Abstract
We present quantitative data on the hindlimb musculature of Pan paniscus, Gorilla gorilla gorilla, Gorilla gorilla graueri, Pongo pygmaeus abelii and Hylobates lar and discuss the findings in relation to the locomotor habits of each. Muscle mass and fascicle length data were obtained for all major hindlimb muscles. Physiological cross-sectional area (PCSA) was estimated. Data were normalized assuming geometric similarity to allow for comparison of animals of different size/species. Muscle mass scaled closely to (body mass)1.0 and fascicle length scaled closely to (body mass)0.3 in most species. However, human hindlimb muscles were heavy and had short fascicles per unit body mass when compared with non-human apes. Gibbon hindlimb anatomy shared some features with human hindlimbs that were not observed in the non-human great apes: limb circumferences tapered from proximal-to-distal, fascicle lengths were short per unit body mass and tendons were relatively long. Non-human great ape hindlimb muscles were, by contrast, characterized by long fascicles arranged in parallel, with little/no tendon of insertion. Such an arrangement of muscle architecture would be useful for locomotion in a three dimensionally complex arboreal environment.
Keywords: architecture, leg, locomotion, morphology, muscle, primate, tendon
Introduction
The evolution of human bipedal walking has fascinated scientists for hundreds of years. While fossils must be our primary source of data, studies of fossil evidence alone are unlikely to identify the functional relationships which underpin the adoption of habitual bipedal gait. A vital supplementary approach is to study the interaction of locomotor morphology and mechanics in living primates. The non-human apes, as our closest living relatives, provide a unique opportunity to investigate those relationships between locomotor form and function which are likely to have existed during the adaptive radiation of the apes and the eventual separation of the lineages leading to common chimpanzees, bonobos and humans.
There are numerous different hypotheses as to the origins of human bipedalism, many of which are based around individual behavioural or physiological features, such as locomotion (Fleagle et al. 1981; Gebo, 1996; Richmond & Strait, 2000), feeding (Jolly, 1970; Rodman & McHenry, 1980; Shipman, 1986), tool use (Fifer, 1987; Knusel, 1992) and temperature regulation (Wheeler, 1991, 1993). However, quantitative data on locomotor behaviour in apes in the wild (e.g. chimpanzee: Doran, 1992a,b; Hunt, 1992, 1994; gorilla: Remis, 1995; Doran, 1997; orang-utan: Cant, 1987; Thorpe & Crompton, 2004; gibbon: Carpenter, 1940; Fleagle, 1974, 1976; Cannon & Leighton, 1994) are fragmented. Field data are often considered in conjunction with data on captive animals (e.g. common chimpanzee: Jenkins, 1972; bonobo: Aerts et al. 2000; D'Août et al. 2002, 2004; Vereecke et al. 2004; gorilla: Isler, 2002, 2005; orang-utan: Tuttle et al. 1978; Stern & Susman, 1981; Payne, 2001; Isler, 2005; gibbon: Ishida et al. 1984; Yamazaki & Ishida, 1984; Isler, 2005; Vereecke et al. 2005). However, captive animals often develop locomotor repertoires that are different to those of their non-captive counterparts (see, e.g. Payne, 2001; Crompton et al. 2003, for orang-utans). In order to improve our understanding of the links between locomotor activity and functional adaptation, we first need to have a better understanding of the locomotor patterns (and postures) performed by living apes and identify both how they are similar to, and differ from, bipedal locomotion in humans. These data should then be considered in conjunction with detailed functionally orientated assessments of anatomy to reveal the functional capacity of the locomotor system.
Locomotor capabilities are determined by a number of different factors including morphology of the bony skeleton and muscle–tendon unit properties. Whole muscle properties are to a large extent determined by the arrangement of the constituent muscle fibres. The proportion of sarcomeres that lie in series to those that lie in parallel is a major determinant of the functional characteristics of a muscle. Muscles with large physiological cross-sectional areas (PCSAs) have a large number of sarcomeres lying in parallel, and this gives such muscles the capacity to generate high force. By contrast, long-fibred muscles have more sarcomeres in series and are able to generate force over a wide range of motion. Such muscles are also able to work at a higher velocity, as the shortening rate of a muscle is a direct function of fascicle length. Thus, of two muscles of equal volume, that with the longest fascicles is optimized for high shortening velocities, whereas that with the largest PCSA is optimized for generating large forces. They will, however, have a similar capacity for power generation as power is directly related to muscle volume (Zajac, 1989, 1992).
Because muscle action is linear, but joint motion rotational, the capacity of a muscle to act on an object (muscle torque) will ultimately depend on how well it transforms linear quantities (force, speed and excursion) into their rotational counterparts. Muscle torque is the product of the muscle force (proportional to PCSA and maximum isometric stress) and muscle moment arm (the shortest perpendicular distance from the instant joint centre of rotation to the line of action of the muscle–tendon unit). Therefore, muscles create different torques depending on their attachment to bone and joint position at the instant of interest, as these two govern moment arm length. Owing to the size and complexity of the data sets, moment arms are not considered in this paper. Instead, ape hindlimb muscle moment arm data are given separately in a companion paper (Payne et al. 2006).
The volume, architecture and geometry of human limb musculature has been well described in the literature (Alexander & Vernon, 1975; Edgerton et al. 1986; Friederich & Brand, 1990; Cutts et al. 1991; Fukunaga et al. 1992; Lieber et al. 1992; Narici et al. 1992; Zajac, 1992). However, although electromyographic studies have been performed on living primates, permitting comparison of patterns of hindlimb muscle activation (Stern & Susman, 1981; Tuttle et al. 1983; Larson & Stern, 1987a,b), and although Thorpe et al. (1999, 2004) have provided information on the limb anatomy of the common chimpanzee, remarkably little information exists concerning the mechanical capabilities of hindlimb muscles in the other extant apes. The aim of this study is to quantify hindlimb muscle architecture in extant apes and discuss the findings in relation to the locomotor habits of each.
Materials and methods
Subject data
The material comprises eight cadavers of adult apes of known age and sex (Table 1). Specifically: one bonobo (Pan paniscus: Pp), two Western lowland gorilla (Gorilla gorilla gorilla: Gj and Gp), one Eastern lowland gorilla (Gorilla gorilla graueri: Gm), three orang-utan (Pongo pygmaeus abelii: Ojf, Ojm and Oam) and one gibbon (Hylobates lar: Haf). All animals were eviscerated during post-mortem examination and then all but one frozen until required for this experiment. The exception was the adult male orang-utan (Oam), which had been preserved in alcohol. Cadavers were obtained from the Anthropological Institute and Museum, Zürich (Ojf, Ojm, Oam Haf and Gp), The North of England Zoological Society (Gj) and The Royal Zoological Society of Antwerp (Pp, Gm). Pre-evisceration body mass of each subject was required for normalization of data; however, this figure had not been recorded in Ojm, Gj or Gp and was therefore estimated using established relationships of limb mass and body mass from the literature (Morbeck & Zihlman, 1988; Zihlman, 2000). Common chimpanzee (Pt) and human (Hs) data were taken directly from Thorpe et al. (1999).
Table 1.
Subject data
| Pt | Pp | Gp | Gj | Gm | Oam | Ojm | Ojf | Haf | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | M | M | M | F | F |
| Age at death (year) | 6 | 29.6 | 35 | 30 | 33 | 30 | 6 | 5 | 16 |
| Mass (kg) | 37 | 64 | 130 | 120 | 120 | 112 | 18.7 | 12.5 | 4.6 |
| Femur (cm) | 29.0 | 28.5 | 39 | 35.7 | 38 | 29 | 19 | 18.6 | 18.3 |
| Tibia (cm) | 24.5 | 27.2 | 32.5 | 32.1 | 43 | 28 | 16.8 | 15.5 | 15.8 |
| Foot length (cm) | – | 28 | 32.5 | 28.7 | 30.5 | 27.2 | 15.6 | 16.8 | 10.2 |
| Cause of death | Peritonitis | CV | CV | CV | CV | CV | CV | Viral | Viral |
Abbreviations: CV indicates death due to cardiovascular problems. Subjects: Pt (Pan troglodytes), Pp (Pan paniscus), Gp (gorilla P), Gj (gorilla J), Gm (gorilla M), Oam (orang-utan adult male), Ojm (orang-utan juvenile male), Ojf (orang-utan juvenile female), Haf (Hylobates lar adult female). Common chimpanzee (Pt) and human (Hs) data are from Thorpe et al. (1999), Thorpe et al. derived the human data (Hs) from several different sources (MRI: Fukunaga et al. 1992; Narici et al. 1992; computer tomography:Cutts et al. 1991 and Cadaveric dissections: Friederich & Brand, 1990) and so are not included in this table.
Measurement of hindlimb muscle dimensions
During dissection of each hindlimb, muscles were removed systematically and measurements of muscle belly mass, muscle belly length and tendon length (not including internal tendon) were recorded. Each muscle was then cut along the line of the tendon to reveal the orientation of its fascicles (where fascicle refers to a bundle of individual muscle fibres and is large enough to be seen by the naked eye). Three separate measurements of muscle fascicle length (to the nearest millimetre) were recorded from different sections of the muscle belly and a mean value was calculated. The external tendon was removed and muscle belly mass was recorded (to the nearest 0.1 g) using a set of electronic scales. Muscle PCSA was estimated as follows:
| (1) |
where m is muscle belly mass in grams, ρ is muscle density (1.06 g cm−3, Mendez & Keys, 1960) and l is muscle fascicle length. PCSA can be directly related to muscle force generation capacity. However, the proportion of muscle force transmitted to the tendon depends on the angle of pennation of the fibres (such that PCSA = m/ρl × cosθ, where θ is the angle of pennation of the fibres with respect to the line of pull of the muscle). Pennation angle was not included in our estimates of PCSA. Thorpe et al. (1999) measured pennation angles in the hindlimb muscles of common chimpanzes and found all angles to be close to 20°. The cosine of 20 is close to one and would thus have little effect on our estimations of PCSA. Further, muscles are complex three-dimensional structures and pennation angle is known to change with muscle contraction, an effect that we were unable to address within the scope of this paper.
There are obvious difficulties associated with comparing muscle dimensions in different species of ape and in individuals of varying age and size (age range of subjects: 5–35 years, body mass range of subjects: 5–160 kg). The data thus require normalization. Although several alternative methods for normalization exist, this is not the place to discuss the merits or otherwise of each. We chose to use a technique based on geometric similarity, testing the vailidity of this assumption in each case. In geometrically similar animals, mass should scale directly to body mass, lengths to (body mass)1/3 and areas to (body mass)2/3 (Alexander et al. 1981). We plotted muscle mass against body mass and muscle fascicle length against body mass for proximal and distal limb muscle groups. Power trend lines were fitted to the data to determine whether the above geometric relationships held true for the subjects studied.
Raw data were normalized by dividing muscle mass by body mass, mean fascicle length by (body mass)1/3 and PCSA by (body mass)2/3. In order that comparisons could be made between functional muscle groups, muscles were grouped as shown in the legend to Table 3. Masses and PCSAs were calculated as group totals, which are the sum of the constituent muscles in one leg. As some muscles form a greater percentage of the total mass of a group than do others, muscle group fascicle length was calculated as a weighted harmonic mean. This was done by weighting each individual muscle's fascicle length by the mass of the muscle. Hence:
| (2) |
where L is the fascicle length for a group of muscles of which the jth member has a mass mj and fascicles of length lj (Alexander et al. 1981). Fascicle length has been shown to vary according to joint angle (Felder et al. 2005). Thus, we measured fascicle lengths with the muscles removed from the skeleton. No attempt was made to normalize the data using sarcomere length as this was deemed to be a level of accuracy beyond that which we were able to achieve with our small and varied population. Functional muscle groups often contain a mixture of uniarticular, biarticular and multiarticular muscles. Hence, the volume of muscle and mean fascicle length will vary according to the joint being crossed. To avoid confusion, the joint in question is specified each time a functional muscle group is mentioned, e.g. hamstrings (hip) does not include the short head of biceps femoris.
Table 3.
Normalized muscle data presented in functional groups
| Subject | Hs | Pt | Pp | Gp | Gj | Gm | Ojm | Ojf | Haf |
|---|---|---|---|---|---|---|---|---|---|
| Muscle mass/(body mass) | |||||||||
| Gluteals | 27.03 | 17.24 | 13.11 | – | 16.34 | 11.83 | 9.37 | – | 11.87 |
| Hamstrings (hip) | 14.86 | 10.05 | 8.23 | – | 8.25 | 8.74 | 14.81 | – | 5.02 |
| Adductors | 15.14 | 11.38 | 13.82 | – | 10.09 | 12.61 | 13.36 | – | 4.59 |
| Hip flexors | – | 2.51 | 4.70 | – | 5.24 | 6.1 | 5.7 | – | 5.5 |
| Quadriceps (knee) | 28.38 | 14.81 | 13.37 | 11.53 | 9.55 | 6.46 | 6.87 | 7.71 | 7.72 |
| Hamstrings (knee) | 14.86 | 12.38 | 10.73 | 9.93 | 9.53 | 8.73 | 15.11 | 12.59 | 7.17 |
| Triceps (knee) | – | 4.24 | 3.85 | 3.28 | 2.12 | 1.54 | 3.03 | 1.72 | 2.48 |
| Triceps (ankle) | 13.51 | 7.70 | 7.30 | 5.82 | 4.00 | 3.16 | 5.33 | 3.30 | 4.13 |
| Pedal digital flexors | 1.49 | 3.30 | 2.54 | 2.09 | 1.66 | 1.42 | 5.42 | 4.57 | 2.22 |
| Dorsiflexors | 4.05 | 2.46 | 2.45 | 1.96 | 2.09 | 1.76 | 3.18 | 3.26 | 2.07 |
| Fascicle length/(body mass)1/3 | |||||||||
| Gluteals | 2.38 | 2.73 | 2.49 | – | 3.24 | 2.49 | 2.30 | – | 3.15 |
| Hamstrings (hip) | 2.31 | 5.99 | 4.78 | – | 5.5 | 4.53 | 4.95 | – | 5.32 |
| Adductors | 2.57 | 6.30 | 4.65 | – | 4.48 | 3.47 | 5.19 | – | 5.11 |
| Hip flexors | – | 2.36 | 2.21 | – | 2.40 | 1.30 | 2.87 | – | 2.30 |
| Quadriceps (knee) | 1.60 | 2.85 | 2.07 | 2.96 | 2.72 | 4.00 | 3.00 | 1.49 | 2.12 |
| Hamstrings (knee) | 2.31 | 3.65 | 3.19 | 3.53 | 3.71 | 3.59 | 4.09 | 3.38 | 2.86 |
| Triceps (knee) | – | 2.42 | 2.40 | 2.55 | 1.88 | 1.75 | 3.11 | 2.69 | 1.67 |
| Triceps (ankle) | 0.67 | 2.01 | 1.87 | 2.01 | 1.53 | 1.68 | 2.98 | 2.56 | 1.56 |
| Pedal digital flexors | 0.81 | 1.81 | 1.87 | 2.33 | 1.58 | 1.58 | 2.39 | 2.42 | 1.57 |
| Dorsiflexors | 0.83 | 3.10 | 2.31 | 2.17 | 1.83 | 1.34 | 2.84 | 2.87 | 1.64 |
| PCSA/(body mass)2/3 | |||||||||
| Gluteals | 10.72 | 6.01 | 4.96 | – | 5.09 | 5.26 | 3.80 | – | 3.43 |
| Hamstrings (hip) | 6.08 | 1.59 | 1.63 | – | 1.41 | 2.14 | 2.79 | – | 0.82 |
| Adductors | 5.56 | 1.72 | 2.80 | – | 2.12 | 4.01 | 2.41 | – | 0.82 |
| Hip flexors | – | 1.02 | 0.80 | – | 2.07 | 0.63 | 0.56 | – | 0.96 |
| Quadriceps (knee) | 16.80 | 5.05 | 6.08 | 3.67 | 3.31 | 2.31 | 2.13 | 4.91 | 3.36 |
| Hamstrings (knee) | 6.08 | 3.23 | 3.18 | 2.66 | 2.43 | 2.69 | 3.46 | 3.53 | 2.29 |
| Triceps (knee) | – | 1.67 | 1.51 | 1.21 | 1.07 | 0.97 | 0.91 | 0.60 | 1.39 |
| Triceps (ankle) | 19.14 | 3.65 | 3.68 | 2.73 | 2.48 | 2.08 | 2.03 | 1.22 | 2.46 |
| Pedal digital flexors | 1.73 | 1.73 | 1.28 | 0.85 | 2.40 | 0.99 | 2.11 | 1.80 | 1.32 |
| Dorsiflexors | 4.59 | 0.76 | 1.00 | 0.85 | 1.08 | 1.46 | 1.06 | 1.09 | 1.14 |
Data have been normalized according to geometric principles using the ratios shown. For a more detailed explanation see Methods. Muscles have been grouped according to function and joint crossed. This is because, for example, gracilis is part of the adductor group at the hip but is part of the hamstrings group (i.e. a flexor) at the knee. M. ischiofemoralis is measured as part of biceps femoris caput longum in orang-utans but as part of gluteus maximus in the African apes and gibbon (Sigmon & Farslow, 1986). Muscle groups: Gluteals (gluteus maximus, gluteus medius, gluteus minimus, scansorius), Adductors (adductor magnus, adductor longus, adductor brevis, gracilis), Hip flexors (rectus femoris and iliacus), Hamstrings at hip (long head of biceps femoris, semitendinosus, semimembranosus), Quadriceps at knee (rectus femoris, vastus medius/intermedius/lateralis), Hamstrings at knee (long and short heads of biceps femoris, semitendinosus, semimembranosus and gracilis), Triceps at knee (gastrocnemius lateralis, gastrocnemius medialis), Triceps at ankle (gastrocnemius lateralis, gastrocnemius medialis, soleus, plantaris), Pedal digital flexors (flexor digitorum tibialis, flexor digitorum fibularis), Dorsiflexors (tibialis anterior, extensor digitorum longus, extensor hallucis longus). Muscles crossing the hip joint muscles were partly missing in Ojf and Gp and were not included here. Hip flexor data and data on individual muscles of the triceps surae muscle group were not published by Thorpe et al. (1999) for Hs. PCSA = physiological cross-sectional area.
Results
Hindlimb muscle dimensions
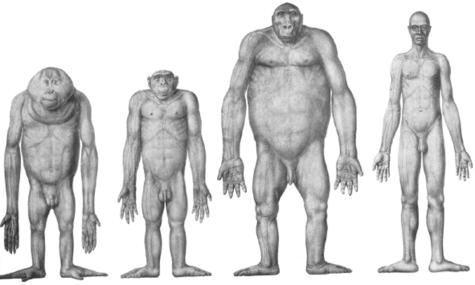
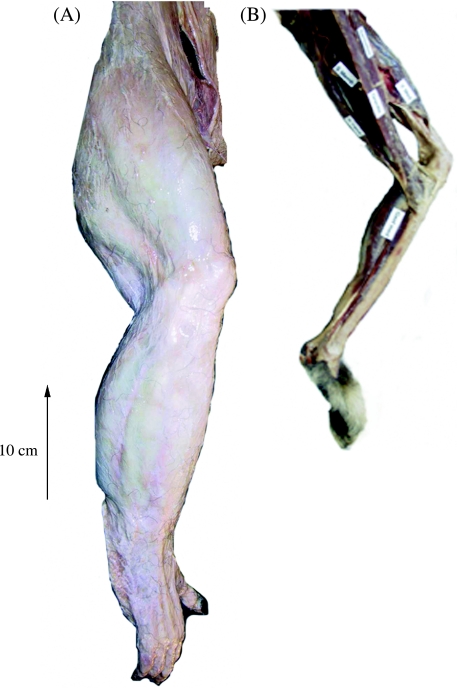
Raw data on the mass and mean fascicle length of individual muscles are provided in Table 2(A,B). Detailed descriptions of hindlimb anatomy have been published for all extant apes (bonobo: Miller, 1952; gorilla: Preuschoft, 1962; orang-utan: Sonntag, 1924; Sigmon, 1974; gibbon: Bisschoff, 1870; Kohlbrügge, 1890/1891). However, some comparative differences, probably related to locomotor function, were observed. For example, distribution of muscle mass along the limb was similar in humans and gibbons (i.e. muscle bulk was located proximally and there was tapering of limb circumference at both the knee and the ankle), but different in the African apes and orang-utans (i.e. little variation in limb circumference from hip to ankle, see Figs 1 and 2).
Table 2A.
Raw muscle data for the bonobo and gorillas (Pp, Gm, Gj and Gp)
| Pp | Gm | Gj | Gp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Mass (g) | FL (cm) | PCSA (cm2) | Mass (g) | FL (cm) | PCSA (cm2) | Mass (g) | FL (cm) | PCSA (cm2) | Mass (g) | FL (cm) | PCSA (cm2) |
| Gluteus maximus | 371.1 | 11 | 31.7 | 747.7 | 14 | 59.2 | 682 | 16 | 40.2 | 439 | 17 | 24.4 |
| Gluteus medius | 402.7 | 11.1 | 34.1 | 495.2 | 14.1 | 38.9 | 1087 | 15.4 | 66.6 | – | – | – |
| Gluteus minimus | 20.6 | 5.2 | 3.8 | 176.7 | 6.5 | 29.9 | 192 | 10.7 | 17 | – | – | – |
| Scansorius | 44.6 | 4.4 | 9.7 | – | 5.5 | – | – | – | – | – | – | – |
| Tensor fascia lata | – | – | – | – | 26.1 | – | 358 | 13.3 | 25.4 | – | – | – |
| Adductor magnus | 582.7 | 20.6 | 26.6 | 1092.4 | 17.5 | 68.9 | 781.8 | 25 | 29.5 | 938 | 24.5 | 36.1 |
| Adductor longus | 43.8 | 13.9 | 3 | 68 | 13.2 | 5.7 | 69.2 | 13.8 | 4.7 | – | – | – |
| Adductor brevis | 111.5 | 10.4 | 10.1 | 85.4 | 9.3 | 10.2 | 158.8 | 13 | 11.5 | – | – | – |
| Pectineus | 24.4 | 7.3 | 3.1 | 36.9 | 11.8 | 3.5 | 29.1 | 11.3 | 2.4 | – | – | – |
| Iliacus | 191.4 | 9.3 | 19.4 | 587.5 | 5.7 | 114.2 | 497 | 11.3 | 41.7 | – | – | – |
| Quadratus femoris | 7.1 | 4.5 | 1.5 | 10.7 | 5.7 | 2.1 | – | – | – | – | – | – |
| Obturator externus | 41.2 | 4.5 | 8.6 | 103.9 | 10.2 | 11.3 | 101.8 | 10.5 | 9.1 | – | – | – |
| Rectus femoris | 109.3 | 8.1 | 12.8 | 145.7 | 10.5 | 15.3 | 131.4 | 14.4 | 8.6 | 202.2 | 15 | 12.7 |
| Vastus | 746.1 | 8.3 | 84.5 | 630 | 24.7 | 40.9 | 1015 | 13.3 | 72 | 1296.2 | 15 | 81.5 |
| Long head biceps | 140.1 | 19.5 | 6.8 | 223.3 | 12.7 | 19.5 | 205.2 | 23.3 | 8.3 | 182.8 | 19.5 | 8.8 |
| Short head biceps | 59.3 | 10 | 5.6 | 81.6 | 34.5 | 2.6 | 100.8 | 20.6 | 4.6 | 160.3 | 20 | 7.6 |
| Gracilis | 146.4 | 27.3 | 5.1 | 267 | 23 | 12.8 | 201.4 | 32 | 5.9 | 205 | 36 | 5.4 |
| Semimembranosus | 100.7 | 18.2 | 5.2 | 179.6 | 18.5 | 10.7 | 202.1 | 20 | 9.5 | 228 | 28 | 7.7 |
| Semitendinosus | 139.6 | 14.7 | 9 | 378.7 | 46.8 | 9 | 381.1 | 34 | 10.6 | 293 | 21.3 | 13 |
| Gastrocnemius lateralis | 105.2 | 9.5 | 10.4 | 58.3 | 12.2 | 5.3 | 104 | 8.7 | 11.3 | 183.6 | 15.5 | 11.2 |
| Gastrocnemius medialis | 141.5 | 9.7 | 13.8 | 126.2 | 7.6 | 18.4 | 150.2 | 9.7 | 14.7 | 243.1 | 11.5 | 19.9 |
| Soleus | 220.2 | 6 | 34.6 | 194.2 | 8 | 27 | 225.3 | 6.2 | 34.3 | 330.3 | 8 | 39 |
| Flexor tibialis | 121.3 | 8 | 14.3 | 67 | 10.1 | 7.3 | 132.7 | 7.1 | 17.6 | 181.5 | 13 | 13.2 |
| Flexor fibularis | 41.5 | 6.3 | 6.2 | 103.9 | 6.8 | 16.8 | 66.1 | 9.7 | 6.4 | 89.8 | 9.9 | 8.5 |
| Peroneus longus | 70.6 | 5.4 | 12.3 | 71.9 | 6.8 | 11.7 | 128.2 | 6.7 | 18.1 | 81.2 | 9.3 | 8.3 |
| Peroneus brevis | 31.4 | 5.4 | 5.5 | – | 12 | – | – | – | – | 52.7 | 10.3 | 4.9 |
| Tibialis anterior | 101.2 | 9.5 | 10.1 | 128.2 | 5.6 | 25.5 | 165.6 | 8.6 | 18.2 | 159.7 | 9.9 | 15.3 |
| Tibialis posterior | 79.3 | 4.4 | 17 | 58.3 | 11.4 | 5.7 | 114.9 | 4.6 | 23.6 | 149.5 | 5.5 | 25.6 |
| Extensor digitorum longus | 43.2 | 9 | 4.5 | 63.1 | 10.5 | 6.7 | 68.1 | 10.7 | 6 | 64.9 | 13 | 4.7 |
| Extensor hallucis longus | 12.1 | 8.3 | 1.4 | 20.4 | 6.6 | 3.4 | 17.6 | 8.2 | 2 | 30 | 15 | 1.9 |
| Flexor digitorum brevis | 10.9 | 2.3 | 0.2 | 10 | 5.5 | 1.8 | – | – | – | 45.5 | 8 | 5.4 |
| Flexor hallucis brevis | 11.5 | 2.0 | 0.2 | 8 | 2.4 | 3.2 | – | – | – | 23.6 | 14 | 1.6 |
| Sartorius | 47.3 | 37 | 1.2 | 58 | 30 | 1.8 | 71.8 | 32 | 2.1 | 293 | 21 | 13.2 |
| Popliteus | 32.2 | 3.1 | 9.7 | 28 | 5 | 5.4 | 72.4 | 12.4 | 5.5 | 84.4 | 8 | 9.9 |
| Obturator internus | 23.0 | 4.5 | 0.2 | – | – | – | – | – | – | – | – | – |
| Psoas Major | 117.2 | 22.8 | 0.2 | – | – | – | – | – | – | – | – | – |
| Psoas Minor | 14.4 | 2.8 | 0.2 | – | – | – | – | – | – | – | – | – |
| Plantaris | 8.7 | 1.8 | 0.2 | – | – | – | – | – | – | – | – | – |
For a variety of reasons, we were not able to measure all muscles in all subjects, in such cases we have used a dash.
FL = fascicle length; PCSA = physiological cross-sectional area.
Table 2B.
Raw muscle data for orang-utans and gibbon (Ojm, Ojf and Haf)
| Ojm | Ojf | Haf | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Mass (g) | FL (cm) | PCSA (cm2) | Mass (g) | FL (cm) | PCSA (cm2) | Mass (g) | FL (cm) | PCSA (cm2) |
| Gluteus maximus | 60.2 | 7.4 | 7.7 | – | – | – | 30.8 | 8.3 | 3.5 |
| Gluteus medius | 81.8 | 5.7 | 13.5 | – | – | – | 20.3 | 4.1 | 4.7 |
| Gluteus minimus | 4.6 | 5.8 | 0.75 | – | – | – | 1.8 | 2.1 | 0.8 |
| Scansorius | 18.3 | 5.7 | 3 | – | – | – | 1.7 | 2.5 | 0.6 |
| Tensor fascia lata | – | – | – | – | – | – | – | – | – |
| Adductor magnus | 150.4 | 14.4 | 9.9 | – | – | – | 12.5 | 11 | 1.1 |
| Adductor longus | 24.6 | 7.6 | 3.1 | – | – | – | – | – | – |
| Adductor brevis | – | – | – | – | – | – | 3.4 | 3.5 | 0.9 |
| Pectineus | 9.8 | 7.1 | 1.3 | – | – | – | 1.7 | 4 | 0.4 |
| Iliacus | 67.3 | 7.1 | 8.9 | – | – | – | 15.4 | 4 | 3.6 |
| Quadratus femoris | 12 | – | – | – | – | – | 1.5 | 2.7 | 0.5 |
| Obturator externus | 15.7 | 3.8 | 3.9 | – | – | – | 3.3 | 2.4 | 1.3 |
| Rectus femoris | 38.5 | 9.2 | 3.9 | 26.4 | 4.2 | 5.9 | 10 | 3.6 | 2.7 |
| Vastus | 89.9 | 7.7 | 11 | 70 | 3.2 | 20.6 | 25.5 | 3.6 | 6.7 |
| Long head biceps | 89.7 | 11.4 | 7.4 | 54.4 | 9.5 | 5.4 | 4.7 | 8.3 | 0.5 |
| Short head biceps | 23.9 | 10.4 | 2.2 | 10.1 | 6.8 | 0 | 3.7 | 6.9 | 0.5 |
| Gracilis | 74.9 | 18.1 | 3.9 | 43 | 8.6 | 4.7 | 5.2 | 16.3 | 0.3 |
| Semimembranosus | 60.4 | 16.1 | 3.5 | 26.3 | 12.3 | 2 | 4.5 | 9.8 | 0.4 |
| Semitendinosus | 51.9 | 10.5 | 4.7 | 45.1 | 6.1 | 7 | 8.7 | 7.2 | 1.1 |
| Gastrocnemius lateralis | 20.5 | 7.8 | 2.5 | 8 | 6.5 | 1.2 | 2.7 | 5 | 0.5 |
| Gastrocnemius medialis | 36.2 | 8.8 | 3.9 | 13.5 | 6 | 2.1 | 8.7 | 2.5 | 3.4 |
| Soleus | 43 | 5.2 | 7.8 | 19.7 | 5.6 | 3.3 | 7.6 | 2.4 | 3 |
| Flexor tibialis | 66.2 | 9 | 6.9 | 34.7 | 6.7 | 4.9 | 7.8 | 2.7 | 2.8 |
| Flexor fibularis | 35.2 | 4.2 | 7.9 | 22.4 | 4.4 | 4.8 | 2.4 | 2.6 | 0.9 |
| Peroneus longus | 17.6 | 4 | 4.2 | 7.7 | 3.9 | 1.9 | – | – | – |
| Peroneus brevis | 10 | 4 | 2.4 | 5.2 | 3.3 | 1.5 | 3.9 | 2.1 | 1.8 |
| Tibialis anterior | 35.2 | 6.7 | 5 | 23.9 | 6.5 | 3.5 | 4.7 | 2.6 | 1.7 |
| Tibialis posterior | 15 | 2.1 | 6.7 | 7.1 | 1.8 | 3.7 | 3 | 1.8 | 1.6 |
| Extensor digitorum longus | 21 | 10 | 2 | 14.2 | 7.5 | 1.8 | 3 | 2.5 | 1.1 |
| Extensor hallucis longus | 3.3 | 8 | 0.4 | 2.7 | 4.5 | 0.6 | 1.8 | 4.5 | 0.4 |
| Flexor digitorum brevis | 5.5 | 3.8 | 1.4 | – | – | – | – | – | – |
| Flexor hallucis brevis | – | – | – | – | – | – | 1.3 | 2.6 | 0.5 |
| Sartorius | 17.1 | 12.5 | 1.3 | M | M | M | 10.4 | 20.5 | 0.5 |
| Popliteus | 13.0 | 5.2 | 2.4 | – | – | – | 1.5 | 2.3 | 0.6 |
| Plantaris | – | – | – | – | – | – | 3.1 | 4.5 | 0.6 |
For a variety of reasons, we were not able to measure all muscles in all subjects, in such cases we have used a dash. If the muscle was missing we used the letter M. Raw data are not provided for Oam as muscle dimensions varied widely from other orang-utan subjects, probably due to preservation methods; see Discussion.
FL = fascicle length; PCSA = physiological cross-sectional area.
Fig. 1.
Comparative limb proportions in hominoids [Figure taken from Schultz AH (1969) The Life of Primates, London: Weidenfield and Nicholson. All attempts at tracing the copyright holder of the figure were unsuccessful].
Fig. 2.
Photographic images of the hindlimb in (A) the bonobo (Pp) and (B) the gibbon (Haf) hindlimb. The dermis has not been removed from the bonobo hindlimb.
Hindlimb muscle anatomy followed the same basic pattern in the apes. However, bonobo (Pp) gluteal anatomy showed some similarities to that of the orang-utans (Oam, Ojm, Ojf) that were not found in the common chimpanzee (Pt). In both species, there was a separate scansorius muscle, almost complete separation of the superior and distal portions of m. gluteus maximus, and a comparatively weak m. tensor fascia lata. In addition, a small anomalous muscle was observed in one of the gorilla cadavers (Gp). It originated from the posterodistal aspect of the tibial shaft and inserted onto the calcaneus, deep to the insertion of the Achilles tendon.
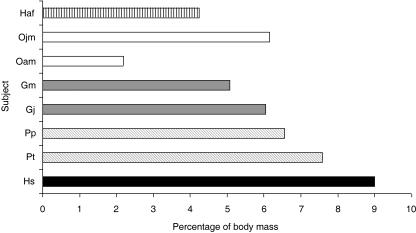
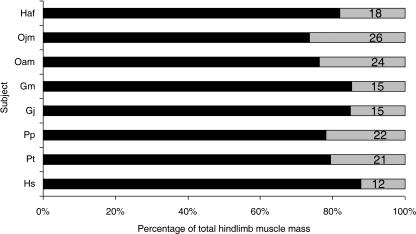
Total hindlimb muscle mass (not including intrinsic hip muscles or intrinsic pedal muscles) as a proportion of total body mass varied within and between species; it was 9% in Hs; 8% in Pt; 7% in Pp; 6% in Gj and Ojm; 5% in Gm; 4% in Haf; and 2% in Oam (see Fig. 3). Differences were also seen in the distribution of muscle volume within the hindlimb (Fig. 4). Humans (Hs) had the lightest distal limb muscles compared with total hindlimb muscle volume (12% of total), followed by the gorillas (both Gm and Gj, 15%), gibbon (18%), common chimpanzee (21%) and bonobo (22%). The adult and juvenile male orang-utans (Oam and Ojm) had relatively the heaviest distal limb muscles (24 and 26%, respectively).
Fig. 3.
Hindlimb muscle mass as a percentage of body mass. Subject name abbreviations are as detailed in Table 1. Total hindlimb muscle mass does not include either small intrinsic hip rotator muscles (piriformis, quadratus femoris, obturator externus, obturator internus, piriformis) or intrinsic pedal muscles. Subjects have been colour coded for ease of comparison: gibbon (black horizontal stripes), orang-utan (white), gorilla (dark grey), chimpanzee (diagonal stripes), human (black). Subjects Gm and Ojf have not been included in this comparison as muscles crossing the hip joint were incomplete in these subjects.
Fig. 4.
Distribution of muscle mass between the proximal and distal hindlimb. Subject name abbreviations are as detailed in Table 1. Percentage of proximal limb muscle mass is depicted in grey and percentage of distal limb muscle mass is depicted in black. Total hindlimb muscle mass does not include the small intrinsic hip rotator muscles (piriformis, quadratus femoris, obturator externus, obturator internus, piriformis) or intrinsic pedal muscles.
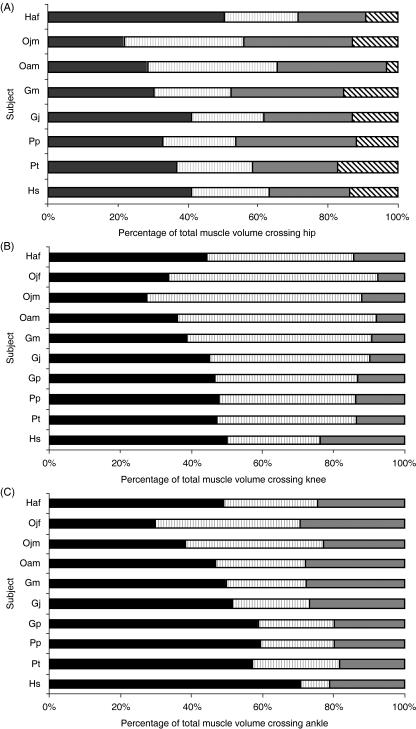
Distribution of muscle volume between the functional muscle groups crossing the hip, knee and ankle joints (Fig. 5) varied both within and between species. The gibbon (Haf) had the highest percentage of total extensor muscle volume crossing the hip joint (71%); hip joint extensor volume ranged from 52 to 62% in humans and the other non-human apes. Knee joint musculature was evenly distributed between flexors and extensors in humans, chimpanzees, gorillas (Gj and Gp) and the gibbon (extensor volume ranged from 44 to 50% of total), but was biased towards flexion in gorilla Gm and the orang-utans (extensor volume ranged from 27 to 38% of total). In humans, the triceps (knee) and hamstrings (knee) contributed almost equally to knee joint flexor volume; this was in direct contrast to the non-human apes where triceps (knee) contributed relatively little. In most subjects, the triceps muscle (ankle) contributed to approximately 60% of total muscle volume crossing the ankle (Fig. 5C). The juvenile orang-utans (Ojm and Ojf) were, however, unique in having an even distribution of muscle volume between triceps (ankle) and the pedal digital flexor muscles.
Fig. 5.
Contribution of functional muscle groups to total muscle volume crossing (A) hip, (B) knee and (C) ankle joints. Muscle groups are coloured coded as follows: (A) gluteals (black), hamstrings (grey vertical stripe), adductors (grey), hip flexors (black diagonal stripe); (B) rectus femoris and vastus (black), hamstrings (grey vertical stripe) and triceps (grey); (C) triceps (black), digital flexors (grey vertical stripe), dorsiflexors (grey). Ojf and Gp did not have a full set of hip joint musculature so they are not included in hip joint analysis. Data were not available for rectus femoris in humans. Instead, proportions were estimated by assuming that human rectus femoris represents the same proportion of the quadriceps muscle group as in the common chimpanzee (17%).
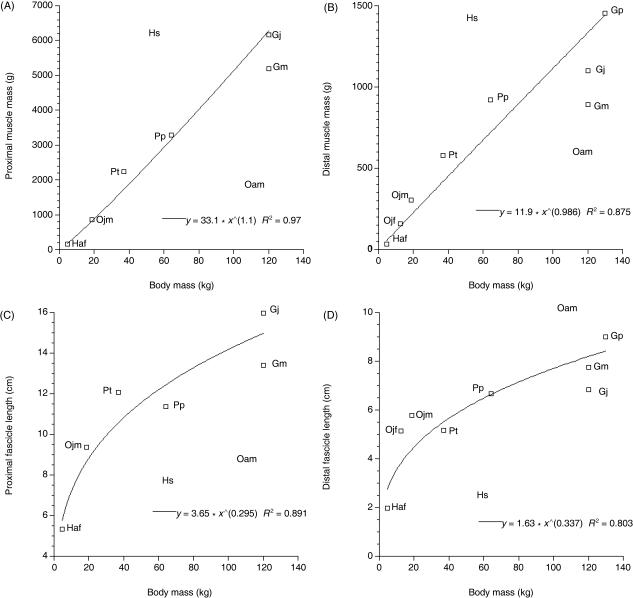
Total muscle mass and mean fascicle length were calculated for proximal and distal limb muscle groups and the data were plotted against body mass (Fig. 6). Power trend lines were fitted to the data. Total proximal limb muscle mass scaled to (body mass)1.1 and total distal limb muscle mass scaled to (body mass)0.99. Mean fascicle length scaled to (body mass)0.30 in the proximal limb and to (body mass)0.34 in the distal limb. The R2 values were significant (P = 0.05) for all correlations. Human and adult orang-utan data were not included in this analysis as their muscle masses and fascicle lengths were very different to those observed in the other subjects. Their position on the plots was, however, marked via the abbreviations Hs and Oam to enable comparison. Humans had heavy muscles per unit body mass and the adult orang-utan (Oam) had light muscles per unit body mass. Humans, the gibbon and the adult orang-utan had short fascicle lengths per unit body mass as compared with the other subjects (Oam; proximal limb muscles only). Differences in adult orang-utan muscle dimensions (see Fig. 3; hindlimb muscle mass represents only 2% of total body mass compared with 6% in Ojm) are probably due to differences in preservation method (i.e. in alcohol rather than fresh frozen). Ward & Lieber (2005) have shown that preservative concentration and muscle tissue rehydration time can effect muscle volume calculations. However, they did not investigate the effect of period of preservation on muscle volume, which may be important in this particular case as the adult orang-utan originated from the Adolph Shultz primate collection (Anthropologisches Institut und Museum, Universität Zürich-Irchel), which was initiated over 30 years ago. For this reason, raw data on mass and fascicle length are not provided for Oam muscles. However, information on the distribution of muscle volume through the hindlimb is still provided for this subject as any effects of preservation on muscle volume distribution are unlikely to have altered these relationships (see Table 4, where distribution of muscle volume in the proximal and distal hindlimb is similar in Oam and Ojm; 24 and 26% of total hindlimb muscle volume was found in the distal limb, respectively).
Fig. 6.
Plots to show the relationship between muscle mass and body mass in proximal (A) and distal (B) limb muscle groups and mean fascicle length and body mass in proximal (C) and distal (D) limb muscle groups. Power trend lines have been fitted to the data and their equations are provided on the plots. All R2 values were significant (P = 0.05). Mean proximal and distal muscle group fascicle lengths were calculated as a weighted harmonic mean (see Methods). Human (Hs) and adult orang-utan (Oam) data were not included in the analysis. However, their position is marked on each plot by name for reference.
Table 4.
Ratio of muscle belly length to total muscle tendon unit length
| Pp | Gp | Gj | Gm | Oam | Ojf | Ojm | Haf | |
|---|---|---|---|---|---|---|---|---|
| Gluteals | 0.92 | – | 1.00 | 0.85 | 0.92 | – | 0.88 | 0.78 |
| Adductors | 0.80 | 0.94 | 0.84 | 0.95 | 0.81 | – | 0.94 | 0.78 |
| Hamstrings | 0.81 | 0.87 | 0.88 | 0.85 | 0.83 | 0.96 | 0.93 | 0.78 |
| Quadriceps | 0.73 | 1.00 | 1.00 | 0.94 | 0.90 | 0.90 | 0.80 | 0.74 |
| Triceps | 0.93 | 0.92 | 1.00 | 1.00 | 0.82 | 0.70 | 0.93 | 0.64 |
| Pedal digital flexors | 0.61 | 0.60 | 0.62 | 0.61 | 0.70 | 0.62 | 0.60 | 0.60 |
| Dorsiflexors | 0.59 | 0.71 | 0.81 | 0.56 | 0.71 | 0.65 | 0.70 | 0.74 |
| Mean | 0.77 | 0.84 | 0.88 | 0.82 | 0.81 | 0.77 | 0.83 | 0.72 |
Ratio was calculated by dividing muscle belly length by total muscle tendon unit length (from origin to insertion). Ratios for individual muscles were averaged to give ratio for the functional group.
Normalized mass, fascicle length and PCSA data for the functional muscle groups of the hip, knee and ankle are provided in Table 3. When normalized, total hindlimb muscle mass was greatest in humans (119 g kg−1 body mass) and smallest in the gibbon (Haf, 53 g kg−1 body mass). The gluteals, adductors, hamstrings (hip), quadriceps (knee), triceps (ankle) and dorsiflexors were all heaviest in humans. The mass of hamstrings (hip and knee) was remarkably similar in humans and orang-utan Ojm. The pedal digital flexor muscles were heaviest in orang-utans Ojm and Ojf. The common chimpanzee and bonobo had the heaviest knee and ankle extensor [quadriceps (knee) and triceps (ankle)] muscles of the non-human apes (they were approximately double the mass of those muscles in the other non-human apes, but half the mass of those muscles in humans). Fascicle lengths were longer in thigh muscles (particularly hamstrings and adductors) than calf muscles. This proximal-to-distal differentiation was greatest in humans. PCSA varied both within and between species. Humans had the largest PCSAs for all muscles except the pedal digital flexors. Of the non-human apes, quadriceps (knee) PCSA was largest in the common chimpanzee and bonobo (due to relatively large muscle volumes) and orang-utan Ojf (due to relatively short fascicles). Total hindlimb normalized muscle PCSA was by far the largest in humans (70.7), followed by the chimpanzees (common chimpanzee, 26.4; bonobo, 26.9).
Table 4 presents the ratio of muscle belly length to total muscle length in non-human primates (these data were not available for humans or common chimpanzees). The ratios ranged from 1.0 (no discernible tendon, e.g. Gj gluteals, quadriceps and triceps) to 0.56 (tendon represents almost half of muscle–tendon unit length, Gm dorsiflexors). Ratios were highest in proximal limb muscles (gluteals, adductors, quadriceps and hamstrings) and lowest in distal limb muscles (pedal digital flexors and dorsiflexors). The exception was the distal limb muscle m. triceps surae, which had a high ratio in all subjects except the gibbon (Haf).
Discussion
Thorpe et al. (1999) observed that human hindlimb muscles were optimized to generate large forces over a narrow range of joint positions, while chimpanzee hindlimb muscles were optimized for generating moderate force over a wide range of joint positions. Our data indicate that this observation holds true for all extant non-human great apes. The functional distinction can be understood if we consider that humans are habitually bipedal, predominantly moving on the horizontal and stable substrate provided by the ground; by contrast, non-human great apes, to a greater or lesser extent, use various combinations of all four limbs, in numerous positions, to traverse an arboreal milieu composed of often unstable supports arranged in a three-dimensionally complex manner.
Sample size
Due to difficulties in obtaining ape cadavers and thus small sample sizes, it was difficult to select a single most appropriate method for comparison of the data. Instead, the data were considered in several different ways. First, raw data were tabulated so that variation in muscle architecture among and between apes of different size, age and species could be seen. Secondly, the proportional distribution of muscle volume within the body, between the proximal and distal hindlimb and across the major joints was compared. Thirdly, the possibility of geometric scaling of muscle mass and fascicle length was investigated so that data could be normalized (see Materials and methods). Although it is difficult to compare hindlimb anatomy across such a varying population, these data are essential for the construction of computer-based models of animal locomotion (van den Bogert et al. 1989; Wilson et al. 2001, 2003). They are particularly useful in the study of the evolution of locomotion in apes because live animals and cadavers are difficult to obtain and modelling locomotion in our early ancestors requires input data from extant apes (e.g. Li et al. 2002; Sellers et al. 2003, 2004; Wang et al. 2003, 2004; Wang & Crompton, 2004a,b).
Comparative hindlimb anatomy
Human and non-human ape hindlimb anatomy adheres to the same basic musculoskeletal plan. However, even prior to skinning, several differences were noted in relative limb proportions. For example, distribution of muscle mass along the limb was similar in humans and gibbons, in that muscle bulk was located proximally and there was tapering of limb circumference at both the knee and the ankle. By contrast, there was little variation in proximal-to-distal limb circumference in the African and Asian great apes (see Figs 1 and 2; Table 1). Humans and gibbons are both adept at bipedal running (sensu lato), which may explain the observed similarities in the shape of their hindlimbs. Proximal-to-distal tapering of the hindlimb has also been noted in other cursorial animals such as birds (Maloiy et al. 1979; Hutchinson, 2004), horses (Payne et al. 2005), dogs (Grand, 1977; Myers & Steudel, 1997) and camels (Alexander et al. 1982), and has been related to the reduction in weight and thus rotational inertia of distal limb segments, which would reduce the cost of swinging the limb (Hildebrand & Hurley, 1985).
Total hindlimb muscle mass as a percentage of body mass varied both within and between species. It was greatest in humans (9% of body mass) and lowest in the gibbon (Haf, < 5% body mass). Bonobo and chimpanzee distal limb muscle proportions (22 and 20%, respectively) were smaller than but similar to the orang-utans. Gibbon and gorilla were smaller still (18 and 15%, respectively). Humans had the lightest distal limb muscles compared with total hindlimb muscle mass (12%). The tendency for primates to be characterized by relatively heavy distal limb segments compared with other quadrupeds, such as dogs, has been related to the importance of grasping hands and feet (Grand, 1977; Alexander et al. 1981; Raichlen, 2004). With this in mind, it is perhaps not surprising that the species that use their feet least for grasping (humans, gibbons and perhaps gorillas) have proportionally the lightest distal limb muscles, and those that use their feet for grasping most (orang-utans, bonobos and common chimpanzees) have the proportionally heaviest distal limb muscles. Heavy distal limb segments have also been linked to fighting ability in dogs and other animals (Carrier, 2002). The functional requirements of display in primates have been linked to the evolution of bipedal stance (Jablonski & Chaplin, 1993). However, the ability to maintain grip on a branch is likely to be an overriding and continual selection pressure in the case of large-bodied arboreal primates.
In spite of the genetic proximity of the African apes, differences were observed in aspects of hindlimb musculature. For example, aspects of bonobo gluteal musculature were similar to those of the orang-utan (e.g. gluteus minimus had a scansorius-like belly and ischiofemoralis was more closely associated with biceps femoris than gluteus maximus). These similarities in muscle design may be linked to comparatively high levels of arboreality in orang-utans and bonobos (Sugardjito, 1986; Doran, 1992a, 1993; Thorpe & Crompton, 2004), as the gluteal muscle design of orang-utans is said to enhance the scanning/circumductive movements of the thigh (Sigmon, 1974). Although the gibbon is also highly arboreal, its gluteal muscles were not arranged in this way. This is likely to be because the gibbon hindlimb is mainly used for jumping and running atop branches (where motion is to a large extent confined to a parasagittal plane), whereas in orang-utans and bonobos the hindlimb is used for climbing, bridging and suspension, all of which are performed with a large degree of limb abduction. Indeed, even when the gibbon uses vertical climbing, the hindlimb is not abducted to the same extent as in the other apes (Isler, 2005). Finally, gorilla Gj had a small anomalous muscle spanning the posterodistal tibial shaft and calcaneus. It would have acted as a pure plantar flexor. This muscle was not found in the other leg, nor was it found in any of the other gorillas or other ape species. The small size of the muscle almost certainly precludes it from having a major role in locomotion.
Distribution of muscle volume between functional muscle groups
The distribution of muscle volume between the functional muscle groups of the hip, knee and ankle varied both within and between the species studied (see Fig. 5). The gluteal muscles represented the greatest proportion of total muscle volume crossing the hip joint in the gibbon. Relatively large glutei probably have a role in forceful extension during bipedal running and jumping. In contrast to gibbons and African apes, orang-utans had small glutei. This is because m. ischiofemoralis is considered to be part of the hamstring group in the orang-utan, but part of the gluteal group in African apes and gibbons (Sigmon & Farslow, 1986). If both hip extensor muscle groups are considered together, their contribution to hip joint muscle volume is similar in non-human apes (i.e. 52–62% of total muscle volume crossing the hip). Unfortunately, psoas major and psoas minor were not complete in all subjects and thus hip flexor volume is likely to have been underestimated. However, even if the muscles were complete, hip joint flexor volume is certain to have been small in comparison with total hip extensor volume in all subjects.
Although the relative volume of flexor/extensor muscle groups crossing the ankle joint was similar among the non-human apes, in the juvenile orang-utans (Ojm and Ojf) a particularly large proportion of the muscle volume crossing the ankle joint was represented by the pedal digital flexors. Digital flexor strength would be particularly useful for securing pedal grip in activities such as quadrumanous climbing and bridging locomotion and of course in clinging to the parent. That the adult orang-utan (Oam) lacked this feature might be an artefact of increased ‘terrestriality’ in captive adult orang-utans (see Crompton et al. 2003) as increased terrestriality appears to be a characteristic of adult great apes in general (orang-utan: Sugardjito, 1986; Cant, 1992; gorilla: Remis, 1995; Doran, 1997; chimpanzee: Doran, 1992a, 1993, 1997). Unfortunately, we were unable to test this relationship in the other species of ape as no juvenile specimens were available for dissection.
In-series elasticity
The role of tendons in the locomotion of non-human apes may be indicated by the ratio of muscle fascicle length to tendon rest length (Table 4). Tendon comprised a greater proportion of the muscle–tendon unit in the gibbon than in the other non-human apes, particularly in triceps surae (ratio = 0.64; see Fig. 2). By contrast, in great apes other than humans, tendons represented a smaller proportion of the entire muscle–tendon unit and in many cases the muscle fascicles inserted directly onto bone. Spring-like distal limbs are exemplified by the ungulates, which generally move on a stable substrate. For an arboreal quadruped, the hypothetical benefits of energy storage in compliant (in the sense of spring-like, rather than posturally compliant, i.e. flexed) limbs are likely in many situations to be offset by the high magnitude of compliance in branches, resulting in net loss of energy to locomotor supports (Alexander, 1991). It may be that limb compliance in non-human great apes is provided primarily by the highly specialized ligaments of the foot (for a detailed description of hominoid pedal anatomy see Vereecke et al. 2005). Ricochetal saltatory motion from supports as rigid as tree trunks, however, may allow internal energy stores to be used, for example by the indriids. Such stores certainly contribute to the saltatory performance of one species of galago (Aerts, 1998). It is as yet unclear whether the relatively long hindlimb tendons of the gibbon function solely to reduce limb mass (and thus rotational inertia, Hildebrand & Hurley, 1985) or whether they are also important as an elastic energy store in bipedal running and/or ricochetal saltatory locomotion between bouts of brachiation.
Scaling of muscle mass and fascicle length
It is difficult to address scaling issues when sample sizes are so small. Therefore, the ‘null hypothesis’ of geometric scaling of muscle mass and fascicle length in apes was investigated before normalization of data. In spite of small sample sizes, muscle masses were found to scale to (body mass)1.1 in proximal limb muscles and to (body mass)0.99 in distal limb muscles. Proximal limb mean fascicle length scaled to (body mass)0.30 and distal limb to (body mass)0.34. Thus, geometric scaling of the data is supported. However, humans had relatively large muscle masses per unit body mass and the adult orang-utan (Oam) and gibbon had relatively small muscle masses per unit body mass. Humans have a relatively short and light trunk compared with the non-human apes: the human lineage appears to have reduced trunk length to minimize forces required at the hip (see Wang & Crompton, 2004b), and may be able to tolerate the consequent reduction in gut volume because of a higher-quality diet whereas other great apes need to accommodate a larger digestive system for processing a greater proportion of plant matter (see Aiello & Wheeler, 1995; Aiello & Wells, 2002). The short trunk of humans may go a long way towards explaining why humans have relatively large muscle masses per unit body mass when compared with non-human apes. Humans and gibbons had short fascicle lengths per unit body mass. This finding could be related to similarities in the locomotor requirements of the hindlimb in humans and gibbons (see above).
Humans have relatively heavy hindlimb muscles with relatively large PCSAs as compared with the non-human apes we studied. This would suggest that humans have a higher capacity for force generation in hindlimb muscles, which may appear counter-intuitive, as ape hindlimbs appear highly muscular (Figs 1 and 2). However, humans also have relatively long hindlimbs compared with non-human apes [intermembral indices (upper limb length/lower limb length × 100): humans: 72; common chimpanzee: 106; bonobo: 102; gorilla: 116; orang-utan: 139; and gibbon: 130 (Fleagle, 1999)], but leg length was not accounted for during normalization of data (see Materials and methods). Relatively short hindlimbs in non-human apes may serve to reduce knee flexion moments by reducing the moment arm of the ground reaction force about the knee joint (Schmitt, 1999). Short hindlimbs additionally bring the centre of motion closer to the substrate, increasing stability.
When normalized, the data showed that all non-human apes were more similar to each other than to humans in hindlimb muscular morphology. Non-human great ape hindlimb muscles had long parallel fascicles and little or no tendon of insertion. Having said this, it was the common chimpanzee and bonobo where knee and ankle extensor muscle dimensions most closely resembled those of humans, being relatively heavy with large PCSAs. This suggests that hindlimb extension is of functional importance in chimpanzee locomotion and may imply increased potential for bipedality (supporting the arguments of Fleagle et al. 1981).
Conclusions
Within the limitations set by a small sample size, the following conclusions were drawn from this study:
Humans stand out among all hominoids in the scale of force-generation capabilities in the key muscles associated with upright bipedalism.
Among the non-human apes, common chimpanzee and bonobo hindlimb muscle architecture was remarkably similar and was characterized by relatively strong muscles of hindlimb extension.
Orang-utans had relatively heavy distal hindlimb muscles and the juveniles had relatively light knee extensor and ankle plantar flexor muscles.
Gibbon hindlimb muscle design was similar to that of humans in that energy storage and/or force transfer may be more important than the ability to generate force over a wide range of motion.
Acknowledgments
We would like to thank Peter Aerts and John R. Hutchinson for providing comments on the manuscript. We also thank The North of England Zoological Society, Bristol Zoo, Zoo Basel, Zoo Zürich and Knie's Kinderzoo Rapperswil for provision of ape cadavers. Individuals who helped greatly in this regard include Mr Nicholas Ellerton (then N.E.Z.S.), at The University of Liverpool: Ray Burniston; at the Institute of Anthropology, Zürich: Bob Martin, Beno Schoh, Marcus Gisi. Daniel Oppliger, Basel, also gave invaluable assistance. This research was funded by The Natural Environment Research Council, The Biotechnology and Biological Sciences Research Council, The Engineering and Physics Research Council, The Royal Society, The L.S.B. Leakey Foundation, The Leverhulme Trust, The University of Liverpool and the Adolph Schultz Foundation, Zürich.
References
- Aerts P. Vertical jumping in Galago senegalensis: the quest for a hidden power amplifier. Phil Trans R Soc Lond. 1998;353:1607–1620. [Google Scholar]
- Aerts P, Van Damme R, Van Elsacker L, Duchene V. Spatio-temporal gait characteristics of hind-limb cycles during voluntary bipedal and quadrupedal walking in bonobos (Pan paniscus). Am J Phys Anthropol. 2000;111:503–517. doi: 10.1002/(SICI)1096-8644(200004)111:4<503::AID-AJPA6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Aiello LC, Wheeler P. The expensive-tissue hypothesis. Curr Anthropol. 1995;36:199–221. [Google Scholar]
- Aiello LC, Wells JCK. Energetics and the evolution of the genus Homo. Annu Rev Anthropol. 2002;31:323–338. [Google Scholar]
- Alexander RM, Vernon A. The dimensions of the knee and ankle muscles and the forces they exert. J Hum Mov Stud. 1975;1:115–123. [Google Scholar]
- Alexander RM, Jayes AS, Maloiy GMO, Wathuta EM. Allometry of the leg muscles of mammals. J Zool (Lond) Series A. 1981;194:227–267. [Google Scholar]
- Alexander RM, Maloiy GMO, Ker RF, Jayes AS, Warui CN. Elasticity in the locomotion of the Camel (Camelus dromedarius) J Zool. 1982;198:293–313. [Google Scholar]
- Alexander RM. Elastic mechanisms in primate locomotion. Z Morph Anthropol. 1991;78:315–320. [PubMed] [Google Scholar]
- Bisschoff LW. Beiträge zur Anatomie des Hylobates leuciscus und zu einer vergleichende Anatomie der Muskeln der Affen und des Menschen. Abd D Mathphys Kl D Königl Bayer Akad D Wiss. 1870;10:199–297. [Google Scholar]
- van den Bogert AJ, Schamhardt HC, Crowe A. Simulation of quadrupedal locomotion using a rigid body model. J Biomech. 1989;22:33–41. doi: 10.1016/0021-9290(89)90182-6. [DOI] [PubMed] [Google Scholar]
- Cannon CH, Leighton M. Comparative locomotor ecology of gibbons and macaques: selection of canopy elements for crossing gaps. Am J Phys Anthropol. 1994;93:505–524. doi: 10.1002/ajpa.1330930409. [DOI] [PubMed] [Google Scholar]
- Cant JGH. Positional behaviour of female Bornean orang-utans (Pongo pygmaeus) Am J Phys Anthropol. 1987;12:71–90. doi: 10.1002/ajp.1350120104. [DOI] [PubMed] [Google Scholar]
- Cant JGH. Positional behaviour and body size of arboreal primates: a theoretical framework for field studies and an illustration of its application. Am J Phys Anthropol. 1992;88:273–284. doi: 10.1002/ajpa.1330880302. [DOI] [PubMed] [Google Scholar]
- Carpenter CR. A field study in Siam of the behaviour and social relations of the gibbon (Hylobates lar). Comp Psych Mo. 1940;16:1–212. [Google Scholar]
- Carrier DR. Functional tradeoffs in specialisation for fighting versus running. In: Aerts P, D'Aout K, Herrel A, Van Damme R, editors. Topics in Functional and Ecological Vertebrate Morphology. Netherlands: Shaker; 2002. pp. 237–255. [Google Scholar]
- Crompton RH, Thorpe SKS, Wang WJ, et al. The biomechanical evolution of erect bipedality. Cour Forsch – Inst Senkenberg. 2003;243:135–146. [Google Scholar]
- Cutts A, Alexander RM, Kerr RF. Ratios of cross-sectional areas of muscles and their tendons in a healthy human forearm. J Anat. 1991;176:133–137. [PMC free article] [PubMed] [Google Scholar]
- D'Août K, Aerts P, De Clerq D, De Meester K, Van Elsacker L. Segment and joint angles of hind limb during bipedal and quadrupedal walking of the bonobo (Pan paniscus). Am J Phys Anthropol. 2002;119:37–51. doi: 10.1002/ajpa.10112. [DOI] [PubMed] [Google Scholar]
- D'Août K, Vereecke E, Schoonaert K, De Clercq D, Van Elsacker L, Aerts P. Locomotion in bonobos (Pan paniscus): differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes. J Anat. 2004;204:353–361. doi: 10.1111/j.0021-8782.2004.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran DM. The ontogeny of chimpanzee and bonobo locomotor behaviour: a case study of paedomorphism and its behavioural correlates. J Hum Evol. 1992a;23:139–157. [Google Scholar]
- Doran DM. Comparison of instantaneous locomotor bout sampling methods: a case study of adult male chimpanzee locomotor behaviour and substrate use. Am J Phys Anthropol. 1992b;89:85–99. doi: 10.1002/ajpa.1330890108. [DOI] [PubMed] [Google Scholar]
- Doran DM. The comparative locomotor behaviour of chimpanzees and bonobos: the influence of morphology on locomotion. Am J Phys Anthropol. 1993;91:99–115. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- Doran DM. Ontogeny of locomotion in mountain gorillas and chimpanzees. J Hum Evol. 1997;32:322–344. doi: 10.1006/jhev.1996.0095. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Apor P. Specific tension of human elbow flexor muscles. In: Saltin B, editor. Biochemistry of Exercise VI. Illinois: Human Kinetics; 1986. pp. 487–500. [Google Scholar]
- Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fibre length in architectural studies. J Exp Biol. 2005;208:3275–3279. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- Fifer FC. The adoption of bipedalism by the hominoids: a new hypothesis. J Hum Evol. 1987;2:135–147. [Google Scholar]
- Fleagle JG. Dynamics of a brachiating siamang (Hylobates symphalangus syndactylus) Nature. 1974;248:259–260. doi: 10.1038/248259a0. [DOI] [PubMed] [Google Scholar]
- Fleagle JG. Locomotion and posture of the Malayan siamang and implications for hominoid evolution. Folia Primatol. 1976;26:245–269. doi: 10.1159/000155756. [DOI] [PubMed] [Google Scholar]
- Fleagle JG, Stern JT, Jungers WL, Susman RL, Vangor AK, Wells JP. Climbing: a biomechanical link with brachiation and with bipedalism. Symp Zool Soc Lond. 1981;48:359–375. [Google Scholar]
- Fleagle JA. Primate Adaptation and Evolution. London: Academic Press; 1999. [Google Scholar]
- Friederich JA, Brand RA. Muscle fascicle architecture in the human lower limb. J Biomech. 1990;23:91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Roy RR, Shellock FG, et al. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res. 1992;10:926–934. doi: 10.1002/jor.1100100623. [DOI] [PubMed] [Google Scholar]
- Gebo DL. Climbing, brachiation, and terrestrial quadrupedalism: historical precursors of hominid bipedalism. Am J Phys Anthropol. 1996;101:55–92. doi: 10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Grand TI. Body weight: Its relation to tissue composition, segment distribution, and motor function. I. Interspecific comparisons. Am J Phys Anthropol. 1977;47:211–240. doi: 10.1002/ajpa.1330470204. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Hurley JP. Energy of the oscillating legs of a fast-moving cheetah, pronghorn, jackrabbit, and elephant. J Morph. 1985;184:23–31. doi: 10.1002/jmor.1051840103. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- Hunt KD. The evolution of bipedality: ecology and functional morphology. J Hum Evol. 1994;26:183–202. [Google Scholar]
- Hutchinson JR. Biomechanical modeling and sensitivity analysis of bipedal running ability. I. Extant taxa. J Morph. 2004;262:421–440. doi: 10.1002/jmor.10241. [DOI] [PubMed] [Google Scholar]
- Ishida H, Kimura T, Okada M, Yamazaki M. Kinesiological aspects of bipedal walking in gibbons. In: Preuschoft H, editor. The Lesser Apes: Evolutionary and Behavioural Biology. Edinburgh: Edinburgh University Press; 1984. pp. 135–145. [Google Scholar]
- Isler K. Characteristics of vertical climbing in African apes. In: Gudo M, Gutmann J, Scholz J, editors. Concepts of Functional, Engineering and Constructional Morphology: Biomechanical Approaches on Fossil and Recent Organisms. Frankfurt: Senkenbergiana Lethaea; 2002. pp. 115–124. [Google Scholar]
- Isler K. 3D-kinematics of vertical climbing in hominoids. Am J Phys Anthropol. 2005;126:66–81. doi: 10.1002/ajpa.10419. [DOI] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G. Origin of habitual terrestrial bipedalism in the ancestor of the Hominidae. J Hum Evol. 1993;24:259–280. [Google Scholar]
- Jenkins FA. Chimpanzee bipedalism: cineradiographic analysis and implications for the evolution of gait. Science. 1972;178:877–879. doi: 10.1126/science.178.4063.877. [DOI] [PubMed] [Google Scholar]
- Jolly CJ. The seed-eaters: a new model of hominid differentiation based upon a baboon analogy. New Sci. 1970;5:5–27. [Google Scholar]
- Knusel CJ. The throwing hypothesis and hominoid origins. J Hum Evol. 1992;7:1–7. [Google Scholar]
- Kohlbrügge JHF. Versuch einer Anatomie des Genus Hylobates. In: Weber M, editor. Zoologische Ergebnisse Einer Reise in Niederländisch Ost-Indien. Leiden: Verlag von EJ Brill; 1890/1891. pp. 211–354. [Google Scholar]
- Larson SG, Stern JT. EMG of chimpanzee shoulder muscles during knuckle-walking: problems of terrestrial locomotion in a suspensory-adapted primate. J Zool Lond. 1987a;212:629–655. [Google Scholar]
- Larson SG, Stern JT. EMG of the hamstrings in chimpanzees and orang-utans. Yearb Phys Anthropol. 1987b;72:223–230. [Google Scholar]
- Li Y, Crompton RH, Gunther M, Wang W, Savage R. Reconstructing the mechanics of quadrupedalism in an extinct hominoid. Z Morph Anthropol. 2002;83:265–274. [PubMed] [Google Scholar]
- Lieber RL, Jacobson MD, Fazeli BM, Abrams RA, Botte MJ. Architecture of selected muscles of the arm and forearm: anatomy and implications for tendon transfer. J Hand Surg [Am] 1992;17:787–798. doi: 10.1016/0363-5023(92)90444-t. [DOI] [PubMed] [Google Scholar]
- Maloiy GMO, Alexander MN, Njau R, Jayes AS. Allometry of the legs of running birds. J Zool. 1979;187:161–167. [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Miller RA. The musculature of Pan paniscus. Am J Anat. 1952;91:183–232. doi: 10.1002/aja.1000910202. [DOI] [PubMed] [Google Scholar]
- Morbeck ME, Zihlman AL. Body composition and limb proportions. In: Schwartz J, editor. Orang-Utan Biology. New York: Oxford University Press; 1988. pp. 285–297. [Google Scholar]
- Myers MJ, Steudel K. Morphological conservation of limb natural pendular period in the domestic dog (Canis familiaris): implications for locomotor energetics. J Morph. 1997;234:183–196. doi: 10.1002/(SICI)1097-4687(199711)234:2<183::AID-JMOR5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Narici MV, Landoni L, Minetti AE. Assessment of human knee extensor muscle stress from in vivo physiological cross-sectional area and strength measurements. Eur J Appl Physiol. 1992;65:438–444. doi: 10.1007/BF00243511. [DOI] [PubMed] [Google Scholar]
- Payne RC. University of Liverpool; 2001. Musculoskeletal adaptations for climbing in hominoids and their role as exaptions for the acquisition of bipedalism. PhD dissertation. [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, Smith NC, Wilson AM. Functional specialisation of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans. II. Moment arms. J Anat. 2006;208:725–742. doi: 10.1111/j.1469-7580.2006.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoft H. Muskeln und gelenke der hinterextremitat des gorilla (Gorilla gorilla) Mor Jarbuch. 1962;101:432–540. [Google Scholar]
- Raichlen DA. Convergence of forelimb and hindlimb natural pendular period in baboons (Papio cynocephalus) and its implication for the evolution of primate quadrupedalism. J Hum Evol. 2004;46:719–738. doi: 10.1016/j.jhevol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Remis M. Effects of body size and social context of lowland gorillas in the Central African Republic. Am J Phys Anthropol. 1995;97:413–433. doi: 10.1002/ajpa.1330970408. [DOI] [PubMed] [Google Scholar]
- Richmond BG, Strait DS. Evidence that humans evolved from a knuckle-walking ancestor. Nature. 2000;404:382–385. doi: 10.1038/35006045. [DOI] [PubMed] [Google Scholar]
- Rodman PS, McHenry HM. Bioenergetics and the origin of hominid bipedalism. Am J Phys Anthropol. 1980;52:103–106. doi: 10.1002/ajpa.1330520113. [DOI] [PubMed] [Google Scholar]
- Schmitt D. Compliant walking in primates. J Zool (Lond) 1999;248:149–160. [Google Scholar]
- Sellers WI, Dennis LA, Crompton RH. Predicting the metabolic energy costs of bipedalism using evolutionary robotics. J Exp Biol. 2003;206:1127–1136. doi: 10.1242/jeb.00205. [DOI] [PubMed] [Google Scholar]
- Sellers WI, Dennis LA, Wang W-J, Crompton RH. Evaluating alternative gait strategies using evolutionary robotics. J Anat. 2004;204:343–351. doi: 10.1111/j.0021-8782.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman P. Scavenging or hunting in early hominids: theoretical framework and tests. Am Anthropol. 1986;88:27–40. [Google Scholar]
- Sigmon BA. A functional analysis of pongid hip and thigh musculature. J Hum Evol. 1974;3:161–185. [Google Scholar]
- Sigmon BA, Farslow DL. The primate hindlimb. In: Swindler DR, Erwin J, editors. Comparative Primate Biology, Systematics, Evolution and Anatomy. I. New York: Alan R Liss Inc; 1986. pp. 671–718. [Google Scholar]
- Sonntag CF. On the anatomy, physiology and pathology of the orang-outan. Proc Zool Soc. 1924;24:24–450. [Google Scholar]
- Stern JT, Susman RL. Electromyography of the gluteal muscles in Hylobates, Pongo, and Pan: implications for the evolution of hominid bipedality. Am J Phys Anthropol. 1981;55:153–166. [Google Scholar]
- Sugardjito J. Age-sex class differences in the positional behaviour of the Sumatran orang-utan (Pongo pygmaeus abelii) in the Gunung Leuser National Park. Indonesia Folia Primatol. 1986;47:14–25. doi: 10.1159/000156260. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH, Günther MM, Ker RF, Alexander RM. Dimensions and moment arms of the hind- and forelimb muscles of common chimpanzees (Pan troglodytes). Am J Phys Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Thorpe SKS, Crompton RH. Locomotor ecology of wild orang-utans (Pongo pygmaeus abelii) in the Gunung Leuser ecosystem, Sumatra, Indonesia: a multivariate analysis using log-linear modelling. Am J Phys Anthropol. 2004;127:58–78. doi: 10.1002/ajpa.20151. [DOI] [PubMed] [Google Scholar]
- Thorpe SK, Crompton RH, Wang W. Stresses exerted in the hindlimb muscles of common chimpanzees (Pan troglodytes) during bipedal locomotion. Folia Primatol. 2004;75:253–265. doi: 10.1159/000078937. [DOI] [PubMed] [Google Scholar]
- Tuttle RH, Basmajian JV, Ishida H. Electromyography of pongid gluteal muscles and hominid evolution. In: Chivers DJ, Joysey KA, editors. Recent Advances in Primatology. London: Academic Press; 1978. pp. 463–468. [Google Scholar]
- Tuttle RH, Velte MJ, Basmajian JV. Electromyography of brachial muscles in Pan troglodytes and Pongo pygmaeus. Am J Phys Anthropol. 1983;61:75–83. doi: 10.1002/ajpa.1330610108. [DOI] [PubMed] [Google Scholar]
- Vereecke EE, D'Aout K, De Clercq D, Van Elsacker L, Aerts P. The relationship between speed, contact time and peak plantar pressure in terrestrial walking of bonobos. Folia Primatol. 2004;75:266–278. doi: 10.1159/000078938. [DOI] [PubMed] [Google Scholar]
- Vereecke EE, D'Aout K, Payne R, Aerts P. Functional analysis of the foot and ankle myology of gibbons and bonobos. J Anat. 2005;206:453–476. doi: 10.1111/j.1469-7580.2005.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Crompton RH, Li Y, Gunther MM. Energy transformation during erect and bent-hip-bent-knee walking by humans with implications for the evolution of bipedalism. J Hum Evol. 2003;44:563–580. doi: 10.1016/s0047-2484(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Crompton RH. Analysis of the human and ape foot during bipedal standing with implications for the evolution of the foot. J Biomech. 2004a;37:1831. doi: 10.1016/j.jbiomech.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Crompton RH. The role of load-carrying in the evolution of modern body proportions. J Anat. 2004b;204:417–430. doi: 10.1111/j.0021-8782.2004.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Crompton RH, Carey TS, et al. Comparison of inverse-dynamics musculo-skeletal models of AL 288-1 Australopithecus afarensis and KNM-WT 15000 Homo ergaster to modern humans, with implications for the evolution of bipedalism. J Hum Evol. 2004;47:453–478. doi: 10.1016/j.jhevol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38:2317–2320. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Wheeler PE. The influences of bipedalism on the energy and water budgets of early hominids. J Hum Evol. 1991;21:117–136. [Google Scholar]
- Wheeler PE. The influences of stature and body form on hominoid energy and water budgets: a comparison of Australopithecus and early Homo physiques. J Hum Evol. 1993;24:13–28. [Google Scholar]
- Wilson AM, McGuigan MP, Su A, Van den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–898. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Watson JC, Lichtwark GA. A catapult mechanism for rapid limb protraction. Nature. 2003;421:35–36. doi: 10.1038/421035a. [DOI] [PubMed] [Google Scholar]
- Yamazaki N, Ishida H. A biomechanical study of vertical climbing and bipedal walking in gibbons. J Hum Evol. 1984;13:563–571. [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- Zajac FE. How muscle-tendon architecture and joint geometry affect the capacity of muscles to move and exert force on objects: a review with application to arm and forearm tendon transfer design. J Hand Surg. 1992;17A:799–804. doi: 10.1016/0363-5023(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Zihlman AL. Body mass in lowland gorillas: a quantitative analysis. Am J Phys Anthropol. 2000;113:61–78. doi: 10.1002/1096-8644(200009)113:1<61::AID-AJPA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]