Abstract
We investigated the early (< 8 weeks) and late (> 8 weeks) postnatal development of the fibre type composition and fibre cross-sectional area in the superficial masseter and digastric muscle of male rabbits. It was hypothesized, first, that due to the transition between suckling and chewing, during early postnatal development the increase in the proportion of slow fibre types and in fibre cross-sectional areas would be larger in the masseter than in the digastric; and second, that due to the supposed influence of testosterone during late postnatal development, the proportion of slow fibre types in both muscles would decrease. Fibre types were classified by immunostaining according to their myosin heavy chain (MyHC) content. The proportion of slow fibre types significantly increased in the masseter, from 7% at week 1 to 47% at week 8, and then decreased to 21% at week 20, while in the digastric it increased from 5% in week 1 to 19% at week 8 and remained the same thereafter. The changes in the proportion of fast fibre types were the opposite. The remarkable increase and decrease in the proportion of slow fibre types in the masseter was attributed predominantly to MyHC-cardiac α fibres. During early development, the cross-sectional area of all fibres in both muscles increased. However, only the fast fibre types in the masseter continued to grow further after week 8. Before weaning, the fast fibre types in the digastric were larger than those in the masseter, but after week 8, they became larger in the masseter than in the digastric. In adult animals, masseter and digastric had the same percentage of fast fibre types, but these fibres were almost twice as large in masseter as in digastric.
Keywords: development, fibre types, jaw muscles, transition
Introduction
In skeletal muscles, various myosin heavy chain (MyHC) isoforms can be identified, including adult isoforms, e.g. MyHC-I, -cardiac α, -IIA, -IIX (or -IID) and -IIB, and developmental isoforms, e.g. MyHC-embryonic and -neonatal (Schiaffino & Reggiani, 1994; Korfage et al. 2005a,b). During postnatal growth the developmental MyHC isoforms are replaced by the adult isoforms. The MyHC content of muscle fibres is well correlated with their unloaded shortening velocity (Bottinelli et al. 1996). This shortening velocity increases in the sequence of fibres expressing MyHC type I, -cardiac α, -IIA, -IIX and -IIB (Kwa et al. 1995; Sciote & Kentish, 1996; Galler et al. 2002). The amount of force that a muscle fibre can produce is proportional to its cross-sectional area. This area is usually related to the fibre type and increases with the amount of resistance that is experienced during contraction (Edgerton et al. 1995; McCall et al. 1996). Hence, determination of the fibre type composition and cross-sectional area can be used to characterize the functional properties and requirements of a muscle.
Muscle fibres have the ability to alter their phenotype by adapting their MyHC isoforms and cross-sectional area. This adaptation can be caused by several environmental influences such as anatomical (Langenbach et al. 1991) or hormonal changes (Izumo et al. 1986; English & Schwartz, 2002). Functional influences also lead to adaptations, as the proportion of slow fibre types has been related the amount of daily activity in limb (Monster et al. 1978; Kernell & Hensbergen, 1998; Kernell et al. 1998) and jaw muscles (Van Wessel et al. 2005c).
During early postnatal development the main function of mammalian jaw muscles changes from suckling to chewing. As a consequence, the amount of activity of the jaw closers during feeding increases relative to that of the jaw openers (Iinuma et al. 1991; Langenbach et al. 1991; Westneat & Hall, 1992), and this might influence the fibre type composition and fibre cross-sectional areas of these muscles differently. Changes in fibre type composition during early postnatal development have been investigated in the masseter of mice (Gojo et al. 2002; Usami et al. 2003; Shida et al. 2005), miniature swine (Anapol & Herring, 2000) and rabbits (Bredman et al. 1992). These studies showed a change from fast to slower fibre types after birth and/or an effect of weaning. By contrast, after the transition from suckling to chewing, a change from slow to fast fibres has been observed during late postnatal development. Eason et al. (2000) found that the masseter of 8- to 12-week-old male and female rabbits had a similar fibre type composition while the masseter of adult male rabbits had less slow fibres than adult female rabbits (English et al. 1999). This late postnatal development therefore has been attributed to the influence of hormones. Testosterone down-regulates the slow isoform associated with MyHC-cardiac α in males starting at the age of 8 weeks and ending when the animals are about 6 months old (d'Albis et al. 1993). The decrease in MyHC-cardiac α by testosterone goes along with an increase in MyHC-IIA (Reader et al. 2001).
The few available studies into the postnatal development of rabbit jaw muscles were limited to 4 weeks after birth (Langenbach et al. 1991, 2001; Bredman et al. 1992) or compared rabbits of 4 weeks (Weijs et al. 1989) or 8 weeks (Eason et al. 2000) with adults. The aim of the present study was to investigate both early (< 8 weeks) and late (> 8 weeks) postnatal changes in fibre type composition and fibre cross-sectional area of two male rabbit jaw muscles, the masseter and the digastric, a jaw closer and a jaw opener, respectively. As suckling is replaced by chewing behaviour, the jaw closers increase their amount of activity and force production relative to the jaw openers. Therefore, it can be hypothesized that both the proportion of slow fibre types and the fibre cross-sectional area increase more in the masseter than in the digastric during early postnatal development in the masseter. Furthermore, because of the possible influence of male hormones we hypothesized a decrease in the proportion of slow fibre types in both muscles during late postnatal development.
Materials and methods
The left masseter and digastric muscle (n = 4 per age group) were dissected from 20 male New Zealand rabbits (Oryctolagus cuniculus) at 1, 4, 8, 14 and 20 weeks after birth (a total of 40 muscles). The animals were weighed (Table 1) and killed by an overdose of pentobarbital (Nembutal, Sanofi Sante, Maassluis, The Netherlands), and the muscles were cut from their attachment sites after they had been exposed. The unfixed muscles were rapidly frozen in liquid-nitrogen-cooled isopentane and stored at −80 °C until required for further processing. The experimental procedure was approved by the Animals Ethics Committee of the Medical School of the University of Amsterdam.
Table 1.
Mean and SD of the weight of the rabbits for the four age groups. For each age group: n = 4
| Age (weeks) | Weight (g) Mean | SD |
|---|---|---|
| 1 | 119 | 20 |
| 4 | 562 | 227 |
| 8 | 1867 | 232 |
| 14 | 2692 | 337 |
| 20 | 3250 | 437 |
Immunohistochemistry
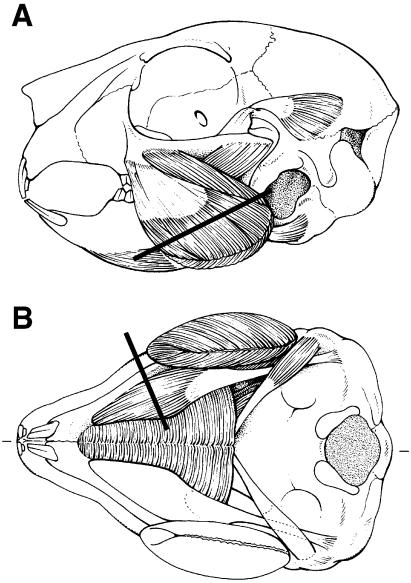
Serial transverse sections of 10 µm were cut in a cryomicrotome (Model HM 500M, Adamas Instruments BV, Leersum, The Netherlands). They were obtained from the belly of the muscles perpendicular to the main direction of the fibres at one-third from the attachment onto the mandible (Fig. 1). The posterior deep portion of the masseter muscle (Weijs et al. 1987) was removed separately and was not considered in the present study.
Fig. 1.
Masseter (A) and digastric (B) muscles in a rabbit of 2 weeks. Lines indicate levels of sections. (A = ventral view, B = lateral view.)
After overnight fixation at −20 °C in a mixture of methanol–acetone–acetic acid–water (35 : 35 : 5 : 25) (Wessels et al. 1988), six consecutive sections of 10 µm were incubated with monoclonal antibodies raised against purified myosin (Bredman et al. 1991; Sant'Ana Pereira et al. 1995). Antibody 219-1D1 recognized MyHC-I, antibody 249-5A4 recognized MyHC-cardiac α, antibody 333-7H1 recognized MyHC-IIA, antibody 340-3B5 recognized all fast MyHC isoforms, and antibody 332-3D4 recognized MyHC-IIA and MyHC-IIX. Because this antibody panel could not distinguish fibres that co-expressed MyHC-IIA, -IIX and/or -IIB from fibres that contained only MyHC-IIA, MyHC-IIX or MyHC-IIB, we realize that there might have been hybrid fibres that co-expessed more than one fast MyHC isoform among fibres which were classified as pure MyHC-IIA, MyHC-IIX and MyHC-IIB fibres. The specificity and characterization of these monoclonal antibodies against MyHC isoforms were demonstrated previously (Wessels et al. 1991; Sant'Ana Pereira & Moorman, 1994; Sant'Ana Pereira et al. 1995). Anti-neonatal MyHC was purchased from Novocastra Laboratories Ltd (UK). The indirect unconjugated immunoperoxidase technique (PAP-technique) was applied to detect the specific binding of the different antibodies. Nickel-DAB was used to visualize the staining (Hancock, 1982).
Fibres were categorized into three groups – fast, slow and intermediate – based on their contraction velocity (Kwa et al. 1995; Galler et al. 2002). All fibres containing MyHC-IIA and/or MyHC-IIX fibres were classified as fast. All fibres containing MyHC-I and/or MyHC-cardiac α were classified as slow. The remaining fibres, containing a combination of myosin types belonging to the fast and slow groups, were classified as intermediate.
Sample method and fibre cross-sectional area measurements
From each muscle, four areas were sampled. A sample area was taken from each quadrant in the muscle section. Each sample was photographed by means of a digital camera attached to a microscope and the fibre area outlines were drawn on a sheet. In each sample area 100–350 fibres (average 182) were analysed. Fibres were classified by means of six consecutive sections. We calculated the cross-sectional area of the fibres by scanning the drawn sheets, together with a grade mark for correction of enlargement, into a personal computer via a flat-bed scanner (Hewlett-Packard, Scanjet 2400). For this purpose, a custom-made program was used that converts the number of pixels into µm2 (Korfage et al. 2000).
Statistics
For each muscle, the proportions and mean cross-sectional areas of the different fibre types were calculated. Mean and standard deviation values were calculated over the four masseter and digastric muscles for each age group. The influence of age on the fibre type proportion and the cross-sectional area was tested by one-way analysis of variance with a post-hoc test (Bonferroni). Differences between the masseter and digastric in each age category were analysed by the Mann–Whitney ranking test. Regional differences within the muscles were analysed by the Friedman ranking test for paired data. The level of significance was set at P < 0.05. Statistical tests were performed using the SPSS statistical software package (SPSS Inc., Chicago, IL, USA), version 10.0.7.
Results
Fibre type composition
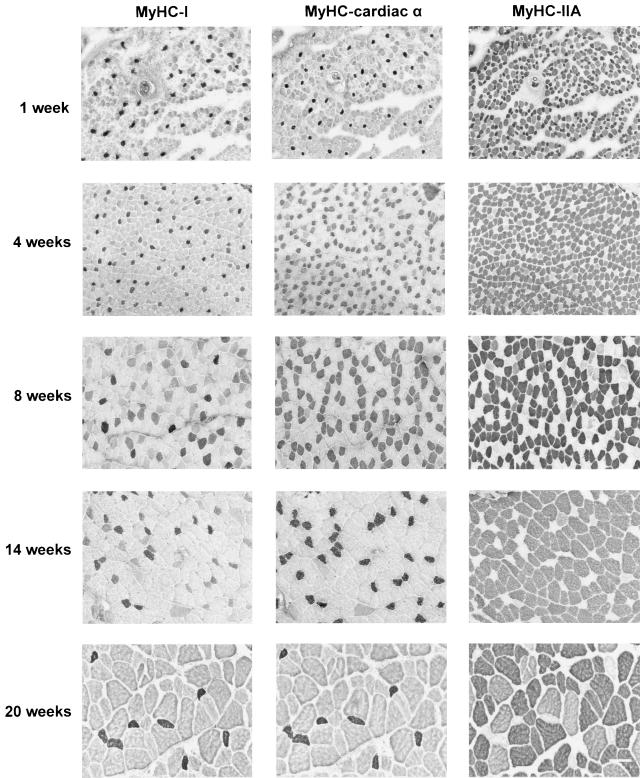
At 1 week after birth, almost all fibres (about 95%) co-expressed MyHC-neonatal. However, at week 4 and older nearly all MyHC-neonatal positive fibres had disappeared. This particular MyHC isoform was therefore not investigated further. No fibres could be classified as pure MyHC-IIB. Examples of consecutive sections of the masseter muscle, incubated with antibodies against MyHC-I, -cardiac α and -IIA, obtained from rabbits of different age of postnatal development are shown in Fig. 2. Note the increase and decrease in the proportion of the MyHC-cardiac α fibres and the increase in cross-sectional areas of the fibres during growth.
Fig. 2.
Examples of three consecutive sections of the masseter incubated with antibodies against MyHC-I (first column), -cardiac α (middle column) and -IIA (right column), from each age group. Bar = 100 µm.
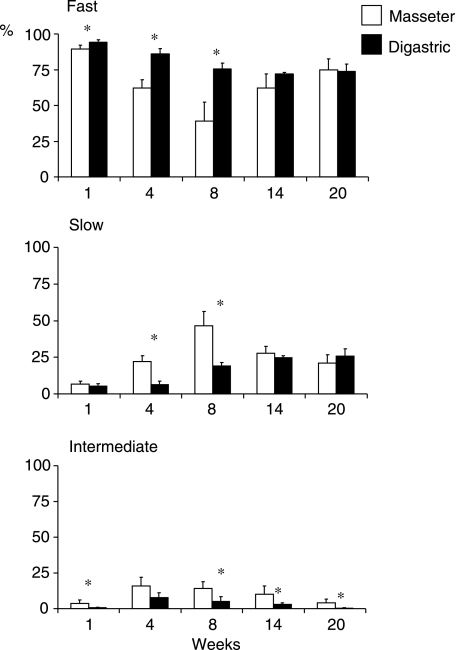
In week 1, the masseter and digastric contained 89 and 94% fast fibre types, respectively (Fig. 3). This proportion was decreased significantly to 47 and 76%, respectively (P < 0.000) at week 8. Thereafter, it increased again to 75% in the masseter at week 20 (P < 0.000) but remained the same in the digastric. The proportion of slow fibre types showed the opposite of what was found for the fast fibre types. There were few intermediate fibre types in week 1 (4 and 1%, respectively, for masseter and digastric). At week 4, this proportion was increased approximately four times (P < 0.003 and P < 0.019, respectively), and at week 20, it was decreased to similar values as in the first week. Comparison of the fibre type proportion between both muscles showed that significantly fewer fast fibre types were found in the masseter than in the digastric at weeks 1, 4 and 8 (P < 0.043, P < 0.021 and P < 0.021, respectively), significantly more slow fibre types at weeks 4 and 8 (P < 0.021 and P < 0.021, respectively), and significantly more intermediate fibre types at weeks 1, 8, 14 and 20 (P < 0.021, P < 0.043, P < 0.021 and P < 0.043, respectively).
Fig. 3.
Mean percentages and standard deviation values for fast, slow and intermediate fibre types of the masseter and digastric. Asterisk, significant difference between the two muscles (P < 0.05).
The most common fast fibre types were those that expressed MyHC-IIA or IIA + IIX, while the most common slow fibre types expressed MyHC-cardiac α (pure and MyHC-cardiac α + I) (Table 2). The changes in the proportion of slow fibre types in the masseter could be attributed almost exclusively to changes in the proportion of pure MyHC-cardiac α fibres (see also Fig. 2), which significantly increased from 1% at week 1 to 29% at week 8 (P < 0.009) and decreased again to 7% at week 20 (P < 0.05).
Table 2.
Mean and SD of individual fibre type proportions (%) in the masseter and digastric for weeks 1, 4, 8, 14, and 20. For each age group: n = 4
| Week 1 | Week 4 | Week 8 | Week 14 | Week 20 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Masseter fibre type | ||||||||||
| IIA/IIA + IIX | 81.42 | 12.26 | 61.97 | 5.56 | 39.17 | 12.96 | 56.51 | 9.45 | 73.26 | 5.49 |
| IIX | 8.05 | 13.22 | 0.20 | 0.18 | 0.06 | 0.11 | 5.62 | 7.12 | 1.55 | 2.88 |
| I | 0.47 | 0.40 | 0.43 | 0.20 | 0.58 | 0.42 | 0.61 | 0.92 | 0.34 | 0.28 |
| Cardiac α | 0.92 | 0.78 | 9.93 | 3.82 | 28.96 | 18.96 | 10.37 | 8.48 | 6.57 | 4.28 |
| Cardiac α + I | 5.44 | 2.26 | 11.58 | 2.49 | 17.08 | 11.78 | 16.69 | 5.50 | 14.07 | 2.72 |
| I + IIA | 0.44 | 0.51 | 0.70 | 0.42 | 0.70 | 1.19 | 0.78 | 1.46 | 0.04 | 0.09 |
| I + IIX | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cardiac α + IIA | 2.70 | 2.67 | 14.64 | 6.48 | 12.79 | 4.49 | 8.10 | 6.86 | 4.06 | 2.49 |
| Cardiac α + IIX | 0.03 | 0.06 | 0.03 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cardiac α + I + IIA | 0.53 | 0.45 | 0.52 | 0.51 | 0.66 | 0.80 | 1.31 | 1.78 | 0.11 | 0.17 |
| Digastric fibre type | ||||||||||
| IIA/IIA + IIX | 93.78 | 2.12 | 85.05 | 2.15 | 73.75 | 2.67 | 69.06 | 4.79 | 73.07 | 4.44 |
| IIX | 0.29 | 0.29 | 0.91 | 1.82 | 1.77 | 3.54 | 3.15 | 4.20 | 0.72 | 0.81 |
| I | 0.10 | 0.20 | 0.22 | 0.23 | 0.20 | 0.32 | 2.46 | 1.80 | 5.66 | 0.86 |
| Cardiac α | 0.17 | 0.33 | 0.06 | 0.11 | 0.07 | 0.08 | 0.29 | 0.21 | 0.04 | 0.08 |
| Cardiac α + I | 5.11 | 1.45 | 6.08 | 2.32 | 18.28 | 2.54 | 21.97 | 2.78 | 20.12 | 4.24 |
| I + IIA | 0.00 | 0.00 | 1.07 | 0.84 | 0.28 | 0.57 | 0.06 | 0.08 | 0.04 | 0.08 |
| I + IIX | 0.03 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cardiac α + IIA | 0.07 | 0.09 | 2.76 | 2.18 | 2.73 | 0.41 | 2.02 | 0.89 | 0.21 | 0.20 |
| Cardiac α + IIX | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cardiac α + I + IIA | 0.45 | 0.52 | 3.86 | 1.53 | 2.75 | 1.93 | 0.98 | 0.56 | 0.15 | 0.30 |
Fibre cross-sectional areas
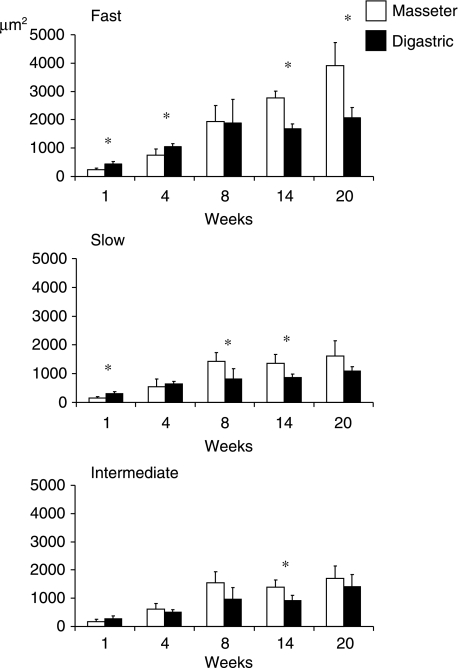
In every age group, fast fibre types had a larger cross-sectional area than slow and intermediate fibre types (Fig. 4). From week 1–8, the cross-sectional area of the fast fibre types in the masseter and digastric increased 8.1 (P < 0.001) and 4.2 (P < 0.002) times, respectively. After the age of 8 weeks, the cross-section of these fibres did not increase further in the digastric, whereas in the masseter they continued to increase. By the age of 20 weeks, they were twice as large as at week 8 (P < 0.000). Both in the slow and in the intermediate fibre groups the fibre cross-sectional area increased significantly between weeks 1 and 8 while it remained unaltered after week 8. Between weeks 1 and 8, the increase for both groups was about 10 times (P < 0.000) in the masseter and 4–5 times (P < 0.014) in the digastric.
Fig. 4.
Mean fibre cross-sectional area and standard deviation values for fast, slow and intermediate fibre types of the masseter and digastric. Asterisk, significant difference between the two muscles (P < 0.05).
Comparison of the fibre cross-sectional areas of the two muscles showed that fast fibre types in the masseter were significantly smaller than those in the digastric at weeks 1 (P < 0.021) and 4 (P < 0.021), but larger at weeks 14 (P < 0.021) and 20 (P < 0.021). Slow fibre types were significantly smaller in the masseter at week 1 (P < 0.021), but larger at weeks 8 (P < 0.043) and 14 (P < 0.021). Intermediate fibre types were only significantly larger in the masseter than in the digastric at week 14 (P < 0.043).
Intramuscular differences could only be observed for the masseter at the age of 20 weeks, i.e. the two posterior muscle portions contained about 10% more MyHC-IIA fibres than the two anterior muscle portions (P < 0.013) and the medio-anterior muscle portion contained about 10% more MyHC-cardiac α + I fibres (P < 0.05) than the other muscle portions.
Discussion
Postnatal MyHC isoform transitions have been investigated in rabbit limb and trunk muscles (Gondret et al. 1996). In general, muscle fibres contain predominantly developmental MyHC isoforms directly after birth and they are rapidly replaced by adult isoforms. All MyHC transitions took place within the first four postnatal weeks, in which the muscles obtained their own specific adult fibre type composition (Gondret et al. 1996). In the jaw muscles, MyHC isoform changes may continue after 4 weeks, because the expression of several muscle-specific transcription factors peaks later in the masseter than in other muscles (Noden et al. 1999; Yamane et al. 2000), and because of the influence of the male hormone testosterone (Eason et al. 2000; Reader et al. 2001). Furthermore, the jaw muscles will still change in length, orientation and functional capacity after 4 weeks (Weijs et al. 1989; Langenbach et al. 1991). The transition between suckling and chewing occurs during early development, between weeks 2 and 6, while during late development (> 8 weeks) sexual maturation takes place, which also might have an influence on the muscle fibres. Therefore, we examined a longer period in the present study.
The transition from suckling to chewing starts with the earliest masticatory movements around the age of 2 weeks (Yardin, 1974; Langenbach et al. 1991). During suckling, activation of the jaw opening muscles is relatively large. At the time the rabbit starts to masticate, the activation of the jaw-closing muscles will gradually increase. It can be expected that after the transition between suckling and chewing has been completed the masseter will be more intensively activated than the digastric. The latter has been confirmed in recent studies in which the daily burst characteristics of jaw muscles in juvenile rabbits have been determined (Van Wessel et al. 2005a,b). Indeed, the result of the present study showed that between weeks 1 and 8 the increase in the proportion of slow fibre types was larger in the masseter than in the digastric, which can be attributed to the increasing amount of activity in the masseter compared with that of the digastric.
At week 8 the fibre type composition of the digastric had reached its adult status. The fibres of the masseter, however, continued to change after week 8. Interestingly, the proportion of slow fibre types decreased again. This remarkable decrease after 8 weeks was not expected from the use of this muscle. Most likely, it is related to the influence of the male hormone testosterone (d'Albis et al. 1993). Such an influence was observed in an experiment in which young adult rabbits were first castrated 8 weeks after birth and then exposed to testosterone (Eason et al. 2000; Reader et al. 2001; English & Schwartz, 2002). The decreased proportion of slow fibre types in the masseter was not seen in female rabbits. We did not find a decrease in slow fibre types in the digastric after 8 weeks. We speculate that the digastric is less susceptible to testosterone than the masseter. Similar differences in the susceptibility to testosterone were found within the masseter (English et al. 1999). However, in contrast to the superficial masseter, the posterior deep masseter did not respond to the influence of testosterone.
The changes in the proportion of slow fibre types in the masseter are predominantly related to changes in the proportion of pure MyHC-cardiac α fibres and not to changes in the other two slow fibre types (Table 2). A temporarily increase of MyHC-cardiac α was also seen during chronic low-frequency stimulation experiments in transforming rabbit limb muscles (Peuker et al. 1998, 1999), and during early development of limb muscles in horse (Dingboom et al. 1999) and pig (Lefaucheur et al. 1995). In these muscles, MyHC-cardiac α was in most cases expressed in hybrid fibres. In the rabbit masseter, however, approximately 30% of the fibres were pure fibres expressing only MyHC-cardiac α at week 8. These particular fibres were not observed in the aforementioned testosterone studies (Eason et al. 2000; Reader et al. 2001; English & Schwartz, 2002), which might be related to the use of different antibodies against MyHC-cardiac α. The presence of pure MyHC-cardiac α fibres, as an intermediate step between slow and fast fibre types, is presumably one of the features in which the jaw muscles are developmentally different from limb muscles (Noden et al. 1999). Furthermore, it should be noted that MyHC-cardiac α remains present in the jaw-closing muscles of rabbit (Bredman et al. 1991; present study), kangaroo (Hoh et al. 2000) and human (Korfage & Van Eijden, 2003). The contraction velocity of MyHC-cardiac α fibres lies between that of MyHC-I and MyHC-IIA fibres (Sciote & Kentish, 1996). However, the contraction velocity of pure MyHC-cardiac α fibres is closer to that of MyHC-I than that of MyHC-IIA (Kwa et al. 1995). Therefore, in the present study we defined the MyHC-cardiac α fibres as a slow fibre type.
Fast fibre types in the masseter continued to increase in size after the age of 8 weeks, which indicates that the masseter is becoming even more powerful. This could be another hormonal effect. The increase in muscle mass by testosterone is associated with an increase in the fibre cross-sectional areas of both slow and fast fibres (Sinha-Hikim et al. 2002). In the masseter, testosterone had an influence on the cross-sectional area of MyHC-IIA fibres, which were larger in the male than in the female masseter (English et al. 1999). The increase in girth is only seen in the fast fibre types of the masseter and not in those of the digastric, another indication that there is a difference in the susceptibility towards testosterone.
The effect of testosterone on jaw muscles could be neurogenic and/or myogenic. Trigeminal motoneurons, which innervate both masseter and digastric, contain abundant androgen receptors (Yu & McGinnis, 2001). As the phenotype of a fibre is controlled by the nerve stimulus, testosterone might influence the expression of MyHC isoforms in the muscle fibres via a difference in stimulation. Testosterone might also act directly on the muscle fibres via androgen receptors (Michel & Baulieu, 1980). Depending on the amount of stimulation, a muscle might increase in girth and change in phenotype differently under the influence of testosterone and the amount of androgen receptors, which might differ between the masseter and digastric. Differences in fibre type proportion and cross-sectional area between the jaw closers and openers could thus be related to either a neurogenic or a myogenic difference, or a combination of both differences. However, the mechanisms by which testosterone acts on the regulation of the MyHC isoform genes and fibre cross-sectional areas are still poorly understood.
Although the masseter and digastric had more or less the same proportion of fast fibre types at the age of 20 weeks, it should be noted that the total area occupied by fast fibres is about twofold greater in the masseter than the digastric, because the cross-sectional area of fast fibre types in the masseter was almost twice those in the digastric and could, thus, produce more force. Such an adaptation was also suggested by the larger number of bursts with a higher activity level in the masseter compared with the digastric (Van Wessel et al. 2005a,b).
Acknowledgments
This research was institutionally supported by the Interuniversity Research School for Dentistry, through the Academic Center of Dentistry Amsterdam. We thank Professor A. F. M. Moorman for the antibodies, and Dr J. H. Koolstra for his critical reading of the manuscript.
References
- Anapol F, Herring SW. Ontogeny of histochemical fiber types and muscle function in the masseter muscle of miniature swine. Am J Phys Anthropol. 2000;112:595–613. doi: 10.1002/1096-8644(200008)112:4<595::AID-AJPA11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force–velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredman JJ, Wessels A, Weijs WA, Korfage JAM, Soffers CAS, Moorman AFM. Demonstration of ‘cardiac-specific’ myosin heavy chain in masticatory muscles of human and rabbit. Histochem J. 1991;23:160–170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Bredman JJ, Weijs WA, Korfage JAM, Brugman P, Moorman AFM. Myosin heavy chain expression in rabbit masseter muscle during postnatal expression. J Anat. 1992;180:263–274. [PMC free article] [PubMed] [Google Scholar]
- d'Albis A, Couteaux R, Janmot C, Mira JC. Opposite regulations by androgenic and thyroid hormones of V1 myosin expression in the two types of rabbit striated muscle: skeletal and cardiac. FEBS Lett. 1993;318:53–56. doi: 10.1016/0014-5793(93)81326-u. [DOI] [PubMed] [Google Scholar]
- Dingboom EG, Dijkstra G, Enzerink E, van Oudheusden HC, Weijs WA. Postnatal muscle fibre composition of the gluteus medius muscle of Dutch Warmblood foals; maturation and the influence of exercise. Equine Vet J Suppl. 1999;31:95–100. doi: 10.1111/j.2042-3306.1999.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Eason JM, Schwartz G, Shirley KA, English AW. Investigation of sexual dimorphism in the rabbit masseter muscle showing different effects of androgen deprivation in adult and young adult animals. Arch Oral Biol. 2000;45:683–690. doi: 10.1016/s0003-9969(00)00030-3. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Zhou M-Y, Ohira Y, et al. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol. 1995;78:1733–1739. doi: 10.1152/jappl.1995.78.5.1733. [DOI] [PubMed] [Google Scholar]
- English AW, Eason J, Schwartz G, Shirley A, Carrasco DI. Sexual dimorphism in the rabbit masseter muscle: myosin heavy chain composition of neuromuscular compartments. Cells Tissues Organs. 1999;164:179–191. doi: 10.1159/000016658. [DOI] [PubMed] [Google Scholar]
- English AW, Schwartz G. Development of sex differences in the rabbit masseter muscle is not restricted to a critical period. J Appl Physiol. 2002;92:1214–1222. doi: 10.1152/japplphysiol.00953.2001. [DOI] [PubMed] [Google Scholar]
- Galler S, Puchert E, Gohlsch B, Schmid D, Pette D. Kinetic properties of cardiac myosin heavy chain isoforms in rat. Pflügers Arch. 2002;445:218–223. doi: 10.1007/s00424-002-0934-6. [DOI] [PubMed] [Google Scholar]
- Gojo K, Abe S, Ide Y. Characteristics of myofibres in the masseter muscle of mice during postnatal growth period. Anat Histol Embryol. 2002;31:105–112. doi: 10.1046/j.1439-0264.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- Gondret F, Lefaucheur L, D'Albis A, Bonneau M. Myosin isoform transitions in four rabbit muscles during postnatal growth. J Muscle Res Cell Motil. 1996;17:657–667. doi: 10.1007/BF00154060. [DOI] [PubMed] [Google Scholar]
- Hancock MB. A serotonin-immunoreactive fiber system in the dorsal columns of the spinal cord. Neurosci Lett. 1982;31:247–252. doi: 10.1016/0304-3940(82)90028-3. [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Kim Y, Sieber LG, Zhong WWH, Lucas CA. Jaw-closing muscles of kangaroos express alpha cardiac myosin heavy chain. J Muscle Res Cell Motil. 2000;21:673–680. doi: 10.1023/a:1005676106940. [DOI] [PubMed] [Google Scholar]
- Iinuma M, Yoshida S, Funakoshi M. Development of masticatory muscles and oral behavior from suckling to chewing in dogs. Comp Biochem Physiol A. 1991;100:789–794. doi: 10.1016/0300-9629(91)90293-l. [DOI] [PubMed] [Google Scholar]
- Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231:597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hensbergen E. Use and fibre type composition in limb muscles of cats. Eur J Morph. 1998;36:288–292. doi: 10.1076/ejom.36.4.288.5821. [DOI] [PubMed] [Google Scholar]
- Kernell D, Hensbergen E, Lind A, Eerbeek O. Relation between fibre composition and daily duration of spontaneous activity in ankle muscles of the cat. Arch Ital Biol. 1998;136:191–203. [PubMed] [Google Scholar]
- Korfage JAM, Brugman P, Van Eijden TMGJ. Intermuscular and intramuscular differences in myosin heavy chain composition of the human masticatory muscles. J Neurol Sci. 2000;178:95–106. doi: 10.1016/s0022-510x(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Van Eijden TMGJ. Myosin heavy chain composition in human masticatory muscles by immunohistochemistry and gel electrophoresis. J Histochem Cytochem. 2003;51:113–119. doi: 10.1177/002215540305100113. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Koolstra JH, Langenbach GEJ, Van Eijden TMGJ. Fibre type composition of the human jaw muscles. Part 1: origin and functional significance of fibre type diversity. J Dent Res. 2005a;84:774–783. doi: 10.1177/154405910508400901. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Koolstra JH, Langenbach GEJ, Van Eijden TMGJ. Fibre type composition of the human jaw muscles. Part 2: Role of hybrid fibres and factors responsible for inter-individual variation. J Dent Res. 2005b;84:784–793. doi: 10.1177/154405910508400902. [DOI] [PubMed] [Google Scholar]
- Kwa SHS, Weijs WA, Jüch PJ. Contraction characteristics and myosin heavy chain composition of rabbit masseter motor units. J Neurophysiol. 1995;73:538–549. doi: 10.1152/jn.1995.73.2.538. [DOI] [PubMed] [Google Scholar]
- Langenbach GE, Weijs WA, Koolstra JH. Biomechanical changes in the rabbit masticatory system during postnatal development. Anat Rec. 1991;230:406–416. doi: 10.1002/ar.1092300313. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, Weijs WA, Brugman P, Van Eijden TMGJ. A longitudinal electromyographic study of the postnatal maturation of mastication in the rabbit. Arch Oral Biol. 2001;46:811–820. doi: 10.1016/s0003-9969(01)00043-7. [DOI] [PubMed] [Google Scholar]
- Lefaucheur L, Edom F, Ecolan P, Butler-Browne GS. Pattern of muscle fiber type formation in the pig. Dev Dyn. 1995;203:27–41. doi: 10.1002/aja.1002030104. [DOI] [PubMed] [Google Scholar]
- McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81:2004–2012. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- Michel G, Baulieu EE. Androgen receptor in rat skeletal muscle: characterization and physiological variations. Endocrinology. 1980;107:2088–2098. doi: 10.1210/endo-107-6-2088. [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan HC, O'Conner D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science. 1978;200:314–317. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, Emerson GP. Differentiation of avian craniofacial muscles. I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn. 1999;216:96–112. doi: 10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Peuker H, Conjard A, Pette D. α-Cardiac-like myosin heavy chain as an intermediate between MHC IIA and MHC Iβ in transforming rabbit muscle. Am J Physiol. 1998;274:C595–C602. doi: 10.1152/ajpcell.1998.274.3.C595. [DOI] [PubMed] [Google Scholar]
- Peuker H, Conjard A, Putman CT, Pette D. Transient expression of myosin heavy chain MHCIα in rabbit muscle during fast-to-slow transition. J Muscle Res Cell Motil. 1999;20:147–154. doi: 10.1023/a:1005482132240. [DOI] [PubMed] [Google Scholar]
- Reader M, Schwartz G, English AW. Brief exposure to testosterone is sufficient to induce sex differences in the rabbit masseter muscle. Cells Tissues Organs. 2001;169:210–217. doi: 10.1159/000047884. [DOI] [PubMed] [Google Scholar]
- Sant'Ana Pereira JAA, Moorman AFM. Do type IIB fibres of human muscle correspond to the IIX/D or to the IIB of rats? J Physiol. 1994;479P:161P–162P. [Google Scholar]
- Sant'Ana Pereira JAA, Wessels A, Nijtmans L, Moorman AFM, Sargeant AJ. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J Muscle Res Cell Motil. 1995;16:21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Kentish JC. Unloaded shortening velocities of rabbit masseter muscle fibres expressing skeletal or alpha cardiac myosin heavy chains. J Physiol. 1996;492:659–667. doi: 10.1113/jphysiol.1996.sp021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida T, Abe S, Sakiyama K, et al. Superficial and deep layer muscle fibre properties of the mouse masseter before and after weaning. Arch Oral Biol. 2005;50:65–71. doi: 10.1016/j.archoralbio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza J, Woodhouse L, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- Usami A, Abe S, Ide Y. Myosin heavy chain isoforms of the murine masseter muscle during pre- and post-natal development. Anat Histol Embryol. 2003;32:244–248. doi: 10.1046/j.1439-0264.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- Van Wessel T, Langenbach GEJ, Brugman P, van Eijden TMGJ. Long-term registration of daily jaw muscle activity in juvenile rabbits. Exp Brain Res. 2005a;162:315–323. doi: 10.1007/s00221-004-2174-9. [DOI] [PubMed] [Google Scholar]
- Van Wessel T, Langenbach GEJ, Kawai N, Brugman P, Tanaka E, van Eijden TMGJ. Burst characteristics of daily jaw muscle activity in juvenile rabbits. J Exp Biol. 2005b;208:2539–2547. doi: 10.1242/jeb.01677. [DOI] [PubMed] [Google Scholar]
- Van Wessel T, Langenbach GEJ, Korfage JAM, et al. Fiber-type composition of rabbit jaw muscles is related to their daily activity. Eur J Neurosci. 2005c;21:2209–2216. doi: 10.1111/j.1460-9568.2005.04466.x. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Brugman P, Klok EM. The growth of the skull and jaw muscles and its functional consequences in the New Zealand rabbit (Oryctolagus cuniculus) J Morph. 1987;194:143–161. doi: 10.1002/jmor.1051940204. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Brugman P, Grimbergen CA. Jaw movements and muscle activity during mastication in growing rabbits. Anat Rec. 1989;224:407–416. doi: 10.1002/ar.1092240309. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JLM, Moorman AFM, Becker AE. Immunohistochemical detection of myosin heavy chain isoforms in large sections of whole human hearts. In: Carrero U, editor. Sarcomeric and Non-Sarcomeric Muscles: Basic and Applied Research Prospects for the 90s. Padova: Unipress; 1988. pp. 311–316. [Google Scholar]
- Wessels A, Vermeulen JLM, Viragh Sz Kalman F, Lamers WH, Moorman AFM. Spatial distribution of ‘tissue specific’ antigens in the developing human heart and skeletal muscle. II. An immunohistochemical analysis of myosin heavy chain isoform expression patterns in the embryonic heart. Anat Rec. 1991;229:355–368. doi: 10.1002/ar.1092290309. [DOI] [PubMed] [Google Scholar]
- Westneat MW, Hall WG. Ontogeny of feeding motor patterns in infant rats: an electromyographic analysis of suckling and chewing. Behav Neurosci. 1992;106:539–554. doi: 10.1037//0735-7044.106.3.539. [DOI] [PubMed] [Google Scholar]
- Yamane A, Ohnuki Y, Saeki Y. Delayed embryonic development of mouse masseter muscle correlates with delayed MyoD family expression. J Dent Res. 2000;79:1933–1936. doi: 10.1177/00220345000790120201. [DOI] [PubMed] [Google Scholar]
- Yardin M. The gnathogram during weaning in the rabbit (Oryctolagus cuniculus) J Biol Buccale. 1974;2:259–268. [PubMed] [Google Scholar]
- Yu WA, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J Neurobiol. 2001;46:1–10. doi: 10.1002/1097-4695(200101)46:1<1::aid-neu1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]