Abstract
In studies of the evolution of avian flight there has been a singular preoccupation with unravelling its origin. By contrast, the complex changes in morphology that occurred between the earliest form of avian flapping flight and the emergence of the flight capabilities of extant birds remain comparatively little explored. Any such work has been limited by a comparative paucity of fossils illuminating bird evolution near the origin of the clade of extant (i.e. ‘modern’) birds (Aves). Here we recognize three species from the Early Cretaceous of China as comprising a new lineage of basal ornithurine birds. Ornithurae is a clade that includes, approximately, comparatively close relatives of crown clade Aves (extant birds) and that crown clade. The morphology of the best-preserved specimen from this newly recognized Asian diversity, the holotype specimen of Yixianornis grabaui Zhou and Zhang 2001, complete with finely preserved wing and tail feather impressions, is used to illustrate the new insights offered by recognition of this lineage. Hypotheses of avian morphological evolution and specifically proposed patterns of change in different avian locomotor modules after the origin of flight are impacted by recognition of the new lineage. The complete articulated holotype specimen of Yixianornis grabaui, from the Early Cretaceous Jiufotang Formation of Liaoning Province, in north-eastern China, arguably the best-preserved basal ornithurine specimen yet discovered, provides the earliest evidence consistent with the presence of extant avian tail feather fanning.
Keywords: birds, flight, morphological evolution, Ornithurae
Introduction
Ornithurine birds comprise comparatively close relatives of crown clade Aves and that clade (Ornithurae Haeckel 1866 is used as a clade name following Gauthier & de Queiroz, 2001). Ornithurines are usually contrasted with parts of a diverse and abundant clade in the Cretaceous, the enantiornithines (Chiappe, 1995a; Chiappe & Walker, 2002). Although numerous enantiornithines and other basal birds have been discovered in recent years, Mesozoic ornithurines have remained rare (e.g. Chiappe, 2002; Clarke & Norell, 2002; Zhou, 2004). Ornithurine taxa are, however, critical to understanding the later period in the evolution of flight and the emergence of the morphologies and unique physiology seen in all living birds. The Early Cretaceous lagerstätte of north-eastern China (e.g. Zhang et al. 2001; Zhou et al. 2003) has recently yielded a particularly rich fauna, the Jehol Biota, including theropod dinosaurs and basal birds, but even in these deposits ornithurine birds remain uncommon (Zhou, 2004).
In addition to the species of the new lineage reported here (Yixianornis, Yanornis, Songlingornis), other Early Cretaceous ornithurines known from more than a single bone include a partial postcranial skeleton from Mongolia (Ambiortus dementjevi; Kurochkin, 1985), a foot from Gansu Province (Gansus yumenensis; Hou & Liu, 1984), and two partial postcrania from the Jiufotang and Yixian formations (Chaoyangia beishanensis; Hou & Zhang, 1993 and Liaoningornis longidigitus; Hou, 1996, respectively). One further Early Cretaceous taxon is from Europe, Enaliornis baretti, known from a large collection of isolated partial elements (Galton & Martin, 2002). Several ornithurine specimens have recently been evaluated from the Late Cretaceous (Chiappe, 1995b, 1996; Clarke & Chiappe, 2001; Norell & Clarke, 2001; Clarke & Norell, 2002, 2004; Dyke et al. 2002; Kurochkin et al. 2002; Clarke et al. 2005). Prior to these finds, Ichthyornis, Hesperornis and their allies, described over 130 years ago from multiple skeletons (Marsh, 1872a, b), provided most of our insight into this part of avian evolution (Norell & Clarke, 2001; Zhou, 2004). Analysis of ornithurine systematics and morphological evolution has also been complicated by the highly apomorphic nature of several of ornithurine (e.g. Hesperornithes; Marsh, 1880), and closely related, taxa (Patagopteryx deferrariisi; Alvarenga & Bonaparte, 1992; Chiappe, 1995b, 1996), associated with the loss of flight.
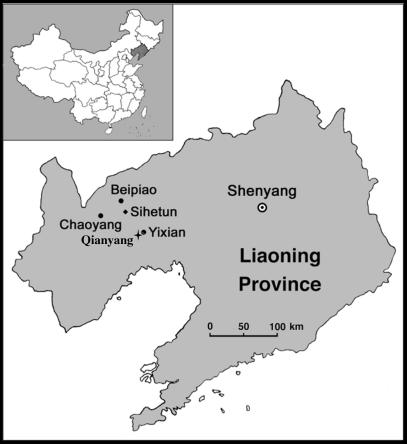
The holotype specimen of Yixianornis grabaui[IVPP V 13631 (IVPP – Institute of Vertebrate Paleontology and Paleoanthropology, Beijing); Zhou & Zhang, 2001], from Liaoning Province, is arguably the best-preserved Mesozoic ornithurine specimen yet discovered, represented by a complete articulated skeleton. It was discovered near the town of Qianyang, approximately 10 miles south-west of Yixian City (Fig. 1) and is from the Jiufotang Formation of the upper Jehol Group (Zhou & Zhang, 2001). The Jiufotang Formation is Early Cretaceous in age (120 Ma; He et al. 2004) and has produced an array of avialan and non-avialan dinosaurs (Zhou et al. 2004). Like many of the Jehol Group specimens, the Yixianornis holotype is exquisitely preserved, with associated wing and tail feather impressions (Figs 1–9). Unlike many specimens from the Jehol Group, the bones are nearly uncrushed and were not split upon discovery. Yixianornis constitutes the only described Mesozoic ornithurine with well-preserved feather impressions (Figs 2 and 9). Two additional specimens from the lineage described here, from the species Yanornis martini, have indications of a feathered outline to the body cavity (Zhou et al. 2002, 2004) but are not known to have wing or tail feathering. Yanornis is one of the rare specimens of Jehol avialans that preserve direct evidence of diet (Zhou et al. 2002, 2004).
Fig. 1.
Map of Liaoning Province, China, showing the Yixianornis grabaui holotype specimen locality near the town of Qianyang, approximately 3 km south-west of Yixian City.
Fig. 9.
Details of the tail feathering preserved in Yixianornis grabaui. Numbers placed at the distal ends of feathers correspond to the eight identified rectrices. Arrows and ‘?’ are explained in the text.
Fig. 2.
The Yixianornis grabaui holotype specimen (IVPP V 13631) with preservation of primary wing and tail feathers. Some body feathers are also preserved close to the ventral edge of the thoracic region and near the neck.
An abbreviated description of Yixianornis grabaui was made at its identification as a new species (Zhou & Zhang, 2001). We (Clarke et al. 2002) then presented our preliminary findings that Yixianornis was phylogenetically placed as most closely related to the Chinese ornithurines, Yanornis martini (Zhou & Zhang, 2001) and Songlingornis linghensis (Hou, 1997) in abstract form.
Here, we detail the morphology of Yixianornis grabaui and present support for recognition of a new clade of Cretaceous ornithurines. We discuss the impact on the perceived pattern of avialan morphological evolution offered by phylogenetic placement of the new clade and the feathers preserved in Yixianornis. Anatomical nomenclature follows Baumel & Witmer (1993).
The morphology of Yixianornis grabaui (Zhou and Zhang, 2001)
Skull
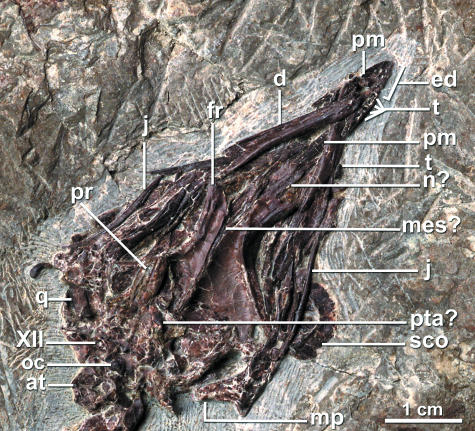
The skull is preserved in ventral view (Fig. 3). The premaxillae comprise less than half the facial margin. They are fused anteriorly, and posteriorly there is an open suture between their frontal (or, plesiomorphically, nasal) processes. Large nutrient foramina dot the anterior premaxillae. Each posterior premaxilla bears four closely spaced teeth, and the anterior third of the extent of the premaxillae along the facial margin is edentulous and pocked with foramina (Table 1, Fig. 3). The maxillary process of the right premaxilla tapers slightly posteriorly toward its articulation with the maxilla. The posterior ends of the frontal processes of the premaxillae are not visible, overlain by other skull elements. However, the frontals bear facets for the articulation of these processes, and the remnants of the processes themselves lie in this facet. Just posterior to the facet, a thin sheet of bone is seen in cross-section between the frontals and is interpreted as the remains of the mesethmoid (Fig. 3). The maxillae are displaced and largely covered by other skull elements. A portion of the left maxilla is slightly exposed between the frontal premaxillary processes and the right dentary. However, no teeth are visibly associated with it. The left nasal is a broad quadrangular element overlying these processes (Fig. 3).
Fig. 3.
The skull of Yixianornis grabaui exposed in ventral view. See Appendix 1 for anatomical abbreviations.
Table 1.
Measurements of the Yixianornis grabaui holotype specimen (in cm)
| Skull | |
| Premaxilla length along facial margin | 0.84 |
| Edentulous portion of premaxilla along facial margin | 0.33 |
| Dentary length, total; from anterior tip to fork of dorsal and ventral processes | 3.2, 1.4 |
| Dentary dorsoventral height at anterior tip | 0.2 |
| Vertebral column | |
| Thoracic vertebra average length | 0.58 |
| Sacrum length | 2.5 |
| Pectoral girdle | |
| Sternum length on midline | 4.31 |
| Sternal fenestra maximum mediolateral diameter | 0.5 |
| Scapula maximum length, breadth just distal to glenoid facet | 4.81, 0.35 |
| Coracoid height, sternal margin length | 2.33, 1.53 |
| Coracoidal lateral process length | 0.3 |
| Furcula: length clavicular ramus (right) | 2.05 |
| Furcula distance between rami just dorsal to apopophysis | 0.42 |
| Furcula shaft diameter at apopophysis | 0.22 |
| Pectoral limb | |
| Humerus maximum length | 4.93 |
| Radius length, midshaft width | 4.8, 0.26 |
| Ulna length, midshaft width | 5.03, 0.32 |
| Carpometacarpus maximum length | 2.56 |
| Metacarpal I length | 0.46 |
| Metacarpal III width | 0.09 |
| Metacarpal II width | 0.28 |
| Phalanx length I:1, I.2 | 1.08, 0.61 |
| Phalanx II:1, II:2, II.3 | 1.25, 1.23, 0.5 |
| Phalanx III:1 | 0.6 |
| Pelvic girdle | |
| Ilium length total, preacetabular, postacetabular | 2.35, 1.2, 1.15 |
| Ischium length (estimated) | 2.05 |
| Pubis length, average shaft diameter, symphysis length | 4.22 est., 0.11, 0.66 |
| Pelvic limb | |
| Femur maximum length, midshaft width | 4.10, 0.42 |
| Tibia maximum length, not including cnemial crest | 5.28, 5.26 |
| Tarsometatarsus maximum length | 2.7 |
| Pedal phalanx I:1 length | 0.78 |
| Pedal phalanx II:1, II:2 | 1.13, 0.94 |
| Pedal phalanx III:1, III:2, III:3 | 1.15, 0.87, 0.83 |
| Pedal phalanx IV:1, IV:2, IV:3, IV:4 | 1.25, 0.58, 0.56, 0.62 |
| Feathers | |
| Rectrices: longest, shortest | 9.2 est., 7.5 est. |
| Remiges: right side, maximum length distal primaries (9 & 8?) | 6.7 est. |
| Remiges: left side, maximum length proximal primary (1–5?) | 5.6 est. |
The mandible is narrow anteriorly and quite delicate overall. The medial mandibular process is very well developed. The dentary is strongly forked posteriorly. Four large mental foramina are visible in a shallow groove near the dorsal margin of this dentary; a fifth large foramen is preserved just anteroventral to these other four (Fig. 3). Small nutrient foramina also lie in a line at the lateroventral edges of the symphysial area.
The premaxillary teeth are socketed. One isolated tooth appears to be associated with the right dentary. Whether the dentary teeth lay in a groove (e.g. as in Hesperornis; Marsh, 1880) or distinct sockets (e.g. Ichthyornis dispar; Marsh, 1880; Clarke, 2004), however, cannot be determined. The dentulous portion of the dentary extended for approximately one-third of the total mandible length and thus was comparatively short. Individual tooth morphology is well preserved in two complete displaced teeth that are exposed next to the left dentary and between the right dentary and the right premaxilla. The tooth crown is unserrated and relatively short and peg-like in morphology. It is slightly constricted at its base, and the crown tip is only slightly deflected (Fig. 3). The tooth crown is less compressed mediolaterally than in Ichthyornis dispar (e.g. Marsh, 1880). The root is conspicuously expanded relative to the crown width. No resorption pits are visible in the exposed tooth roots.
The large, relatively domed frontals are visible in ventral view. An open ventral suture between the right and left frontals indicates they were not completely fused to each other. The suture with the crushed remnants of the parietals appears to be open as well, but this area is poorly preserved. A notch in the posterolateral margin of the left frontal may indicate some complexity in this contact (frontal/parietal).
The jugals, visible on both sides of the specimen, are narrow rod-like elements that lacked an ascending process. The otic process of the right quadrate is visible, and there does not appear to be an incisure separating the capitula. The parasphenoid rostrum, basisphenoid plate and occipital condyle are exposed but badly crushed. The basicranial pterygoid articulations are relatively unprojected facets (Fig. 3). One foramen anterolateral to the occipital condyle on the right side of the skull is tentatively identified as that of n. hypoglossi (XII).
A displaced ring of scleral ossicles is preserved near the right side of the skull. The inner margins of the ossicles are deflected, making their exposed lateral surface slightly concave.
Vertebral column, ribs and gastralia
The complete vertebral series is preserved in articulation with the exception of several anterior caudal vertebrae (Fig. 2). Twenty-two presacral vertebrae are present. The atlas is preserved in articulation with the odontoid process of the axis (Fig. 3). Twelve vertebrae are anterior to the first with an associated elongate free rib. Transverse foramina partially enclosed by fused ribs are best exposed in the third to seventh vertebrae. Mid-series cervical vertebrae have well-arched post-zygopophyses. The centra of the twelfth vertebra is short and has a blade-like hypapophysis similar to that developed on the eleventh, whereas a more diminutive hypapophysis is developed on the tenth. Evaluation of cervical centra articulations across Avialae is problematic (Clarke, 2004). It appears that whereas some anterior cervical vertebrae may have a degree of heterocoely or ‘incipient’ heterocoely, the posterior cervicals are best described as completely amphyplatous.
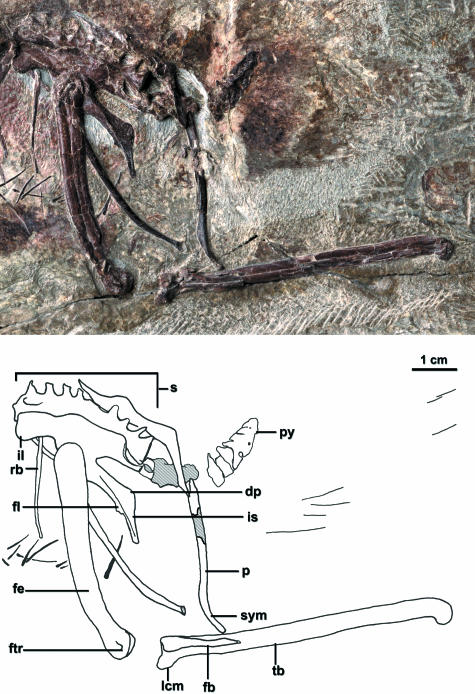
Large lateral excavations are present in presacral vertebrae 11–22 as well as in the first sacral vertebra, although these are not as deep as in some other basal ornithurine taxa (e.g. Ichthyornis dispar; Clarke, 2004; Fig. 4). The estimated ten thoracic vertebrae have centra that are longer than wide with articular surfaces that are amphyplatous. Their parapophyseal facets are located near the anterior margin of these centra. There are nine sacral vertebrae (Fig. 5). The ankylosed sacral vertebrae and their costal processes are approximately of the same length and evenly spaced, respectively; the vertebrae in the middle of the series are not abbreviated anteroposteriorly (see character commentary in Clarke, 2004; Fig. 5). Five free caudal vertebrae are inferred to comprise the poorly preserved series. Four vertebrae are visibly incorporated into the short ploughshare-shaped pygostyle; their centra are fused, but portions of the neural spines remain distinguishable (Fig. 5).
Fig. 4.
Presacral vertebrae and pectoral girdle of Yixianornis grabaui. See Appendix 1 for anatomical abbreviations.
Fig. 5.
Sacral vertebrae, pelvic girdle and limb of Yixianornis grabaui. See Appendix 1 for anatomical abbreviations. Dashed regions in the line drawing indicate broken or crushed areas.
Approximately 15 partial or complete delicate components of the gastral basket are roughly in life position (Fig. 4). From their position and orientation, 6–8 sets of gastralia are estimated. In Velociraptor mongoliensis (Norell & Makovicky, 1997) 12 are present, and in Confuciusornis sanctus eight are known (Chiappe et al. 1999). Elongate slender uncinate processes cross two ribs (Fig. 4). The proximal ends of these processes are expanded and blocky. It is unclear whether these processes were fused to the ribs.
Pectoral girdle and limb
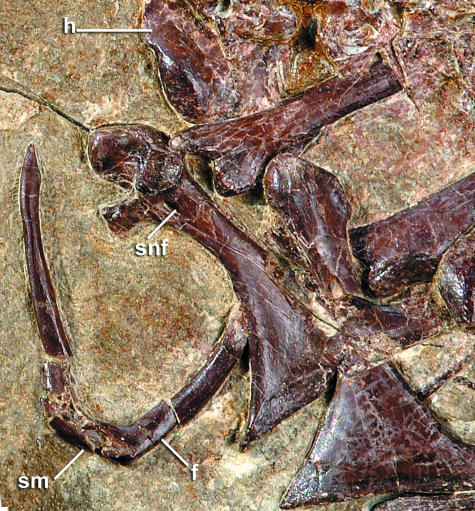
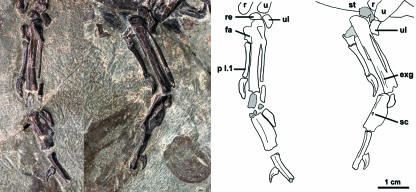
The sternum is exposed in left lateral view (Fig. 4). It has a well-projected keel with the apex slightly posteriorly displaced relative to the sternal rostrum. On the lateral sternal margin, a well-projected sternocoracoid process and small zyphoid process are visible. The posterior sternal margin bears a posterolateral process with an expanded tip and a posteromedial process that curves to fuse with the sternal midline bounding an ovoid fenestra (Fig. 4). The keel extends to the posterior terminus of the sternum. The furcula is exposed in anterior view (Figs 4 and 6). Its ventral (or sternal) margin at the apophysis is straight rather than smoothly curved or pointed (Fig. 6, sm; Fig. 10, inset). Unfortunately, this part of the furcula was lost (compare Figs 4 and 6) while the specimen was in transit for exhibition.
Fig. 6.
Furcula of Yixianornis grabaui prior to damage. See Appendix 1 for anatomical abbreviations.
Fig. 10.
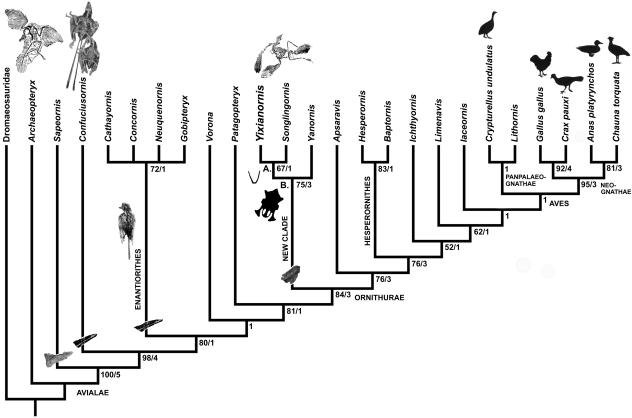
Strict consensus cladogram of the two most parsimonious trees resulting from analysis of the characters and taxa in Appendices 2 and 3[length: 422, CI: 0.63, RI 0.82, RC 0:51 (PIC only)]. Bootstrap support for those nodes recovered in greater than 50% of the 1000 replicates performed and Bremer (1988) support values are reported to the right of the node to which they apply (Format: Bootstrap/Bremer). Mesozoic avialans with tail feathering known are pictured, Protopteryx is shown for Enantiornithes as it is basally placed within that group. No Enantiornithes are known to possess more than two elongate rectrices, although some possess none at all (Zhang & Zhou, 2004). Pygostyle morphology is illustrated for Sapeornis, Confuciusornis, Iberomesornis and Yixianornis and shown in insets. One unambiguous synapomorphy supports the clade Yixianornis + Songlingornis (Node A). Monophyly of new clade of Chinese ornithurines (Node B) is supported by four unambiguously optimized synapomorphies (numbers refer to characters and states listed in Appendix 2): 53:0, cervical vertebrae not completely heterocoelous; 94:1, presence of a procoracoid process; 99:1, coracoid without groove at medial opening of n. supracoracoideus foramen; 79:2, medial posterior process of sternum joined to sternal midline to enclose sternal fenestra; 85:1, base of furcula with a truncate or squared base. Seven unambiguous synapomorphies support placement of the clade as more closely related to Aves than Patagopteryx: 55:2, ten or fewer thoracic vertebrae; 95:1, intermuscular line on ventral surface of the coracoid; 107:1, humeral head domed or globose; 143:1, metacarpal III narrow compared with diameter of metacarpal II; 152:1, phalanx II.1 strongly dorsoventrally compressed; 160:1, antitrochanter posterodorsally located with respect to acetabulum; 193:1, metatarsal III displaced posterior to II and IV proximally. Apsaravis is placed more closely to Aves than this new lineage by six unambiguous synapomorphies: 62:4, ten sacral vertebrae; 67:1, non contacting pre- and postzygopophyses in tail; 146:1, metacarpal I articulation developed as a shelf; 159:2, ischium and pubis subparallel and appressed; 169:1, pubis compressed mediolaterally; 188:2, posterior portion of distal articular surface of tibiotarsus for tibial cartilage with well-developed wings.
The right coracoid is preserved in dorsal view and the left in ventral view (Fig. 4). Both strut-like coracoids bear visible lateral processes. The scapular cotyla is deeply concave, and the procoracoid process is broad and well projected with a slightly expanded distal end. The scapular cotyla extends slightly sternal to the glenoid facet. It is teardrop shaped, and its medial edge is slightly posteriorly projected. The supracoracoid nerve foramen penetrates the base of the procoracoid process where it meets the shaft, and the dorsal surface of the coracoid is flat (Figs 4 and 6). The articular surface for the furcula is projected medially. The right scapula is preserved in ventral view and the left in dorsal view. The scapula is approximately of the same length as the humerus (Fig. 4; Table 1), and the scapular blade is recurved and tapers posteriorly. The acromion extends anterior to the pronounced hemispherical coracoid tubercle, but it is not as extremely elongate as in other basal ornithurines (e.g. Apsaravis ukhaana, Norell & Clarke, 2001; Iaceornis marshi, Clarke, 2004).
The right and left humeri are preserved in posterior view (Figs 2 and 4). They are approximately of the same length as the ulnae. Morphologies of their proximal ends are obscured by breakage and overlying elements. The humeral head is globose, and the remains of the deltopectoral crest on the right side indicate that it projected dorsally. This crest extends distally for more than one-third of the total humerus length. The ventral tubercle and bicipital crest are obscured although the capital incisure is visibly open. The flexor process/ventral epicondyle is projected distally only as far as the ventral condyle, and an olecranon fossa is visible. The m. scapulotriceps groove on the distal humerus does not appear to be developed.
The right ulna is exposed in ventral view. A prominent bicipital tubercle is developed. The distal end is rather blunt but has a semilunate trochlear surface. The width of the radius is just slightly greater than half the width of the ulna. The ulnare is box-shaped with little differentiation into dorsal and ventral rami (incisure metacarpalis undeveloped). It is approximately of the same size as the radiale.
Proximally, metacarpals I, II and III are fused to each other and to the semilunate carpal (Fig. 7). There is no sign of an unincorporated third free carpal as is present in more basal taxa; it is inferred that this carpal has been fused in formation of the carpometacarpus. Metacarpal I, although ankylosed proximally, is not fused to metacarpal II (Fig. 7) distally; this morphology is also seen in Yanornis martini (IVPP V13358). There is no indication that the specimens in which this condition is observed represent subadult individuals (e.g. all bone epiphyses are fully ossified, late-stage ontogenetic events such as fusion of the cervical ribs to enclose transverse foramina have occurred and fine muscle scars are visible). The articulation surface developed on its distal end is robust and strongly ginglymoid. Metacarpals II and III are fused to each other distally and subequal in distal extent. Metacarpal II is slightly shorter than III, and its area of fusion to III is less extensive than in most taxa with distal fusion. The extensor groove extends straight down the ventral surface of metacarpal II, with a slight scar for the distal retinacular restraint near its distal terminus. This projected scar is not as large as in Ichthyornis, where this feature is developed as a pronounced tubercle (Clarke, 2004). The pisiform process is connected by a ridge to proximal metacarpal III where a tubercle is developed.
Fig. 7.
Pectoral limb of Yixianornis grabaui. See Appendix 1 for anatomical abbreviations. Dashed regions in the line drawing indicate broken or crushed areas. The dashed area near the sternal margin in the left arm has been repaired with glue.
Phalanx I.1 is bowed and extremely elongate (Fig. 7). Its distal end has deep flexor pits associated with the articulation of phalanx I.2, which is developed as a robust, highly recurved claw more than half the length of I.1. Phalanx II.1 is flat and lacks an internal indicus process. On the distal anterodorsal surface (of the pila cranialis), a slight tubercle is developed as in Ichthyornis (Clarke, 2004). Phalanx II.2 is approximately of the same length as II.1. Phalanx III.1 has a diminutive flexor process not as well developed as in Ichthyornis (Clarke, 2004).
Pelvic girdle and limb
The pelvic bones are fused; the ilium and ischium are visibly ankylosed on the right side, and the ischium and pubis on the left (Fig. 5). The ilium, ischium and pubis are not subparallel, as the pubis and ischium angle clearly ventrally to the other pelvic elements. The pre- and post-acetabular portions of the ilium are subequal in length. Preparation of the ventrolateral surface of the ilium confirmed the absence of an m. cuppedicus fossa or shelf. The anterior end of the ilium may have overlapped a free set of ribs. The ischium is much shorter than the pubis, extending a short distance past the posterior terminus of the sacrum; in this extent it approximates the condition in Confuciusornis (Chiappe et al. 1999) and other basal birds such as Enantiornithes (Chiappe & Walker, 2002). It has a slight ridge on its lateral surface, and its posterior tip tapers to a point. A dorsal process is developed that is similar in shape, size and distal position to that developed in Ichthyornis (Marsh, 1880). A small flange projects off the ventral margin of the ischium (Fig. 5). The pubes are elongate, relatively robust elements that are ovoid in cross-section rather than compressed mediolaterally. They would have contacted posteriorly in a short symphysis (Fig. 5). The symphysial area of the right pubis is preserved with an abraded tip. Their distal terminus would not have been expanded into a boot.
The femora are exposed in lateral view (Fig. 5). They are only slightly bowed and longer than the tarsometatarsi. Their greater and lesser trochanters are fused to form a trochanteric crest. A small fibular trochlea is developed. From the conformation of the incompletely exposed distal end, a patellar groove is not inferred to be present. The proximal tarsals are fused to the tibia. The left tibiotarsus is exposed in lateral view, and the fibula is visible lying along its proximal surface (Fig. 5). The right tibiotarsus is twisted with the proximal end in a somewhat more oblique anterolateral view, and the distal end is in posterior view. The lateral cnemial crest is exposed best on the left tibiotarsus, and a prominence that may be an anterior cnemial crest is visible on the right tibiotarsus. The fibular crest, best seen on the right side, is well projected. On the right side, the sulcus cartilaginous tibialis extends onto the posterior tibia and is demarcated medially and laterally by small alae. These wings are not as well projected as in Apsaravis (Norell & Clarke, 2001). The m. iliofibularis tubercle is visible on the left fibula (Fig. 5). The fibula does not contact the distal tarsals.
The distal tarsals are fused to the metatarsals, and the metatarsals are co-osified proximally and distally to enclose a distal vascular foramen (Fig. 8). Metatarsal V is not present. A well-projected globose intercotylar prominence is lacking, and a hypotarsus with grooves or ridges is either absent or was only weakly projected, as in Apsaravis, Hesperornis and Patagopteryx (Chiappe, 1996; Clarke & Norell, 2002). Metatarsal III is extended furthest distally, metatarsal IV is just slightly shorter than III, and metatarsal II is the shortest. Medial and lateral plantar grooves are developed (Fig. 8). Metatarsal I is clearly visibly swung back to the plantar surface but does not appear to be conspicuously twisted. The phalanges are delicate. Flexor pits at their distal ends are deep. Pedal digit III is the longest with digit IV approaching it in length. Digit II is notably shorter than digits III and IV (Zhou & Zhang, 2001).
Fig. 8.
The feet of Yixianornis grabaui: the right is exposed in ventral view (top) and the left is exposed in dorsolateral view (bottom). See Appendix 1 for anatomical abbreviations.
Feathers
A minimum of eight elongate tail feathers (rectrices) is represented (Fig. 9). Additionally, there is an area with light brown apparently carbonized material extending for approximately 10 mm on either side of the pygostyle. The tips of five rectrices are well preserved. Black linear impressions of the raches of individual feathers lie in the area of the right foot and between the distal feather tips and the pygostyle. Many of these align with the distal, better-preserved feather tips (e.g. two arrows in Fig. 9). One extremely short feather (possibly a ventral covert) or incompletely preserved feather is visible just below the two rachis impressions indicated by arrows (indicated with a ‘?’) in Fig. 9. Additionally, close to the right foot, one short, black, lineate impression subparallel to a comparatively well-preserved feather numbered seven in Fig. 9 may indicate the presence of one more rectrix (‘?’ in Fig. 9).
The tail feathers are slightly graduated, the outer-most being shorter than the inner (Fig. 9; Table 1). However, the shortest feather is also the most ventrally situated with respect to the orientation of the pygostyle, and at least four rectrices approximate the longest measured in length, suggesting that the tail may be considered to be rounded by some definitions (see Fitzpatrick, 1999). The tail is just over three times the tarsometatarsus length and approaches, but is slightly shorter than, the body length (from the set of four most elongate rectrices; Table 1).
Five elongate primary feathers (remiges) are associated with the distal right wing, and the remains of six are associated with the left (Fig. 2). The primary feathers associated with the right wing are broad and asymmetrically vaned. They have correspondingly broad, rounded tips that do not taper distally and are un-notched (Fig. 2). The distal-most primary is slightly shorter than that just proximal to it (Fig. 2; Table 1); that this represents a preservational artefact, however, cannot be completely excluded.
Phylogenetic analyses
We investigated the phylogenetic position of Yixianornis, Yanornis and Songlingornis using a dataset modified from that of Clarke & Norell (2002). This dataset was revised by adding five additional characters (listed in Appendix 2) to address morphologies present in the new taxa and not encompassed by previous characters/character descriptions (e.g. sternal fenestra). Two deliberately redundant characters from that dataset representing previous wordings were removed (see Clarke & Norell, 2002, for the original rationale for inclusion of these characters). The modified character list is given in Appendix 2. Taxon sampling follows that of Clarke & Norell (2002) with the addition of two recently described taxa, Iaceornis marshi (Clarke, 2004) and Sapeornis chaoyangensis (Zhou & Zhang, 2002). The former taxon is scored from the holotype specimen (YPM 1734) and the latter taxon is scored from the holotype specimen (IVPP V12698) as well as from two referred specimens (IVPP V13275, IVPP V13276; Zhou & Zhang, 2003). Five species exemplars were used for Aves (sensuGauthier, 1986; Gauthier & de Queiroz, 2001). These exemplars were chosen to sample both basal divergences (Crypturellus, Chauna and Crax) and deeply nested taxa (Anas and Gallus) from within the three included avian subclades based on previous phylogenetic hypotheses (see Clarke, 2002, for further explanation of exemplar choice). Outgroup taxa were Archaeopteryx lithographica and Dromaeosauridae, the latter represented by Deinonychus antirrhopus, Dromaeosaurus albertensis and Velociraptor mongoliensis. The material scored for these taxa was given in Clarke & Norell (2002). Yanornis martini was evaluated from all specimens referred to this taxon (IVPP V12558, IVPP V12444, IVPP V13259, IVPP V13358; Zhou et al. 2002, 2004). Songlingornis linghensis was scored from the holotype specimen (IVPP V10913) as was Yixianornis.
Analysis of 191 parsimony informative characters (PIC) of 205 total (38 ordered) evaluated for 25 taxa was conducted using PAUP*4.08b [Swofford, 2002; branch and bound search, amb- (collapsing minimum 0 length branches), polymorphism differentiated from ambiguity]. Two most parsimonious trees resulted [length: 422, CI: 0.63, RI 0.82, RC 0 : 51 (PIC only); length: 434, CI: 0.64, RI 0.82, RC 0 : 52 for all 205 characters]. Figure 10 is the strict consensus cladogram of the two trees. One thousand bootstrap replicates (addition sequence, furthest; initial upper bound, computed via stepwise addition; all other search settings the same in the initial analyses) were used to evaluate support for recovered nodes. Bootstrap and Bremer support values (Bremer, 1988) are reported (Fig. 10).
When all characters were run unordered, there were 22 resultant most parsimonious trees [length: 410, CI: 0.63, RI 0.80, RC 0.51 (PIC only); length: 426, CI: 0.65, RI 0.80, RC 0.52 for all 205 characters]. The strict consensus cladogram of these 22 trees recovers the monophyletic Chinese ornithurine clade, although some basal avialan relationships become unresolved. The recovered relationships are consistent with previous analyses of avialan relationships with similar sampling (e.g. Chiappe, 2002; Clarke, 2004; Clarke et al. 2005).
The monophyly of a new clade of Chinese ornithurines, recovered with significant bootstrap support (76% of replicates), is supported by four unambiguously optimized synapomorphies (Fig. 10 caption) including the presence of a sternal fenestra (Fig. 10 inset at node, illustrated from Songlingornis). A partially edentulous premaxilla is uniquely known from the clade (preserved for both Yanornis and Songlingornis; the latter contra Hou, 1997) within Avialae. Although this morphology may additionally support monophyly, it was allowed to be a potentially intermediate state [toward the complete loss of teeth in the premaxilla seen later in Ornithurae (Martin, 1983; Chiappe, 1991, 2002; Clarke, 2004)] and elsewhere in Avialae. One unambiguous synapomorphy, the ventral (or sternal) margin of the furcula (at the apophysis) truncate or with a squared base (rather than a smooth curve or pointed), supports the clade Yixianornis + Songlingornis (character state 85:1 of Appendix 2; Figs 6 and 10: inset at node, illustrated from Yixianornis).
Amended differential diagnosis of Yixianornis
A differentia of Yixianornis from Yanornis, Songlingornis and other Early Cretaceous ornithurines was provided in Zhou & Zhang (2001). Here we provide a further differentia. Yixianornis is distinguished from other parts of the new clade by a greater extent of zyphoid process along the sternal margin than in either Yanornis or Songlingornis. The dentulous portion of the dentary in Yixianornis (inferred as maximally the length from the anterior tip to the divergence of the dentary posterior processes) is also shorter than in Yanornis or Songlingornis. The width of the ‘U’ shape of the furcular rami appears narrower in Yixianornis than in Songlingornis. One character is unambiguously optimized as a local autapomorphy of Yanornis martini: scapular length less than that of the humerus (character 103:0, Appendix 2) whereas in the other taxa of the new clade the scapula is approximately the length of the humerus as well as approaching the length of the complete thoracic series. In Songlingornis, the distal expansion of the lateral posterior processes of the sternum is greater than in the other two taxa. The presence of a sternal fenestra was earlier remarked for Songlingornis by Hou (1996b: fig. 32), who considered it to be possibly unique for the taxon; here it is recognized as a synapomorphy of the new clade (Fig. 10).
No unambiguous autapomorphies of Yixianornis were recovered in the phylogenetic analysis although it is easily differentiated by the combination of characters described above. New character data for closely related species will allow unambiguously optimized autapomorphies to be recovered in future analyses. For example, character 144:2, concerning greater projection of the extensor process on metacarpal I in Yixianornis relative to Yanornis, is under some character optimizations such an autapomorphy of Yixianornis but it is ambiguously optimized due to missing data for Songlingornis and more basally divergent outgroups of the new clade.
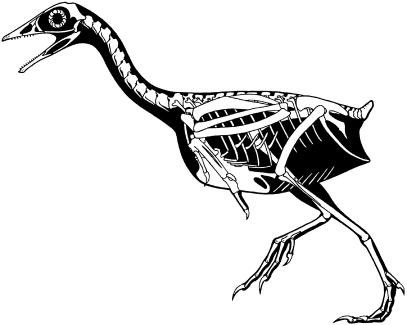
A skeletal reconstruction of Yixianornis grabaui by G. S. Paul is shown in Fig. 11.
Fig. 11.
Skeletal reconstruction of Yixianornis grabaui by G. S. Paul.
Discussion
Morphological evolution and the origin of avian tail fanning
Yixianornis provides understanding of the diversity of basal ornithurines (Zhou & Zhang, 2001) and morphological evolution across Avialae. The recovery of the new clade suggests for the first time the possibility of Early Cretaceous endemism within the earliest part of Ornithurae. The broad wing tips, relatively elongate tail and slightly shorter distal-most primary in Yixianornis are consistent with a taxon manoeuvring in a close or densely vegetated environment (Saville, 1957; Rayner, 1988; Keast, 1996), an ecology not previously remarked for non-crown clade ornithurines (e.g. review and citations in Clarke & Norell, 2002).
All three species in the new clade evidence forelimb morphologies (e.g. lateral process, globose humeral head) previously conceived as originating closer to crown clade Aves (Zhou & Zhang, 2001), and thus previously optimized as phylogenetically later in avian evolution (e.g. Chiappe, 2002; Clarke & Norell, 2002). All three species also retain gastralia, a pubic symphysis and other pelvic morphologies previously optimized as having been lost earlier (Chiappe, 2002; Clarke & Norell, 2002). This novel combination, or apparent mosaicism of retaining primitive pelvic morphologies and derived forelimb morphologies, seems to correspond with prior hypotheses of avialan morphological change.
Chiappe (1991, 1995a, b, 1996, 2002) concluded that evolution of the modern flight apparatus preceded evolution of the modern bird mechanism of terrestrial locomotion. Under this hypothesis, given the phylogenetic position of the new clade, it would be predicted that most modifications of the forelimb toward the condition seen in living birds would have already occurred by the divergence of the Chinese ornithurines, and that many modifications of the hind limb would not yet have occurred. Only additional data and taxon sampling will illuminate this proposed pattern. Homoplasy and missing data at the base of Ornithurae now make many of these characters ambiguously optimized (e.g. pubic symphysis loss). Furthermore, an array of hind limb morphologies (e.g. a posteriorly displaced metatarsal III) are now seen phylogenetically earlier, co-present in the new clade with the plesiomorphic pelvic morphologies. These optimizations suggest an earlier origin for hind limb traits as well.
Chiappe (2002: 460) noted a relatively peak-less track of changes in the tail characters, concluding that it might suggest a ‘progressive’ change in avian tail after the origin of flight. Nevertheless, an array of traits related to ‘modern’ avian tail function or fanning have all been inferred present at, and associated with, any fusion of distal caudal vertebrae to form a pygostyle, including those unambiguously optimized as non-homologous with the pygostyle in Aves (e.g. see treatment of Nomingia, Barsbold et al. 2000).
Extant birds possess specialized adipose tissue structures lying on either side of the pygostyle encasing the base of the tail feathers and surrounded by muscle. These structures are called the bulbi rectricium, or rectrical bulbs (Baumel, 1988; Gatesy & Dial, 1996a: fig. 4) and have been identified as crown avian neomorphs (e.g. Gatesy, 2001). Gatesy & Dial (1993) showed that tail fanning, spreading tail feathers from a closed resting condition to extension of the fan (parallel to radial array; Gatesy, 2001), is controlled solely by this bulbi rectricium.
Prior to the discovery of the rod-shaped pygostyles of basal avialans, Baumel (1988) hypothesized that the pygostyle could have co-evolved with the bulbi rectricium (Gatesy & Dial, 1996a). However, Gatesy & Dial (1996a, b) noted that the structure of these rod-shaped pygostyles, distinct from that seen in living birds (originally noted in the enantiornithine Iberomesornis; Gatesy & Dial, 1996a), may or may not be so correlated. Here we bring to bear evidence from recent fossil discoveries for variation in the morphologies of the pygostyle and tail feathering further to explore evolution of the locomotor function of the tail seen in living birds. We find that the hypothesis that any fused distal caudal vertebrae (commonly called a pygostyle wherever present in Theropoda) correlates with the presence of a bulbi rectricium and tail fanning (e.g. Baumel, 1988) is not supported by available evidence. By contrast, the ploughshare-shaped morphology first seen in Yixianornis and other basal ornithurines may be so correlated. The rod-shaped pygostyles homologous with those in Aves in basal avialans may best be interpreted as associated simply with tail reduction (Gatesy, 2001); they are only currently known to be associated with the presence of two rectrices (Zhang & Zhou, 2004).
In Archaeopteryx an elongate, feathered tail is present (Fig. 10, inset). In Sapeornis distal caudal vertebrae fuse to form a mediolaterally broad and peg-like pygostyle, but no feathers are known (Fig. 10, inset; Zhou & Zhang, 2003). A more elongate and rod-like pygostyle, as long or longer than the free caudal segment of the tail, is present in Confuciusornithidae (Chiappe et al. 1999) and Enantiornithes (Fig. 10, insets) with up to 12 incorporated vertebrae (e.g. Gatesy, 2002). All known Enantiornithines preserving tail feathering (Eoenantiornis buhleri, Hou et al. 1999; Protopteryx fengningensis, Zhang & Zhou, 2000; Longirostravis hani, Hou et al. 2004) have at most two elongate tail feathers, or rectrices. These two rectrices may be present in only one morph or sex for Confuciusornis sanctus (Hou et al. 1996; Chiappe et al. 1999). In specimens lacking these elongate feathers, nothing fitting the description of rectrices (i.e. elongate tail feathers) is known. There is just a short tuft of feathers around the tail (e.g. Zhang & Zhou, 2004). By contrast, eight elongate rectrices are associated with the short, ploughshare-shaped tail in Yixianornis, the only known non-crown clade Ornithurine with preserved tail feathers.
Gatesy (2001) considered the possibility that a theropod-shaped pygostyle in Confuciusornis, and basal and derived Enantiornithes could simply be a vestige of tail shortening representing reduction and terminal fusion (or lack of differentiation) of feather-associated caudal segments rather than being already associated with derived function related to the presence of a manoeuvrable rectricial fan. Zhang & Zhou (2004) commented on the evidence for evolution of tail feathering, noting the presence of a tail with, at most, a single pair of elongate tail feathers in the Confuciusornithidae and Enantiornithes. They hypothesized that a pair of tail feathers could represent the primitive condition (Zhang & Zhou, 2004) presumably for a part of Avialae.
We suggest that the two tail feathers in these basal taxa may be homologues of the two attaching to the pygostyle in living birds (Baumel, 1988; Gatesy & Dial, 1996b, and citations therein). These two feathers might have been vestigial and free to evolve into the aerodynamically costly, extremely elongate feathers seen in the Confuciusornithidae and Enantiornithes acted on primarily through sexual selection. That the pygostyle-associated tail feathers in Confuciusornis (e.g. Chiappe et al. 1999), for example, did not contribute much aerodynamically may be suggested by the extremely elongate wings in that taxon. Compensatory increases in wing length may be seen in species with aerodynamically costly tails associated with signalling and sexual selection in living birds (e.g. Fitzpatrick, 1999). We further believe that the hypothesis that sexual selection, key in affecting tail shape in living birds (e.g. Evans & Thomas, 1993; Balmford et al. 1993; Barbosa & Møller, 1999; Fitzpatrick, 1999; Buchanan & Evans, 2000; Lowe et al. 2001), may have been as central as locomotor function early in avialan evolution in affecting tail morphology merits further attention.
The earliest presence of a pygostyle morphology associated with a rectrical bulb in extant birds and the earliest presence of multiple, radially arranged rectrices are optimized as phylogenetically contemporaneous, at the most recent common ancestor of the new clade including Yixianornis and Aves. The change in pygostyle morphology to a mediolaterally compressed upturned ploughshare shape seen in Yixianornis may be associated with the origin of a bulbi rectriculum and tail fanning. Only further evidence, such as discovery of feathered specimens of Sapeornis, the phylogenetically earliest taxon with fused distal caudal vertebrae homologous with a pygostyle in Aves, will resolve whether the presence of at most two elongate tail feathers represents convergence in the Confuciusornithidae and Enantiornithes or is primitive for part of Avialae. These two optimizations are currently equally parsimonious.
Conclusions
The complete articulated holotype specimen of Yixianornis grabaui (Zhou & Zhang, 2001) is arguably one of the best-preserved Mesozoic ornithurine specimens ever discovered and the only such specimen with well-preserved feather impressions. Yixianornis is identified as most closely related to other Chinese ornithurines, Yanornis and Songlingornis. Because of its phylogenetic placement, the component species of the new clade inform morphological diversity and optimization of ancestral character states for one of the most poorly sampled parts of avialan evolution. The new clade shows combinations of morphologies not presented by any other Mesozoic avialans. Plesiomorphic pelvic morphologies and the presence of touchstone characters for ‘advanced’ avian function of the forelimb, like a globose humeral head or lateral process on the coracoid, in the new clade seem to fit the proposed timing of the evolution of these parts of avian locomotion (Chiappe, 1991, 1995a, b, 1996, 2002).
Feathering and tail morphology in Yixianornis and the placement of the new clade suggest a new perspective on sequence of events in the evolution of the tail or caudal locomotor module (Gatesy & Dial, 1996a) in avialan evolution. The first pygostyles, rod-like and elongate, are not justifiably inferred to be associated with the avian neomorph, the bulbi rectriculum, and its associated tail fanning function in avian aerial locomotion (Gatesy & Dial, 1996a, b). Thus, the evolutionary timing of this novelty in avian aerial locomotion is identified as arising later than previously proposed. With this argument, we want to spur new consideration of the morphology of the rectrices with respect to that of the pygostyle and of the potentially important role of sexual selection in affecting tail shape in basal avialans.
Acknowledgments
We would like to thank Xiaolin Wang, Kevin Middleton, Mark Norell, Luis Chiappe, Jacques Gauthier and one anonymous reviewer for comments that improved the manuscript. Many thanks also to Gregory Paul for undertaking the skeletal reconstuction as well as to Mick Ellison for photographs of the specimen and Kaitlin Strickland for figure preparation. The AAAS WISC programme, NSFC-40121202-Chinese Academy of Sciences kzcx3-sw-142, AMNH Division of Paleontology/Frick Fund and North Carolina State University are gratefully acknowledged for financial support.
Appendix 1
Anatomical abbreviations used in the figures
XII, cranial nerve XII (n. hypoglossi); at, atlas; c, coracoid; crv, cervical vertebrae; d, dentary; df, distal foramen; dp, dorsal process; ed, edentulous region; exg, extensor groove; f, furcula; fa, fused area; fb, fibula; fe, femur; fen, fenestra; fl, flange; fr, frontal; ftr, fibular trochlea; g, gastralia; h, humerus; ha, hypotarsal area; il, ilium; is, ischium; j, jugal; k, keel; lcm, lateral cnemial crest; le, lateral excavation; mes, mesethmoid; mp, medial process; mt 1, metatarsal 1; n, nasal; oc, occipital condyle; p, pubis; pc, plantar crests; pI. 1, phalanx I. 1; pm, premaxilla(e); pr, parasphenoid rostrum; pta?, basicranial pterygoid articulation; py, pygostyle; q, quadrate; r, radius; rb, rib; re, radiale; s, sacrum; sa, sternal apex; sc, scar; sco, scleral ossicles; scp, scapulocoracoid process; sk, sternal keel; sm, sternal margin; snf, supracoracoid nerve foramen; st, sternum; sym, symphysial region; t, tooth/teeth; tb, tibiotarsus; tv, thoracic vertebrae; u, ulna; ul, ulnare; up, uncinate process.
Appendix 2
The 205 morphological characters used in the phylogenetic analysis. Characters 3, 79 (ordered), 85, 116 and 159 (ordered) were added to the Clarke et al. (2002) dataset; 79 and 86 of those authors are eliminated (see text).
Premaxillae: (0) unfused in adults; (1) fused anteriorly in adults, posterior nasal [frontal] processes not fused to each other; (2) frontal processes completely fused as well as anterior premaxillae (Ordered).
Premaxillary teeth: (0) present, (1) absent.
Premaxillae at least partially edentulous: (0) absent, (1) present.
Maxillary teeth: (0) present, (1) absent.
Dentary teeth: (0) present, (1) absent.
Tooth crown serration: (0) present, (1) vestigial or absent.
Dentaries: (0) joined proximally by ligaments, (1) joined by bone.
Mandibular symphysis, two strong grooves forming an anteriorly opening ‘v’ in ventral view: (0) absent, (1) present.
Facial margin: (0) primarily formed by the maxilla, with the maxillary process of the premaxilla restricted to the anterior tip; (1) maxillary process of the premaxilla extending 1/2 facial margin; (2) maxillary process of the premaxilla extending more than 1/2 of facial margin (Ordered).
Nasal [frontal] process of premaxilla: (0) short, (1) long, closely approaching frontal.
Nasal process of maxilla, dorsal ramus: (0) prominent, exposed medially and laterally; (1) absent or reduced to slight medial, and no lateral, exposure.
Nasal process of maxilla, participation of ventral ramus in anterior margin of antorbital fenestra in lateral view: (0) present, extensive; (1) small dorsal projection of the maxilla participates in the anterior margin of the antorbital fenestra, descending process of the nasals contacts premaxilla to exclude maxilla from narial margin; (2) no dorsal projection of maxilla participates in anterior margin of the antorbital fenestra (Ordered).
Osseous external naris: (0) considerably smaller than the antorbital fenestra, (1) larger.
Ectopterygoid: (0) present, (1) absent.
Articulation between vomer and pterygoid: (0) present, well developed; (1) reduced, narrow process of pterygoid passes dorsally over palatine to contact vomer; (2) absent, pterygoid and vomer do not contact.
Palatine and pterygoid: (0) long, anteroposteriorly overlapping, contact, (1) short, primarily dorsoventral, contact.
Palatine contacts: (0) maxillae only, (1) premaxillae and maxillae.
Vomer contacts premaxilla: (0) present, (1) absent.
Coronoid ossification: (0) present, (1) absent.
Projecting basisphenoid articulation with pterygoid: (0) present, (1) absent.
Basipterygoid processes: (0) long, (1) short (articulation with pterygoid subequal to, or longer than, amount projected from the basisphenoid rostrum).
Basisphenoid–pterygoid articulations: (0) located basal on basisphe.oid, (1) located markedly anterior on basisphenoid (parasphenoid rostrum) such that the articulations are subadjacent on the narrow rostrum.
Basisphenoid/pterygoid articulation, orientation of contact: (0) anteroventral, (1) mediolateral, (2) entirely dorsoventral.
Pterygoid, articular surface for basisphenoid: (0) concave ‘socket’, or short groove enclosed by dorsal and ventral flanges; (1) flat to convex; (2) flat to convex facet, stalked, variably projected (Ordered).
Pterygoid, kinked: (0) present, surface for basisphenoid articulation at high angle to axis of palatal process of pterygoid; (1) absent, articulation in line with axis of pterygoid.
Osseous interorbital septum (mesethmoid): (0) absent, (1) present.
Osseous interorbital septum (mesethmoid): (0) restricted to posterior or another just surpassing premaxillae/frontal contact in rostral extent does not surpass posterior edge of external nares in rostral extent; (1) extending rostral to posterior extent of frontal processes of premaxillae and rostral to posterior edge of external nares.
Eustachian tubes: (0) paired and lateral; (1) paired, close to cranial midline; (2) paired and adjacent on midline or single anterior opening.
Eustachian tubes ossified: (0) absent, (1) present.
Squamosal, ventral or ‘zygomatic’ process: (0) variably elongate, dorsally enclosing otic process of the quadrate and extending anteroventrally along shaft of this bone, dorsal head of quadrate not visible in lateral view; (1) short, head of quadrate exposed in lateral view.
Orbital process of quadrate, pterygoid articulation: (0) pterygoid broadly overlapping medial surface of orbital process (i.e. ‘pterygoid ramus’); (1) restricted to anteromedial edge of process.
Quadrate, orbital process: (0) pterygoid articulates with anterior-most tip; (1) pterygoid articulation does not reach tip; (2) pterygoid articulation with no extent up orbital process, restricted to quadrate corpus (Ordered).
Quadrate/pterygoid contact: (0) as a facet, variably with slight anteromedial projection cradling base; (1) condylar, with a well-projected tubercle on the quadrate.
Quadrate, well-developed tubercle on anterior surface of dorsal process: (0) absent, (1) present.
Quadrate, quadratojugal articulation: (0) overlapping, (1) peg and socket articulation.
Quadrate, dorsal process, articulation: (0) with squamosal only, (1) with squamosal and prootic.
Quadrate, dorsal process, development of intercotylar incisure between prootic and squamosal cotylae: (0) absent, articular surfaces not differentiated; (1) two distinct articular facets, incisure not developed; (2) incisure present, ‘double headed’.
Quadrate, mandibular articulation: (0) bicondylar articulation with mandible; (1) tricondylar articulation, additional posterior condyle or broad surface.
Quadrate, pneumaticity: (0) absent, (1) present.
Quadrate, cluster of pneumatic foramina on posterior surface of the tip of dorsal process: (0) absent, (1) present.
Quadrate, pneumatization, large, single pneumatic foramen: (0) absent, (1) posteromedial surface of corpus.
Articular pneumaticity: (0) absent, (1) present.
Dentary strongly forked posteriorly: (0) unforked, or with a weakly developed dorsal ramus; (1) strongly forked with the dorsal and ventral rami approximately equal in posterior extent.
Splenial, anterior extent: (0) splenial stops well posterior to mandibular symphysis; (1) extending to mandibular symphysis, though noncontacting; (2) extending to proximal tip of mandible, contacting on midline.
Mandibular symphysis, anteroposteriorly extensive, flat to convex, dorsal-facing surface developed: (0) absent, concave, (1) flat surface developed.
Mandibular symphysis, symphysial foramina: (0) absent, (1) present.
Mandibular symphysis, symphysial foramen/foramina: (0) single, (1) paired.
Mandibular symphysis, symphysial foramen/foramina: (0) opening on posterior edge of symphysis, (1) opening on dorsal surface of symphysis.
Meckel's groove: (0) not completely covered by splenial, deep and conspicuous medially; (1) covered by splenial, not exposed medially.
Anterior external mandibular fenestra: (0) absent, (1) present.
Jugal/postorbital contact: (0) present, (1) absent.
Frontal/parietal suture (0) open, (1) fused.
Cervical vertebrae: (0) variably dorsoventrally compressed, amphicoelous (‘biconcave’: flat to concave articular surfaces); (1) anterior surface heterocoelous (i.e. mediolaterally concave, dorsoventrally convex), posterior surface flat; (2) heterocoelous anterior (i.e. mediolaterally concave, dorsoventrally convex) and posterior (i.e. mediolaterally convex, dorsoventrally concave) surfaces (Ordered).
Thoracic vertebrae (with ribs articulating with the sternum), one or more with prominent hypapophyses: (0) absent, (1) present. (This character does not address the presence of hypapophyses on transitional vertebrae, or ‘cervicothoracics’, that do not have associated ribs that articulate with the sternum (e.g. Gauthier, 1986; Chiappe, 1996). In contrast, in Aves, well-developed hypapophyses are developed well into the thoracic series, on vertebrae with ribs articulating with the sternum.)
Thoracic vertebrae, count: (0) 12 or more, (1) 11, (2) 10 or fewer (Ordered).
Thoracic vertebrae: (0) at least part of series with subround, central articular surfaces (e.g. amphicoelous/opisthocoelous) that lack the dorsoventral compression seen in heterocoelous vertebrae; (1) series completely heterocoelous.
Thoracic vertebrae, parapophyses: (0) rostral to transverse processes, (1) directly ventral to transverse processes (close to midpoint of vertebrae).
Thoracic vertebrae, centra, length, and midpoint width: (0) approximately equal in length and midpoint width, (1) length markedly greater than midpoint width.
Thoracic vertebrae, lateral surfaces of centra: (0) flat to slightly depressed; (1) deep, emarginate fossae; (2) central ovoid foramina.
Thoracic vertebrae with ossified connective tissue bridging transverse processes: (0) absent, (1) present.
Notarium: (0) absent, (1) present.
Sacral vertebrae, number ankylosed: (0) less than 7, (1) 7, (2) 8, (3) 9, (4) 10, (5) 11 or more, (6) 15 or more (Chiappe, 1996) (Ordered).
Sacral vertebrae, series of short vertebrae, with dorsally directed parapophyses just anterior to the acetabulum: (0) absent; (1) present, three such vertebrae; (2) present, four such vertebrae (Ordered).
Free caudal vertebrae, number: (0) more than 8, (1) 8 or less.
Caudal vertebrae, chevrons, fused on at least one anterior caudal: (0) present, (1) absent.
Free caudals; length of transverse processes: (0) subequal to width of centrum, (1) significantly shorter than centrum width.
Anterior free caudal vertebrae: (0) elongate pre/postzygapophyses; (1) pre- and postzygapophyses short and variably noncontacting; (2) prezygapophyses clasping the posterior surface of neural arch of preceding vertebra, postzygapophyses negligible (Ordered).
Distal caudals: (0) unfused, (1) fused.
Fused distal caudals, morphology: (0) fused element length equal or greater than 4 free caudal vertebrae; (1) length less than 4 caudal vertebrae; (2) less than 2 caudal vertebrae in length (Ordered).
Ossified uncinate processes: (0) absent, (1) present and unfused to ribs, (2) fused to ribs (Ordered).
Gastralia: (0) present, (1) absent.
Ossified sternal plates: (0) unfused; (1) fused, flat; (2) fused, with slightly raised midline ridge; (3) fused with projected carina (Ordered).
Carina or midline ridge: (0) restricted to posterior half of sternum, (1) approaches anterior limit of sternum.
Sternum, dorsal surface, pneumatic foramen (or foramina): (0) absent, (1) present.
Sternum, pneumatic foramina in the depressions (loculi costalis; Baumel & Witmer, 1993) between rib articulations (processi articularis sternocostalis; Baumel & Witmer, 1993): (0) absent, (1) present.
Sternum, coracoidal sulci spacing on anterior edge: (0) widely separated mediolaterally, (1) adjacent, (2) crossed on midline.
Sternum, number of processes for articulation with the sternal ribs: (0) three, (1) four, (2) five, (3) six, (4) seven or more (Ordered).
Sternum: raised, paired intermuscular ridges (linea intermuscularis; Baumel & Witmer, 1993) parallel to sternal midline: (0) absent, (1) present.
Sternum, posterior margin, distinct posteriorly projected medial and/or lateral processes: (0) absent (directly laterally projected zyphoid processes developed but not considered homologues as these are copresent with the posterior processes in the new clade); (1) with distinct posterior processes; (2) midpoint of posterior sternal margin connected to medial posterior processes to enclose paired fenestra (Ordered).
Clavicles: (0) fused, (1) unfused.
Interclavicular angle (clavicles elongate): (0) greater than, or equal, to 90 degrees, (1) less than 90 degrees.
Furcula, hypocleideum: (0) absent, (1) a tubercle, (2) an elongate process (Ordered).
Furcula, laterally excavated: (0) absent, (1) present.
Furcula, dorsal (omal) tip: (0) flat or blunt tip, (1) with a pronounced posteriorly pointed tip.
Furcula, ventral margin of apophysis: (0) curved, angling; (1) with a truncate or squared base.
Scapula and coracoid: (0) fused, (1) unfused.
Scapula and coracoid articulation: (0) pit-shaped scapular cotyla developed on the coracoid, and coracoidal tubercle developed on the scapula (‘ball and socket’ articulation); (1) scapular articular surface of coracoid convex; (2) flat.
Coracoid, procoracoid process: (0) absent, (1) present.
Coracoid: (0) height approximately equal mediolateral dimension; (1) height more than twice width, coracoid ‘strut-like’.
Coracoid, lateral margin: (0) straight to slightly concave, (1) convex.
Coracoid, dorsal surface (= posterior surface of basal maniraptoran theropods): (0) strongly concave, (1) flat to convex.
Coracoid, pneumatized: (0) absent, (1) present.
Coracoid, pneumatic foramen: (0) proximal, (1) distal.
Coracoid, lateral process: (0) absent, (1) present.
Coracoid, ventral surface, lateral intermuscular line or ridge: (0) absent, (1) present.
Coracoid, glenoid facet: (0) dorsal to, or at approximately same level as, acrocoracoid process/‘biceps tubercle’, (1) ventral to acrocoracoid process.
Coracoid, acrocoracoid: (0) straight, (1) hooked medially.
Coracoid, n. supracoracoideus passes through coracoid: (0) present, (1) absent.
Coracoid, medial surface, area of the foramen n. supracoracoideus (when developed): (0) strongly depressed, (1) flat to convex.
Angle between coracoid and scapula at glenoid: (0) more than 90 degrees, (1) 90 degrees or less.
Scapula, posterior end: (0) wider or approximately the same width as proximal dorsoventral shaft width, (1) tapering distally.
Scapula: (0) straight, (1) dorsoventrally curved.
Scapula, length: (0) shorter than humerus, (1) as long as or longer than the humerus.
Scapula, acromion process: (0) projected anteriorly to surpass the articular surface for coracoid (facies articularis coracoidea; Baumel & Witmer, 1993), (1) projected less anteriorly than the articular surface for coracoid.
Scapula, acromion process: (0) straight, (1) laterally hooked tip.
Humerus and ulna, length: (0) humerus longer than ulna, (1) ulna and humerus approximately the same length, (2) ulna significantly longer than humerus (Ordered).
Humerus, proximal end, head in anterior or posterior view: (0) strap-like, articular surface flat, no proximal midline convexity; (1) head domed proximally.
Humerus, proximal end, proximal projection: (0) dorsal edge projected farthest, (1) midline projected farthest.
Humerus, ventral tubercle and capital incisure: (0) absent, (1) present.
Humerus, capital incisure: (0) an open groove, (1) closed by tubercle associated with a muscle insertion just distal to humeral head.
Humerus, anterior surface, well-developed fossa on midline making proximal articular surface appear v-shaped in proximal view: (0) absent, (1) present.
Humerus, ‘transverse groove’: (0) absent, (1) present, developed as a discreet, depressed scar on the proximal surface of the bicipital crest or as a slight transverse groove.
Humerus, deltopectoral crest: (0) projected dorsally (in line with the long axis of humeral head), (1) projected anteriorly.
Humerus, deltopectoral crest: (0) less than shaft width, (1) same width, (2) dorsoventral width greater than shaft width (Ordered).
Humerus, deltopectoral crest, proximoposterior surface: (0) flat to convex, (1) concave.
Humerus, deltopectoral crest: (0) not perforate, (1) with a large fenestra.
Humerus, bicipital crest, pit-shaped scar/fossa for muscular attachment on anterodistal, distal or posterodistal surface of crest: (0) absent, (1) present.
Humerus, bicipital crest, pit-shaped fossa for muscular attachment: (0) anterodistal on bicipital crest, (1) directly ventrodistal at tip of bicipital crest, (2) posterodistal, variably developed as a fossa.
Humerus, bicipital crest: (0) little or no anterior projection, (1) developed as an anterior projection relative to shaft surface in ventral view, (2) hypertrophied, rounded tumescence (Ordered).
Humerus, proximal end, one or more pneumatic foramina: (0) absent, (1) present.
Humerus, distal condyles: (0) developed distally, (1) developed on anterior surface of humerus.
Humerus, long axis of dorsal condyle: (0) at low angle to humeral axis, proximodistally orientated, (1) at high angle to humeral axis, almost transversely orientated.
Humerus, distal condyles: (0) subround, bulbous; (1) weakly defined, ‘strap-like’.
Humerus, distal margin: (0) approximately perpendicular to long axis of humeral shaft, (1) ventrodistal margin projected significantly distal to dorsodistal margin, distal margin angling strongly ventrally (sometimes described as a well-projected flexor process).
Humerus, distal end, compressed anteroposteriorly and flared dorsoventrally: (0) absent, (1) present.
Humerus, brachial fossa: (0) absent; (1) present, developed as a flat scar or as a scar-impressed fossa.
Humerus, ventral condyle: (0) length of long axis of condyle less than the same measure of the dorsal condyle, (1) same or greater’.
Humerus, demarcation of muscle origins (e.g. m. extensor metacarpi radialis in Aves) on the dorsal edge of the distal humerus: (0) no indication of origin as a scar, a pit, or a tubercle; (1) indication as a pit-shaped scar or as a variably projected scar-bearing tubercle or facet.
Humerus, distal end, posterior surface, groove for passage of m. scapulotriceps: (0) absent, (1) present.
Humerus, m. humerotricipitalis groove: (0) absent, (1) present as a ventral depression contiguous with the olecranon fossa.
Ulna, cotylae: (0) dorsoventrally adjacent, (1) widely separated by a deep groove.
Ulna, dorsal cotyla convex: (0) absent, (1) present.
Ulna, distal end, dorsal condyle, dorsal trochlear surface developed as a semilunate ridge: (0) absent, (1) present.
Ulna, distal end, dorsal condyle, dorsal trochlear surface, extent along posterior margin: (0) less than transverse measure of dorsal trochlear surface, (1) approximately equal in extent.
Ulna, bicipital scar: (0) absent, (1) developed as a slightly raised scar, (2) developed as a conspicuous tubercle.
Ulna, brachial scar: (0) absent, (1) present.
Radius, ventroposterior surface: (0) smooth, (1) with muscle impression along most of surface, (2) deep longitudinal groove.
Ulnare: (0) absent, (1) present.
Ulnare: (0), ‘heart-shaped’, little differentiation into short dorsal and ventral rami, (1) V-shaped, well-developed dorsal and ventral rami.
Ulnare, ventral ramus (crus longus, Baumel & Witmer, 1993): (0) shorter than dorsal ramus (crus brevis), (1) same length as dorsal ramus, (2) longer than dorsal ramus.
Semilunate carpal and metacarpals: (0) no fusion, (1) incomplete proximal fusion, (2) complete proximal fusion, (3) complete proximal and distal fusion (Ordered).
Semilunate carpal, position relative to metacarpal I: (0) over 1/2 or more of proximal surface, (2) over less than 1/2 proximal surface (Ordered).
Metacarpal III, anteroposterior diameter as a percent of same dimension of metacarpal II: (0) approximately equal or greater than 50%, (1) less than 50%.
Metacarpal I, anteroproximally projected muscular process: (0) absent no distinct process visible; (1) small knob at anteroproximal tip of metacarpal; (2) tip of process just surpasses the distal articular facet for phalanx 1 in anterior extent; (3) tip of extensor process conspicuously surpasses articular facet by approximately half the width of facet, producing a pronounced knob; (4) tip of extensor process conspicuously surpasses articular facet by approximately the width of facet, producing a pronounced knob (Ordered).
Metacarpal I, anterior surface: (0) roughly hourglass-shaped proximally, at least moderately expanded anteroposteriorly, and constricted just before flare of articulation for phalanx 1, (1) anterior surface broadly convex.
Metacarpal I, distal articulation with phalanx I: (0) ginglymoid, (1) shelf.
Pisiform process: (0) absent, (1) present.
Carpometacarpus, ventral surface, supratrochlear fossa deeply excavating proximal surface of pisiform process: (0) absent, (1) present.
Intermetacarpal space (between metacarpals II and III) (0) reaches proximally as far as the distal end of metacarpal I, (1) terminates distal to end of metacarpal I.
Carpometacarpus, distal end, metacarpals II and III, articular surfaces for digits: (0) metacarpal II subequal or surpasses metacarpal III in distal extent; (1) metacarpal III extends further.
Intermetacarpal process or tubercle: (0) absent, (1) present as scar, (2) present as tubercle or flange (Ordered).
Manual digit II, phalanx 1: (0) subcylindrical to subtriangular; (1) strongly dorsoventrally compressed, flat caudal surface.
Manual digit II, phalanges: (0) length of phalanx II-1 less than or equal to that of II-2, (1) longer.
Manual digit II, phalanx 2, internal index process on posterodistal edge: (0) absent, (1) present (Clarke & Chiappe, 2001).
Ilium, ischium, pubis, proximal contact in adult: (0) unfused, (1) partial fusion (pubis not ankylosed), (2) completely fused (Ordered).
Ilium/ischium, distal co-ossification to completely enclose the ilioischiadic fenestra: (0) absent, (1) present.
Ischium: (0) forked (dorsal process present); (1) straight, no dorsal process.
Ischium, dorsal process: (0) does not contact ilium, (1) contacts ilium.
Ischium and pubis: (0) not subparallel, pubis directed nearly directly ventrally; (1) subparallel, pubis posteriorly directed; (2) pubis appressed to ischium (Ordered).
Laterally projected process on ischiadic peduncle (antitrochanter): (0) directly posterior to acetabulum, (1) posterodorsal to acetabulum.
Preacetabular pectineal process (Baumel & Witmer, 1993): (0) absent, (1) present as a small flange, (2) present as a well-projected flange (Ordered).
Preacetabular ilium: (0) approach on midline, open, or cartilaginous connection, (1) co-ossified, dorsal closure of ‘iliosynsacral canals’.
Preacetabular ilium extends anterior to first sacral vertebrae: (0) no free ribs overlapped, (1) one or more ribs overlapped.
Postacetabular ilium: (0) dorsoventrally orientated, (1) mediolaterally orientated.
Postacetabular ilium, ventral surface, renal fossa developed: (0) absent, (1) present.
Ilium, m. cuppedicus fossa as broad, mediolaterally orientated surface directly anteroventral to acetabulum: (0) present; (1) surface absent, insertion variably marked by a small entirely lateral fossa anterior to acetabulum.
Ischium, posterior demarcation of the obturator foramen: (0) absent; (1) present, developed as a small flange or raised scar contacting/fused with pubis and demarcating the obturator foramen distally.
Ischium, length relative to that of pubis: (0) 1/3 or greater total pubis length extends posterior to end of ishium, (1) less than 1/3 pubis extends further than end of ishium.
Pubis: (0) suboval in cross section, (1) compressed mediolaterally.
Pubes, distal contact: (0) contacting, variably co-ossified into symphysis, (1) noncontacting.
Distal end of pubis: (0) expanded, flared; (1) straight, subequal, in proportion with rest of pubis.
Femur, fossa for insertion of lig. capitis femoris: (0) absent, (1) present.
Femur, posterior trochanter: (0) present, developed as a slightly projected tubercle or flange; (1) hypertrophied, ‘shelf-like’ conformation (in combination with development of the trochanteric shelf; see Hutchinson, 2001); (2) absent (Chiappe, 1991) (Ordered).
Femur, lesser and greater trochanters: (0) separated by a notch, (1) developed as a single trochanteric crest.
Femur, patellar groove: (0) absent, (1) present.
Femur: (0) ectocondylar tubercle and lateral condyle separated by deep notch, (1) ectocondylar tubercle and lateral condyle form single trochlear surface.
Femur, posterior projection of the lateral border of the distal end, continuous with lateral condyle: (0) absent, (1) present.
Laterally projected fibular trochlea: (0) absent; (1) present, developed as small notch; (2) a shelf-like projection (Ordered).
Femur, popliteal fossa: (0) a groove open distally and bounded medially and laterally by narrow condyles, (1) closed distally by expansion of both condyles (primarily the medial).
Calcaneum and astragalus: (0) unfused to each other or tibia in adult, (1) fused to each other, unfused to tibia, (2) complete fused to each other and tibia (Ordered).
Tibia, cnemial crest(s): (0) lateral crest only, (1) lateral and anterior crests developed.
Tibia/tarsal formed condyles: (0) medial condyle projecting further anteriorly than lateral, (1) equal in anterior projection.
Tibia/tarsal formed condyles, extensor canal: (0) absent, (1) an emarginate groove, (2) groove bridged by an ossified supratendinal bridge (Ordered).
Tibia/tarsal formed condyles, tuberositas retinaculi extensoris (Baumel & Witmer, 1993) indicated by short medial ridge or tubercle proximal to the condyles close to the midline and a more proximal second ridge on the medial edge: (0) absent, (1) present.
Tibia/tarsal formed condyles, mediolateral widths: (0) medial condyle wider, (1) approximately equal, (2) lateral condyle wider (Ordered).
Tibia/tarsal formed condyles: (0) gradual sloping medial constriction of condyles, (1) no medial tapering of either condyle.
Tibia/tarsal formed condyles, intercondylar groove: (0) mediolaterally broad, approximately 1/3 width of anterior surface, (1) less than 1/3 width of total anterior surface.
Tibia, extension of articular surface for distal tarsals/tarsometatarsus: (0) no posterior extension of trochlear surface, or restricted to distal-most edge of posterior surface; (1) well-developed posterior extension, sulcus cartilaginis tibialis of Aves (Baumel & Witmer, 1993), distinct surface extending up the posterior surface of the tibiotarsus; (2) with well-developed, posteriorly projecting, medial and lateral crests (Ordered).
Tibia, distal-most mediolateral width: (0) wider than mid-point of shaft, giving distal profile a weakly developed triangular form, (1) approximately equal to shaft width, no distal expansion of whole shaft, although condyles may be variably splayed mediolaterally.
Fibula: (0) reaches tarsal joint articulating into distinct socket formed between the proximal tarsals and the tibia, (1) reduced in length, does not reach tarsal joint.
Distal tarsals and metatarsals, fusion: (0) distal tarsals fuse to metatarsals, (1) distal tarsals fuse to metatarsals and proximal metatarsals co-ossify, (2) distal tarsals fuse to metatarsals, and metatarsals fuse to each other proximally and distally, (3) extreme distal fusion, distal vascular foramen closed (Martin, 1983; Cracraft, 1986) (Ordered).
Metatarsal V: (0) present, (1) absent.
Metatarsal III: (0) proximally in plane with II and IV, (1) proximally displaced plantarly, relative to metatarsals II and IV.
Tarsometatarsus, intercotylar eminence: (0) absent; (1) well developed, globose.
Tarsometatarsus, projected surface or grooves on proximoposterior surface (associated with the passage of tendons of the pes flexors in Aves; hypotarsus): (0) absent, (1) developed as posterior projection with flat posterior surface, (2) projection, with distinct crests and grooves, (3) at least one groove enclosed by bone posteriorly (Ordered).
Tarsometatarsus, proximal vascular foramen (foramina): (0) absent, (1) one, between metatarsals III and IV, (2) two (Ordered).
Metatarsal I: (0) straight; (1) curved or distally deflected but not twisted, ventral surface convex ‘J shaped’; (2) deflected and twisted such that the ventromedial surface is concave proximal to trochlear surface for phalanx I (Ordered).
Metatarsal II tubercle (associated with the insertion of the tendon of the m. tibialis cranialis in Aves): (0) absent; (1) present, on approximately the center of the proximodorsal surface of metatarsal II; (2) present, developed on lateral surface of metatarsal II, at contact with metatarsal III or on lateral edge of metatarsal III (Ordered).
Metatarsal II, distal plantar surface, fossa for metatarsal I (fossa metatarsi I; Baumel and Witmer, 1993): (0) absent, (1) shallow notch, (2) conspicuous ovoid fossa (Ordered).
Metatarsal II, articular surface for first phalanx: (0) ginglymoid, (1) rounded.
Metatarsals, relative mediolateral width: (0) metatarsal IV approximately the same width as metatarsals II and III, (1) metatarsal IV narrower than MII and MIII, (2) metatarsal IV greater in width than either metatarsal II or III.
Metatarsals, comparative trochlear width: (0) II approximately the same size as III and/or IV, (1) II wider than III and/or IV, (2) II narrower than III and/or IV.
Distal vascular foramen: (0) simple, with one exit, (1) forked, two exits (plantar and distal) between metatarsals III and IV.
Metatarsal III, trochlea in plantar view, proximal extent of lateral and medial edges of trochlea: (0) absent, trochlear edges approximately equal in proximal extent; (1) present, lateral edge extends further.
Metatarsal II, distal extent of metatarsal II relative to metatarsal IV: (0) approximately equal in distal extent; (1) metatarsal II shorter than metatarsal IV, but reaching distally further than base of metatarsal IV trochlea, (2) metatarsal II shorter than metatarsal IV, reachin.
Appendix 3
Datamatrix (& = polymorphism; / = uncertainty)
References
- Alvarenga HMF, Bonaparte JF. A new flightless bird from the Cretaceous of Patagonia. Nat Hist Mus Los Angeles, Sci Series. 1992;36:51–64. [Google Scholar]
- Balmford A, Jones IL, Thomas ALR. On avian asymmetry: evidence of natural selection for symmetrical tails and wings in birds. Proc R Soc Lond. 1993;252B:245–251. [Google Scholar]
- Barbosa A, Møller AP. Aerodynamic costs of long tails in male barn swallows Hirundo rustica and the evolution of sexual size dimorphism. Behav Ecol. 1999;10:128–155. [Google Scholar]
- Barsbold R, Currie PJ, Myhrvold NP, Osmólska H, Tsogtbaatar K, Watabe M. A pygostyle from a non-avian theropod. Nature. 2000;403:155. doi: 10.1038/35003103. [DOI] [PubMed] [Google Scholar]
- Baumel JJ. Functional morphology of the tail apparatus of the pigeon (Columba livia) Adv Anat Embryol Cell Biol. 1988;110:1–115. [PubMed] [Google Scholar]
- Baumel JJ, Witmer LM. Osteologia. In: Baumel JJ, King AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of Avian Anatomy: Nomina Anatomica Avium. Cambridge, MA: Publications of the Nuttall Ornithological Club; 1993. pp. 45–132. [Google Scholar]
- Bremer K. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution. 1988;42:795–803. doi: 10.1111/j.1558-5646.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Evans MR. The effect of tail streamer length on aerodynamic performance in the barn swallow. Behav Ecol. 2000;11:228–238. [Google Scholar]
- Chiappe LM. Cretaceous avian remains from Patagonia shed new light on the early radiation of birds. Alcheringa. 1991;15:333–338. [Google Scholar]
- Chiappe LM. The first 85 million years of avian evolution. Nature. 1995a;378:349–355. [Google Scholar]
- Chiappe LM. The phylogenetic position of the Cretaceous birds of Argentina: Enantiornithes and Patagopteryx deferrariisi. Cour Forsch Senckenberg. 1995b;181:55–63. [Google Scholar]
- Chiappe LM. Late Cretaceous birds of southern South America: anatomy and systematics of Enantiornithes and Patagopteryx deferrariisi. Muench Geowiss Abd (A) 1996;30:203–244. [Google Scholar]
- Chiappe LM, Ji S, Ji Q, Norell MA. Anatomy and systematics of the Confuciusornithidae (Theropoda: Aves) from the late Mesozoic of northeastern China. Bull Am Mus Nat Hist. 1999;242:1–89. [Google Scholar]
- Chiappe LM. Basal bird phylogeny: problems and solutions. In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 448–472. [Google Scholar]
- Chiappe LM, Walker CA. Skeletal morphology and systematics of the Cretaceous Euenantiornithes (Ornithothoraces: Enantiornithes) In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 240–267. [Google Scholar]
- Clarke JA. The morphology and taxonomy of Ichthyornis Marsh and the phylogenetic relationships of basal Ornithurae. 2002. Doctoral dissertation, Yale University.
- Clarke JA, Chiappe LM. A new carinate bird from the Late Cretaceous of Patagonia (Argentina) Am Mus Novit. 2001;323:1–22. [Google Scholar]
- Clarke JA, Norell MA. The morphology and phylogenetic position of Apsaravis ukhaana from the Late Cretaceous of Mongolia. Am Mus Novit. 2002;3387:1–46. [Google Scholar]
- Clarke JA, Norell MA, Zhou Z, Zhang F. An ornithurine from the Early Cretaceous of China. J Vert Paleontol. 2002;22:45A. [Google Scholar]
- Clarke JA. Morphology, phylogenetic taxonomy, and systematics of Ichthyornis and Apatornis (Avialae: Ornithurae) Bull Am Mus Nat Hist. 2004;286:1–179. [Google Scholar]
- Clarke JA, Norell MA. New avialan remains from the Late Cretaceous of Mongolia and a review of the known avifauna of the Nemegt Formation. Am Mus Novit. 2004;3447:1–12. [Google Scholar]
- Clarke JA, Tambussi CPJI, Erickson GM, Ketcham RA. First definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. [DOI] [PubMed] [Google Scholar]
- Cracraft JL. The origin and early diversification of birds. Paleobiology. 1986;12:383–399. [Google Scholar]
- Dyke GJ, Dortangs RW, Jagt JW, Mulder EW, Schulp A, Chiappe LM. Europe's last Mesozoic bird. Naturwissenshaften. 2002;89:408–411. doi: 10.1007/s00114-002-0352-9. [DOI] [PubMed] [Google Scholar]
- Evans MR, Thomas ALR. The aerodynamic and mechanical effects of elongated tails in the scarlet-tufted malachite sunbird: measuring the cost of a handicap. Anim Behav. 1992;43:337–347. [Google Scholar]
- Fitzpatrick S. Tail length in birds in relation to tail shape, general flight ecology and sexual selection. J Evol Biol. 1999;12:49–60. [Google Scholar]
- Galton PM, Martin LD. Enaliornis, an Early Cretaceous hesperornithiform bird from England, with comments on other Hesperornithiformes. In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 317–338. [Google Scholar]
- Gatesy SM, Dial KP. Tail muscle activity patterns in walking and flying pidgeons. Columba livia. J Exp Biol. 1993;176:55–76. [Google Scholar]
- Gatesy SM, Dial KP. Locomotor modules and the evolution of avian flight. Evolution. 1996a;50:331–340. doi: 10.1111/j.1558-5646.1996.tb04496.x. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Dial KP. From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution. 1996b;50:2037–2048. doi: 10.1111/j.1558-5646.1996.tb03590.x. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. The evolutionary history of the theropod caudal locomotor module. In: Gauthier J, Gall LF, editors. New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John HOstrom. New Haven, CT: Peabody Museum (Natural History); 2001. pp. 237–254. [Google Scholar]
- Gatesy SM. Locomotor evolution on the line to modern birds. In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley, CA: University of California Press; 2002. pp. 432–447. [Google Scholar]
- Gauthier J. Saurischian monophyly and the origin of birds. Mem Cal Acad Sci. 1986;8:185–197. [Google Scholar]
- Gauthier J, de Queiroz K. Feathered dinosaurs, flying dinosaurs, crown dinosaurs and the name ‘Aves’. In: Gauthier J, Gall LF, editors. New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John HOstrom. New Haven, CT: Peabody Museum (Natural History); 2001. pp. 7–41. [Google Scholar]
- Haeckel E. Generelle Morphologie der Organismen. Berlin: Georg Reimer; 1866. [Google Scholar]
- He H, Wang X, Zhou Z, Wang F, Boven A, Shi G, Zhu R. Timing of the Jiufotang Formation (Jehol Group) in Liaoning, northeastern China and its implications. Geophys Res Let. 2004;31:L12605. [Google Scholar]
- Hou L, Liu Z. A new fossil bird from the Lower Cretaceous of Gansu and early evolution of birds. Sci Sinica (B) 1984;27:1296–1302. [Google Scholar]
- Hou L, Zhang J. A new fossil bird from the Lower Cretaceous of Liaoning. Vert Pal Asiat. 1993;31:217–224. [Google Scholar]
- Hou L. The discovery of a Jurassic carinate bird in China. Sci Bull. 1996;41:1861–1864. [Google Scholar]
- Hou L, Martin LD, Zhou Z, Feduccia A. Early adaptation of birds-evidence from fossils from Northeastern China. Science. 1996;27:1164–1167. doi: 10.1126/science.274.5290.1164. [DOI] [PubMed] [Google Scholar]
- Hou L. Mesozoic Birds of China. Taiwan: Nan Tou; 1997. Taiwan Provincial Feng Huang Ku Bird Park. [Google Scholar]
- Hou L, Martin LD, Zhou Z, Feduccia A, Zhang F. A diapsid skull in a new species of the primitive bird Confuciusornis. Nature. 1999;399:679–682. [Google Scholar]
- Hou L, Chiappe LM, Zhang F, Chuong CM. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 2004;91:22–25. doi: 10.1007/s00114-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JR. The evolution of femoral osteology and soft tissues on the line to extant birds (Neornithes) Zoo J Linnean Soc. 2001;131:169–197. [Google Scholar]
- Keast A. Wing shape in insectivorous passerines inhabiting New Guinea and Australian rain forests and Eucalypt forest/Eucalypt woodlands. Auk. 1996;113:94–104. [Google Scholar]
- Kurochkin EN. Lower Cretaceous birds from Mongolia and their evolutionary significance. Proc XVIII Int Cong Ornithol Acta. 1985;1:191–199. [Google Scholar]
- Kurochkin EN, Dyke GJ, Karhu AA. A new presbyornithid bird (Aves, Anseriformes) from the Late Cretaceous of southern Mongolia. Am Mus Novit. 2002;3386:1–11. [Google Scholar]
- Lowe LV, Evans MR, Buchanan KL. The function and evolution of the tail streamers in hirundines. Behav Ecol. 2001;12:157–163. [Google Scholar]
- Marsh OC. Preliminary description of Hesperornis regalis, with notices of four other new species of Cretaceous birds. Am J Sci, 3rd Ser. 1872a;3:359–365. [Google Scholar]
- Marsh OC. Notice of a new and remarkable fossil bird. Am J Sci, 3rd Ser. 1872b;4:344. [Google Scholar]
- Marsh OC. Odontornithes. A Monograph on the Extinct Toothed Birds of North America. Washington, DC: U.S. Government Printing Office; 1880. [Google Scholar]
- Martin LD. The origin and early radiation of birds. In: Brush AH, Clark GA Jr, editors. Perspectives in Ornithology. New York: Cambridge University Press; 1983. pp. 291–338. [Google Scholar]
- Norell MA, Mackovicky P. Important features of the dromaeosaur skeleton: information from a new specimen. Am Mus Novit. 1997;3215:1–28. [Google Scholar]
- Norell MA, Clarke JA. Fossil that fills a critical gap in avian evolution. Nature. 2001;409:181–184. doi: 10.1038/35051563. [DOI] [PubMed] [Google Scholar]
- Rayner JMV. Form and function in avian flight. Curr Ornith. 1988;5:1–66. [Google Scholar]
- Saville DBO. Adaptive evolution in the avian wing. Evolution. 1957;11:212–224. [Google Scholar]
- Swofford DL. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer Associates; 2002. Version 4.0. [Google Scholar]
- Thomas ALR. On the aerodynamics of birds’ tails. Phil Trans Royal Soc Lond. 1993;340:361–380. [Google Scholar]
- Zhang F, Zhou Z. A primitive enantiornithine bird and the origin of feathers. Science. 2000;290:1955–1959. doi: 10.1126/science.290.5498.1955. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhou Z. Leg feathers in an Early Cretaceous bird. Nature. 2004;431:925. doi: 10.1038/431925a. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen P, Wang Y-Q, Wang Y. Shanghai: Shanghai Science and Technology Press; 2001. The Jehol Biota – The Emergence of Feathered Dinosaurs, Beaked Birds and Flowering Plants. [Google Scholar]
- Zhou Z, Zhang F. Two new ornithurine birds from the Early Cretaceous of western Liaoning, China. Chinese Sci Bull. 2001;46:1258–1264. [Google Scholar]
- Zhou Z, Zhang F. Largest bird from the Early Cretaceous and its implications for the earliest avian ecological diversification. Naturwissenschaften. 2002;89:34–38. doi: 10.1007/s00114-001-0276-9. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can J Earth Sci. 2003;40:731–747. [Google Scholar]
- Zhou Z, Clarke JA, Zhang F. Archaeoraptor's better half. Nature. 2002;420:285. doi: 10.1038/420285a. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Barrett PM, Hilton J. An exceptionally preserved Lower Cretaceous ecosystem. Nature. 2003;421:807–814. doi: 10.1038/nature01420. [DOI] [PubMed] [Google Scholar]
- Zhou Z. The origin and early evolution of birds: discoveries, disputes, and perspectives from fossil evidence. Naturwissenschaften. 2004;91:455–471. doi: 10.1007/s00114-004-0570-4. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Clarke JA, Zhang F, Wings O. Gastroliths in Yanornis: an indication of the earliest radical diet-switching and gizzard plasticity in the lineage leading to living birds? Naturwissenschaften. 2004;91:571–574. doi: 10.1007/s00114-004-0567-z. [DOI] [PubMed] [Google Scholar]