Abstract
The amygdaloid complex was investigated in 36 serially sectioned staged human embryos, including 20 impregnated with silver. This is the first such account based on graphic reconstructions, 28 of which were prepared. Significant findings in the human include the following. (1) The medial (first) and (then) lateral ventricular eminences arise independently at stages 14 and 15, and unite only at stage 18 to form the floor of the lateral ventricle. (2) The future amygdaloid region is discernible at stage 14 and the amygdaloid primordium at stage 15. (3) The anterior amygdaloid area and the corticomedial and basolateral complexes appear at stage 16. (4) These three major divisions arise initially from the medial ventricular eminence, which is diencephalic. (5) Individual nuclei begin to be detectable at stages 17–21, the central nucleus at stage 23 and the lateral nucleus shortly thereafter. (6) The ontogenetic findings in the human embryonic period accord best with the classification used by Humphrey (J Comp Neurol 132, 135–165, 1968). (7) The lateral eminence, which is telencephalic, contributes to the cortical nucleus at stage 18. (8) The primordial plexiform layer develops independently of the cortical nucleus. (9) Spatial changes of the nuclei within the amygdaloid complex and of the complex as a whole begin in the embryonic period and continue during the fetal period, during the early part of which the definitive amygdaloid topography in relation to the corpus striatum is attained. (10) The developing amygdaloid nuclei are closely related to the medial forebrain bundle, which has already appeared in stage 15. (11) Fibre connections develop successively between the amygdaloid nuclei and the septal, hippocampal and diencephalic formations, constituting the beginning of the limbic system before the end of the embryonic period. Although the nucleus accumbens also appears relatively early (stage 19), connections between it and the amygdaloid complex are not evident during the embryonic period. (12) Influence of the olfactory bulb and tubercle on initial amygdaloid development, as postulated for rodents, is unlikely in the human. The findings exemplify the necessity of beginning developmental studies with the embryonic period proper.
Keywords: ‘ganglionic’ eminences, amygdaloid complex, corpus striatum, limbic system
Introduction
The amygdaloid nucleus [body] was described, illustrated and named by Burdach in 1819–1826, and the subdivisions of the amygdaloid complex, which were slowly elaborated upon early in the twentieth century, became clarified in 1923 by Johnston, on whose studies subsequent classifications (e.g. by Crosby & Humphrey, 1941) were based (Meyer, 1971) and were adopted by others, although an alternative was more recently proposed (Stephan & Andy, 1977). Important studies of the development of the human amygdaloid complex have appeared for both the embryonic (Humphrey, 1968) and the fetal (Macchi, 1951; Humphrey, 1972) period.
A difficulty in the study of the amygdaloid complex of the human embryo is the totally different arrangement from the definitive topography. Furthermore, the temporal pole of the early embryonic cerebral hemispheres cannot be reliably localized as a landmark.
The amygdaloid complex is ‘shown to be involved in the modulation of neuroendocrine function, visceral effector mechanisms, and complex patterns of integrated behavior, such as defense, ingestion, aggression, reproduction, memory and learning’ (de Olmos, 2004). Afferents from the olfactory tract to the amygdaloid complex develop during the fetal period, but its nucleus is ‘highly variable’ (Humphrey, 1972) or even unidentifiable (Macchi, 1951). The amygdaloid complex as a key component of the limbic system is involved in processes such as learning, memory and emotion, and in a number of human neurogenerative diseases, including Alzheimer's disease, Huntington's chorea and neuronal ceroid lipofuscins (de Olmos, 2004). Neuropathological changes are observed also in schizophrenia and autism.
The present investigation was undertaken: (1) to control the initial appearance of the amygdaloid nuclei, which lack sharp boundaries, and to establish the most appropriate initial classification; (2) to verify the origin, sequence of appearance and derivatives of the medial and lateral ventricular eminences, especially the amygdaloid nuclei and the globus pallidus; (3) to trace the unexpected displacement of diencephalic structures (amygdaloid complex and globus pallidus) into telencephalic territory; (4) to determine whether signs of ‘rotation’ can be detected during the embryonic period and to assess a possible influence of the inferior horn of the expanding lateral ventricle; (5) to study the neural connections of the various nuclei; (6) to use precise reconstructions rather than isolated photomicrographs or drawings of individual sections; and (7) to arrange the findings in terms of morphological stages, so that the information can be integrated in the ever-expanding compendium of staged data.
Materials and methods
The amygdaloid region was investigated at stages 14–23 (4.5–8 weeks) in 36 serially sectioned human embryos (six of stage 14, three of 15, five of 16, six of 17, seven of 18, four of 19, two of 20, three of 23) and one fetus of the Carnegie Collection. The sections were in various planes and were stained with alum cochineal, haematoxylin and eosin, or azan (modified Mallory's trichrome method). Twenty of the embryos and the fetus were impregnated with silver (Bodian method). Twenty-eight precise graphic reconstructions were prepared by the authors, using the point-plotting technique (basically that of Gaunt & Gaunt, 1978). The reconstructions are the basis for the schematic representations of the nuclei in figures that show their relationships. The internationally accepted Carnegie staging system based on morphological criteria (O’Rahilly & Müller, 1987) was used throughout, and the ages given, unless otherwise specified, are post-fertilizational. The imprecise term gestational is avoided (O’Rahilly & Müller, 2000).
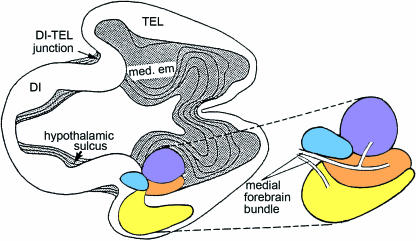
Three topographical landmarks help to localize the amygdaloid nuclei: (1) the internal di-telencephalic boundary (which leads from the preoptic groove to the velum transversum, a tranverse fold in the roof of the di-telencephalic roof), (2) the medial and (3) the lateral ventricular eminence (Fig. 1). The dividing line between diencephalic and telencephalic territories aids in appreciating the changing topography of the medial ventricular eminence, which is responsible for the bulk of the amygdaloid complex.
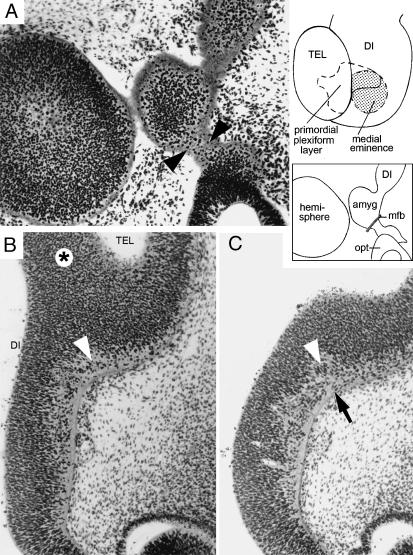
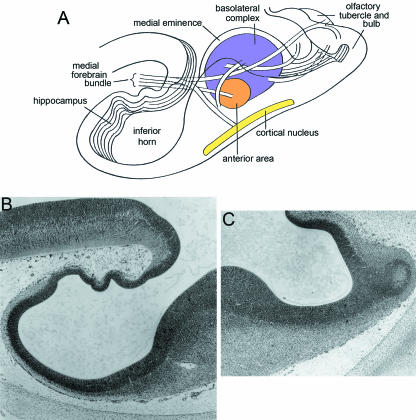
Fig. 1.
Reconstructions of the medial and lateral ventricular eminences and the site of the future amygdaloid complex of the left side, seen from inside the hemisphere at 5–6 weeks. The region where the primordial plexiform layer has developed at the periphery is indicated by an asterisk. (A) Stage 15. The medial eminence (med. em) is the basal part of the torus hemisphericus that separates telencephalon from diencephalon. The lateral eminence (lat. em) is beginning to form but is clearly separated from the medial eminence. (B) Stage 16. A widely open interventricular foramen (outlined by an interrupted line) is detectable. (C) Stage 17. The medial eminence is still partly diencephalic. It belongs to the longitudinal diencephalic zone of which the ventral thalamus is a part. The two eminences are connected by a primordial plexiform layer.
Several topographical criteria for the identification of the nuclei were used in the present study: (1) the anterior amygdaloid area (a term that refers to a relatively undifferentiated region not allocated to any specific nucleus) is situated inferocaudally at first and is traversed by fibres from the medial forebrain bundle; (2) the cortical nucleus develops at the surface, whereas the medial is occipital and close to the dentate gyrus; and (3) the basolateral complex is adjacent to the ventricular layer.
Results
Stage 14 (c. 5–7 mm)
The di-telencephalic sulcus was noted in one-third of the embryos. The surface of the future amygdaloid region possesses fibres and tangentially arranged cells that indicate the beginning of the primordial plexiform layer.
Eminences
The medial ventricular eminence is present and is exclusively diencephalic, as indicated by its relationship to the di-telencephalic sulcus.
Stage 15 (c. 7–9 mm; c. 5 post-fertilizational weeks)
What may now be termed the amygdaloid primordium is characterized by an advanced surface organization that is continuous with that of the ventral thalamus (Fig. 1A), where at the di-telencephalic sulcus an especially well-developed intermediate layer is present. The torus hemisphericus is a well-demarcated internal ridge that passes from the velum transversum towards the medial ventricular eminence. Its relationship with the amygdaloid region and the medial eminence is shown in Fig. 2(A) (insets). The cerebral hemispheres possess a primordial plexiform layer caudally. Loosely arranged cells (Fig. 2A) belong to the primordial plexiform layer and possibly represent elements of the amygdaloid primordium.
Fig. 2.
Sites of early cellular production and migration around the time of formation of the amygdaloid nuclei. (A) Sagittal section at stage 15 showing part of the amygdaloid region of the left side. The arrowheads point to faint, silver-impregnated fibres of the medial forebrain bundle (mfb, see lower inset), which descends through the ventral thalamus. The medial eminence is shaded grey in the upper inset. amyg, amydaloid primordium; opt, optic stalk. (B,C) Transverse sections at stage 16 showing the di-telencephalic sulcus at the periphery of the torus hemisphericus (asterisk). The white arrowheads point to the initial formation of the anterior amygdaloid area. The black arrow in C separates the diencephalic marginal layer from the telencephalic primordial plexiform layer.
Eminences
The newly arisen lateral ventricular eminence (Fig. 1A) is separated from the medial eminence and therefore from the amygdaloid primordium.
Connections
Fibres of the medial forebrain bundle run from the mesencephalon through the diencephalon towards the amygdaloid region (Figs 1A and 2A).
Stage 16 (c. 8–11 mm; c. 51/2 weeks)
The three major divisions (anterior amygdaloid area and corticomedial and basolateral complexes) are discernible. The torus hemisphericus is still a well-demarcated ridge (Figs 1B and 2C,D). The differentiation of the amygdaloid region is as advanced as that of the hypothalamic cell cord, the chiasmatic plate and the ventral thalamus. A primordial plexiform layer is noticeable along the periphery of the intermediate layer in the caudal rim of the cerebral hemispheres (di-telencephalic sulcus) where the tangentially arranged cells contrast with the cell columns of the ventricular layer (Fig. 2C). At this early stage, the primordial plexiform layer can be distinguished from the anterior amygdaloid area, where an accumulation of taller cells begins (Fig. 2B,C, arrowheads). The cells of the anterior amygdaloid area and the corticomedial complex are further apart from each other than are those of the basolateral primordium, which is at the periphery of the ventricular layer and has an irregular external surface (Fig. 3D). Although the corticomedial primordium is recognizable (Fig. 3C,D), its two components may already be beginning to separate (Fig. 3A,B). A topographical scheme is provided here as Fig. 3(B), which is based on a reconstruction (Fig. 3A) made from a series of sections, two examples of which are reproduced (Fig. 3C,D).
Fig. 3.
Major divisions in the early development of the amygdaloid region (stage 16). (A) Reconstruction of the future occipital part of the left cerebral hemisphere showing the anterior amygdaloid area, the basolateral and corticomedial complexes, and the medial ventricular eminence. (B) Scheme of the amygdaloid divisions of the left side of the brain in a caudal view. (C,D) Examples of photomicrographs used in preparing the reconstructions in A. The white arrowheads point to the medial nucleus.
Eminences
The medial ventricular eminence has begun to invade telencephalic territory in two out of six embryos. The lateral ventricular eminence is not connected with the medial.
Connections
Fibres of the medial forebrain bundle can be followed to the amygdaloid region and they continue to the olfactory tubercle and bulb.
Stage 17 (c. 11–14 mm; c. 6 weeks)
This important stage is characterized by the first appearance of individually recognizable amygdaloid nuclei. The periphery of the amygdaloid region is more developed than the remaining hemispheric regions (Fig. 4). It is rich in fibres and tangentially arranged cells, whereas in the frontal part of the brain most of the superficial cells are radially orientated, and no or only short neurites are recognizable. In the basal, olfactory part of the telencephalon only some tangentially orientated neurons appear and also a few fibres.
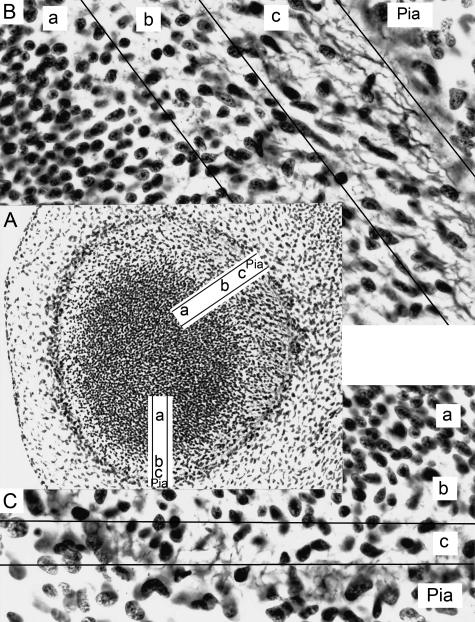
Fig. 4.
The primordial plexiform layer at the periphery of the amygdaloid region at stage 17. (A) Tangential section of the left hemisphere showing ventricular (a), intermediate (b) and primordial plexiform (c) layers. Rostral is to the left. (B) Enlargement of the amygdaloid region. The plexiform layer contains nerve fibres and elongated cells with their longitudinal axes parallel or tangential to the surface. (C) Enlargement of the olfactory region. The primordial plexiform layer is less developed than that in B.
The amygdaloid region, rostral to and slightly above the optic stalk, is still clearly related to the medial ventricular eminence (Fig. 1C). Cellular agglomerations for the three main amygdaloid parts are easily recognizable (Fig. 5A): (1) the anterior amygdaloid area, near the di-telencephalic sulcus, (2) the corticomedial complex and (3) the basolateral complex, adjacent to the ventricular layer. The corticomedial complex has split into cortical (Fig. 5A) and medial (Fig. 5B) nuclei. The cortical nucleus is wrapped around the occipital hemispheric surface, extends to the di-telencephalic sulcus, reaches as far as the olfactory tubercle and occupies about one-third of the basal surface. It covers the more deeply located anterior amygdaloid area. The medial nucleus, which is situated at the di-telencephalic sulcus and at the transition to the dentate gyrus, is best seen in sagittal sections (Fig. 5B). The basolateral complex is still intimately related to the ventricular layer.
Fig. 5.
The amygdaloid nuclei in the medial eminence at stage 17. (A) Transverse section showing the di-telencephalic transition. The hippocampal region and medial eminence protrude into the telencephalic ventricle. The key shows three of the four amygdaloid components that are now distinguishable. (B) Sagittal section in which the medial nucleus is visible adjacent to the future dentate region. Anterior and close to the medial nucleus are the basolateral complex and the anterior amygdaloid area. The cortical nucleus is present in more lateral sections.
Eminences
Medial and lateral eminences are still separated from each other (Fig. 1C). The medial eminence occupies almost the whole caudobasal telencephalic wall (Fig. 5A). Its rich capillary network penetrates the ventricular layer.
Connections
The anterior amygdaloid area is distinctly and directly connected to the medial forebrain bundle. From here nerve fibres reach the cortical nucleus.
Stage 18 (c. 13–17 mm)
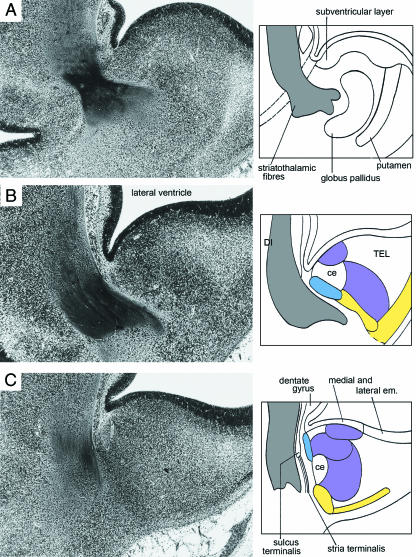
An overall view of the two amygdaloid complexes in their relationship to hippocampal and septal formations is shown in Fig. 6(A). The parts of the amygdaloid complex described for stage 17 have expanded in size, but their fundamental topography has remained (Fig. 7): the basolateral complex occupies a great part of the medial ventricular eminence at the periphery of the ventricular layer. A slight subdivision can be observed in it. It is clearly separated from the anterior area and the corticomedial complex. The cortical nucleus is wrapped around the basolateral hemispheric surface and seems to form a medial and a lateral part. The globus pallidus externus begins to occupy the di-telencephalic border zone. The lateral forebrain bundle separates it from the amygdaloid region.
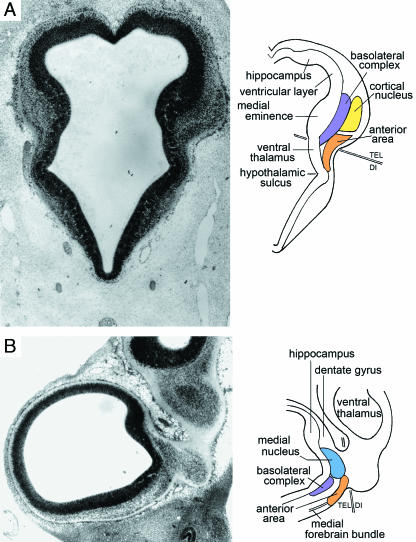
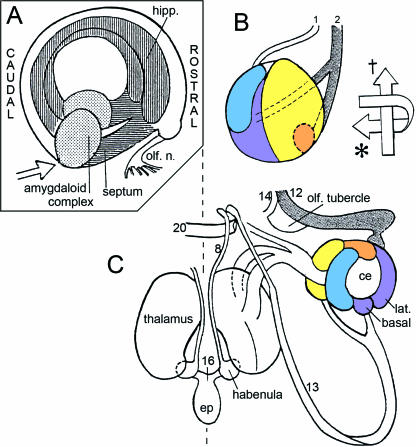
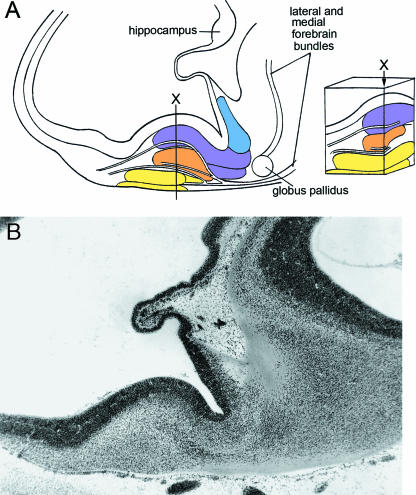
Fig. 6.
Relationship of the amygdaloid complex to other parts of the developing limbic system at 61/2–7 weeks. Colours as in Fig. 3. (A) Three-dimensional right lateral reconstruction of the amygdaloid complex, hippocampal (hipp.) primordium, septal area, and olfactory bulb of the right and left sides at stage 18. The amygdaloid complex is at the future temporal pole of the cerebral hemisphere. (B) Schematic representation of the amygdaloid complex at stage 19, seen from the direction of the broad arrow in A. Two principal neural connections are found: (1) between olfactory bulb via olfactory tubercle and the medial nucleus; and (2) between olfactory bulb and the basolateral complex and anterior area. The arrows to the right indicate caudal-to-rostral movement (dagger) and lateral-to-medial (anticlockwise) rotation (asterisk). (C) Posterosuperior view of the adult amygdaloid nuclei showing that the definitive arrangement differs from the embryonic (simplified scheme after Nieuwenhuys et al. 1988). The identity of nos. 8–20 is given in Table 1. ce, central nucleus; ep, epithalamus.
Fig. 7.
The amygdaloid nuclei and their innervation at stage 18. Colours as in Fig. 3. Dorsal view of a reconstructed block of transverse sections showing telencephalon and diencephalon. The ventricular surfaces are stippled and the four amygdaloid components are in colour. In the enlargement at the right the innervation is by ramifications of the medial longitudinal bundle. The medial eminence is now in a telencephalic position, and the rostral part of the cortical nucleus arises from the lateral ventricular eminence.
Eminences
With the partial exception of the cortical nucleus, the amygdaloid cell groups are located exclusively in the region of the medial ventricular eminence (Figs 7 and 8). The cortical nucleus extends into the lateral eminence.
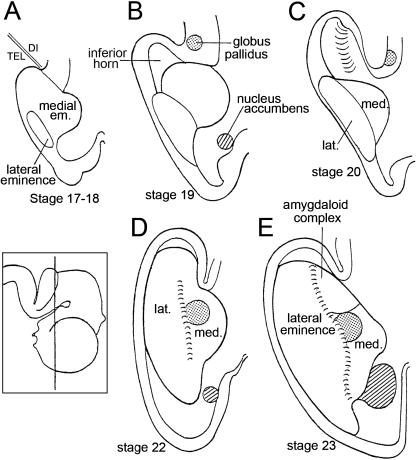
Fig. 8.
The relationship of the ventricular eminences to the parts of the lateral ventricle in dorsal view. (A) In stages 17 and 18 the basal part of the cerebral hemisphere is almost completely occupied by the medial ventricular eminence, whereas the lateral eminence is relatively small. (B) In stage 19 the lateral eminence grows medially and appears to ‘push’ the medial eminence towards the median plane. The inferior horn of the lateral ventricle begins to develop. (C) In stage 20 the inferior horn is better defined. (D) In stage 22 the lateral and medial eminences lie alongside each other and form the floor of the lateral ventricle. The lateral eminence reaches more caudally than the medial and occupies the floor of the inferior horn. The position of the lateral eminence is closer to the definitive topography than is that of the medial eminence. The intereminential sulcus is indicated by short parallel lines. (E) In stage 23 the lateral eminence is larger than the medial. The globus pallidus (stippled) is rostral to the amygdaloid complex. The nucleus accumbens is indicated by oblique hatching. The inset shows the plane of section of D and E.
Connections
The innervation of the anterior amygdaloid area spreads to the medial and cortical nuclei and to the basolateral complex. The fibres seen between amygdaloid nuclei and olfactory tubercle and bulb (Fig. 6B) seem to be continuations from the same source (medial forebrain bundle). These fibres are (1) between olfactory bulb via olfactory tubercle and the medial nucleus, and (2) between olfactory bulb and the basolateral nuclei and the anterior amygdaloid area. An early link to the dorsal thalamus, namely the lateral forebrain bundle, is now present as fibres of the striatothalamic bundle.
Stage 19 (c. 16–18 mm; c. 61/2 weeks)
The anterior amygdaloid area, the basolateral complex and the medial nucleus are still related mostly to the medial ventricular eminence. The basal part of the cortical nucleus becomes subdivided, judging by the size and density of the cells (Fig. 9A). Tall neurons are present in the anterior amygdaloid area and in the medial nucleus. The external globus pallidus is diencephalic in position and the material of the future nucleus accumbens projects as a thickening into the lateral ventricle (Fig. 8B).
Fig. 9.
The subdivision of some of the amygdaloid nuclei at stage 19. (A) Lateral view of the medial eminence within the left cerebral hemisphere. Fibres of the medial forebrain bundle pass through the basolateral complex and the anterior amygdaloid area. The basolateral complex and cortical nucleus each appear split in two parts. The globus pallidus is still diencephalic, and not yet in its definitive telencephalic position. The three-dimensional view to the right is centred along the line X in the main drawing. Colours as in Fig. 3. (B) Photomicrograph of one of the silver-impregnated sagittal sections used for the reconstruction in A.
Eminences
The recess between the medial ventricular eminence and the occipital hemispheric wall seen in Fig. 9(A) is part of the developing inferior horn. The lateral eminence extends rostrally (Fig. 8B).
Connections
The innervation by the medial forebrain bundle is striking. The globus pallidus at the transition to the diencephalon (Fig. 9A) is surrounded by fibres of the stria medullaris thalami and fibres of the lateral forebrain bundle. Connections (most probably descending) are found between the basolateral amygdaloid nucleus and the lateral habenular nucleus. These fibres run together with those of the stria medullaris thalami (see Fig. 11B). Furthermore, a connection to the interpeduncular nucleus via the habenulo-interpeduncular tract is present. The lateral forebrain bundle began to develop in stage 18; the fibres of the stria terminalis appear in stage 19.
Fig. 11.
The fibres and tracts within the limbic system numbered in order of their appearance in staged embryos, as shown in Table 1. Based on the authors’ studies. (A) At stage 17 (6 weeks). (B) At stage 19 (61/2 weeks). The stria medullaris thalami (no. 8) runs ‘on top’ of the dorsal thalamus. Fibres of no.14 are present, although not shown here; their existence in the adult is disputed. (C) In an early fetus near stage 23. The stria terminalis (no. 13), which crosses over the fibres of the lateral forebrain bundle (no. 11), will change its position later. The fornix, the longitudinal striae and the corpus callosum are not yet present.
Stage 20 (c. 18–22 mm; c. 7 weeks)
The amygdaloid complexes now lie between the inferior horn of the lateral ventricle and the interventricular foramen (Fig. 10). The external globus pallidus is still diencephalic in position (Fig. 8C).
Fig. 10.
The inferior horn of the lateral ventricle and the position of the medial ventricular eminence at stage 20. (A) Reconstructed block of transverse sections showing part of the right cerebral hemisphere in dorsal view. The main neural connections between the medial forebrain bundle, the amygdaloid nuclei, and olfactory structures are represented. The innervation of the cortical nucleus is detectable. (B,C) Examples of photomicrographs used for the reconstruction in A.
Eminences
The medial ventricular eminence has come to occupy entirely telencephalic territory (Fig. 8C).
Connections (Fig. 10)
Strands of fibres of the medial forebrain bundle enter the telencephalon caudally from the ventral aspect. They innervate the olfactory tubercle and bulb, as well as the three major subdivisions of the amygdaloid complex. Connections between the complex and the nucleus accumbens are not evident.
Stages 21 and 22 (c. 22–28 mm; c. 71/2 weeks)
At stage 21 the basolateral complex begins to divide into the accessory basal and the basal nuclei. The amygdalo-thalamic transitional area is visible in the form of cell strands connecting the two regions.
At stage 22 the external globus pallidus enters the medial ventricular eminence (Fig. 8D). The region ventral to it is occupied by a relatively loose group of cells, namely the anterior amygdaloid area.
Eminences
The two eminences form the floor of the lateral ventricle. The lateral eminence extends caudally into the inferior horn (Fig. 8D). The intereminential sulcus appears in some embryos.
Connections
The tracts are shown in Fig. 11, and the numbers assigned are indicated in Table 1. Di-telencephalic connections of the lateral forebrain bundle to the dorsal thalamus (No. 11) become more numerous. Connections are present also between the amygdaloid nuclei and the lateral habenular nucleus (No. 9), and continue via the habenulo-interpeduncular tract (No. 5) to the interpeduncular nucleus.
Table 1.
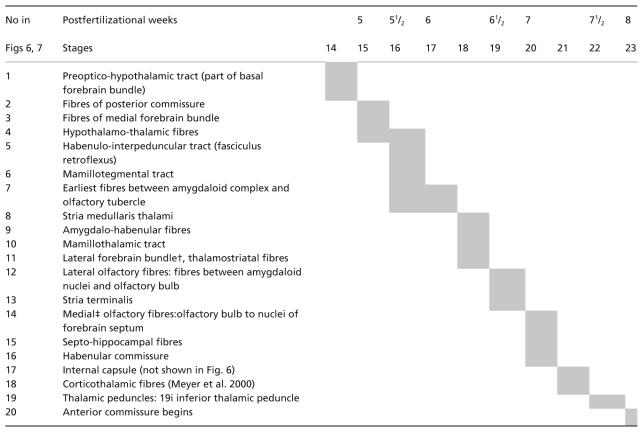 |
Included are secondary connections: (1) from olfactory bulb to olfactory tubercle and to amygdaloid body. (2) centrifugal: diagonal nucleus to olfactory bulb; locus caeruleus to olfactory bulb; anterior olfactory nucleus to olfactory bulb (Akert, 1994).
Fibres from the amygdaloid region, especially from the basolateral nucleus, to the diencephalon; thalamostriatal tract; fibres do not continue to the telencephalic pallium.
Terminology of adult olfactory striae from Kuhlenbeck (1977).
Stage 23 (c. 27–31 mm; c. 8 weeks)
Some of the amygdaloid nuclei are shown in a rostrocaudal succession of coronal sections in Fig. 12. The topography of the amygdaloid complex within the lateral ventricle is represented in Fig. 8(E).
Fig. 12.
Silver-impregnated coronal sections at stage 23 from rostral to caudal. Colours as in Fig. 3. (A) The future corpus striatum and striato-thalamic nerve fibres. The globus pallidus has now moved into its definitive telencephalic position. Between it and the putamen is the boundary between medial and lateral ventricular eminences; an intereminential sulcus is not yet present in this embryo. ce, central nucleus. (B) The rostral part of the amygdaloid region. (C) The medial nucleus here is near the dentate gyrus, and the cortical nucleus at the ventral surface.
New features are (1) the first appearance of the central nucleus (Fig. 12B,C) and the limitation of the cortical nucleus to the mediobasal periphery of the temporal pole (Fig. 13C). The anterior amygdaloid area is evident. (2) The amygdalo-hippocampal transitional area is visible. The lateral nucleus was not yet identified as a separate entity in the present series, and no indication of a nucleus of the lateral olfactory tract was seen.
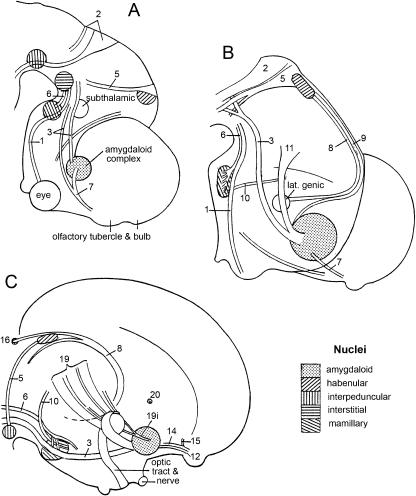
Fig. 13.
A scheme to show the positional changes within the amygdaloid complex of the left side in caudal views. In each drawing lateral is to the left, medial to the right. A–C are based on reconstructions. Colours as in Fig. 3. (A) At stage 17 the cortical and medial nuclei are situated laterally and medially, respectively. (B) At stages 19 and 20 the cortical nucleus has moved ventrally. (C) At stage 23 the medial nucleus retains its medial position. (D) In trimester 3 (modified from Bayer & Altman, 2004) the cortical nucleus is now medial in position and the central nucleus has moved dorsalwards. (E) In the newborn (Kuhlenbeck, 1977). (F) In the adult (reconstructed from Mai et al. 1997). (G) In the Chinese hamster (after Lammers, 1976). (H) In the rat (after Bayer, 1980). ce, central nucleus.
Eminences
The ventricular eminences are not separated by an intereminential sulcus in the embryo shown in Fig. 12, but they can easily be distinguished from each other, the medial having a thinner ventricular layer. The external globus pallidus occupies the medial eminence (Figs 8E and 12A), and the primordium of the putamen occupies the lateral eminence (Fig. 12A).
Connections
Already shortly after stage 23 (in a fetus of 33 mm) the central connections to other regions of the limbic system are elaborate (Fig. 11C), although the arrangement is still different from that of the adult.
Discussion
Terminology and classification
The main criteria are topographical and ontogenetic, and the classifications of relevance to this study are those of Humphrey (1968, 1972), followed here, and Stephan & Andy (1977), who did not cite the 1968 study by Humphrey. The chief basis of the newer classification is that in insectivores and primates ‘the cortical amygdaloid nucleus … is more closely related to the basolateral than the medial amygdaloid complex based on spatial and architectural relationships’ (Stephan & Andy, 1977). These features, however, are not characteristic of very early human development and, when stages are taken into consideration, the present findings accord better with the Humphrey scheme, as can be seen from Table 2 and Fig. 13. Moreover, even the times of neuronal origin in the mouse ‘could not aid … in the subdivision of the amygdala’ (McConnell & Angevine, 1983). Further terminological distinctions and many more subdivisions, particularly in the adult, including the ‘extended amygdala’, are discussed in detail by de Olmos (2004).
Table 2.
Amygdaloid divisions and subdivisions in the human embryo
F, fetal period.
Previous investigations
The only detailed work on the embryonic development of the human amygdaloid complex is the excellent study by Humphrey (1968), which, although staging was not used, is an essential starting point. Unfortunately, however, reconstructions were not prepared, and isolated photomicrographs and drawings are inadequate for the study of complicated and changing relationships. Furthermore, although most of the information concerning the timing and sequence of appearance of the amygdaloid nuclei has been confirmed here, a number of points needed to be clarified and even corrected, for example the distinctions between the two ventricular eminences. Furthermore, the anterior amygdaloid area, said to be converted into the accessory basal nucleus, is present alongside the latter and is found in the fetus (Humphrey, 1972) and in the adult (cross-sections in Mai et al. 1997).
The fetal development of the human amygdaloid complex was included in Macchi's (1951) extensive study of the olfactory telencephalon. Five nuclear masses were distinguished at 110–160 mm, as well as the anterior amygdaloid area, but no nucleus of the lateral olfactory tract was found. A further publication by Humphrey (1972) expanded the study to 216 mm. More recent works on the fetal development continue to appear (e.g. Ulfig et al. 1998, 2003).
In the rhesus monkey, neurogenesis in the amygdaloid nuclear complex was studied by autoradiography by Kordower et al. (1992), who concluded that the first neurons destined for the amygdaloid complex are ‘among the earliest postmitotic neurons in the telencephalon.’ It was also emphasized that the developmental pattern of many telencephalic regions in primates occurs earlier in prenatal life than their homologues in non-primate species, and this is not restricted to the forebrain.
In the insectivorous bat, the development of the amygdaloid complex was examined by Brown (1967). That of the Chinese hamster Cricetulus griseus was studied thoroughly by Lammers (1976), who included autoradiographic analysis and who prepared reconstructions. He investigated the germinative layer and the ventricular eminences in some detail. Subsequently, studies of amygdaloid neurogenesis in the same species (ten Donkelaar et al. 1979) and in the mouse (McConnell & Angevine, 1983) revealed a rostrocaudal nuclear gradient and seemingly provided support for the nuclear classification of Stephan & Andy (1977).
Development
Embryonic period
After the arrival of the amygdaloid primordium at stage 15 (5 weeks), the anterior amygdaloid area, the corticomedial complex and the basolateral complex appear almost simultaneously at stage 16 (51/2 weeks). More precisely their succession follows that order. This early differentiation of the three fundamental parts is said to reflect ‘the ontogenetic expression of their phylogenetic constance’, whereas ‘the subsequent pattern of differentiation of the individual nuclei and their fiber connections parallels, in general, the development of other … diencephalic structures with which fiber connections are established’ (Brown, 1967). Individual nuclei begin to be detectable at stages 17–21 (6–7 weeks), and the central nucleus by stage 23 (8 weeks). Humphrey found the central nucleus at 22.2 mm, but the degree of differentiation of the embryo indicates that that length is too small. The lateral nucleus, recognized by Humphrey at 20.7 mm, is characteristic rather of the fetal period.
Fetal period
The lateral nucleus is present in fetuses of approximately 65 mm (Nikolic and Kostovic). Growth of the amygdaloid complex (Ulfig et al. 1998) continues by migration of cells from the ventricular eminences, which now appear as a single structure. It should be mentioned in passing that ‘ontogeny’ does not begin with ‘fetal development’ (e.g. Brana et al. 1995; Ulfig et al. 2003). Neurons expressing neuropeptide genes have been studied in the fetal striatum (caudate-putamen), but without explicit reference to an eminence (Brana et al. 1995). Differences exist between the fetal lateral eminence and the globus pallidus, whereas a resemblance has been found between the medial eminence and the globus pallidus, in which fetal grafts from the lateral (but not the medial) eminence reconstitute striatal morphology (Pakzaban et al. 1993).
The identification of the nucleus of the lateral olfactory tract ‘is uncertain until relatively late in development’, beginning as a cell cluster at 38.2 mm and becoming recognizable ‘in later fetal life’, as well as in the adult, but ‘its location is highly variable’ (Humphrey, 1972). However, even the very ‘existence as such’ of a nucleus of the lateral olfactory tract (in the adult) has been ‘firmly questioned’ (de Olmos, 2004).
From a phylogenetic viewpoint the corticomedial complex is considered to be the oldest, followed by the basolateral, although both appear ontogenetically at the same stage. The central nucleus appears late in both ontogeny and phylogeny, and the nucleus of the lateral olfactory tract is ‘the latest of all’ (Humphrey, 1972).
A peri-amygdaloid cortex has not yet appeared in the embryonic period, but the primordial plexiform layer was found to be independent of the cortical nucleus. The fetal development of the peri-amygdaloid cortex was studied by Macchi (1951), who also believed it to be independent of the cortical nucleus, and this applies particularly to primates (Stephan & Andy, 1977). Similarly, de Olmos (2004) considered the amygdaloid-piriform area to be a ‘separate anatomical entity from the ventral cortical nucleus’. Sidman & Rakic (1982), however, believed that the cells at the brain surface in the peri-amygdaloid area ‘never become completely separated from the underlying components of the amygdaloid complex’. Many years ago Obersteiner even considered the amygdaloid complex as a thickening of the temporal cortex (Humphrey, 1968).
The ventricular eminences and their derivatives
The ventricular eminences, also termed striatal ridges or elevations, and inaccurately ‘ganglionic’ hillocks (Ganglienhügel), are two in number: medial and lateral (Table 3). The two are separated by an intereminential sulcus, also termed interstitial and incorrectly strio-caudate sulcus.
Table 3.
Comparison between medial and lateral ventricular eminences
| Medial ventricular eminence (Archistriatum) | Lateral ventricular eminence: caudate nucleus and putamen of corpus striatum (Neostriatum) |
|---|---|
| Primordium, stage 14 | Primordium, stage 15 |
| Diencephalic in origin1 | Telencephalic in origin, joins medial eminence in stages 18 and 19 |
| Forms most of the early amygdaloid nuclei | Invaded by cortical amygdaloid nucleus in and after stage 19 |
| Cells migrate to the | Forms neocortical GABA-expressing interneurons (in the mouse) |
| (1) telencephalic cortex, | |
| (2) corpus striatum (Marin et al. 2001, in the mouse) | |
| Scant striatum-like tissue | Striatum-like tissue (striosomes) acetylcholinesterase-positive and dopamine-receptive |
| Short proliferative phase (Brana et al. 1955) | Long proliferative phase (Brana et al. 1955) |
| Gen expression Gbx-2 in mouse embryo (Rubenstein et al. 1994) | Gen expression Dlx-2 in mouse embryo (Rubenstein et al. 1994) |
| Globus pallidus (Paleostriatum) | |
| Appears in stage 18 | |
| Subthalamic in origin | |
| Reaches its final position by ‘intussusception’ (Richter, 1965) | |
H. Spatz (Lammers, 1976).
Two eminences develop, so that it is inappropriate to write of the eminence (e.g. Ulfig, 2002; Ulfig & Chan, 2004). Kahle (1969) and Sidman & Rakic (1982) distinguished rightly between medial and lateral.
Even when the two eminences have joined to form a common floor for the lateral ventricle, each can be clearly distinguished early in the fetal period (37 mm, Richter, 1965, fig. 9f; 46.5 mm, Hochstetter, 1919, plate 7, figure 41, and plate 19). However, it has been assumed incorrectly that both of the combined eminences are telencephalic (Ulfig et al. 2003; Ulfig & Chan, 2004). The correct interpretation, that the medial ventricular eminence is diencephalic in origin, was appreciated already in the 1920s by Spatz (Lammers, 1976). The medial eminence up to stage 15 belongs to neuromere D1 (Müller & O’Rahilly, 1997), moves towards the telencephalic neuromere in stage 16, and is in a telencephalic position in stage 17 (Fig. 2C). Hence, the early origin of the amygdaloid nuclei is clearly diencephalic.
Contrary to the findings of Humphrey (1968), the medial eminence appears first. Hence, during initial development all cells of the amygdaloid complex are derived not ‘from the lateral striatal ridge, the only one present’ (Humphrey, 1968) but from the medial. Nevertheless, the amygdaloid complex is admitted by Humphrey to be (or to become) archistriatum. The claim that the medial eminence develops ‘caudalward from its area of origin near the interventricular foramen’ (Humphrey, 1972) is not in agreement with the studies based on reconstructions by the present authors. In the absence of three-dimensional reconstructions incorrect interpretations can easily arise. For example, what has been identified as ‘primordial neostriatum’ (Humphrey, 1968, 1972, fig. 4B) is actually diencephalic wall and corresponds to a part of the medial eminence.
The derivation of the amygdaloid nuclei from mainly the medial ventricular eminence is confirmed by the observation that a high expression of epidermal growth factor is found in both the medial eminence and the amygdaloid nuclei (Mai & Ashwell, 2004). Moreover, distinctions in gene expression have been investigated in the mouse (Rubenstein et al. 1994).
In agreement with Humphrey (1968), no evidence of cortical infolding or migration from the surface was observed in the present series. The hypothesis of infolding (revived by Molnár & Butler, 2002) is not supported in ontogeny. On the contrary, practically all of the amygdaloid nuclei develop by migration from the germinal layer of the medial ventricular eminence, as attested to also by Lammers (1976) and ten Donkelaar et al. (1979).
Positional changes in and of the amygdaloid complex
Developmental alterations in the spatial orientation of the amygdaloid complex ‘as reflected in phylogeny and ontogeny’ occur in simians (Stephan & Andy, 1977). These alterations, generally referred to as ‘rotation’, are shown here in Figs 6 and 13. They begin already in the embryonic period and continue in the fetal period.
Changes within the amygdaloid complex
A comparison between the embryonic topography (Fig. 6B) and the adult configuration (Fig. 6C) shows that some rearrangement of the nuclei occurs within the amygdaloid complex (Fig. 13). These are attributed to differential growth of the nuclei.
The anterior amygdaloid area, at first caudoventral (stage 17), becomes more rostral (stage 23) and remains so in the adult (photomicrographs in Mai et al. 1997).
The cortical nucleus, which is variable and not clearly delineated, is at first lateral (stage 17, Fig. 13A) and remains so in the rat, then ventral (stage 23) and remains so in the hamster and ‘in mammals with a smooth hemisphere’ (Humphrey, 1972), and finally medial in the human fetus (Fig. 13D), newborn (Fig. 13E) and adult (Fig. 13F);
The medial nucleus, which is more obvious, maintains its position, possibly because of its relationship to the dentate area.
The central nucleus, seen later (stage 23), shifts from a central to a dorsal position in the fetus, newborn and adult.
The basolateral complex maintains a central position.
Changes of the entire amygdaloid complex
These are attributed to increased development of the temporal lobe. Two components are involved. (1) A caudorostral positional change of the amygdaloid complex as a unit occurs during the embryonic period (stages 19–23). The complex moves rostrally (Figs 8 and 10) as the medial eminence appears to be ‘pushed’ rostrally by the enlarging inferior horn of the lateral ventricle. (2) The corpus striatum appears superimposed on the amygdaloid complex early in the fetal period, so that both can be seen in a single coronal section, e.g. at 79 mm (Humphrey, 1972, fig. 13), 155 mm (Feess-Higgins & Larroche, 1987, fig. 17(F)3), and also in the adult (excellent photomicrographs in Mai et al. 1997).
Phylogenetic aspects of amygdaloid rotation were discussed by Humphrey (1972) and Stephan & Andy (1977). A lateral-to-medial rotation (approximately 130–140°) occurs ‘from lower mammals to man without any essential change in the relative position of the individual nuclei’ (Humphrey, 1972, fig. 20).
Neural connections in the limbic system
In Table 1 the tracts are listed in the order of their appearance. In Fig. 11 they are illustrated for stages 17 and 19, and for an early fetus.
The early innervation of the amygdaloid nuclei is by the medial forebrain bundle. At stage 17 ‘the human embryo already has general outlines of the hypothalamic CA [catecholaminergic] cell groups found by the end of gestation and in the adult brain’ (Zecevic & Verney, 1995).
An influence from olfactory fibres is highly unlikely. Most of the axons that leave the olfactory bulb are from the mitral cells. Although their early development is difficult to follow without special techniques, from comparison with rodents it would seem that efferent fibres could arise near the end of the embryonic period (Müller & O’Rahilly, 2004). Hence, the first connections of the amygdaloid complex with the olfactory bulb in stage 17, in the absence of mitral cells, would be afferent via the medial forebrain bundle (Fig. 11A, no. 7). An early olfactory–amygdaloid input (as suggested for the rat by Bayer, 1980) had already been excluded for the human by Humphrey (1968). From stage 19 onwards (Fig. 11B) connections between the olfactory bulb and the corticomedial complex and olfactory tubercle, and between the olfactory tubercle and the amygdaloid complex would still be afferent. The forebrain septum, which is the basal part of the medial telencephalic wall, extends from the olfactory bulb to the commissural plate and hence includes the olfactory tubercle.
The hippocampal primordium grows rostrocaudally and its dentate area is closely related to the medial nucleus already in stage 17 (Fig. 5B).
A connection between the amygdaloid complex and the habenular nucleus is seen in stages 18 and 19 (Fig. 11B, no. 9). The stria terminalis arises towards the end of the embryonic period (Fig. 12C, no. 13) and is already well developed in an early fetus (Fig. 11C). The present authors’ reconstructions of the fibre tracts in Fig. 11(A–C) are confirmed by immunocytochemistry (Verney et al. 1991; Zecevic & Verney, 1995). Configurational differences between the tracts prenatally (Fig. 11A–C) and those of the adult concern the fornix, stria medullaris thalami (no. 8) and stria terminalis (no. 13), the change to the definitive pattern being related to the volumetric expansion of the components of the forebrain. The connections of the amygdaloid nuclei via the medial forebrain bundle (no. 3) with the locus caeruleus, ventral tegmental area and substantia nigra (Fig. 11A) allow noradrenergic and dopaminergic influences, and monoamines are important in the morphogenesis of the brain (Zecevic & Verney, 1995).
Many features that are constituents of, or are associated with, the limbic system appear during the first 7 weeks. For example, the hypothalamus and the medial forebrain bundle (recognizable at stage 15), the hippocampus (stages 15–18), the amygdaloid complex (stage 16), the mesencephalic tegmentum and the mamillary body (stage 17), the septal area (stages 18, 19), the stria terminalis and the nucleus accumbens (stage 19).
References
- Akert K. Limbisches System. In: Drenckhahn D, Zenker W, editors. Benninghoff Anatomie. 15. Vol. 2. München: Urban and Schwarzenberg; 1994. pp. 603–625. [Google Scholar]
- Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. The Human Brain During the Third Trimester. Boca Raton: CRC Press; 2004. [Google Scholar]
- Brana C, Charron G, Aubert D, et al. Ontogeny of the striatal neurons expressing neuropeptide genes in the human fetus and neonate. J Comp Neurol. 1995;360:488–505. doi: 10.1002/cne.903600310. [DOI] [PubMed] [Google Scholar]
- Brown JW. The development of the amygdaloid complex in insectivorous bat embryos. Ala J Med Sci. 1967;4:399–415. [PubMed] [Google Scholar]
- Crosby EC, Humphrey T. Studies of the vertebrate telencephalon. II. The nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J Comp Neurol. 1941;74:309–352. [Google Scholar]
- ten Donkelaar HJ, Lammers GJ, Gribnau AAM. Neurogenesis in the amygdaloid nuclear complex in a rodent (the Chinese hamster) Brain Res. 1979;165:348–353. doi: 10.1016/0006-8993(79)90568-7. [DOI] [PubMed] [Google Scholar]
- Feess-Higgins A, Larroche J-C. Le Développement Du Cerveau Foetal Human. Atlas Anatomique. Paris: INSERM-CNRS, Masson; 1987. [Google Scholar]
- Gaunt PN, Gaunt WA. Three Dimensional Reconstruction in Biology. Tunbridge Wells: Pitman Medical; 1978. [Google Scholar]
- Hochstetter F. Beiträge zur Entwicklungsgeschichte des Menschlichen Gehirns. Vienna: Deuticke; 1919. Teil I. [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132:135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdaloid complex. In: Eleftheriou BE, editor. The Neurobiology of the Amygdala. New York: Plenum; 1972. pp. 21–80. [Google Scholar]
- Kahle W. Die Entwicklung der menschlichen Grosshirnhemisphäre. Schriftenreihe Neurologie. 1969;1:1–116. [PubMed] [Google Scholar]
- Kordower JH, Piecinski P, Rakic P. Neurogenesis of the amygdaloid nuclear complex in the Rhesus monkey. Dev Brain Res. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-o. [DOI] [PubMed] [Google Scholar]
- Kuhlenbeck H. The Central Nervous System of Vertebrates. Pt 1. Vol. 5. Basel: Karger; 1977. pp. 686–859. [Google Scholar]
- Lammers GJ. On the Development of the Strio-Amygdaloid Complex in the Chinese Hamster, Cricetulus griseus. Nijmegen: Krips Repro Meppel; 1976. [Google Scholar]
- Macchi G. The ontogenetic development of the olfactory telencephalon in man. J Comp Neurol. 1951;95:245–305. doi: 10.1002/cne.900950203. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego: Academic Press; 1997. [Google Scholar]
- Mai JK, Ashwell KWS. Fetal development of the central nervous system. In: Paxinos G, Mai JK, editors. The Human Nervous System. Amsterdam: Elsevier; 2004. pp. 49–86. [Google Scholar]
- Marin O, Yaron A, Bagri A, et al. Sorting of striatal and cortical interneurons regulated by semaphorin–neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- McConnell J, Angevine JB. Time of neuron origin in the amygdaloid complex of the mouse. Brain Res. 1983;272:150–156. doi: 10.1016/0006-8993(83)90372-4. [DOI] [PubMed] [Google Scholar]
- Meyer A. Historical Aspects of Cerebral Anatomy. London: Oxford University Press; 1971. [Google Scholar]
- Molnár Z, Butler A. The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays. 2002;24:530–541. doi: 10.1002/bies.10100. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The timing and sequence of appearance of neuromeres and their derivatives in staged human embryos. Acta Anat. 1997;158:83–99. [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. Olfactory structures in staged human embryos. Cells Tissues Organs. 2004;178:93–116. doi: 10.1159/000081720. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen Ch. The Human Central Nervous System. 3. Berlin: Springer; 1988. [Google Scholar]
- O’Rahilly R, Müller F. Developmental Stages in Human Embryos Including a Revision of Streeter's ‘Horizons’ and a Survey of the Carnegie Collection. Washington, DC: Carnegie Institute; 1987. Publication 637. [Google Scholar]
- O’Rahilly R, Müller F. Prenatal ages and stages: measures and errors. Teratology. 2000;61:382–384. doi: 10.1002/(SICI)1096-9926(200005)61:5<382::AID-TERA10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- de Olmos JS. Amygdala. In: Paxinos G, Mai JK, editors. The Human Nervous System. Amsterdam: Elsevier; 2004. pp. 739–868. [Google Scholar]
- Pakzaban P, Deacon TW, Burns LH, Isacson O. Increased proportion of acetylcholinesterase-rich zones and improved morphological integration in host striatum of fetal grafts derived from the lateral but not the medial ganglionic eminence. Exp Brain Res. 1993;97:13–22. doi: 10.1007/BF00228813. [DOI] [PubMed] [Google Scholar]
- Richter E. Die Entwicklung des Globus pallidus und des Corpus subthalamicum. Monogr Gesamtgeb Neurol Psych. 1965;108:1–131. [PubMed] [Google Scholar]
- Rubenstein JLR, Martinez S, Shimamura K, et al. The embryonic vertebrate forebrain: the prosomeric model. Science. 1994;266:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Development of the human central nervous system. In: Sidman RL, Rakic P, Haymaker W, Adams RD, editors. Histology and Histopathology of the Nervous System. Springfield: Thomas; 1982. pp. 3–145. [Google Scholar]
- Stephan H, Andy OJ. Quantitative comparison of the amygdala in insectivores and primates. Acta Anat. 1977;98:130–153. doi: 10.1159/000144789. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Transient architectonic features in the basolateral amygdala of the human fetal brain. Acta Anat. 1998;163:99–112. doi: 10.1159/000046489. [DOI] [PubMed] [Google Scholar]
- Ulfig N. Ganglionic eminence of the human fetal brain- new vistas. Anat Rec. 2002;267:191–195. doi: 10.1002/ar.10104. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann NY Acad Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Chan WY. Expression patterns of PSA-NCAM in the human ganglionic eminence and its vicinity: role of PSA-NCAM in neuronal migration and axonal growth? Cells Tissues Organs. 2004;177:229–236. doi: 10.1159/000080136. [DOI] [PubMed] [Google Scholar]
- Verney C, Zecevic N, Nikolic B, Alvarez C, Berger B. Early evidence of catecholaminergic cell groups in 5- and 6-week-old human embryos using tyrosine hydroxylase and dopamine-β-hydroxylase immunocytochemistry. Neurosci Lett. 1991;131:121–124. doi: 10.1016/0304-3940(91)90351-s. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Verney C. Development of the catecholamine neurons in human embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J Comp Neurol. 1995;351:509–535. doi: 10.1002/cne.903510404. [DOI] [PubMed] [Google Scholar]