Abstract
Dietary protein is a limiting factor in mammalian growth, significantly affecting the non-linear trajectories of skeletal growth. Young females may be particularly vulnerable to protein malnutrition if the restriction is not lifted before they become reproductive. With such early malnutrition, limited amino acids would be partitioned between two physiological objectives, successful reproduction vs. continued growth. Thus, the consequences of protein malnutrition could affect more than one generation. However, few studies have quantified these cross-generational effects. Our objective was to test for differences in skeletal growth in a second generation of malnourished rats compared with rats malnourished only post-weaning, the first generation and with controls. In this longitudinal study we modelled the growth of 22 craniofacial measurements with the logistic Gompertz equation, and tested for differences in the equation's parameters among the diet groups. The female offspring of post-weaning malnourished dams did not catch up in size to the first generation or to controls, although certain aspects of their craniofacial skeleton were less affected than others. The second generation's growth trajectories resembled the longer and slower growth of the first malnourished generation. There was a complex interaction between developmental processes and early nutritional environment, which affected variation of adult size.
Keywords: body size, craniofacial skeleton, nutrition, ontogeny, variation
Introduction
The availability of dietary protein is a limiting factor in mammalian reproductive success (Stewart et al. 1975; Desai et al. 1996; Reichling, 1999), development (Cameron & Eshelman, 1996; Gomez et al. 1996; da Silva Faria et al. 2004; Fortman et al. 2005) and gross somatic growth (Stewart et al. 1975; Edozier & Switzer, 1978; Pond & Wu, 1981; Miller & German, 1999; Reichling & German, 2000). Restriction of dietary protein affects skeletal growth trajectories, the non-linear patterns of size changes over time. Even seasonal fluctuations in protein-rich food sources can significantly delay achievement of adult stature compared with well-nourished individuals (McAdam & Boutin, 2003). Young females are vulnerable to inadequate protein because they may reproduce while still growing. A limited supply of amino acids would then be partitioned between two physiological processes, successful reproduction and continued growth. Thus, the consequences of protein malnutrition could affect more than one generation.
Multigenerational protein malnutrition can result when protein resources are depleted due to ecological cycles of available plant material or regional differences in nutrient availability (Sinclair et al. 1988; Cameron & Eshelman, 1996; Herbert et al. 2002; McAdam & Boutin, 2003). Despite the importance of understanding how protein malnutrition affects growth in female mammals and their offspring, few controlled studies have investigated these effects through successive generations. One notable exception is in the work of Stewart et al. (1975, 1980). Using a colony of rats maintained on a protein-deficient diet for 12 generations, these researchers documented the effects of suboptimal dietary protein levels on behaviour, life-history traits and growth. That study, however, had a significant design problem. Rather than using a measure of body size or skeletal maturity, they ‘regarded [rats] as adult at 24–26 weeks of age, and very few were allowed to live beyond this time’ (Stewart et al. 1975). Other work demonstrated that rats maintained post-weaning on a low-protein diet eventually reach control adult skeletal size, although it is not achieved until well after 40 weeks of growth (Miller & German, 1999; Reichling & German, 2000). Stewart and colleagues also generated growth curves by simply reporting groups' averaged body masses, rather than individuals' values, for each sex and diet (control and low-protein) group through time. This approach neglects variation within the groups, variation that may change as animals age, and prohibits quantitative comparisons of growth trajectories (German, 2004). The consequences of their definition of adult size are evident in the growth curves that Stewart et al. (1975) presented for average body masses of the low- and high-protein groups, combined for all 12 generations. These curves, plotting weight as a function of age, indicate that the malnourished animals are not maximum size at 24 weeks because the weight values do not reach an asymptote when the experiments end. In particular, the low-protein animals are still growing at a significant rate, with no sign of slowing. So although the protein malnutrition in Stewart and colleagues' colony lasted for many generations, these growth trajectories are incomplete and suggest incorrect conclusions about the impact of protein malnutrition.
Other studies of the effects of protein malnutrition on offspring growth place the maternal generation on a low-protein diet only shortly before conception, at the detection of pregnancy, or at parturition (Pond & Wu, 1981; Desai et al. 1996; El-Khattabi et al. 2003). The females in such studies are well nourished until pregnancy and/or lactation. Compared with females placed on a low-protein diet before reaching reproductive maturity, animals in these studies presumably have accumulated greater muscle protein content and fat stores, both of which may be catabolized to support fetal growth and supplement the higher energy costs of milk production. Their offspring are thus not comparable with those of females who had been protein malnourished during their own post-weaning growth phase.
Suboptimal levels of dietary protein impact the physiological systems that regulate the pace and timing of skeletal growth and maturation. Short-term protein restriction results in a decrease in amplitude or concentration of growth hormone (GH) release, but not the normal 3.3-h cycles of release, as well as a decrease in plasma IGF-I and insulin concentrations (Harel & Tannenbaum, 1993; Thissen et al. 1994; Underwood et al. 1994; Bourrin et al. 2000; El-Khattabi et al. 2003; Jimenez-Gancedo et al. 2004; Martin et al. 2004). In cultured bone marrow cells from malnourished rats, cell generation times are greater than in controls due largely to a four-fold increase in time spent in the meiosis I and gap1 phases, although the synthesis phase was shorter than in controls (Gomez et al. 1996). There is further evidence that protein restriction affects rates of DNA and protein synthesis on a tissue-specific basis (Masanes et al. 1999; Proud, 2002; El-Khattabi et al. 2003). These effects on cell-cycle and synthesis rates may be attributed to the regulation of mRNA translation and the production of ribosomal constituents by nutrients, particularly proteins (Proud, 2002; Kimball & Jefferson, 2004). Finally, protein restriction potentially affects DNA methylation patterns, which would have later consequences with respect to growth of the whole organism (Kuzawa, 2005).
Over several generations, the potential impact of protein malnutrition on these cellular or physiological processes could affect growth of the whole organism in several ways. First, if protein malnutrition increases the duration of body growth, the longer duration may compensate for expected slower bone growth rates. This would result in animals that are not smaller than well-nourished animals (Miller & German, 1999; Reichling & German, 2000). Alternatively, perturbation of ‘normal’ skeletal ontogeny due to marginal levels of dietary protein throughout the animals' entire lives could fundamentally change the trajectory of growth. In such a case individuals may never achieve normal overall size or proportion. Examining only final adult sizes in populations subjected to this environmental insult will, however, not provide the data necessary to understand how protein malnutrition affects skeletal growth in individuals, or variation in the pattern of growth within a population. Examination of the entire growth trajectory is necessary in order to appreciate fully the effects of limited protein during animals' entire lives.
The goal of this study was to test for differences in skeletal growth due to life-long protein malnutrition (i.e. pathological malnutrition suffered from conception through post-weaning development) relative to animals malnourished only post-weaning and to control animals on an optimal protein diet. We focused on the craniofacial skeleton, and limited our analyses to females. We addressed three specific questions. Do second-generation malnourished females retain the capacity to achieve control adult size if allowed to continue to grow until growth naturally ceases? We expected sizes of craniofacial measurements at weaning to be smaller in the second generation, which had been malnourished in utero, than in the first, which had only been malnourished post-weaning (Stewart et al. 1975; Reichling, 1999; Jimenez-Gancedo et al. 2004). Regardless of this predicted initial difference, are the overall trajectories of skeletal growth in the second generation similar to those of controls, of post-weaning malnourished rats, or different from both? Finally, does a low-protein diet across successive generations increase the within-generation (group) variation in rates of skeletal growth and final sizes? This was a longitudinal study, and we modelled growth over time for a number of skeletal aspects. We were therefore also able to examine the differential effects of protein malnutrition on growth of functional skeletal systems, i.e. the neuro- vs. viscero-craniums, and to speculate about what features of the skeletal growth trajectory, e.g. rates and durations, were more resistant or susceptible to perturbation.
Materials and methods
Diets
The diets (Dyets, Bethlehem, PA, USA, #111146, 111147) were based on AIN-93G rodent diet standards for growth, pregnancy and lactation (Reeves et al. 1993). These diets were identical to those used in previous studies (Miller & German, 1999; Reichling & German, 2000). The control higher protein diet had 27.6% protein in the form of casein by weight (Table 1). This diet supported maximum growth rates associated with increased protein uptake (Edozier & Switzer, 1978). The isocaloric low-protein diet had 4.6% protein. This diet was not detrimental to post-weaning malnourished rats' health (Edozier & Switzer, 1978; Cameron & Eshelman, 1996), but had sufficiently less protein to make differences in growth rates detectable. The diets were complete with respect to amino acid composition, and contained the same number of calories, 3.4 kcal/kg. Calcium and potassium levels were modified in the low-protein diet. Caloric intake for each animal was determined by data from same-aged control animals allowed to eat ad libitum (Miller & German, 1999). All rats were fed an equal number of calories per gram body mass so that only protein consumption varied between the groups (Table 2).
Table 1.
Composition of control and experimental low-protein diets. Both diets have 3.4 kcal kg−1. All quantities are in milligrams of ingredient per gram of food
| Ingredient | Control diet 27.6% protein | Low-protein diet 4.6% protein |
|---|---|---|
| Casein | 276.00 | 46.00 |
| Cornstarch | 329.07 | 502.66 |
| DYETROSE®* | 109.69 | 167.55 |
| Sucrose | 100.00 | 100.00 |
| Cellulose | 50.00 | 50.00 |
| Soybean oil | 70.00 | 70.00 |
| t-Butylhdroquinone | 0.01 | 0.01 |
| Salt Mix #213266 | 35.00 | 35.00 |
| Calcium phosphate dibasic | 4.08 | 11.66 |
| Calcium carbonate | 9.49 | 3.91 |
| Vitamin mix #310025 | 10.00 | 10.00 |
| l-Cystine | 4.10 | 0.70 |
| Choline bitartrate | 2.50 | 2.50 |
| Blue dye | 0.05 | 0.00 |
DYETROSE® (Dyets, Bethlehem, PA, USA) is a selectively depolymerized food-grade cornstarch that can be substituted for cornstarch without any detectable dietary effects.
Table 2.
Daily food allocation for females on the low-protein diet, calculated based on consumption, per gram body mass, of same-aged controls that had ad libitum access to the high-protein diet
| Body mass (g) | Low-protein diet (g) |
|---|---|
| ≤ 59 | 8.5 |
| 60–69 | 10.5 |
| 70–79 | 13.5 |
| 80–89 | 15.5 |
| 90–99 | 16.5 |
| 100–109 | 17.0 |
| 110–119 | 18.0 |
| 120–129 | 20.0 |
| 130–139 | 21.0 |
| 140–149 | 21.5 |
| 150–189 | 23.0 |
| 190–259 | 25.0 |
| ≥ 260 | 26.0 |
Animals and dietary treatments
Animals were Sprague–Dawley strain of Rattus norvegicus, originally obtained from Zivic Miller (Pittsburgh, PA, USA) and maintained for several generations at the University of Cincinnati. Animal care and husbandry in the current study were identical to those previously described (Miller & German, 1999; Reichling & German, 2000) and conformed to University of Cincinnati IACUC (protocols #91-05-27-01 and #01-02-26-01). After weaning, all animals had ad libitum. access to water, were maintained on a 12/12-h light–dark cycle, and were housed individually. Initially, rats were housed in plastic shoebox cages but were later moved to wire-bottomed cages in order to prohibit their consumption of the cob bedding. University of Cincinnati IACUC approved this change in husbandry. Two to 4 weeks after rats reached adult size they were deeply anaesthetized with isoflurane gas (Henry Schein, Port Washington, NY, USA) and were then killed with an injection of pentobarbital (Henry Schein). Only females were used in the current study. The male offspring of protein-malnourished females suffered a variety of health problems, including uretor calcification, bladder stones (cystolith) and kidney stones (nephrolithiasis). More than half of the sample of males were killed for humane reasons before reaching adult size, and therefore males were excluded from the study.
This study compared the pattern of body mass increase and craniofacial skeletal growth in three groups of female rats. Two groups, the control animals (CP) and animals that experienced only post-weaning protein malnutrition (LP1), were from Miller & German's (1999) study. Females that experienced life-long protein malnutrition (LP2) were generated separately, and data collected independently, for the current study. Control protein rats (CP, n = 10) were the offspring of breeders (male and female) maintained on a standard rat chow diet in our colony (Miller & German, 1999). At weaning, age 21 days, the CP animals were placed on the 27.6% protein diet. First-generation low-protein rats (LP1, n = 10) were also born to breeders. At weaning the LP1 animals were placed on the 4.6% protein diet. Second-generation low-protein females (LP2, n = 24) were the offspring of breeder males and post-weaning protein-malnourished females used by Fortman et al. (2005). The dams that produced the LP2 generation were the equivalent of the LP1 groups in the current study. At weaning (28 days) the LP2 females were placed on the low-protein diet. Because their dams had been raised on a low-protein diet, the LP2 animals were protein restricted through all phases of growth. All animals were maintained on their respective diets for the duration of the experiments.
Females in the CP and LP1 groups were weaned at age 21 days; females in the LP2 treatment were significantly smaller and underdeveloped at 21 days and therefore weaning was delayed until age 28 days. As others have noted, viable weaning of the offspring of chronically malnourished dams at 21 days is not possible due to their slower development (Stewart et al. 1975; Reichling, 2000). Therefore, we used age at weaning as T0 in our modelling of growth trajectories (described below).
Data collection
Beginning on the day of weaning, all animals were weighed daily (Ohaus Navigator Balance, Ohaus Scale, Florham Park, NJ, USA) and were radiographed in the dorsoventral and lateral planes 2–3 times per week. Immediately prior to radiography sessions, rats were lightly anaesthetized with isoflurane gas in a small induction chamber using an Ohio 4000 Compact Anesthesia Machine at 2.75–3.5% isoflurane per litre of oxygen for less than 5 min. Animals recovered from anaesthesia within minutes of being removed from the induction chamber. Radiographs were taken with Kodak MRM-film (24 × 30 cm) using low-dosage radiation from a Bennett Mammography Machine (Bennett X-ray, Copiague, NY, USA) set for 0.25 s at 75 mA and 30–40 kV, depending on the size of the rat. Measurement distortion was minimized by placing animals directly on the film cassette at a standardized focal-film distance of ∼80 cm, and a field of view encompassing only the film cassette. In addition, all rats were orientated on the cassette in identical fashion. Neither the anaesthetic nor the radiographic methods adversely affect the health or growth of the animals (Fiorello & German, 1997).
As growth slowed, the frequency of radiography sessions was reduced and concluded when individuals reached adult size, as determined by an asymptote in body mass and long bone length increase, and/or closure of the skeletal sutures. Because of variation among individuals, adult age, and thus the number of radiographs taken of each rat, varied among individual rats and treatment groups.
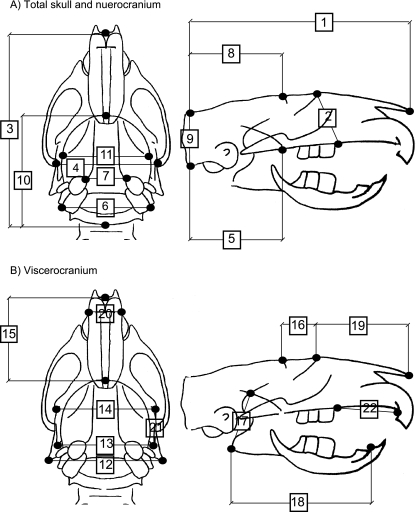
Thirty anatomical landmarks, repeatable on all radiographs and identical to those in Miller & German (1999), were converted to Cartesian coordinates using a Numonics AccuGrid Digitizing Tablet (Numonics, Montgomeryville, PA, USA; accuracy of 0.127 mm) and the program DIGIT (written by David Hertwek). For each rat at each radiography session, the linear distances between pairs of landmarks were calculated to generate 22 craniofacial measurements (Table 3). Figure 1 shows the landmarks and measurements. Additionally, the quality of each radiograph was assessed based on skeletal alignment and resolution. Approximately 35 LP2 radiographs, out of nearly 1750, were not used because of poor skeletal alignment and/or resolution. One of us (S.L.L.) digitized all radiographs of the LP2 females. Jeffery Miller (Miller & German, 1999) digitized all radiographs of CP and LP1 rats. Prior to the start of the current analyses we confirmed that intra- and interpersonal variation in locating and digitizing the anatomical landmarks would not affect our analyses.
Table 3.
Descriptions of the 22 craniofacial measurements and the landmarks used to record those measurements. Lateral and dorsoventral refer to radiograph view from which the measurement was obtained
| Total skull |
| 1. Skull length (lateral): anterior tip of nasal bone – posterior edge of nuchal crest |
| 2. Skull height (lateral): suture between nasal and frontal bone – posteriormost point of upper diastema |
| 3. Skull length (dorsoventral): the anterior tip of nasal bone – posterior edge of occipital bone |
| 4. Skull width (dorsoventral): right zygomatic and temporal bone suture – left zygomatic and temporal bone suture |
| Neurocranium |
| 5. Occipitopalatal length (lateral): edge of occipital condyle – posterior edge of palatine bone |
| 6. Distance between mastoid processes (dorsoventral): right mastoid process – left mastoid process |
| 7. Distance between tympanic bullae (dorsoventral): anterior/medial edge of right tympanic bulla – anterior/medial edge of left tympanic bulla |
| 8. Neurocranium length (lateral): lateral ridge of frontal bone – posterior edge of nuchal crest |
| 9. Neurocranium height (lateral): posterior edge of nuchal crest – edge of occipital condyle |
| 10. Neurocranium length (dorsoventral): posterior edge of cribiform plate – posterior edge of occipital bone |
| 11. Neurocranium width (dorsoventral): right temporal line of the parietal bone – left temporal line of the parietal bone |
| Viscerocranium |
| 12. Distance between angles (dorsoventral): right mandibular angle – left mandibular angle |
| 13. Distance between condyles (dorsoventral): right mandibular condyle – left mandibular condyle |
| 14. Distance between coronoids (dorsoventral): right coronoid process of mandible – left coronoid process of mandible |
| 15. Facial length (dorsoventral): anterior tip of nasal bone – posterior edge of cribiform plate |
| 16. Frontal length (lateral): suture between nasal and frontal bone – lateral ridge of frontal bone |
| 17. Mandible height (lateral): posteriormost point on mandibular angle – superior most point of condyle |
| 18. Mandible length (lateral): posteriormost point of mandibular angle – anteriormost point of lower diastema |
| 19. Nasal bone length (lateral): anterior tip of nasal bone – suture between nasal and frontal bone |
| 20. Nasal width (dorsoventral): right most maxilla-incisive bone suture – left most maxilla-incisive bone suture |
| 21. Right mandibular notch length (lateral): right coronoid process of mandible – right condylar process of mandible |
| 22. Upper diastema length (lateral): anteriormost point of upper diastema – posterior most point of upper diastema |
Fig. 1.
Schematic of adult rat skull. (A) Linear measurements of the total skull and neurocranium digitized from the dorsoventral (left) and lateral (right) radiographic views. (B) Measurements of the viscerocranium digitized from the dorsoventral (left) and lateral (right) radiographic views. Measurement numbers and anatomical descriptions of points digitized correspond to those in Table 3.
Data analysis
We modelled the body mass and skeletal growth of each animal with the non-linear logistic Gompertz equation. The Gompertz equation provides one of the best-fit models for the characteristically sigmoidal nature of mammalian growth (Laird et al. 1965, 1968; Laird, 1966; Jolicoeur & Heusner, 1986; Lebeau et al. 1986). It is a flexible sigmoidal curve, and provides a good empirical fit to the data, as well as parameters that permit biological interpretation of growth (Fiorello & German, 1997; Miller & German, 1999; Reichling & German, 2000; Farmer & German, 2004). A further discussion of the history of modelling of growth can be found in Zeger & Harlow (1987). We fit a Gompertz equation to body mass and the 22 longitudinal skeletal measurements using two algebraically equivalent forms:
| (1) |
| (2) |
Both forms are useful because they provide estimates of different parameters with biological meaning (Laird et al. 1965; Miller & German, 1999). Five parameters can be obtained from equations (1) and (2): A is an estimate of final size, or the asymptote of the sigmoidal curve; b describes a lag in initial growth – a unit increase in b delays a unit increase in the size of y, the measurement of interest (Farmer & German, 2004); k measures growth decay and estimates how fast growth slows as animals approach adult size; w is an estimate of initial size; and I (which is equal to b*k) estimates the instantaneous initial rate of growth at time = 0 (in these analyses, age at weaning) (Laird et al. 1965; Miller & German, 1999; Reichling & German, 2000). In these analyses we did not test for differences in b, the parameter that describes the delay in initial growth, because b is a function of I, the instantaneous rate of growth, and k, the rate of growth decay and is thus not independent of their values: b = I/k (Farmer & German, 2004).
The first derivative (Eq. 3) of the Gompertz equation was calculated to determine the rate of growth over time. From this equation, two additional, useful biological parameters were separately calculated. Rmax is the maximum rate of growth, calculated as the maximum of the first derivative. Tf represents the time at which growth slows to 5% of its maximum growth rate (Rmax) and we used it as an estimate of growth duration, or the length of time until the animal reached adult size.
| (3) |
The data were analysed using the NONLIN module of systat10 (Wilkinson, 2000). The unit of analysis was the individual rat. That is, we fit a curve to each individual animal's growth trajectory for 22 skeletal measurements and body mass. A one-way anova was used to test for significant differences among treatments (CP, LP1, LP2) for six of the Gompertz parameters describing growth of the craniofacial skeleton and body mass increase: w, I, Rmax, k, Tf, and A. Due to the number of comparisons being made, groups were considered significantly different if the P-value was < 0.001 and marginally different if the P-value was < 0.01.
We were also interested in learning if a low-protein diet across successive generations increased the within-generation (group) variation in sizes and rates of skeletal growth. However, given the structure of our data, it was not possible to test for statistical differences in variation among the diet groups. Therefore, we qualitatively assessed variation by visually comparing groups' ranges of values for each Gompertz parameter and each measurement using box and dot density plots. The range or spread of values is suggestive of within-group variation. We noted if any particular diet group appeared different from the others, and if there was a tendency, within a measurement or parameter, for one group to be represented more frequently than the others.
Results
The Gompertz model fit the data for all skeletal measurements in the CP rats with an average mean corrected R2 = 0.968, and the LP1 data with an average mean corrected R2 = 0.962 (Miller & German, 1999). The mean corrected R2, averaged across 22 measurements of the LP2 data, was 0.843. However, three measurements were excluded from further analyses because of poor fit in the LP2 data: mandible height (R2 = 0.503), neurocranium width (R2 = 0.571) and tympanic bulla width (R2 = 0.661). Figure 2 provides examples of curves with good and poor fit for an individual rat for two different measurements. The model fit the remaining 19 LP2 measurements with mean corrected R2 values ranging from 0.801 (frontal length) to 0.979 (total skull height) and an average mean corrected R2 = 0.885.
Fig. 2.
Example of good- and poor-fit Gompertz curves. (A) Good-fit curve for skull length (lateral view) in a second-generation protein-malnourished (LP2) individual. Mean corrected R2 = 0.910. (B) Poor-fit curve for mandible height in the same individual. Mean corrected R2 = 0.503. This measurement was excluded from further analysis. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
Initial sizes (w) and rates of growth (I)
There was a significant difference among the diet groups in the estimated initial body mass (w) at weaning (21 days for animals in the CP and LP1 treatments, 28 days for animals in the LP2 treatment). As expected, posthoc pair-wise comparisons showed that animals in the CP and LP1 groups were equal, and weighed significantly more at weaning than animals in the LP2 group (Table 4). The actual recorded body mass of rats in the LP2 group was approximately 50% less than animals well nourished in utero and during suckling (Table 5). The instantaneous rate of weight gain (I) in controls was significantly greater than in both groups of malnourished animals (Table 4).
Table 4.
Comparison of groups' average values of growth parameters as determined by Gompertz model. C, controls (CP treatment); 1, first-generation low protein (LP1 treatment); 2, second-generation (LP2 treatment). ‘Typical’ was the most frequent result from post-hoc pair-wise comparisons between treatments, for all measurements, for a given parameter. Both marginal (P < 0.01) and significant (P < 0.001) differences between treatments are indicated by > and < ; no difference by =. A typical result for a given measurement and parameter is indicated by a blank cell. Results that were not typical are noted. Skeletal measurements and definitions are as in Table 3 and Fig. 1. Measurements from the lateral view are indicated by (lat); from dorsoventral view, by (dv)
| Typical | w initial size (C = 1) > 2 | I initial growth rate C > (1 = 2) | Rmax maximum growth rate C > (1 = 2) | k growth decay C > (1 = 2) | Tf duration of growth C < (1 = 2) | A final size (C = 1) > 2 |
|---|---|---|---|---|---|---|
| Body mass | ||||||
| Total skull | ||||||
| 1. Length (lat) | ||||||
| 2. Height | C = 1 = 2 | C > 1 > 2 | C > 1 > 2 | C > 1 > 2 | C < 1 < 2 | C = 1 = 2 |
| 3. Length (dv) | C = 1, C = 2, 1 > 2 | |||||
| 4. Width | ||||||
| Neurocranium | ||||||
| 5. Occipitopalatal length | (C = 1) < 2 | (C = 1) < 2 | ||||
| 6. Distance between mastoid processes | C > 1 > 2 | |||||
| 8. Length (lat) | C < 1 < 2 | C = 1 = 2 | ||||
| 9. Height | C = 1, C = 2, 1 > 2 | |||||
| 10. Length (dv) | C = 1, C = 2, 1 > 2 | |||||
| Viscerocranium | ||||||
| 12. Distance between angles | C = 1 = 2 | C = 1 = 2 | ||||
| 13. Distance between condyles | ||||||
| 14. Distance between coronoids | C < 1 < 2 | C = 1 = 2 | ||||
| 15. Facial length | (C = 1) < 2 | C = 1 = 2 | ||||
| 16. Frontal length | C = 1 = 2 | C > 1 > 2 | C < 1 < 2 | (C = 1) < 2 | ||
| 18. Mandible length | C = 1 = 2 | C = 1, 1 = 2, C > 2 | C = 1, 1 = 2, C > 2 | |||
| 19. Nasal bone length | C > 1 > 2 | C = 1, 1 = 2, C < 1 | ||||
| 20. Nasal width | ||||||
| 21. Mandibular notch length | C = 1, C = 2, 1 > 2 | C > 1 > 2 | C > 1 > 2 | (C = 1) < 2 | ||
| 22. Upper diastema length | C > 1 > 2 | C = 1, 1 = 2, C < 2 |
Table 5.
Percentage differences between treatments for average actual measured weaning and final sizes for body mass and 19 skeletal measurements *. For each two-group comparison, percentage differences were calculated by subtracting the second group from the first, and dividing that difference (positive or negative) by the first group. Positive percentages indicate that the first group is larger than the second group in the comparison. Negative percentages indicate that the first group is smaller. C, controls (CP treatment); 1, first-generation low-protein (LP1 treatment); 2, second-generation (LP2 treatment). Skeletal measurements and definitions are as in Table 3 and Fig. 1. Measurements from the lateral view are indicated by (lat); from the dorsoventral view, by (dv). Figures 4 and 10 provide graphical comparisons of groups' average actual measurements at weaning and at adult size, respectively
| Percentage difference | ||||||
|---|---|---|---|---|---|---|
| Weaning | Final | |||||
| Body mass | C vs. 1 1.0 | 1 vs. 2 50.3 | C vs. 2 49.8 | C vs. 1 15.8 | 1 vs. 2 19.2 | C vs. 2 31.9 |
| Total skull | ||||||
| 1. Length (lat) | −0.4 | 10.3 | 9.9 | 3.1 | 5.1 | 8.0 |
| 2. Height | −0.8 | 4.2 | 3.5 | 3.5 | 1.5 | 5.0 |
| 3. Length (dv) | 1.2 | 10.3 | 11.3 | 2.8 | 10.5 | 13.0 |
| 4. Width | −0.2 | 8.6 | 8.5 | 1.8 | 8.0 | 9.6 |
| Neurocranium | ||||||
| 5. Occipitopalatal length | 0.8 | −23.7 | −22.8 | 0.9 | −21.7 | −20.6 |
| 6. Distance between mastoids | −0.8 | 15.8 | 15.1 | 1.9 | 15.0 | 16.5 |
| 8. Length (lat) | −1.3 | 9.4 | 8.2 | 1.0 | 4.0 | 5.0 |
| 9. Height | −1.1 | 15.3 | 14.4 | 3.2 | 7.0 | 10.0 |
| 10. Length (dv) | 1.2 | 9.9 | 11.0 | 2.1 | 6.2 | 8.2 |
| Viscerocranium | ||||||
| 12. Distance between angles | −0.7 | 5.7 | 5.0 | 2.8 | 7.1 | 9.7 |
| 13. Distance between condyles | −1.1 | 9.8 | 8.8 | 4.3 | 7.1 | 11.1 |
| 14. Distance between coronoids | 0.0 | 7.0 | 7.0 | 1.9 | 2.8 | 4.6 |
| 15. Facial length | 0.4 | −8.0 | −7.5 | 4.2 | −7.0 | −2.4 |
| 16. Frontal length | 0.5 | −1.8 | −1.4 | 6.1 | −24.3 | −16.7 |
| 18. Mandible length | −0.8 | 7.2 | 6.5 | 5.8 | 1.3 | 7.1 |
| 19. Nasal bone length | 1.1 | 33.3 | 34.1 | 3.6 | 29.9 | 32.4 |
| 20. Nasal width | 1.0 | 36.5 | 37.2 | 0.8 | 25.3 | 25.9 |
| 21. Mandibular notch length | 5.7 | 7.3 | 12.6 | 7.3 | 12.6 | 19.0 |
| 22. Upper diastema length | 1.0 | 10.7 | 11.5 | 4.7 | 8.1 | 12.5 |
Actual weaning size is equivalent to estimated initial size (w) in the model. Actual final size is equivalent to estimated final size (A) in the model.
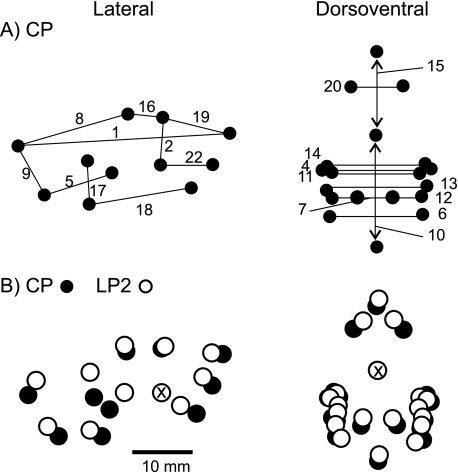
When testing for differences in initial sizes (w) of the skeletal measurements, the typical result (i.e. the result for 10 of 19 measurements; e.g. Fig. 3a) was that animals in the CP and LP1 groups were of equal size, and marginally or significantly larger than those in the LP2 group (Table 4). In the four instances where differences among diet groups were not significant, i.e. all treatments were the same, three were from the viscerocranium: total skull height, mandible length, distance between the angles, and frontal bone length (Fig. 3b). By contrast, the initial occipitopalatal and facial lengths (Fig. 3c) in the LP2 treatment were significantly larger than in controls and first-generation malnourished rats. As illustrated in Fig. 3, for nearly all estimates of initial size, there was a greater range of values within the LP2 group than within the CP or LP1 groups. Table 5 shows the percentage difference among the groups in actual sizes of skeletal measurements at weaning. Figure 4 shows and compares the location of landmarks digitized from radiographs of a CP and an LP2 individual on the day of weaning.
Fig. 3.
Box plots of estimated size at weaning (w). The second-generation protein-malnourished group had a greater range of values for w in all three measurements. (A) Controls and the LP1 treatment had larger distances between the mastoid processes than the LP2 group. Average mean corrected R2 of CP group = 0.993, LP1 group = 0.991, LP2 group = 0.919. (B) There were no differences in initial frontal lengths among the treatments. Average mean corrected R2 of CP group = 0.907, LP1 group = 0.855, LP2 group = 0.801. (C) LP2 rats had longer facial lengths than controls and the LP2 group. Average mean corrected R2 of CP group = 0.970, LP1 group = 0.981, LP2 group = 0.824. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
Fig. 4.
Landmark locations illustrating average actual measurements on the day of weaning. (A) Control with linear distances between landmarks (measurements) from the lateral (left) and dorsoventral (right) radiographic views noted, as in Fig. 1 and Table 3. (B) Average LP2 landmark locations (open circles) compared with the control (black circles). The two comparable sets of points from the lateral view are overlaid and aligned at the posteriormost point of the upper diastema, indicated by the X through the point. Points from dorsoventral view are overlaid and aligned at the posterior edge of the cribiform plate. Refer to Table 5 for actual percentage differences between groups for each measurement.
The initial rates of skeletal growth (I) were always significantly faster in controls, and typically (17 of 19 measurements) the LP1 and LP2 treatments had equal rates of growth at weaning (Table 4). Total skull height and mandibular notch length initial growth rates were marginally or significantly greater in the LP1 group than the LP2 group (Table 4). Although there were some exceptions (data not shown), females in the CP group tended to have more variation in instantaneous rates of growth than did malnourished females.
Maximum rates of growth (Rmax)
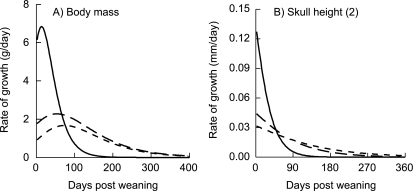
The estimated maximum rate of body mass increase was significantly greater in CP animals than in the LP1 or LP2 groups (Fig. 5a). Females in the control treatment had an average maximum rate of growth (scaled to estimated final body mass: Rmax/A) of 0.014 (SD = 0.004) g/day; both LP1 and LP2 females had a scaled Rmax = 0.005 (SD = 0.001) g/day.
Fig. 5.
Estimated rates of growth for (A) body mass increase over time, and (B) skull height, calculated from the 1st derivative of the Gompertz model (Eq. 2). Solid line is CP group, long dash is LP1 treatment, short dash is LP2 group. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
The estimated maximum rates of growth for all measurements of the craniofacial skeleton were significantly greater in animals in the CP treatment than in the LP1 or LP2 groups. Posthoc pair-wise comparisons of Rmax for skeletal measurements typically showed no differences between the LP1 and LP2 treatments. Four of the 19 measurements were not typical. The LP1 animals had either marginally or significantly faster rates of growth than the LP2 animals in total skull height (Fig. 5b), one measurement of the neurocranium and three of the viscerocranium (Table 4). Scaling maximum rates of growth to estimated final sizes (Rmax/A) showed that the fastest growing bones in all treatments were almost exclusively in the viscerocranium (Table 6). Neurocranium height was one of the slowest growing measurements in all groups (Table 6).
Table 6.
Comparison of scaled * maximum rates of skeletal growth (Rmax) among treatments. For each treatment, measurements are ordered from highest to lowest scaled Rmax. CP, control treatment; LP1, first-generation low-protein; LP2, second-generation low-protein. Measurements from the lateral view are indicated by (lat); from the dorsoventral view, by (dv). Rmax/A is a group's average scaled Rmax; SD, standard deviation. All units are (mm × 10−3) day−1
| CP | LP1 | LP2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Measurement | Rmax/A | (SD) | Measurement | Rmax/A | (SD) | Measurement | Rmax/A | (SD) |
| Between condyles | 20.3 | (4.5) | Frontal length | 8.1 | (3.1) | Facial length | 5.8 | (1.8) |
| Frontal length | 17.6 | (4.0) | Nasal bone length | 7.7 | (3.2) | Skull length (dv) | 5.8 | (1.9) |
| Nasal bone length | 17.1 | (3.7) | Skull length (dv) | 7.4 | (3.4) | Neurocranium length (dv) | 5.5 | (2.2) |
| Skull width | 16.5 | (1.6) | Upper diastema length | 6.8 | (3.3) | Upper diastema length | 5.3 | (2.0) |
| Nasal bone width | 16.5 | (2.0) | Mandibular notch length | 6.6 | (1.6) | Nasal bone length | 5.0 | (1.9) |
| Skull length (dv) | 16.4 | (3.9) | Neurocranium length (dv) | 6.5 | (3.9) | Skull width | 4.9 | (1.6) |
| Neurocranium length (dv) | 15.8 | (4.3) | Facial length | 6.1 | (1.9) | Mandible length | 4.9 | (1.8) |
| Between coronoids | 15.5 | (2.4) | Between angles | 5.9 | (1.5) | Between condyles | 4.8 | (2.6) |
| Between mastoids | 15.1 | (1.8) | Between mastoids | 5.7 | (1.2) | Between mastoids | 4.7 | (1.1) |
| Upper diastema length | 14.7 | (0.7) | Skull length (lat) | 5.5 | (1.3) | Between angles | 4.6 | (1.3) |
| Neurocranium length (lat) | 14.7 | (1.6) | Mandible length | 5.3 | (0.8) | Occipitopalatal length | 4.6 | (1.3) |
| Skull height | 14.6 | (1.4) | Occipitopalatal length | 5.2 | (1.8) | Skull length (lat) | 4.6 | (1.1) |
| Skull length (lat) | 14.6 | (0.9) | Skull height | 5.2 | (1.4) | Between coronoids | 4.6 | (1.6) |
| Facial length | 14.5 | (2.2) | Between coronoids | 4.9 | (1.1) | Neurocranium length (lat) | 4.5 | (1.8) |
| Between angles | 14.3 | (1.8) | Between condyles | 4.9 | (2.2) | Frontal length | 3.6 | (1.2) |
| Occipitopalatal length | 13.2 | (4.0) | Skull width | 4.5 | (1.5) | Nasal bone width | 3.6 | (2.0) |
| Mandibular notch length | 12.8 | (3.0) | Nasal bone width | 4.5 | (1.1) | Skull height | 3.5 | (1.1) |
| Neurocranium height | 12.4 | (3.2) | Neurocranium length (lat) | 3.9 | (1.8) | Mandibular notch length | 3.4 | (1.2) |
| Mandible length | 11.9 | (1.7) | Neurocranium height | 3.8 | (1.6) | Neurocranium height | 3.4 | (1.5) |
Scaled maximum rates of growth were calculated by the formula (Rmax/A) for each measurement.
For most measurements, the CP treatment exhibited the greatest rage of values for maximum rates of growth, and for many, the LP2 treatment the least. However, no clear pattern of variation between the neruo- vs. visceroskeleton emerged, as shown in Fig. 6.
Fig. 6.
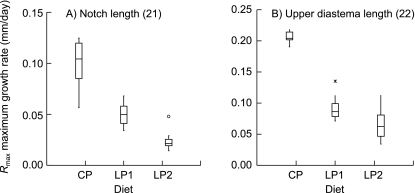
Box plots of estimated maximum rates of growth (Rmax) for two measurements of the viscerocranium. The CP group had (A) greater Rmax in both mandibular notch length, and (B) upper diastema length increase than malnourished groups, although there was a greater range of values in rate of notch length increase among the control treatment. Average mandibular notch length mean corrected R2 of CP group = 0.942, LP1 group = 0.927, LP2 group = 0.828. Average upper diastema length mean corrected R2 of CP group = 0.985, LP1 group = 0.982, LP2 group = 0.904. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
Growth decay (k)
For all skeletal measurements and for body mass, growth slowed significantly faster in well-nourished females as they approached adult size than it did in either of the malnourished groups. Only total skull height and frontal length growth decayed faster in the LP1 animals than in the LP2 animals. For all other measurements the rates of decay were the same between animals in the LP1 and LP2 groups. As with Rmax, although the animals in the CP group tended to have a larger spread of values in more measurements, this was not always the case and no clear pattern emerged.
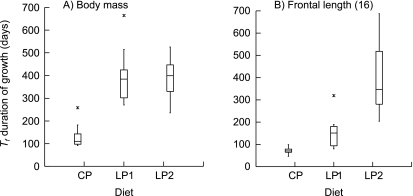
Durations of growth (Tf) and final sizes (A)
There were marginal or significant differences in growth duration among the diet groups for all skeletal measurements and for body mass. The duration of growth in body mass for the animals on the control diet was shorter than for both groups on the low-protein diet (Fig. 7a). There was no difference between the two malnourished groups. Average estimated Tf for the CP group was 132 days (SD = 52), for the LP1 group 397 days (SD = 119) and for the LP2 group 397 days (SD = 82).
Fig. 7.
Box plots of estimated growth duration (Tf) for (A) body mass, and (B) frontal bone length. Average body mass mean corrected R2 of CP group = 0.983, LP1 group = 0.996, LP2 group = 0.995. Average frontal bone length mean corrected R2 of CP group = 0.907, LP1 group = 0.855, LP2 group = 0.801. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
The typical result among all craniofacial skeletal measurements (11 of 19) was for both groups on the low-protein diet to increase in size marginally or significantly longer than controls, and for Tf in LP1 and LP2 treatments to be equal (Table 4). However, the majority of viscerocranial measurements were not typical (Table 4). For five viscerocranium measurements the second-generation low-protein treatment grew longer than first-generation or control females (e.g. Fig. 7b). There was a tendency among measurements for controls to have reduced variation in this growth parameter and for the second generation of malnourished females to have increased variation. The animals in the LP2 group did not show the greatest range of values for Tf in only one measurement of the viscerocranium (distance between condyles).
There was a significant difference among the groups' average estimated final body masses (A). The average final body mass of the LP2 group (352 g, SD = 50) was significantly less than that of the CP (467 g, SD = 59) and LP1 (495 g, SD = 84) groups, which were not statistically different. The actual measured adult body mass of rats in the control group was ∼30% greater than those in the LP2 treatment (Table 5).
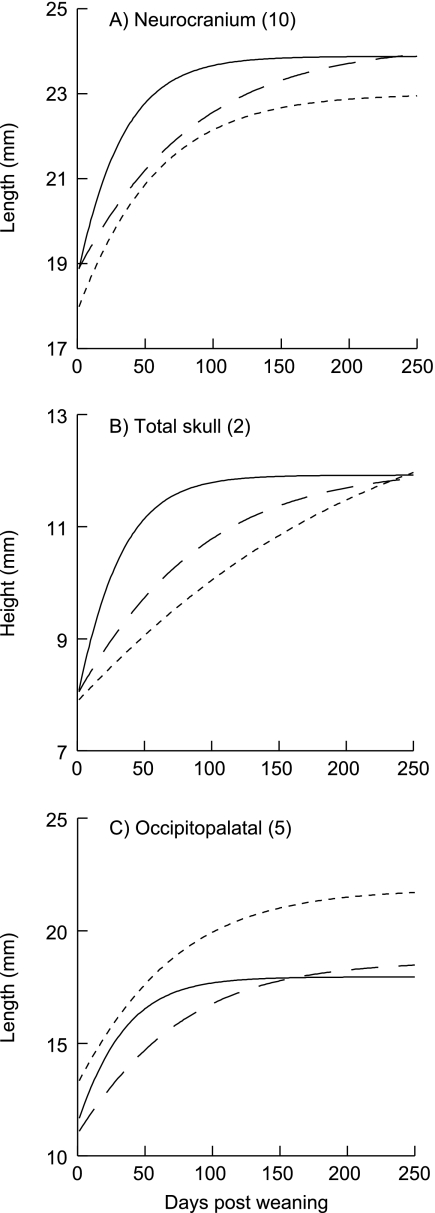
All estimated final sizes of skeletal measurements were equal between the control and LP1 treatments, and 10 of 19 measurements were marginally or significantly less in the LP2 animals compared with controls and animals malnourished only post-weaning (LP1) (Table 4). Figure 8(a) shows this typical result for A. No differences between the LP1 and LP2 treatments were found in total skull height (Fig. 8b), lateral neurocranium length, and three measurements of the viscerocranium: distances between angles of the mandible, between the coronoid processes, and facial length (Table 4). Posthoc pair-wise comparisons indicated that only average estimated occipitopalatal (Figs 8c and 9a) and frontal lengths were larger in the LP2 treatment than in the CP or LP1 treatments. The tendency of controls to have less variation in measures of the neurocranium is illustrated in Fig. 9. With respect to the viscerocranium, there was a tendency for second-generation low-protein females to have a greater range of final sizes than other groups (e.g. Fig. 9b). The actual recorded final skeletal measurements of rats in the LP2 group ranged from ∼30% less to ∼5% greater than those in the CP treatment (Table 5). Figure 10 shows and compares the location of landmarks digitized from the final radiograph of an adult rat representative of each diet group.
Fig. 8.
Gompertz curves show A, estimated final sizes, for an individual from each diet treatment. Solid line is CP group, long dash is LP1 treatment, short dash is LP2 group. (A) As in all skeletal measurements, final neurocranium lengths (dorsoventral view) were the same between controls and first-generation low-protein rats, and as was typical (10 of 19 measurements), the LP2 group was significantly smaller. Average mean corrected R2 of CP group = 0.959, LP1 group = 0.948, LP2 group = 0.863. (B) Final total skull height did not differ among animals in the three treatments. Average mean corrected R2 of CP group = 0.987, LP1 group = 0.980, LP2 group = 0.979. (C) Final occipitopalatal distance in the LP2 treatment was greater than in the control and LP1 animals. Average mean corrected R2 of CP group = 0.950, LP1 group = 0.968, LP2 group = 0.909. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
Fig. 9.
Box plots of A, estimated final sizes. (A) Occipitopalatal length was a typical result, and as with all measures of the neurocranium, the control group had less variation compared with both groups of malnourished rats. Average mean corrected R2 of CP group = 0.950, LP1 group = 0.968, LP2 group = 0.909. (B) Nasal bone length was also a typical result and illustrates the tendency for the LP2 group to have greater variation in measurements of the viscerocranium. Average mean corrected R2 of CP group = 0.954, LP1 group = 0.945, LP2 group = 0.852. Numbers in parentheses correspond to measurement numbers in Fig. 1 and Table 3.
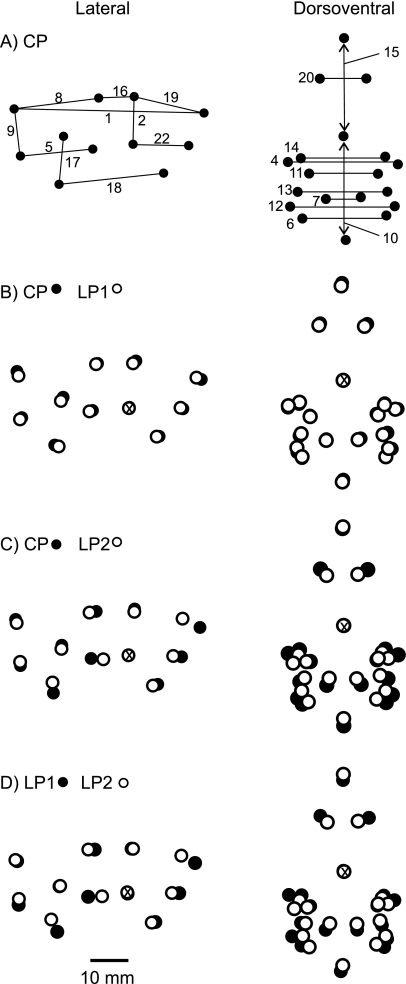
Fig. 10.
Landmark locations illustrating average actual measurements at adult size. (A) Control with linear distances between landmarks (measurements) from the lateral (left) and dorsoventral (right) radiographic views noted, as in Fig. 1 and Table 3. (B) Average LP1 landmark locations (open circles) compared with the control (black circles). (C) Average LP2 landmark locations (open) compared with the control. (D) LP2 landmarks (open) compared with LP1 landmarks (black). In B–D the two comparable sets of points from the lateral view are overlaid and aligned at the posteriormost point of the upper diastema, indicated by the X through the point. Points from dorsoventral view are overlaid and aligned at the posterior edge of the cribiform plate. Refer to Table 5 for actual percentage differences between groups for each measurement.
Discussion
There was a significant impact of life-long protein malnutrition on the patterns of growth that generate final adult sizes of the craniofacial skeleton, relative to rats malnourished only post-weaning and to rats on a control diet. Over most craniofacial measurements, the female offspring of post-weaning malnourished females did not catch up in size to the first generation or to controls, although certain aspects of their craniofacial skeleton showed more resistance to the effects of protein restriction than others. The second generation's growth trajectories more closely resembled the longer and slower growth of the first generation.
Catch up growth: neuro- vs. viscerocranium
Previous work from our laboratory demonstrates that post-weaning malnourished female rats can catch up in skeletal size to controls (Miller & German, 1999; Reichling & German, 2000). This was not true for females malnourished from conception, through fetal life, suckling and post-weaning development, despite the fact that the animals were allowed to continue to grow until they naturally stopped increasing in size. However, the impact of protein malnutrition in the second generation malnourished animals differed in the neurocranium relative to the viscerocranium. This result is consistent with previous studies (Pucciarelli, 1980, 1981; Fields, 1991; Lightfoot & German, 1998). In Miller & German (1999), measurements of the viscerocranium are the only measurements in which the final size of the first generation is smaller than that of the controls (these differences were no longer present when the second-generation animals were added to the analyses).
Although there are no differences in initial sizes (w) between controls and animals malnourished post-weaning (Miller & German, 1999), we hypothesized that the offspring (LP2) of post-weaning malnourished dams would be smaller than the offspring (LP1) of well-nourished dams (Naismith, 1969; Sasaki et al. 1982; Fortman et al. 2005). The measurements for which the LP2 animals were equal or larger than controls and/or LP1 females at weaning (total skull height and length; occipitopalatal and neurocranium lengths; distance between the angles; frontal, mandible, facial and mandibular notch lengths) suggest that these bones have a developmental priority with respect to the allocation of amino acids and possibly energy resources in utero, and during suckling (Chowdhury & Orskov, 1997; Masanes et al. 1999; Horton, 2005). On the other hand, measurements for which the LP2 rats were smaller indicated that growth of those bones is more responsive to trans-generational fluctuations in nutrient resources.
Priorities, however, may change through ontogeny. A comparison of initial (w) to final (A) sizes in the LP2 treatment shows that some measurements were not affected by gestational or post-weaning malnutrition: both w and A in the LP2 treatment were the same as controls and/or the first generation. Other measurements of the second generation were affected only after weaning. For example, in the neurocranium, only one measurement of the second generation was equal to or greater than controls or the first-generation low-protein rats at weaning and at adult size. By contrast, in the viscerocranium four of ten measurements were equal among the groups at weaning and at adult size. However, there were ultimately more measurements of the neurocranium (three of five) that caught up in size to controls.
The findings of Fortman et al. (2005) support the idea that different aspects of craniofacial skeletal growth are affected differently during ontogeny. They radiographed a large sample of offspring from dams on either the control or the low-protein diet. These infants are the equivalent of our CP/LP1 (no pre-weaning malnutrition) and LP2 (pre-weaning malnutrition) groups, and the digitized anatomical landmarks and measurements correspond directly to the current study. Fortman et al. reported that at each of the three ages they examined, 9, 16 and 22 days, offspring of low-protein females always have smaller cranial measurements. However, there is a significant age × diet interaction in six of their nine measurements. That is, growth during the suckling phase differs between treatments and measurements. In fact, two of the three measurements that we found to be equal between control and LP2 groups at weaning (total skull and neurocranium lengths) are the two measurements that have increased rates of growth in low-protein offspring (compared with controls) between ages 16 and 22 days in Fortman et al. (Note that they killed all of the offspring at 22 days, whereas we weaned our LP2 group at 28 days.) Fortman et al. (2005) and others also report that eye and brain weights, scaled to body mass, are significantly greater in the offspring of low-protein dams compared with controls (Desai et al. 1996; Opperman, 2000; Reichling & German, 2000). All of these results suggest not only that tissues themselves respond differently to protein restriction, but also that the mechanisms that govern cell differentiation and tissue development may be affected differently during the formation of cartilage, intramembranous ossification, and organogenesis. Tissue- or lower-level analyses would be necessary to test these hypotheses.
Patterns of growth
The growth trajectories of the second malnourished generation, graphically or measured by the Gompertz parameters, more closely resembled the longer and slower growth of first-generation malnourished females. In nearly all comparisons of growth rate parameters (I, Rmax, k) the first and second generations were equal, and significantly different from controls. Visually, this was equivalent to the flatter, longer and slower growth curve that Miller & German (1999) describe. Even though the LP1 animals had just been placed on the low-protein diet, and the LP2 animals had been protein restricted in utero and through suckling, the instantaneous rates of skeletal growth at weaning (I) were very similar between the two malnourished groups. This suggests that the effects of protein restriction are almost immediate in the LP1 generation, and that initial growth rates are highly susceptible to perturbation. The additional stores of protein that the LP1 generation received from their well-nourished dams did not provide a protection against the reduction in protein at weaning, and there was no lag in time of the impact of this dietary effect.
The physiological mechanisms contributing to such an immediate effect are not completely understood. Harel & Tannenbaum (1993) report that in protein-malnourished (4% protein diet) juvenile male rats, plasma GH concentrations are significantly reduced after 4 days of protein restriction. However, there are no differences in the 3.3 h cycling of GH release. They show that plasma insulin concentrations are likewise reduced, but glucose levels are unaffected. In 21.5-day fetuses of short-term malnourished females (8% protein diet at mating), circulating IGF-1 levels are significantly reduced although IGF-binding protein-1 levels are as in controls (El-Khattabi et al. 2003). Gomez et al. (1996) report that cultured bone marrow cells from severely malnourished 21-day-old rats have significantly longer cell-cycle times owing to increased durations of synthesis, and gap-1 and meiosis I phases. This result corresponds to the finding of El-Khattabi et al. (2003) that DNA synthesis was markedly reduced in cells cultured from malnourished fetuses. All of these results are consistent with, and may in part explain, significantly lower instantaneous rates of initial growth in the first and second generations of malnourished animals.
The duration of growth (Tf) was dramatically influenced by protein malnutrition, in both post-weaning and life-long malnourished animals. Post-weaning malnourished females reached a control final size because, despite slower growth rates, they had durations of growth nearly three times longer than control animals. Growth of life-long malnourished rats did not show further compensatory growth duration: most measurements stopped increasing at the same time as LP1 animals, although three measurements of the viscerocranium continued to grow longer than in LP1 animals. The measurements in the LP2 animals that did catch up, or were larger than the other treatments, grew for a longer period of time than controls and first-generation animals, and many of these parts of the skull had initial sizes at weaning that were not as affected by the prenatal/suckling malnutrition. However, being larger at weaning, and growing significantly longer, did not necessarily predict that LP2 final adult size would be the same, as was the case in mandibular notch length.
The cellular level mechanisms that signal the switch from growth to maintenance in the normally growing rat involve the GH axis and its downstream targets, as well as other endocrine, paracrine, neural and genetic factors. How life-long protein malnutrition affects these factors is unclear, especially as studies that report the effects of protein restriction on these factors test for differences in offspring of short-term malnourished females. Recent work suggests that the pathways regulating new protein synthesis (DNA transcription and mRNA translation) are themselves regulated by nutrient levels, and there is cross-talk with pathways and events associated with GH, IGF-1, and other endocrine factors involved with growth/maintenance (Proud, 2002, 2004a,b; Beugnet et al. 2003).
Variation in growth parameters
We hypothesized that a low-protein diet across successive generations would increase the variation in sizes, and in rates of skeletal growth, in life-long malnourished females compared with controls and first-generation malnourished animals. This would be consistent with the results of Jones & German (2005). They generate a sample of variation for each Gompertz parameter using 19 craniofacial measurements and the data collected by Miller & German (1999), i.e. the control and post-weaning groups in the current study. They then test for differences between the sexes and two diet groups in the coefficients of variation. They find that across all measurements of the craniofacial skeleton, animals on the low-protein diet have increased variation in all parameters except w, initial size. Their result for initial size at weaning is consistent with ours, as it was the LP2 group that had a greater range of values for w compared with controls and the LP2 group.
Graphical comparisons of initial (w) and adult (A) sizes, and durations of growth (Tf), showed that for most measurements controls had reduced variation and life-long malnourished females a greater range of values for these parameters than other groups. However, in apparent contrast to Jones & German (2005), our graphical comparisons of the spread of data for each measurement and parameter showed that the control group tended to have increased variation in the rates of skeletal growth (I, Rmax, k). Increased variation within this group is indicative of greater developmental plasticity in the mechanisms through which rates of growth are governed. This suggests the processes that regulate rates of normal growth are less canalized (Hallgrimsson et al. 2002); there can be greater variation within these processes than within those that regulate final outcomes (e.g. durations of growth and final sizes) without affecting growth per se. The reverse seems to be true for the LP2 animals. Increased variation in initial sizes, durations of growth and final sizes of life-long malnourished animals suggests greater developmental plasticity in the mechanisms that generate these features of the overall growth trajectory. The consequences of these patterns of variation in the face of early nutritional insult are a matter of considerable debate (Ozanne & Hales, 1999; Crespi & Denver, 2005; Horton, 2005; Jones, 2005; Kuzawa, 2005; Lampl, 2005).
Conclusion
Some of the results from this study differed from those of Miller & German (1999) and Reichling & German (2000). Significant differences in final skeletal sizes between the controls and first-generation animals were no longer significant when a third treatment (the LP2 animals) was added to the analysis. Discrepancies were due to the partitioning of variance in the models: differences between the LP2 group and the other two treatments were so great that the differences between the controls and first-generation animals were no longer significant. With respect to the multigenerational effect of protein malnutrition on variation within the craniofacial skeleton, or within a given parameter describing growth, our results point to a complex interaction between females' genetically regulated developmental processes and their early nutritional environment.
This study was the first to quantify the effects of a pathological low-protein diet on growth of the craniofacial skeleton in an animal model, under conditions that more closely approximate what we would find in nature during prolonged periods of nutritional stress.
Acknowledgments
We appreciate the conscientious animal care, feeding and data collection of Joe Weber, James Fortman and Doug Bauman for the LP2 animals. We thank Meredith Farmer, Guy Cameron and the late Tom Kane for their time and input, Donna Jones for her thoughtful criticisms of earlier drafts, and Jeffery Miller and Tim Reichling for the use of their data. Caroline Cooper extensively modified Fig. 1. S.L.L. received support from the Department of Biological Sciences' Wendell-Weiman fund. This research was conducted by S.L.L. as partial fulfillment of the requirements of a Masters degree in Biological Sciences at the University of Cincinnati.
References
- Beugnet A, Tee AR, Taylor PM, Proud CG. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J. 2003;372:555–566. doi: 10.1042/BJ20021266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourrin S, Ammann P, Bonjour JP, Rizzoli R. Dietary protein restriction lowers plasma insulin-like growth factor I (IGF-I), impairs cortical bone formation, and induces osteoblastic resistance to IGF-I in adult female rats. Endocrinology. 2000;141:3149–3155. doi: 10.1210/endo.141.9.7633. [DOI] [PubMed] [Google Scholar]
- Cameron G, Eshelman B. Growth and reproduction in hispid cotton rats (Sigmodon hispidus) in response to naturally occurring levels of dietary protein. J Mammal. 1996;77:220–231. [Google Scholar]
- Chowdhury S, Orskov E. Protein energy relationships with particular reference to energy undernutrition: a review. Small Ruminant Res. 1997;26:1–7. [Google Scholar]
- Crespi E, Denver R. Ancient origins of human developmental plasticity. Am J Human Biol. 2005;17:44–54. doi: 10.1002/ajhb.20098. [DOI] [PubMed] [Google Scholar]
- Desai M, Crowther N, Lucuc A, Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br J Nutr. 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- Edozier J, Switzer B. Influence of diet on growth in the rat. J Nutr. 1978;108:282–290. doi: 10.1093/jn/108.2.282. [DOI] [PubMed] [Google Scholar]
- El-Khattabi I, Gregoire F, Remacle C, Reusens B. Isocaloric maternal low-protein diet alters IGF-I, IGFBPs, and hepatocyte proliferation in the fetal rat. Am J Physiol Endocrinol Metabolism. 2003;285:E991–E1000. doi: 10.1152/ajpendo.00037.2003. [DOI] [PubMed] [Google Scholar]
- Farmer MA, German RZ. Sexual dimorphism in the craniofacial growth of the guinea pig (Cavia porcellus) J Morph. 2004;259:172–181. doi: 10.1002/jmor.10180. [DOI] [PubMed] [Google Scholar]
- Fields HW. Craniofacial growth from infancy through adulthood. Pediatr Clin North Am. 1991;38:1053–1088. doi: 10.1016/s0031-3955(16)38189-5. [DOI] [PubMed] [Google Scholar]
- Fiorello C, German R. Heterochrony in conspecific mammals: craniofacial growth in giant, standard, and dwarf rabbits. Evolution. 1997;51:250–261. doi: 10.1111/j.1558-5646.1997.tb02406.x. [DOI] [PubMed] [Google Scholar]
- Fortman J, Reichling T, German R. Maternal protein malnutrition affects pre-weaning skeletal growth in offspring, but not all visceral organs in Rattus norvegicus. Growth Dev Aging. 2005;69:39–52. [PubMed] [Google Scholar]
- German R. The ontogeny of sexual dimorphism: the implications of longitudinal vs. cross-sectional data for studying heterochrony in mammals. In: Anapol F, German R, Jablonski N, editors. Shaping Primate Evolution: Form, Function, and Behavior. Cambridge: Cambridge University Press; 2004. pp. 11–23. [Google Scholar]
- Gomez JL, Campos C, Rangel P, Ortiz R. Cell cycle phase duration in bone marrow cells from malnourished rats during suckling. Mutat Res. 1996;352:57–60. doi: 10.1016/0027-5107(95)00228-6. [DOI] [PubMed] [Google Scholar]
- Hallgrimsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Am JPhys Anthropol Suppl. 2002;35:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel Z, Tannenbaum GS. Dietary protein restriction impairs both spontaneous and growth hormone-releasing factor-stimulated growth hormone release in the rat. Endocrinology. 1993;133:1035–1043. doi: 10.1210/endo.133.3.8103447. [DOI] [PubMed] [Google Scholar]
- Herbert C, Shutt J, Ball R. Plasma amino acid concentrations as an indicator of protein availability to breeding herring gulls (Larus argentatus) Auk. 2002;119:185–200. [Google Scholar]
- Horton TH. Fetal origins of developmental plasticity: Animal models of induced life history variation. Am J Human Biol. 2005;17:34–43. doi: 10.1002/ajhb.20092. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gancedo B, Agis-Torres A, Lopez-Oliva ME, Munoz-Martinez E. Dietary protein concentration correlates in a complex way with glucose metabolism and growth performance in pregnant rats. Domest Anim Endocrinol. 2004;26:277–289. doi: 10.1016/j.domaniend.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Heusner A. Log-normal variation belts for growth curves. Biometrics. 1986;42:785–794. [PubMed] [Google Scholar]
- Jones D, German R. Variation in ontogeny. In: Hallgrimsson B, Hall B, editors. Variation: a Hierarchical Examination of a Central Concept in Biology. New York: Elsevier; 2005. pp. 55–62. [Google Scholar]
- Jones J. Fetal programming: adaptive life-history tactics or making the best of a bad start? Am J Human Biol. 2005;17:22–33. doi: 10.1002/ajhb.20099. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Amino acids as regulators of gene expression. Nutr Metab. 2004;1:3. doi: 10.1186/1743-7075-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Human Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- Laird AK. Postnatal growth of birds and mammals. Growth. 1966;30:349–363. [PubMed] [Google Scholar]
- Laird AK, Sylvanus AT, Barton AD. Dynamics of normal growth. Growth. 1965;29:233–248. [PubMed] [Google Scholar]
- Laird AK, Barton AD, Tyler SA. Growth and time: an interpretation of allometry. Growth. 1968;32:347–354. [PubMed] [Google Scholar]
- Lampl M. Cellular life history and bow tie biology. Am J Human Biol. 2005;17:66–80. doi: 10.1002/ajhb.20094. [DOI] [PubMed] [Google Scholar]
- Lebeau B, Jolicoeur P, Pageau G. Asymptotic growth, egg production and trivariate allometry in Esox masquinongy mitchill. Growth. 1986;50:185–200. [PubMed] [Google Scholar]
- Lightfoot P, German R. The effects of muscular dystrophy on craniofacial growth in mice: a study of heterochrony and ontogenetic allometry. J Morph. 1998;235:1–16. doi: 10.1002/(SICI)1097-4687(199801)235:1<1::AID-JMOR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Martin MA, Fernandez E, Pascual-Leone AM, Escriva F, Alvarez C. Protein calorie restriction has opposite effects on glucose metabolism and insulin gene expression in fetal and adult rat endocrine pancreas. Am J Physiol Endocrinol Metabolism. 2004;286:E542–E550. doi: 10.1152/ajpendo.00242.2003. [DOI] [PubMed] [Google Scholar]
- Masanes R, Fernandez-Lopez JLA, Iemany M, Remesar X, Rafecas I. Effect of dietary protein content on tissue protein synthesis rates in Zucker lean rats. Nutrition Res. 1999;19:1017–1026. [Google Scholar]
- McAdam AG, Boutin S. Effects of food abundance on genetic and maternal variation in the growth rate of juvenile red squirrels. J Evol Biol. 2003;16:1249–1256. doi: 10.1046/j.1420-9101.2003.00630.x. [DOI] [PubMed] [Google Scholar]
- Miller J, German R. Protein malnutrition affects the growth trajectories of the craniofacial skeleton in rats. J Nutr. 1999;129:2061–2069. doi: 10.1093/jn/129.11.2061. [DOI] [PubMed] [Google Scholar]
- Naismith DJ. The foetus as a parasite. Proc Nutr Soc. 1969;28:25–31. [PubMed] [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ozanne S, Hales CN. The long-term consequences of intra-uterine protein malnutrition for glucose metabolism. Proc Nutrition Soc. 1999;58:615–619. doi: 10.1017/s0029665199000804. [DOI] [PubMed] [Google Scholar]
- Pond W, Wu J. Mature body weight and life-span of male and female progeny of primiparous rats fed a low protein of adequate diet throughout pregnancy. J Nutr. 1981;111:1949–1954. doi: 10.1093/jn/111.11.1949. [DOI] [PubMed] [Google Scholar]
- Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269:5338–5349. doi: 10.1046/j.1432-1033.2002.03292.x. [DOI] [PubMed] [Google Scholar]
- Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophysics Res Comm. 2004a;313:429–436. doi: 10.1016/j.bbrc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004b;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- Pucciarelli HM. The effects of race, sex, and nutrition on craniofacial differentiation in rats. A multivariate analysis. Am J Phys Anthropol. 1980;53:359–368. doi: 10.1002/ajpa.1330530307. [DOI] [PubMed] [Google Scholar]
- Pucciarelli HM. Growth of the functional components of the rat skull and its alteration by nutritional effects. A multivariate analysis. Am J Phys Anthropol. 1981;56:33–41. doi: 10.1002/ajpa.1330560104. [DOI] [PubMed] [Google Scholar]
- Reeves P, Nielsen F, Fahey G. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Reichling T. The impact of protein malnutrition on the reproductive success of female rats. Am Zool. 1999;39:101A. [Google Scholar]
- Reichling T. The Impact of Protein Malnutrition on the Reproductive Success of Female Rats. Cincinnati, OH: Department of Biological Sciences, University of Cincinnati; 2000. [Google Scholar]
- Reichling T, German R. Bones, muscles and organs of protein malnourished rats (Rattus norvegicus) grow more slowly but for longer durations to reach normal final size. J Nutr. 2000;130:2326–2332. doi: 10.1093/jn/130.9.2326. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Nakagawa I, Kajimoto M. Effect of protein nutrition throughout gestation and lactation on growth, morbidity, and life span of rat progeny. J Nutr Sci Vitaminol (Tokyo) 1982;28:543–555. doi: 10.3177/jnsv.28.543. [DOI] [PubMed] [Google Scholar]
- da Silva Faria T, da Fonte Ramos C, Sampaio F. Puberty onset in the female offspring of rats submitted to protein or energy restricted diet during lactation. J Nutritional Biochem. 2004;15:123–127. doi: 10.1016/j.jnutbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Sinclair A, Kerebs C, Smith J, Boutin S. Population biology of snowshoe hares. III. Nutrition, plant secondary compounds and food limitation. J Anim Ecol. 1988;57:787–806. [Google Scholar]
- Stewart R, Preece R, Sheppard H. Twelve generations of marginal protein deficiency. Br J Nutr. 1975;33:233–253. doi: 10.1079/bjn19750027. [DOI] [PubMed] [Google Scholar]
- Stewart R, Sheppard H, Preece R, Waterlow J. The effect of rehabilitation at different stages of development of rats marginally malnourished for ten to twelve generations. Br J Nutr. 1980;43:403–412. doi: 10.1079/bjn19800108. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers J, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- Underwood LE, Thissen JP, Lemozy S, Ketelslegers JM, Clemmons DR. Hormonal and nutritional regulation of IGF-I and its binding proteins. Horm Res. 1994;42:145–151. doi: 10.1159/000184187. [DOI] [PubMed] [Google Scholar]
- Wilkinson L. SYSTAT 10. Chicago: Systat, Inc; 2000. [Google Scholar]
- Zeger S, Harlow S. Mathematical models from laws of growth to tools for biologic analysis: fifty yeras of growth. Growth. 1987;51:1–21. [PubMed] [Google Scholar]