1914. doi: 10.1152/japplphysiol.00084.2004. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Green S, Bülow J, Skovgaard D, Kjær M. Blood flow and oxygenation in peritendinous tissue and calf muscle during dynamic exercise in humans. J Physiol. 2000a;524:305–313. doi: 10.1111/j.1469-7793.2000.t01-2-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen JL, Bülow J, Kjær M. Blood flow determination in muscle and tendon with the use of indocyanine green – near infrared spectroscopy (ICG-NIRS) at rest and during exercise. J Appl Physiol. 2000b;89:1868–1878. doi: 10.1152/jappl.2000.89.5.1868. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen JL, Gonzales-Alonso J, Bülow J, Kjær M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, et al. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Risum N, Kjær M. Regulation of blood flow by prostaglandins. Curr Vasc Pharm. 2004;2:191–197. doi: 10.2174/1570161043476410. [DOI] [PubMed] [Google Scholar]
- Crameri R, Langberg H, Jensen CH, Teisner B, Schrøder HD, Kjær M. Activation of satellite cells in human skeletal muscle after a single bout of exercise. J Physiol. 2004a;558:333–340. doi: 10.1113/jphysiol.2004.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri R, Langberg H, Teisner B, et al. Synchronous disruption of the extracellular matrix and mechanical tenderness in skeletal muscle after a single bout of eccentric loading in humans. Matrix Biol. 2004b;23:259–264. doi: 10.1016/j.matbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Hannukainen J, Nuutila P, Fujimoto T, et al. Glucose uptake in human tendon during exercise using positron emission tomography (PET) Int J Sports Med. 2005;26:727–731. doi: 10.1055/s-2005-837458. [DOI] [PubMed] [Google Scholar]
- Hansen P, Aagaard P, Kjær M, Larsson B, Magnusson SP. Effect of habitual Achilles tendon load–deformation properties and cross-sectional area. J Appl Physiol. 2003;95:2375–2380. doi: 10.1152/japplphysiol.00503.2003. [DOI] [PubMed] [Google Scholar]
- Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjær M, Magnusson SP. Region specific mechanical properties of the human patellar tendon. J Appl Physiol. 2005;98:1006–1012. doi: 10.1152/japplphysiol.00482.2004. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Olesen JL, Kjær M. Role of transforming growth factor beta in relation to exercise induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Langberg H, Ryberg AK, et al. The effect of dynamic knee-extension exercise on patellar tendon and quadriceps femoris muscle glucose uptake in humans studied by positron emission tomography. J Appl Physiol. 2005;98:1020–1032. doi: 10.1152/japplphysiol.00283.2005. [DOI] [PubMed] [Google Scholar]
- Kjær M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Koskinen SOA, Wang W, Ahtikoski AM, et al. Turnover of basement membrane type IV collagen in exercise-induced skeletal muscle injury. Am J Physiol. 2001;280:R1292–R1300. doi: 10.1152/ajpregu.2001.280.5.R1292. [DOI] [PubMed] [Google Scholar]
- Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjær M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon related connective tissue. J Appl Physiol. 2004;96:861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bülow J, Kjær M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiol Scand. 1998;163:149–153. doi: 10.1046/j.1365-201X.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bülow J, Kjær M. Standardized intermittent static exercise increases peritendineous blood flow in human leg. Clin Physiol. 1999a;19:89–93. doi: 10.1046/j.1365-2281.1999.00148.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bülow J, Kjær M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999b;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Asp S, Kjær M. Time pattern of exercise induced changes in type-1 collagen turnover after prolonged endurance exercise in humans. Calc Tissue Int. 2000;67:41–44. doi: 10.1007/s00223001094. [DOI] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjær M. Training induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Olesen JL, Gemmer C, Kjær M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, but not in muscle, following prolonged exercise in humans. J Physiol. 2002a;542:985–990. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Bjørn C, Boushel R, Hellsten Y, Kjær M. Exercise induced increase in interstitial bradykinin and adenosine concentration of skeletal muscle and peritendinous tissue in humans. J Physiol. 2002b;542:977–983. doi: 10.1113/jphysiol.2002.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Boushel R, Skovgaard D, Risum N, Kjær M. Cyclooxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading. J Physiol. 2003;551:683–689. doi: 10.1113/jphysiol.2003.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33:85–92. doi: 10.1002/mus.20441. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, et al. Differential strain pattern of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Kjær M. Region specific differences in Achilles tendon cross-sectional area in runners and non-runners evaluated by MR imaging. Eur J Appl Physiol. 2003;90:549–553. doi: 10.1007/s00421-003-0865-8. [DOI] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;175:593–600. [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Barbraj J, et al. Muscle and tendon collagen and non-collagen protein synthesis rates are synchronized after strenuous exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Hansen M, Olesen JL, et al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol Endocrinol Matab. 2006;290:E163–E168. doi: 10.1152/ajpendo.00300.2005. [DOI] [PubMed] [Google Scholar]
- Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. A Mol Integr Physiol. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548:971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjær M, Magnusson SP. Load–displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12:90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Trappe T, Williams R, Carrithers J, et al. Influence of age and resistance exercise on skeletal muscle proteolysis: a microdialysis approach. J Physiol. 2004;554:803–813. doi: 10.1113/jphysiol.2003.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]