Abstract
Until now there has been no definitive anatomical study describing the area where the parotid duct enters the buccinator muscle. In this study, we performed anatomical and histological examinations to investigate the relationship between the parotid duct and the buccinator muscle. Thirty specimens (including the buccinator and the terminal portion of the parotid duct) were obtained from embalmed Korean cadavers. Dissection was performed on 22 of these specimens, and the remaining eight specimens were prepared for histological examination and stained with haematoxylin–eosin or Gomori trichrome. In all specimens, small, distinct muscle fibres originating from the buccinator muscle extended to and inserted into the terminal portion of the parotid duct. The topography of these fibres varied, and we classified them into three categories according to where they originated. Type I buccinator muscle fibres, which inserted into the terminal portion of the parotid duct, originated simultaneously from the anterior and posterior aspects of the duct (ten cases, 45.5%). Type II fibres originated from the anterior aspect of the duct and inserted into the anterior side of the duct (seven cases, 31.8%). Type III fibres originated from the posterior aspect of the parotid duct and ran anteriorly toward the duct (five cases, 22.7%). These results were confirmed in the histological examination of all eight specimens. Based on these findings, we have proposed a tentative description of the physiological role of the buccinator muscle fibres in salivary secretion and in the formation of the sialoliths.
Keywords: buccinator, muscle fibres, parotid duct, terminal portion
Introduction
The human parotid duct (Stensen's duct) is a single duct arising from the anterior border of the parotid gland. In adults, the parotid duct is about 6–8 cm long. It crosses the masseter muscle and turns medially at almost a right angle to traverse the buccal fat pad and buccinator muscle. The parotid duct then passes through the buccal fat pad, runs obliquely forward for a short distance between the buccinator muscle and the mucous membrane of the oral cavity, and finally opens into the oral cavity opposite the maxillary second molar (Standring, 2005).
There have been some studies of the area where the parotid duct penetrates the buccinator muscle. Investigators have reported the existence of small muscle fibres originating from the buccinator that extend to and insert into the distal portion of the parotid duct. The buccinator muscle fibres play a functional role here during oral motor behaviour; they regulate physiological saliva secretion from the parotid gland (Guerrier & Bolonyi, 1948; Courbier & Richelme, 1955; Couly et al. 1976). The buccinator muscle fibres surrounding the distal portion of the parotid duct were once considered to act as a passive sphincter system (Cerruti, 1939; Guerrier & Bolonyi, 1948; Couly et al. 1976). However, there have been no conclusive anatomical and physiological descriptions in recent literature or in anatomy textbooks about the area where the parotid duct pierces the buccinator muscle.
The aim of this study was to confirm the existence of the buccinator muscle fibres that extend to and insert into the terminal portion of the parotid duct. We also studied their topographic relationships and patterns of distribution, thereby providing critical data to enhance current understanding of the physiological mechanism underlying saliva secretion from the parotid gland.
Materials and methods
Anatomical and histological examinations were performed to investigate the relationship between the parotid duct and the buccinator muscle. Thirty specimens (16 right and 14 left sides) from 19 Korean adult cadavers (14 males, five females; age 41–94 years), perfused with formaldehyde fixative, were used.
After removing the facial skin, subcutaneous fat and facial muscles, including the superficial musculoaponeurotic system, the origin of the buccinator muscle was detached and the buccinator – comprising the oral mucosa, the buccinator muscle, the distal portion of the parotid duct, a part of the buccal fat pad and other surrounding structures – was removed en bloc.
Microdissection was performed on the specimens from 22 cases with the aid of a surgical microscope (Carl Zeiss, Germany). Through careful and detailed dissections using both internal and external approaches, the terminal portion of the parotid duct, the muscle fibres of the buccinator and several adjacent anatomical structures were exposed. Using these dissected specimens, we examined the anatomical relationship between the buccinator muscle fibres and the distal portion of the parotid duct.
To obtain histological sections, we harvested the terminal portion of the parotid duct including the surrounding buccinator muscle tissue and the parotid duct orifice from the eight undissected specimens. These specimens were post-fixed for 72 h with 4% paraformaldehyde and then embedded in paraffin wax. Transverse and longitudinal 5-µm-thick sections were cut along the parotid duct, mounted on glass slides, and then stained with haematoxylin–eosin and Gomori trichrome. Histological observations were performed with the aid of a light microscope, and photographs were taken with a Spot RT digital camera (Leica, DFC300FX, Germany).
No distinction was made between the male and female cadavers. All photographs and diagrams in this article are of structures viewed from the left side of the specimen.
Results
Topographic relationships between the terminal portion of the parotid duct and the buccinator muscle fibres
In all cases, the parotid duct pierced the maxillary portion of the buccinator muscle and entered the oral cavity at the parotid papilla. Histological examination also revealed that the external layer (the tunica adventitia) of the parotid duct was continuous with the buccinator fascia (a part of the buccopharyngeal fascia).
Through meticulous dissection, we isolated and observed distinct, small muscle fibres originating from the buccinator that extended to and inserted into the external layer of the terminal portion of the parotid duct (Fig. 1). These fibres varied in length from 3 to 10 mm and arose mainly from the superficial or deep layer of the buccinator muscle, although in some specimens they originated from both the superficial and the deep layers (Fig. 2).
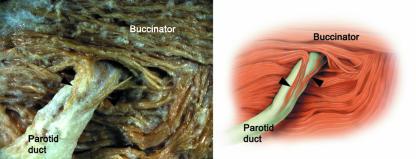
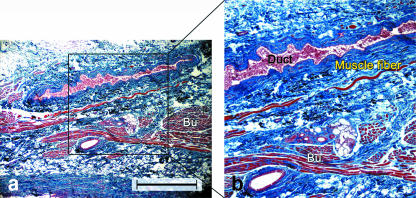
Fig. 1.
Photograph and corresponding illustration of the small muscle fibres (arrowheads) originating from the buccinator that extend to the terminal portion of the parotid duct.
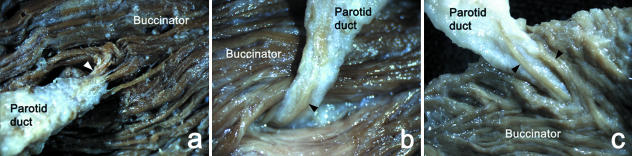
Fig. 2.
Photographs showing the patterns of origin of the buccinator muscle fibres (arrowheads) that extend to the terminal portion of the parotid duct. The muscle fibres originated from the superficial (a), deep (b) or both superficial and deep layers (c) of the buccinator muscle.
The muscle fibres that extend to the parotid duct originated from different locations. Three categories were defined here based upon the anterior-to-posterior position of muscle fibre origin (Fig. 3). Type I buccinator muscle fibres, which were observed in ten cases (45.5%), originated simultaneously from the anterior and posterior aspects of the duct and extended to the terminal portion of the parotid duct. In four cases within this group (18.3%), the muscle fibres only originated from the anterior and posterior aspects of the duct. In the other cases, additional buccinator muscle fibres originated from the superior (five cases, 22.7%) and inferior (one case, 4.5%) aspects of the parotid duct.
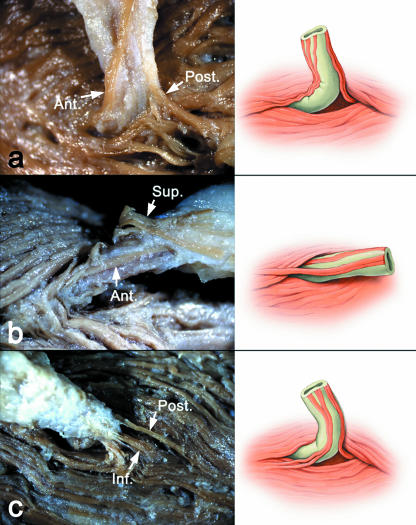
Fig. 3.
Photographs and corresponding illustrations of three categories of fibre characterized according to their origin and location (arrows). The muscle fibres simultaneously originated (a) from the anterior and posterior aspects of the duct, (b) from the anterior and superior aspects of the duct and then inserted into the anterior aspect of the duct, and (c) from the posterior and inferior aspects of the duct. Ant., anterior; Post., posterior; Sup., superior; Inf., inferior.
Type II buccinator muscle fibres originated from the anterior aspect of the duct and inserted into the anterior aspect of the duct (seven cases, 31.8%); however, they were always accompanied by other muscle fibres with different locations of origin. In six cases (27.3%), the additional muscle fibres originated from the superior aspect of the parotid duct, and in one case (4.5%) they originated from the inferior aspect of the parotid duct.
Type III buccinator muscle fibres originated from the posterior aspect of the parotid duct and ran posteriorly to the duct (five cases, 22.7%). Those fibres originating from the posterior aspect of the duct always accompanied other fibres with a different origin. In three cases (13.7%) additional muscle fibres originated simultaneously from the superior and inferior aspects of the duct. There was one case (4.5%) in which the additional fibres originated from either the superior aspect or the inferior aspect of the duct. The results of the various insertion patterns of the muscle fibres are given in Table 1.
Table 1.
Classifications of the extension and insertion patterns of the buccinator muscle fibres into the terminal portion of the parotid duct
| Classification | Origin and location of muscle fibres into the parotid duct | No. of specimens (%) | Total (%) |
|---|---|---|---|
| Type I | From A and P | 4 (18.3%) | 10 (45.5%) |
| From A, P, and S | 5 (22.7%) | ||
| From A, P, and I | 1 (4.5%) | ||
| Type II | From A only | 0 (0%) | 7 (31.8%) |
| From A and S | 6 (27.3%) | ||
| From A and I | 1 (4.5%) | ||
| Type III | From P only | 0 (0%) | 5 (22.7%) |
| From P and S | 1 (4.5%) | ||
| From P and I | 1 (4.5%) | ||
| From P, S, and I | 3 (13.7%) | ||
| Total | 22 (100%) |
A, anterior aspect (in front) of the parotid duct; P, posterior aspect of (behind) the parotid duct; S, superior aspect of the parotid duct; I, inferior aspect of the parotid duct.
Histological observations of the terminal portion of the parotid duct
Cross-sectional analysis of the parotid duct revealed that it has three components: the mucosa, the muscle layer and the adventitia (the outer connective tissue layer, a structure normally seen in excretory ducts). The lumen of the duct had a round-to-oval shape, and its diameter varied between different cross-sections, and even within the same specimen. The muscular layer consisted of small smooth muscle fibre bundles running in a longitudinal direction (Fig. 4). Oblique muscle fibres were often seen intermingled with longitudinal fibres. There was no circular muscle fibre observed in any section.
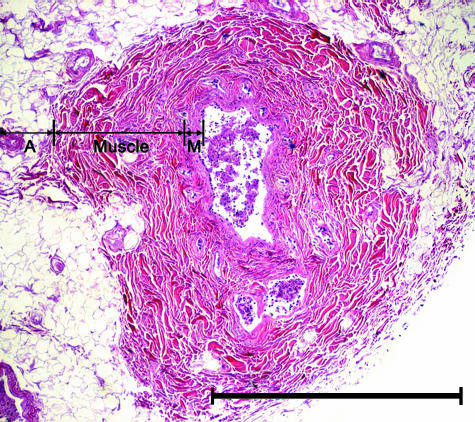
Fig. 4.
Light micrograph of a transverse section of the parotid duct. The duct was composed of three layers: the adventitia (A), muscle layer (Muscle) and mucosa (M). Haematoxylin–eosin. Scale bar = 1 mm.
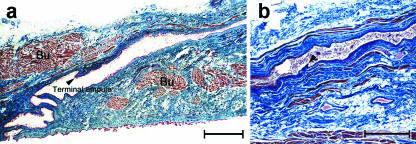
We also made longitudinal sections along the axis of the parotid duct and the papilla to investigate the morphology of the junction between the terminal portion of the parotid duct and the buccinator muscle (Figs 5 and 6). The terminal portion of the parotid duct within the buccinator muscle was not straight, and instead displayed a tortuous course with a few terminal siphons (Fig. 6a). In some cases, a valve-like structure within the lumen of the terminal duct of the parotid was observed (Fig. 6). Small but distinct muscle fibres originating from the buccinator muscle ran parallel to the duct (Figs 5 and 6). These muscle fibres extended to the terminal portion of the duct from the outermost layer of the buccinator, and varied in length from 4 to 10 mm. As the duct approached the oral mucosa within the buccinator muscle, these muscle fibres disappeared. Other muscle fibres from the buccinator wrapped around the terminal portion of the parotid duct in a spiral fashion. These observations were in accordance with the results shown in our detailed dissections (Fig. 3).
Fig. 5.
(a) Light micrograph of a longitudinal section along the axis of the parotid duct. (b) Higher magnification view of the boxed area in (a) showing distinct muscle fibres originating from the buccinator and running parallel to the duct (Duct). Bu, buccinator. Gomori trichrome. Scale bar = 100 µm.
Fig. 6.
(a,b) Light micrographs of longitudinal sections along the axis of the parotid duct showing the valve-like structure (arrowheads) within the lumen of the terminal duct of the parotid. Bu, buccinator. Gomori trichrome. Scale bar = 100 µm (a), 50 µm (b).
Discussion
Although several previous studies have examined the position of the terminal portion of the parotid duct relative to the buccinator muscle (Cerruti, 1939; Guerrier & Bolonyi, 1948; Courbier & Richelme, 1955; Couly et al. 1976), recent literature and anatomy textbooks pay little attention to the detailed anatomy of this critical structure. The parotid duct has been considered a static rather than a dynamic organ, and researchers have failed to notice the functional and physiological roles of the buccinator muscle fibres that extend to the terminal portion of the parotid duct. In this study, we confirmed their existence and described their topography. It is assumed that these fibres play an important physiological role by regulating secretions from the parotid duct.
Functional electromyographic (EMG) studies have claimed that the buccinator is activated on the working side during mastication intervals (Lundquist, 1955). Blanton et al. (1970) reported that no EMG activity was detected in the central portion of the buccinator when slowly opening and closing the mouth, and this central portion is located around the first molar and the orifice of the parotid duct. Perkins et al. (1977) found that the orbicularis oris and the buccinator were particularly active in the initial stage of swallowing, and they concluded that these muscles initiate swallowing by producing a peristalsis-like wave of contraction originating in the oral cavity and continuing on to the pharynx.
In the present study, we were able to identify in all specimens the existence of distinct, small muscle fibres originating from the buccinator and extending to the terminal portion of the parotid duct. This suggests that the buccinator muscle fibres play a functional role in saliva secretion from the parotid duct. In previous studies (Cerruti, 1939; Guerrier & Bolonyi, 1948; Courbier & Richelme, 1955; Couly et al. 1976), the anatomical structure of the terminal portion of the parotid duct was referred to as fibrous tissue. However, based on the anatomical and histological examinations performed here, we conclude that it is not fibrous tissue, but instead a distinct bundle of skeletal muscle fibres from the buccinator. The sequential movements during the contraction and relaxation of the buccinator muscle may be transferred to the parotid duct through these small muscle fibres, thus regulating the filled and empty states of the parotid gland with saliva. We propose that when the buccinator is relaxed, the small muscle fibres that extend to the terminal portion of the parotid duct stretch the wall of the duct in various directions, widening the lumen of the duct and forming a terminal ampule. During this stage, while the orifice of the parotid is closed, saliva is collected in this widened terminal ampule. By contrast, upon contraction of the buccinator, the muscle fibres around the parotid duct orifice are thickened, squeezing and lengthening the bulged area (terminal tubule) of the terminal portion of the parotid duct. These sequential events may result in saliva being stored and secreted. Based on this tentative description of their physiological functions, these small muscle fibres from the buccinator may thus act as a dilator of the terminal portion of the parotid duct. They do not function as the sphincter system, as has previously been suggested (Guerrier & Bolonyi, 1948; Couly et al. 1976). Although further study on this mechanism is required, we believe that this striking system involving saliva secretion is an acceptable explanation of the function of the buccinator muscle fibres that extend to the terminal portion of the parotid duct.
The dynamic relationship between the buccinator and the parotid duct resembles the ureterovesical junction. In the bladder, the muscle bundles of the ureteral sheath (Waldeyer's sheath) extend from the outer bladder wall and function as a part of a preventive mechanism against the vesicoureteral reflux. This is accomplished by compressing the intravesical ureter through independent contraction during the voiding and filling phases (Tanagho & Pugh, 1963). Noordzij & Dabhoiwala (1993) reported that the juxtavesical and intramural ureter are enclosed by a fibromuscular sheath, and the muscular fibres of this sheath seem to be predominantly a continuation of the detrusor muscle. According to their results, the ureteral sheath (Waldeyer's) is firmly anchored to the adventitia of the juxtavesical ureter, but permits axial sliding of the ureter. This description is similar to our observations that show the continuation of the muscle fibres from the buccinator muscle to the adventitia layer of the terminal parotid duct.
Based on the histological examinations performed in the present study, we observed two structures in the terminal portion of the parotid duct where it pierces the buccinator muscle: broad terminal siphons and valve-like structures. These oppose salivary flow within the lumen of the terminal siphons of the parotid duct. From the configuration of such anatomical structures, we could speculate that they may play an important role in controlling salivary flux or preventing salivary reflux. The distinct, small muscle fibres that originate from the buccinator and extend to the parotid duct, along with the valve-like structures within the terminal siphons, may both be integral components of the system that controls salivary secretion. These components may expel saliva or prevent the reflux of residual saliva in the terminal portion of the parotid duct.
Sialolithiasis is the most common disease of the salivary glands in middle-aged patients. Its etiology includes local chemical changes in salivary components, infectious factors, secretory disturbances, ductal anomalies of the salivary glands and foreign bodies. Simultaneous sialolithiasis in several salivary glands is rare; in most cases, it occurs in a single gland, and particularly in the submandibular gland. The frequent occurrence of sialolithiasis in the submandibular gland can be explained by the high mucin content of submandibular saliva, which adheres to foreign particles (Shafer et al. 1983). In addition, the anatomy of the submandibular duct (Wharton's duct), which is long and irregular in its course, also provides another risk factor for sialolithiasis. In the context of diagnostic sialendoscopy, Marchal et al. (2001) suggested that the presence of a sphincter system in Wharton's duct was another possible risk factor for sialolithiasis. However, Teymoortash et al. (2003) reported that Wharton's duct had no anatomical correlation with the presence of a sphincter and did not support the involvement of sphincter-like structures during the formation of sialoliths. In contrast to Wharton's duct, the presence of functional muscle fibres on the parotid duct together with the bulging of the terminal portion of the parotid gland suggests that it plays an important role in the formation of sialoliths. In such a situation, the remnants of saliva could make a nidus for the sialolith. This mechanism may explain the relatively frequent occurrence of parotid sialolithiasis in the terminal portion near the orifice. Further morphological and physiological studies are required to clarify this point.
In conclusion, based on our anatomical and histological examinations, we have tentatively described a physiological role for the buccinator muscle fibres that extend to the terminal portion of the parotid gland. In this study, these distinct, small muscle fibres were observed in all specimens, and extended to the terminal portion of the parotid duct, suggesting that they act as a dilator of the duct. In addition, the tortuous luminal structures related to the longitudinal smooth muscle layer of the duct also participate in the control of salivary flux (peristaltic activity). The valve-like structures could also play a role in the prevention of salivary reflux. Finally, the presence of the terminal ampule, which is associated with the muscle fibres surrounding the parotid duct, could play a role in the localization of sialolithiasis.
Acknowledgments
This study was supported by the Yonsei University College of Dentistry fund, 2005.
References
- Blanton PL, Biggs NL, Perkins RC. Electromyographic analysis of the buccinator muscle. J Dent Res. 1970;49:389–394. doi: 10.1177/00220345700490023201. [DOI] [PubMed] [Google Scholar]
- Cerruti H. A parte terminal do ductus parotidicus no humen (pesquisas morfo-histologicus) Rev Ass Paulista Med. 1939;14:345–412. [Google Scholar]
- Couly G, Guilbert F, Descrozailles JM. Functional anatomy and terminal tubules of Stensen's duct. Rev Stomatol Chir Maxillofac. 1976;77:645–652. [PubMed] [Google Scholar]
- Courbier R, Richelme H. Note sur l’existence d’une gaine musculaire au niveau de la partie terminale du canal de STENON. Travaux l’Institut d’Anat Fac Med Marseille Fasc. 1955–56;16:61–62. [Google Scholar]
- Guerrier Y, Bolonyi F. L’architecture du muscle buccinateur et ses rapports avec le canal de STENON. Ann Oto-Laryngologie. 1948;65:106–108. [Google Scholar]
- Lundquist DO. An electromyographic analysis of the function of the buccinator muscle as an aid to denture retention and stabilization. J Pros Dent. 1955;9:44–52. [Google Scholar]
- Marchal F, Kurt AM, Dulguerov P, Lehmann W. Retrograde theory in sialolithiasis formation. Arch Otolaryngol Head Neck Surg. 2001;127:66–68. doi: 10.1001/archotol.127.1.66. [DOI] [PubMed] [Google Scholar]
- Noordzij JW, Dabhoiwala NF. A view on the anatomy of the ureterovesical junction. Scand J Urol Nephrol. 1993;27:371–380. doi: 10.3109/00365599309180449. [DOI] [PubMed] [Google Scholar]
- Perkins RE, Blanton PL, Biggs NL. Electromyographic analysis of the ‘buccinator mechanism’ in human beings. J Dent Res. 1977;56:783–794. doi: 10.1177/00220345770560071301. [DOI] [PubMed] [Google Scholar]
- Shafer WG, Hine MK, Levy BM. A Text Book of Oral Pathology. 4. Philadelphia: W.B. Saunders; 1983. [Google Scholar]
- Standring S. Gray's Anatomythe Anatomical Basis of Clinical Practice. 39. New York: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- Tanagho EA, Pugh RC. The anatomy and function of the ureterovesical junction. Br J Urol. 1963;35:151–165. doi: 10.1111/j.1464-410x.1963.tb02610.x. [DOI] [PubMed] [Google Scholar]
- Teymoortash A, Ramaswamy A, Werner JA. Is there evidence of a sphincter system in Wharton's duct? Etiological factors related to sialolith formation. J Oral Sci. 2003;45:233–235. doi: 10.2334/josnusd.45.233. [DOI] [PubMed] [Google Scholar]