Abstract
Iliotibial band (ITB) syndrome is a common overuse injury in runners and cyclists. It is regarded as a friction syndrome where the ITB rubs against (and ‘rolls over’) the lateral femoral epicondyle. Here, we re-evaluate the clinical anatomy of the region to challenge the view that the ITB moves antero-posteriorly over the epicondyle. Gross anatomical and microscopical studies were conducted on the distal portion of the ITB in 15 cadavers. This was complemented by magnetic resonance (MR) imaging of six asymptomatic volunteers and studies of two athletes with acute ITB syndrome. In all cadavers, the ITB was anchored to the distal femur by fibrous strands, associated with a layer of richly innervated and vascularized fat. In no cadaver, volunteer or patient was a bursa seen. The MR scans showed that the ITB was compressed against the epicondyle at 30° of knee flexion as a consequence of tibial internal rotation, but moved laterally in extension. MR signal changes in the patients with ITB syndrome were present in the region occupied by fat, deep to the ITB. The ITB is prevented from rolling over the epicondyle by its femoral anchorage and because it is a part of the fascia lata. We suggest that it creates the illusion of movement, because of changing tension in its anterior and posterior fibres during knee flexion. Thus, on anatomical grounds, ITB overuse injuries may be more likely to be associated with fat compression beneath the tract, rather than with repetitive friction as the knee flexes and extends.
Keywords: bursa, enthesis, enthesopathy, fat, iliotibial tract
Introduction
The iliotibial band (ITB) or tract is a lateral thickening of the fascia lata in the thigh. Proximally it splits into superficial and deep layers, enclosing tensor fasciae latae and anchoring this muscle to the iliac crest (Standring, 2004). It also receives most of the tendon of gluteus maximus. The ITB is generally viewed as a band of dense fibrous connective tissue that passes over the lateral femoral epicondyle and attaches to Gerdy's tubercle on the anterolateral aspect of the tibia. ITB friction syndrome is an overuse injury well recognized as a common cause of lateral knee pain. It is particularly common in runners and cyclists, though it also occurs in weightlifters, skiers and soccer players (Orava, 1978; Noble, 1979; McNicol et al. 1981; Martens et al. 1989; Orchard et al. 1996). The incidence is reported to be as high as 12% of all running-related, overuse injuries (for a review see Fredericson & Wolf, 2005). The diagnosis of ITB friction syndrome is based on clinical examination – patients typically present with tenderness over the lateral femoral epicondyle and report a sharp, burning pain when the practitioner presses on the lateral epicondyle during knee flexion and extension (Ekman et al. 1994). The pain is particularly acute when the knee is at 30° of flexion (Orchard et al. 1996; Fredericson & Wolf, 2005). ITB syndrome is viewed by many as a failed healing response that results from repetitive friction between the ITB and the lateral femoral epicondyle, as the fibrous band ‘rolls over’ the epicondyle during knee movements (Orchard et al. 1996; Richards et al. 2003; Fredericson & Wolf, 2005).
The purpose of the present study is to present a re-evaluation of the clinical anatomy of the region. On this basis, the common view that the ITB moves in an anterior–posterior direction over the lateral femoral epicondyle and thus that ITB syndrome is a ‘friction syndrome’ is challenged. We have shown that the ITB is firmly anchored to the distal femur, precluding its anterior–posterior translation. It is thus more likely that the movement of the ITB is in a medial–lateral direction so that the band is compressed against the epicondyle during knee flexion.
Materials and methods
Gross anatomy
The ITB was examined by gross inspection in the region of the lateral epicondyle in five dissecting-room cadavers (both sexes; mean age 78 years) donated to Cardiff University for the purposes of anatomical investigation under the provision of the 1984 Anatomy Act and the 1961 Human Tissues Act. The presence of fat deep to the ITB, fibrous connections anchoring the tract to the femur and any bursae were noted. A well-defined surface anatomy model, who gave full written informed consent, was asked to perform a series of knee squats from full extension to 90° of flexion. Photographs were captured at 10 frames s−1 with a Konica Minolta Z3 digital camera used in continuous drive mode at a resolution of 1280 × 960 pixels in−1.
Histology
The distal part of the ITB, together with the adjacent lateral femoral epicondyle, was removed from ten additional, formalin-fixed, dissecting-room cadavers. The specimens were post-fixed in 10% neutral buffered formalin, decalcified with 5% nitric acid, dehydrated with graded alcohols, cleared in xylene and embedded in paraffin wax. Serial sections were cut at 8 µm along the long axis of the ITB and 12 sections were mounted on glass slides at 1-mm intervals. Slides were stained with the Hall–Brunt quadruple stain (Hall, 1986), Masson's trichrome, Weigert's elastic stain and toluidine blue and subsequently viewed with a Leica DMRB photomicroscope.
Magnetic resonance (MR) imaging of volunteers
Six healthy volunteers (31–60 years, five male, one female, all gave full written consent) were studied on a 1.5-T MR scanner (Siemens, Erlangen, Germany) in the Department of Radiology at the University of California San Diego. In order to determine whether the ITB was compressed against the lateral femoral epicondyle at 30° of knee flexion, the knees were imaged in this position and in full extension, both when the quadriceps muscles were maximally contracted and when they were completely relaxed. Multi-slice, T1-weighted conventional spin echo sequences (TR = 500 ms, TE = 16 ms) as well as proton density (TR = 2500 ms, TEeff = 12 ms) and T2-weighted fast spin echo (TR = 2500 ms, TEeff = 92), oblique coronal sequences of 3 mm slice thickness were performed. The matrix size was 512 × 384 with a 16-cm field of view and a surface coil was used adjacent to the lateral surface of the knee.
Case studies
Two athletes presented to the Pentwyn Hospital, Cardiff, with pain in the region of the lateral epicondyle and gave their full written approval to participate in this study. The first patient was a 22-year-old, elite, male, track and field athlete and the second a 23-year-old, female, recreational marathon runner. Both individuals had clinical signs of ITB syndrome, including a positive Ober's test (Adkins & Figler, 2000). However, neither had any marked gait abnormality or history of previous injury to the ITB. The MR scans were taken on a 1.5-T MR scanner, using a Medical Advances quadrature, lower extremity coil. A cod liver oil capsule was placed over the symptomatic area (to act as a reference mark visible in the MR scans) and three sets of sequences were performed in the coronal and axial planes, at a slice thickness of 3 mm: multislice proton density (TR = 2800 ms, TEeff = 14 ms), proton density fat-saturated (TR = 3700 ms, TEeff = 14 ms) and gadolinium-enhanced (0.2 mL kg−1) T1-weighted fat-saturated, conventional spin echo (TEeff = 460 ms, TEeff = 12 ms). The matrix size was 320 × 224 and the field of view was 14 cm.
Results
Gross anatomy
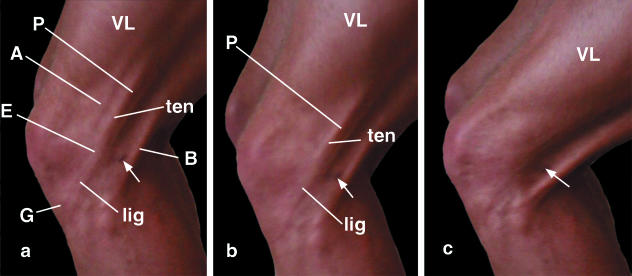
In a well-defined surface anatomy model (an elite 22-year-old male track and field athlete), the ITB was clearly visible in the region of the knee, both when the subject stood on one leg in full extension and particularly when the knee was progressively flexed (Fig. 1a,b). Furthermore, it was possible to distinguish two regions of the ITB: a ‘tendinous’ part proximal to the lateral femoral epicondyle and a ‘ligamentous’ part between the epicondyle and Gerdy's tubercle (Fig. 1a,b). The ITB was tense when the limb was loaded, both in the neutral position and when the tibia was internally or externally rotated. Furthermore, different parts of the ITB were under tension at different positions of knee joint flexion (Fig. 1a–c). As the knee was progressively flexed, tension shifted from anterior to posterior fibre bundles of the ITB (compare Fig. 1a,b). As the knee flexes, the bands of fascia lata attached to the patella (which are contiguous with the ITB) come under tension as the patella moves around the femoral condyle, while the ligamentous part of the ITB is tensioned in turn as the tibia moves posteriorly.
Fig. 1.
The appearance of the ITB in a well-defined, surface anatomy model (22-year-old, elite male track and field athlete) at progressively increasing angles of knee flexion (a–c). The small skin blemish (arrow) is a convenient landmark for evaluating the changing appearance of the ITB at different angles of knee flexion. Note that at lesser knee flexion angles (a,b), the ‘ligamentous’ (lig) and ‘tendinous’ (ten) parts of the tract are both visible, proximal and distal to the lateral femoral epicondyle (E). The ligamentous part attaches to Gerdy's tubercle (G). As the knee is flexed, the tension in the ITB shifts from its anterior (A) to its posterior (P) fibres. This is aided by the increasing posterior ‘bowstringing’ of the biceps tendon (B). VL, vastus lateralis.
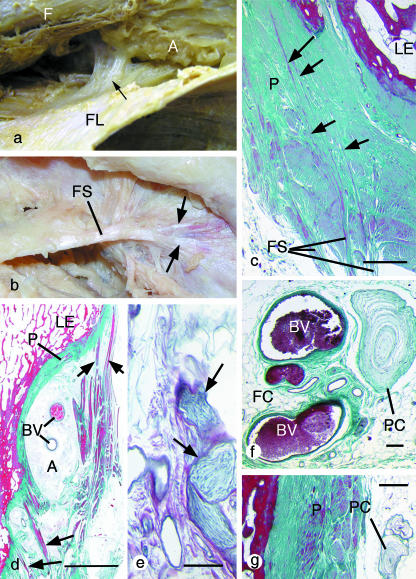
In contrast to the clarity with which the ITB stood out through the skin in a good surface anatomy model, it was impossible to identify such a discrete band with corresponding anterior and posterior margins in any dissecting-room cadaver. The ITB was simply a thickening in the lateral part of the fascia lata. This layer of deep fascia completely ensheathed the thigh and was continuous with the strong lateral intermuscular septum, which was firmly anchored to the linea aspera of the femur. Furthermore, dissections showed that the ITB was consistently anchored to the femur in the region of the lateral epicondyle by strong fibrous strands that were often obliquely orientated (Fig. 2a,b). Although the attachment was occasionally to the epicondyle itself, the strands were more usually attached immediately proximal to this site and fanned out as they approached the bone. Closely associated with the fibrous strands and intervening between the ITB itself and the femur was a region of adipose tissue (Fig. 2a). No bursa was identified in any of the cadavers.
Fig. 2.
Gross and microscopic anatomy of the fibrous strands linking the ITB to the femur. All histological sections are stained with Masson's trichrome. (a) A gross anatomical view of the fascia lata (FL), which has been cut longitudinally and reflected laterally to reveal a strong fibrous band (arrow) that connects the ITB to the femur (F) immediately above the lateral epicondyle. Adipose tissue (A) fills the space between the two structures, but has been partly removed in order to display the fibrous strands. (b) A gross anatomical view of a thick fibrous strand (FS) extending from the ITB to the femur and attaching to the latter in the manner of a tendon enthesis, i.e. fanning out (arrows) as it reaches the bone. (c) A histological section through the attachment of a collection of fibrous strands (FS) that anchor the ITB to the femur adjacent to the lateral epicondyle (LE). Note how the strands do not stop at the surface of the thickened periosteum (P), but pass through it towards the bone (arrows). (d) A histological section cut in the coronal plane showing the attachment of the fibrous strands (arrows) to the thick periosteum on the femur. The fibrous strands splay out to attach both to the lateral epicondyle (LE) and to a region of the femur immediately proximal to it. Note the adipose tissue that lies deep to the strands and which contains conspicuous blood vessels (BV), and the anisotropy of the trabeculae at the lateral epicondyle. Scale bar, 500 µm. (e) A bundle of nerve fibres (arrows) typical of those traversing the adipose tissue deep to the ITB. Scale bar, 100 µm. (f) A Pacinian corpuscle (PC) and numerous blood vessels of various sizes surrounded by fat cells (FC) in the adipose tissue between the ITB and the femur. Scale bar, 100 µm. (g) A Pacinian corpuscle on the surface of the periosteum in the insertional angle deep to a fibrous band extending from the ITB to the femur. Scale bar, 500 µm.
Histology
The region between the ITB (or the fibrous strands passing to the femur) and the lateral aspect of the femur was filled with a highly vascularized and richly innervated mass of adipose tissue that in some specimens contained Pacinian corpuscles and bundles of myelinated and non-myelinated nerve fibres (Fig. 2e–g). The epicondyle itself was covered with a greatly thickened, though largely fibrous, periosteum (Fig. 2c). The fibrous strands that linked the ITB to the femur consisted of dense, regular, fibrous connective tissue (Fig. 2c,d) in which the sparse cells were elongate fibroblasts. Elastic fibres were inconspicuous. The strands typically approached the bone at an oblique angle and some fibres could be traced through the periosteum, as a series of distinct bundles, to attach to the bone itself (Fig. 2c). At the bony enthesis, there was variable evidence of fibrocartilage differentiation and the trabeculae in the underlying bone were anisotropic and aligned largely along the long axis of the strands (Fig. 2d).
Magnetic resonance imaging
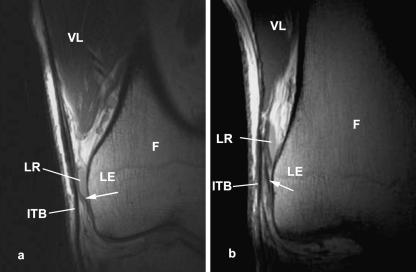
The fibrous links between the ITB and the femur were evident in all asymptomatic volunteers (Fig. 3) and in the two patients with ITB syndrome. So, too, was the adipose tissue between the ITB and the femur, and the presence and quantity of fat at this location were independent of the adiposity of the subjects (Fig. 4). The lateral recess of the knee was clearly visible in the proton density (PD) and T2 images as uniform areas of lower (PD) or higher (T2) signal than the adjacent fat (Fig. 3a,b). No bursa was identified in any of the asymptomatic volunteers.
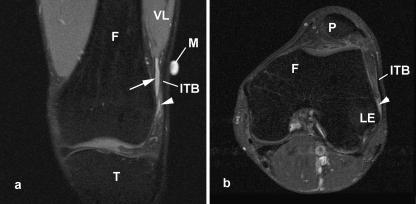
Fig. 3.
Coronal plane MR scans (proton density weighted) of the iliotibial tract (ITB) showing the fibrous strands (arrows) linking the tract to the lateral epicondyle (LE) of the femur (F) in an asymptomatic volunteer. Note the presence of the lateral recess (LR) of the knee, which can be mistaken for a bursa. VL, vastus lateralis.
Fig. 4.
Coronal plane, T1-weighted MR scans of individuals with large (a) and small (b) quantities of subcutaneous adipose tissue (arrows) in the region of the knee. Note that the quantity of fat deep to the ITB does not differ between the two individuals.
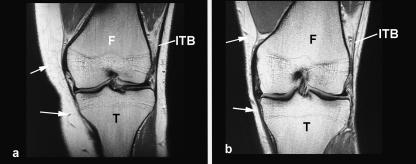
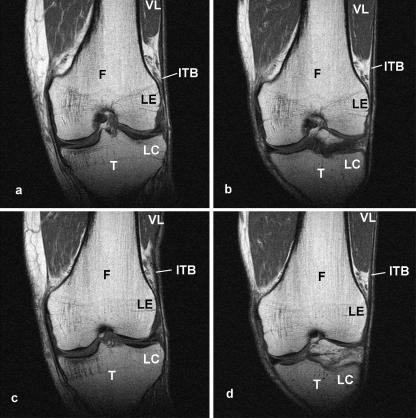
There was a difference in the position of the ITB relative to the lateral femoral epicondyle and the lateral supracondylar ridge in knees imaged in full extension and at 30° of flexion, both when the quadriceps muscles were contracted and when they were relaxed. Figure 5 shows that the site of the attachment of the ITB to Gerdy's tubercle was more lateral relative to the femur in full extension than at 30° of flexion. Consequently, the ITB was compressed against the lateral epicondyle at 30° of flexion, but was pulled away from it in full extension (Fig. 5). The area beneath the ITB that was occupied by fat, immediately superior to the epicondyle, was less extensive in knees flexed to 30° than in full extension (Fig. 5).
Fig. 5.
Coronal plane, T1-weighted MR scans of the knee of a 61-year-old volunteer with the quadriceps muscles relaxed (a,b) and contracted (c,d). In ‘a’ and ‘c’, the knee is fully extended, whereas in ‘b’ and ‘d’ it is flexed to 30°. The images have been selected from a complete series of scans through the knee, so that the appearance of the femur is matched in ‘a’ and ‘b’ and in ‘c’ and ‘d’. In an extended knee (‘a’ and ‘c’), the iliotibial band (ITB) slopes laterally as it passes from the lateral epicondyle (LE) of the femur (F), to the lateral condyle (LC) of the tibia, but at 30° of flexion (‘b’ and ‘d’) it slopes medially. Consequently, the ITB is compressed against the lateral epicondyle at 30° of flexion. The more distal extension of vastus lateralis (VL) in a flexed knee reduces the space occupied by the fat deep to the ITB. This in turn compresses the fat. Note that in a fully extended knee, the ITB lies closer to the femur (i.e. acts as a brace) when the quadriceps muscles are contracted than when they are relaxed (compare ‘a’ and ‘c’).
Case studies
The gadolinium-enhanced MR scans of the track and field athlete demonstrated that the symptomatic area was associated with an increased signal deep to the ITB in the region of the lateral femoral epicondyle (Fig. 6). There was no evidence of a bursa and no enhanced signal within the distal part of the vastus lateralis. Post-gadolinium, there was soft tissue enhancement in the adipose tissue deep to the ITB.
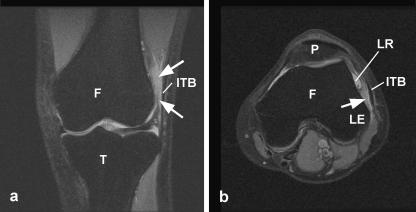
Fig. 6.
Coronal (a) and axial (b) plane, fat-saturated T2-weighted MR scans of the knee of the 22-year-old male track and field athlete with ITB syndrome. A skin marker (M) has been placed adjacent to the symptomatic area, i.e. inferior to vastus lateralis (VL). Note the region of moderately high signal (arrowheads) in the adipose tissue deep to the iliotibial band (ITB) in both figures. Arrow, lateral recess; F, femur; LE, lateral epicondyle; P, patella; T, tibia.
The MR scans of the recreational runner demonstrated pronounced inflammation immediately deep to the ITB in the region of the lateral femoral epicondyle (Fig. 7). There was evidence of oedema in the fatty tissue deep to the fascia lata. The abnormal region was immediately proximal to the site of femoral attachment of the fibrous strands from the ITB. There was no evidence of any bursa or any indication of inflammation in the fascia itself.
Fig. 7.
Coronal (a) and axial (b) plane, fat-saturated T2-weighted MR scans of the knee of the 22-year-old female recreational marathon runner with ITB syndrome. Here, there is an extensive region of high signal (arrows) in the region occupied by adipose tissue deep to the ITB, indicative of oedema or inflammation in the region. F, femur; LE, lateral epicondyle; LR, lateral recess; P, patella; T, tibia.
Discussion
The term ‘ITB friction syndrome’ suggests that there is a shear movement of a fascial plane relative to the lateral femoral epicondyle. This has led to the belief that a bursa is present between the opposing surfaces (Ekman et al. 1994; Drogset et al. 1999) and that bursitis is inherent in the syndrome. However, on anatomical grounds, we suggest that the injury may not be the consequence of friction of the ITB over the epicondyle, but of compression against a layer of highly innervated fat that intervenes between the ITB and the epicondyle. That the ITB is compressed against the femur reflects its role as a brace that reduces bending stresses on the bone, by converting tensile to compressive loading along its lateral aspect (Tichy & Tillmann, 1989; Fetto et al. 2002). Our coronal MR scans show that the ITB is drawn medially towards the epicondyle at 30° of flexion, probably as a consequence of passive tibial rotation during flexion and extension of the knee. It is also possible that the greater distal extension of the vastus lateralis evident in a flexed knee, moves the fat deep to the ITB and thereby adds to its compression.
The prominence of a tensed ITB in a thin individual gives the impression that the tract has distinctive anterior and posterior borders. However, our cadaveric dissections confirm that the ITB is simply a thickened, lateral part of the fascia lata. It completely surrounds the thigh, is anchored to the femoral shaft by the lateral intermuscular septum and is continuous with the patellar retinacula (Joseph, 1976; Muhle et al. 1999; Standring, 2004). Furthermore, we have shown that the ITB is firmly attached to the distal femur by strong, obliquely orientated, fibrous strands that can be regarded as tendon entheses. Thus, the ITB is unlikely to roll forwards and backwards during flexion and extension of the knee, but could move slightly in a medial–lateral direction. The impression of a rolling movement is likely to be an illusion created by a sequential shifting of tensile load within the ITB. Its fibres are tensioned in sequence from anterior to posterior as the knee flexes. Although our finding of distal fibrous strands of the ITB agrees with descriptions of normal anatomy in standard texts (e.g. Standring, 2004), such strands have been interpreted by others as pathological adhesions (e.g. Fredericson & Wolf, 2005). However, we have shown that they pass through the periosteum, rather than merely attaching to its surface.
The presence of fat deep to the ITB in the region of its fibrous attachments to the femur can be compared with the fat present at many tendon and ligament entheses (Benjamin et al. 2004) and its rich vascularity could explain the localized oedema often seen in patients diagnosed with ITB syndrome. The presence of numerous blood vessels should not be interpreted as a sign of inflammation. That Pacinian corpuscles can be present in the adipose tissue supports the view that the fat is subject to compression and that it may have a proprioceptive role. It is also worth noting that hypertrophy of Pacinian corpuscles has been associated with pain (Jones & Eadie, 1991; Bas et al. 1993; Reznik et al. 1998). Hence, together with the other nerve fibres we have demonstrated in the fat, Pacinian corpuscles may be implicated in the pain associated with ITB syndrome. The finding that there is no relationship between the depth of subcutaneous adipose tissue and the quantity of fat deep to the ITB suggests that the fat is a constant feature analogous to Hoffa's pad in the knee or the fat pads in the palms and soles.
The case studies that we have presented of the two runners with acute ITB syndrome demonstrate MR signal changes in the fatty tissue rather than within the tract itself, and that no bursa could be identified. Together these observations suggest that oedema within the region does not necessarily indicate bursitis – a finding that needs to be confirmed in a larger cohort of patients. The absence of a bursa is consistent with the MR and cadaveric studies of Muhle et al. (1999) and the sonographic study of Goh et al. (2003). It is most likely that synovium deep to the ITB is part of the lining of the knee joint, which appears in this region as the lateral recess (Nemeth & Sanders, 1996).
Finally, the presence of fibrous strands anchoring the ITB to the femur has implications for understanding the functions of tensor fasciae latae. Their presence divides the ITB into a proximal ‘tendinous’ and a distal ‘ligamentous’ part. As only the latter crosses the knee joint, it is difficult to envisage how tensor fasciae latae could externally rotate the knee (Standring, 2004). Indeed, we are unaware of reports suggesting that this movement is impaired following the use of the ITB as an anterior cruciate ligament replacement (Ellison, 1979). We suggest, however, that the ligamentous part of the ITB could contribute to limiting internal rotation of the tibia. In our view, the proximal region of the ITB should be considered as part of a musculotendinous unit that has its origin on the pelvic girdle and its insertion at the lower end of the femur. Thus, ITB syndrome could be a form of enthesopathy – a suggestion that merits further consideration.
References
- Adkins SB3rd, Figler RA. Hip pain in athletes. Am Fam Phys. 2000;61:2109–2118. [PubMed] [Google Scholar]
- Bas L, Oztek I, Numanoglu A. Subepineural hyperplastic Pacinian corpuscle: an unusual cause of digital pain. Plast Reconstr Surg. 1993;92:151–153. doi: 10.1097/00006534-199307000-00024. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Redman S, Milz S, et al. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis. 2004;63:1549–1555. doi: 10.1136/ard.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogset JO, Rossvoll I, Grontvedt T. Surgical treatment of iliotibial band friction syndrome. A retrospective study of 45 patients. Scand J Med Sci Sports. 1999;9:296–298. doi: 10.1111/j.1600-0838.1999.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Ekman EF, Pope T, Martin DF, Curl WW. Magnetic resonance imaging of iliotibial band syndrome. Am J Sports Med. 1994;22:851–854. doi: 10.1177/036354659402200619. [DOI] [PubMed] [Google Scholar]
- Ellison AE. Distal iliotibial-band transfer for anterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1979;61:330–337. [PubMed] [Google Scholar]
- Fetto J, Leali A, Moroz A. Evolution of the Koch model of the biomechanics of the hip: clinical perspective. J Orthop Sci. 2002;7:724–730. doi: 10.1007/s007760200130. [DOI] [PubMed] [Google Scholar]
- Fredericson M, Wolf C. Iliotibial band syndrome in runners: innovations in treatment. Sports Med. 2005;35:451–459. doi: 10.2165/00007256-200535050-00006. [DOI] [PubMed] [Google Scholar]
- Goh LA, Chhem RK, Wang SC, Chee T. Iliotibial band thickness: sonographic measurements in asymptomatic volunteers. J Clin Ultrasound. 2003;31:239–244. doi: 10.1002/jcu.10168. [DOI] [PubMed] [Google Scholar]
- Hall BK. The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J Embryol Exp Morph. 1986;93:133–152. [PubMed] [Google Scholar]
- Jones NF, Eadie P. Pacinian corpuscle hyperplasia in the hand. J Hand Surg [Am] 1991;16:865–869. doi: 10.1016/s0363-5023(10)80151-0. [DOI] [PubMed] [Google Scholar]
- Joseph J. Locomotor system. In: Hamilton WJ, editor. Textbook of Human Anatomy. London: MacMillan; 1976. pp. 19–200. [Google Scholar]
- Martens M, Libbrecht P, Burssens A. Surgical treatment of the iliotibial band friction syndrome. Am J Sports Med. 1989;17:651–654. doi: 10.1177/036354658901700511. [DOI] [PubMed] [Google Scholar]
- McNicol K, Taunton JE, Clement DB. Iliotibial tract friction syndrome in athletes. Can J Appl Sport Sci. 1981;6:76–80. [PubMed] [Google Scholar]
- Muhle C, Ahn JM, Yeh L, et al. Iliotibial band friction syndrome: MR imaging findings in 16 patients and MR arthrographic study of six cadaveric knees. Radiology. 1999;212:103–110. doi: 10.1148/radiology.212.1.r99jl29103. [DOI] [PubMed] [Google Scholar]
- Nemeth WC, Sanders BL. The lateral synovial recess of the knee: anatomy and role in chronic iliotibial band friction syndrome. Arthroscopy. 1996;12:574–580. doi: 10.1016/s0749-8063(96)90197-8. [DOI] [PubMed] [Google Scholar]
- Noble CA. The treatment of iliotibial band friction syndrome. Br J Sports Med. 1979;13:51–54. doi: 10.1136/bjsm.13.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orava S. Iliotibial tract friction syndrome in athletes – an uncommon exertion syndrome on the lateral side of the knee. Br J Sports Med. 1978;12:69–73. doi: 10.1136/bjsm.12.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard JW, Fricker PA, Abud AT, Mason BR. Biomechanics of iliotibial band friction syndrome in runners. Am J Sports Med. 1996;24:375–379. doi: 10.1177/036354659602400321. [DOI] [PubMed] [Google Scholar]
- Reznik M, Thiry A, Fridman V. Painful hyperplasia and hypertrophy of Pacinian corpuscles in the hand: report of two cases with immunohistochemical and ultrastructural studies, and a review of the literature. Am J Dermatopathol. 1998;20:203–207. doi: 10.1097/00000372-199804000-00019. [DOI] [PubMed] [Google Scholar]
- Richards DP, Alan Barber F, Troop RL. Iliotibial band Z-lengthening. Arthroscopy. 2003;19:326–329. doi: 10.1053/jars.2003.50081. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy: the Anatomical Basis of Clinical Practice. 39. Edinburgh: Elsevier/Churchill Livingstone; 2004. [Google Scholar]
- Tichy P, Tillmann B. The tension band effect of the iliotibial tract. Unfallchirurg. 1989;92:240–244. [PubMed] [Google Scholar]