Abstract
As in monotreme mammals, the pectoral apparatus of basal (fossil) amniotes includes two coracoid elements, the procoracoid and metacoracoid. Among extant reptiles the metacoracoid has long been assumed lost; this notion is herein challenged. A comprehensive review of data from numerous sources, including the fossil record, experimental embryology, genetic manipulations and an analysis of morphology at the level cell condensations, supports the conclusion that the metacoracoid gives rise to the majority of the reptilian coracoid. By contrast, the reptilian procoracoid remains as a rudiment that is incorporated as a process of the (meta)coracoid and/or the glenoid region of the scapula early during development, prior to skeletogenesis. Application of this integrated approach corroborates and enhances previous work describing the evolution of the pectoral apparatus in mammals. A revised scenario of amniote coracoid evolution is presented emphasizing the importance of considering cell condensations when evaluating the homology of a skeletal complex.
Keywords: cell condensation, metacoracoid, procoracoid, skeletal complex, sternum
Introduction
Among limbed (and many limbless) amniotes, elements of the shoulder girdle and sternum partially encircle the ribcage to form a structural yoke that indirectly connects the forelimbs to the axial skeleton. Herein referred to as the pectoral apparatus, this skeletogenous nexus demonstrates enormous taxonomic variation and multiple (independent) instances of elements appearing to have been lost and/or insensibly combined. Furthermore, the pectoral apparatus includes both dermal and replacement bones. These features underline the difficulty of determining homology for various pectoral components.
Historical observations from both the fossil record and ontogenetic studies initially provided a well-structured scenario of the morphological transformation of mammals from the earliest stem synapsids. However, subtle yet important evolutionary details are only now being understood through the use of experimental methods and detailed developmental analyses at the level of the cell.
Among reptiles, sister group to mammals, the situation is less clear. Despite an increased knowledge of embryology and phylogenetics, and an abundance of new palaeontological data, the prevalent hypothesis regarding the homology of the reptilian coracoid has remained largely unchanged for more than 80 years. Unlike in mammals, there has been little consideration given to the development of the reptilian pectoral apparatus as a whole. This paper has three main objectives: (1) to review the morphology and development of the pectoral apparatus in both extinct and extant amniotes; (2) to re-address homology of the reptilian coracoid; and (3) to re-evaluate the scenario of coracoid evolution in amniotes.
Early evolution of the pectoral apparatus – a brief review
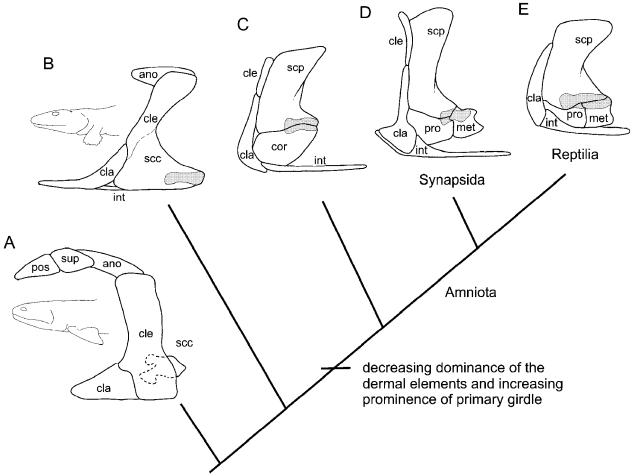
Except for the sternum, most pectoral elements were present in osteichthyians well before the appearance of limbs. Among non-digit-bearing members (Fig. 1A) this region is dominated by dermal elements, including the cleithrum, clavicle and unpaired interclavicle. These components are linked to the caudalmost portion of the head skeleton overlying the gill chamber. Articulation with the humerus occurs at the relatively small and laterally obscured primary girdle, the undivided scapulocoracoid. [The primary girdle includes those elements preforming in cartilage that develop before the dermal pectoral contributions. This includes the scapulocoracoid and all derivatives thereof.]
Fig. 1.
Evolution of the tetrapod pectoral apparatus. Among basalmost non-digit-bearing tetrapods (A), the pectoral apparatus is dominated by dermal elements; the scapulocoracoid is small and obscured laterally. With the advent of digits (B), taxa demonstrate a trend towards enlargement of the scapulocoracoid while diminishing the dermal contributions. Characteristically, many of these early digit-bearing taxa fuse the scapulocoracoid with the cleithrum (indicated by dashed line). Among sister taxa to amniotes (C), the once unified scapulocoracoid is partitioned into a dorsal scapula and ventral coracoid, with the glenoid nested at the caudal border between the two. Basal members of both major amniote lineages, Synapsida (D) and Reptilia (E), demonstrate a three-part primary girdle consisting of a scapula, procoracoid and metacoracoid. Abbreviations: ano (anocleithrum), cla (clavicle), cle (cleithrum), cor (coracoid), int (interclavicle), met (metacoracoid), pos (post-temporal), pro (procoracoid), scp (scapula), scc (scapulocoracoid), sup (supracleithrum). Glenoid shaded. Phylogeny based on Ruta et al. (2003). Sources for images: (A) †Eusthenopteron modified from Jarvik (1980); (B) †Acanthostega reproduced by permission of the Royal Society of Edinburgh and M. I. Coates from Transactions of the Royal Society of Edinburgh: Earth Sciences, volume 87 (1996), pp. 363–421; (C) †Seymouria modified from Williston (1925); (D) †Dimetrodon modified from Romer & Price (1940) and reproduced with fair use permission from the Geological Society of America; (E) †Captorhinus modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History. Not to scale.
With the evolution of digit-bearing limbs (Fig. 1B) elements linking the head skeleton with the pectoral apparatus were lost, resulting in a decoupling of the dermal girdle from the skull (Clack, 2002). Furthermore, the once modest scapulocoracoid adopted a more prominent and laterally facing profile. Further reduction of the dermal girdle (in particular the cleithrum) and enlargement of the primary girdle characterizes more deeply nested non-amniote tetrapods (Fig. 1C). Among these and more derived tetrapods, the singular scapulocoracoid is partitioned into two elements, a dorsal scapula and a ventral coracoid.
Among basal amniotes (Fig. 1D,E) the lone coracoid is replaced by two discrete components, a cranial procoracoid and a caudal metacoracoid (see Table 1 for a listing of coracoid synonyms). For modern taxa, the scapula is the most widely conserved element of the pectoral apparatus, absent only in some lepidosauromorphs (e.g. ophidians). Monotremes are unique in retaining both the procoracoid and the metacoracoid, whereas therian mammals (marsupials and eutherians) and reptiles (including birds) each maintain only one of the two elements; in therians the procoracoid no longer forms a discrete entity and the metacoracoid is a rudiment that fuses with the scapula, giving rise to the coracoid process. Conversely, in reptiles it has long been assumed that the metacoracoid has disappeared and only the procoracoid remains. The procoracoid is understood to be equivalent to the coracoid of non-amniote tetrapods. Consequently, modern authors typically refer to this element in reptiles as simply the coracoid (e.g. McGonnell, 2001), a designation adopted hereafter to avoid unnecessary confusion.
Table 1.
Synonyms (and source listings) of the cranialmost and caudalmost coracoid elements present in basal amniotes and monotremes, and their inferred homologues in therians and reptiles
| Coracoid | Amniote | |||
|---|---|---|---|---|
| Source | Cranialmost coracoid | Caudalmost coracoid | Therian coracoid process | Reptilian coracoid |
| Parker, 1868 | epicoracoid | coracoid | coracoid | coracoid |
| Flower, 1876 | epicoracoid | coracoid | coracoid | coracoid |
| Howes, 1887 | epicoracoid | coracoid | epicoracoid | coracoid |
| Howes, 1893 | epicoracoid | metacoracoid | epicoracoid | coracoid* |
| Lydekker, 1893 | coracoid | metacoracoid | coracoid | metacoracoid |
| Broom, 1899 | precoracoid | coracoid | coracoid | coracoid |
| Broom, 1912 | precoracoid | coracoid | coracoid | precoracoid |
| Williston, 1911, 1925 | procoracoid | metacoracoid | coracoid (=procoracoid) | procoracoid |
| Watson, 1917 | procoracoid | metacoracoid | metacoracoid | procoracoid |
| Gregory & Camp, 1918† | epicoracoid | coracoid | coracoid | coracoid |
| Romer, 1922 | epicoracoid | coracoid | coracoid | epicoracoid |
| Romer, 1956 | procoracoid | coracoid | coracoid | procoracoid |
| Klima, 1973, 1987 | procoracoid | metacoracoid | metacoracoid | procoracoid |
| Current hypothesis | procoracoid | metacoracoid | metacoracoid | metacoracoid‡ |
Howes regards the extant reptile coracoid as a combined epicoracoid and metacoracoid (before the two elements have separated from one another).
Gregory suggests that a third coracoid, the metacoracoid, was present in basal synapsids and that a neomorphic “subcoracoid” was also also present in placentals.
including minor contributions from the procoracoid.
In comparison with the rest of the pectoral apparatus, the sternum is often poorly preserved or entirely absent from fossil taxa. This observation is usually explained in the context of histology; unlike other pectoral components that ossify, the sternum often remains cartilaginous. Notwithstanding its poor palaeontological representation, the sternum is considered characteristic of virtually all tetrapods [including lissamphibians and most amniotes, with the exception of turtles (although see below) and ophidians], with preserved material dating back to at least the Late Permian (256–248 million years ago; Vaughn, 1955).
A question of homology – the coracoid conundrum
Questions concerning the homology of various pectoral elements rank as some of the oldest in comparative anatomy. In a celebrated series of woodcuts, Belon (1555) compared the skeletons of a man and a bird. Using a series of labels, Belon accurately identified the majority of the homologous elements between the two specimens. One of his few misinterpretations was to pair the avian coracoid with the human clavicle. Although this particular hypothesis has long since become abandoned, the homology of the coracoid element(s) remained as a topic of considerable debate for early anatomists.
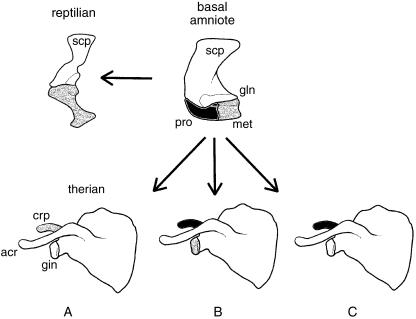
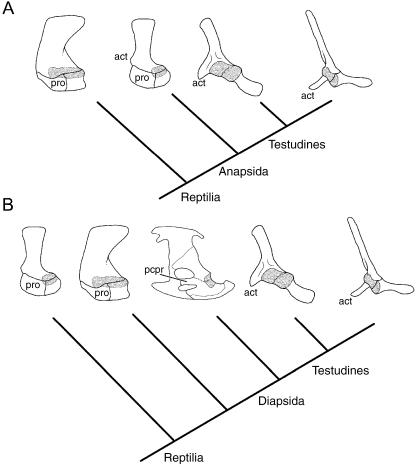
Prior to the late 1800s, it was commonly held that of the two coracoid elements present in basal amniotes and monotremes, only the caudalmost element, the metacoracoid, was retained by modern reptiles and therians (e.g. Parker, 1868; Flower, 1876; Fig. 2A). The cranialmost element, the procoracoid, was assumed to be lost. Soon, however, new developmental and palaeontological evidence began to confuse the issue. Howes (1887, 1893) observed that the developing scapula of a generic rabbit consisted of three discrete centres – one giving rise to the blade and scapular spine, one forming the coracoid process and one contributing to the glenoid. He interpreted these centres as corresponding to the scapula, procoracoid and metacoracoid of monotremes, therefore suggesting that the procoracoid was not lost (Fig. 2B).
Fig. 2.
Competing hypotheses of coracoid homology. (A) Among many early workers (e.g. Parker, 1868) the procoracoid of basal taxa was assumed to be lost in more deeply nested forms. Consequently, the coracoid process of therians and the coracoid element of modern reptiles were both considered homologous with the metacoracoid. (B) Howes (1887, 1893) later argued that in therians the procoracoid gave rise to the coracoid process while the metacoracoid contributed to the glenoid. (C) Alternatively, Lydekker (1893) suggested that the procoracoid formed the coracoid process of therians and the metacoracoid was the equivalent of the reptilian coracoid. Abbreviations: acr (acromion), cpr (coracoid process), gln (glenoid), met (metacoracoid [grey]), pro (procoracoid [black]), scp (scapula [white]). Sources for images: basal amniote: †Captorhinus modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History; therian: Bathyergus modified from Parker (1868); reptile: Alligator modified from Mook (1921) and reproduced with permission from the American Museum of Natural History. Not to scale.
An alternative opinion was expressed by Lydekker (1893), who compared the development of the coracoid process of a sloth with the dual coracoid elements of what was then considered a basal reptile (namely †Dicynodon sp., now considered to be a basal synapsid). He concluded that the procoracoid was homologous with the therian coracoid process of the scapula, and that the caudalmost element, what he named the metacoracoid, was the only coracoid element of reptiles (Fig. 2C). This was later rebutted by Broom (1899), who returned to the notion that modern amniotes, with the exception of monotremes, had lost the procoracoid.
Although aspects of the debate continued, e.g. Gregory & Camp (1918; see also Hanson, 1920) argued that basal amniotes originally had three coracoid elements – the epicoracoid, coracoid and metacoracoid, the work of Williston (1911, 1925) is often cited as the basis for our modern interpretation of reptilian coracoid homology (Broom, 1912; Case & Williston, 1913; Hanson, 1920; Romer, 1922, 1956). Williston examined a variety of what were then considered to be fossil ‘reptilian’ taxa (most of which have since been recollected as Amniota outgroups) and noted that whereas the scapula and procoracoid were generally fused, the metacoracoid, if present at all, was often poorly sutured or completely unattached. Williston and others (e.g. Broom, 1912; Case & Williston, 1913) interpreted this as evidence for the anagenetic disappearance of the metacoracoid among modern reptiles.
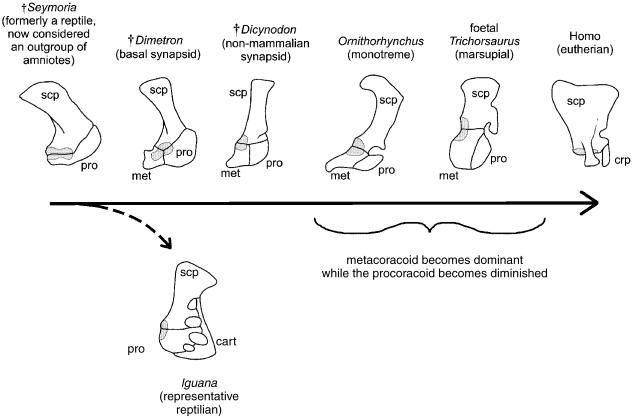
Adopting the interpretation of Williston, Romer (1956; see also 1922) expanded the argument. Observing that non-amniote tetrapods (e.g. †Seymouria spp., lissamphibians), therians and modern reptiles have only one coracoid element, whereas some basal amniotes (e.g. †Dimetrodon spp.; see below) had two. Romer formulated an evolutionary scenario (Fig. 3) in which the procoracoid was the primitive element. This procoracoid was retained by reptiles and some synapsids (e.g. monotremes). Among basal synapsids a second more caudal element, the metacoracoid, developed. In therians the procoracoid disappears whereas the metacoracoid is retained, albeit in a reduced form as the coracoid process of the scapula. Among those fossil reptiles that demonstrated both coracoid elements, Romer suggested that for some taxa this reflected a proximate genealogy with synapsids. For others he stated ‘[w]e must assume either that the general reptile stem early acquired a second coracoid element which survived only in synapsids, other reptiles rapidly losing it again, or that parallelism occurred, with the development of a second coracoid in two or more lines. The second assumption is the more reasonable, but the situation is far from clear.’ (Romer, 1956, p. 309). Indeed, the situation remains uncertain, for as alluded to above, revised phylogenetic hypotheses of Amniota (Fig. 4) have weakened some of the fundamental underpinnings to the theory of reptilian coracoid homology as established by Williston (1911, 1925).
Fig. 3.
Romer's interpretation of amniote primary girdle evolution. Building on the work of Williston (1911, 1925), Romer hypothesized that the primitive coracoid of non-amniote tetrapods such as †Seymouria, the procoracoid, was retained as the coracoid of modern reptiles. Among synapsids a second element evolved, the metacoracoid. Whereas synapsids eventually lost the primitive reptilian procoracoid, the newly evolved metacoracoid was retained by therians as the coracoid process. Although this hypothesis has long been accepted, it is not supported by the data presented herein. Abbreviations: cart (epicoracoidal and suprascapula cartilages), cpr (coracoid process), met (metacoracoid), pro (procoracoid), scp (scapula). Glenoid shaded. Source for image: modified from The comparison of mammalian and reptilian coracoids, A. S. Romer (1922), ©Journal of Morphology); reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. Not to scale.
Fig. 4.
Revised amniote phylogeny based on the work of Brochu (2001), Lee (1996), Luo et al. (2002), Rieppel & Reisz (1999) and Ruta et al. (2003). As the phylogenetic position of turtles remains confused, both competing hypotheses are presented (i.e. turtles as diapsids and turtles as proganosaurs). Nodes: 1, Tetrapoda; 2, Amniota; 3, Synapsida; 4, Reptilia; 5, Proganosauria; 6, Diapsida; 7, Archosauromorpha. Sources for images, from left to right: †Eusthenopteron modified from Jarvik (1980); †Acanthostega reproduced by permission of the Royal Society of Edinburgh and M. I. Coates from Transactions of the Royal Society of Edinburgh: Earth Sciences, volume 87 (1996), pp. 363–421; †Seymouria modified from Williston (1925); Priodontes modified from Flower (1876); Tachyglossus reproduced by permission of F. A. Jenkins, Jr. from The postcranial remains of Eozostrodon, Megazostrodon and Erythrotherium, fig. 19 (p. 419), 1976, published by the Royal Society; †Dimetrodon modified from Romer & Price (1940) and reproduced with fair use permission from the Geological Society of America; Macroclemys modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History; †Scutosaurus reproduced by permission of M. S. Y. Lee from The homologies and early evolution of the shoulder girdle in turtles, fig. 2 (p. 113), 1996, published by the Royal Society; †Captorhinus modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History; Shinisaurus modified from Postcranial anatomy of Shinisaurus crocodilurus (Squamata: Anguimorpha), J. L. Conrad (in press, ©Journal of Morphology); reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.; Alligator modified from Mook (1921) and reproduced with permission from the American Museum of Natural History; Sturnus modified from Jenkins (1993) ‘The evolution of the avian shoulder joint’, and reprinted by permission of the American Journal of Science. Not to scale.
Cell condensations
Elucidating the evolution and development of a morphological complex such as the pectoral apparatus is a daunting task. Variation in the embryological origin, adult phenotype and overall number of constituent elements often leads to confusion regarding each component's anatomical and phylogenetic identity. Atchley & Hall (1991) created a model for understanding complex structures that begins with the identification of fundamental developmental units. At the cellular level, these fundamental developmental units are cell condensations characterized as ‘… the raw material of morphology …’ (Hall & Miyake, 1992, p. 108). All organs, bones included, begin as aggregations of cells (Atchley & Hall, 1991; Eames et al. 2003), with those condensations presaging the skeleton referred to (collectively) as the membranous skeleton (Grüneberg, 1963; Hall & Miyake, 1992). Unlike adult skeletal elements, which may fail to form or become insensibly assimilated into other bones and cartilages, cell condensations are present (at least briefly) as discretely recognizable units. During both development and evolution, cell condensations may experience variation as a result of changes in mitosis (cell proliferation), identity (differentiation), the size of the aggregation, and/or localized heterochrony (Atchley & Hall, 1991; Hall & Miyake, 1992; Dollé et al. 1993). Consequently, cell condensations provide a useful means for establishing homology of elements in a multipartite structure (Hall & Miyake, 1992; see also Klima, 1973, 1987), and are critical for the study of the evolution and development of the pectoral apparatus.
The synapsid pectoral apparatus
Extant development and morphology
In order to address the question of reptilian coracoid homology effectively, it is first necessary to review the pectoral apparatus of mammals. As will be demonstrated, in mammals the transformation of the pectoral apparatus from a multipartite complex (characteristic of basal forms and monotremes) to one of relatively few elements (such as therians) is achieved by a reorganization of cell condensations. Elements are not lost per se, but have become insensibly integrated and thus are no longer discretely identifiable. Lessons drawn from the mammalian case study are subsequently used to underpin the revised scenario of coracoid homology in reptiles and illustrate the overall trend among amniotes towards morphological evolution of the pectoral apparatus.
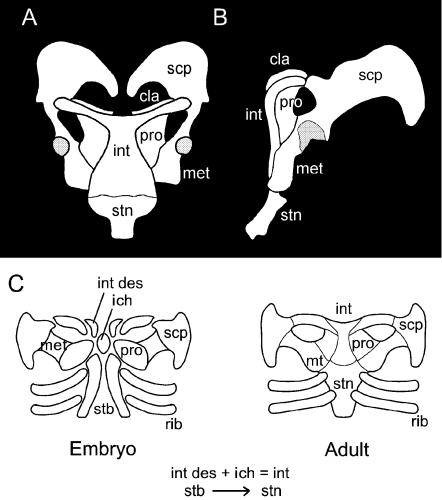
Unique among modern amniotes, adult monotremes retain each of the scapula, procoracoid, metacoracoid, clavicle, interclavicle and sternum (Fig. 5A,B). Despite the rarity of available material, morphogenesis of the monotreme pectoral apparatus has been well documented (Klima, 1973, 1985). During development, each ipsilateral scapula, procoracoid and metacoracoid arises from a common homogeneous endochondral condensation, the coracoid–scapular plate (Fig. 5C; Klima, 1973). The glenoid (for articulation with the humerus) develops at the intersection of the scapula and metacoracoid, to the exclusion of the procoracoid. Midventrally, these elements are bridged by a midline T-shaped interclavicle.
Fig. 5.
Monotreme pectoral apparatus. An adult Ornithorhynchus anatinus pectoral apparatus in ventral (A) and left lateral (B) views. Among skeletally mature monotremes, the pectoral apparatus is composed of six elements: an unpaired sternum and interclavicle, and bilateral clavicles, scapulae, procoracoids and metacoracoids. The glenoid for articulation with the humerus is positioned between the scapula and metacoracoid at the exclusion of the procoracoid. Early during skeletogenesis (C) the cellular condensations (membranous skeleton) giving rise to the pectoral apparatus include a median unpaired pars chondralis interclaviculae and bilateral pars desmalis interclaviculae, clavicular condensations, coracoid–scapular plates, sternal bands and rib primordia. The singular pars chondralis interclaviculae and paired pars desmalis interclaviculae coalesce to form the skeletally mature interclavicle. The majority of the coracoid–scapular plate forms the scapula, with more medial portions becoming segregated to form the procoracoid and metacoracoid (see text for details). Glenoid shaded. Abbreviations: cla (clavicle), gln (glenoid), int (skeletally mature interclavicle), ich (pars chondralis interclaviculae), int des (pars desmalis interclaviculae), met (metacoracoid), pro (procoracoid), rib (rib), scp (scapula), stb (sternal band), stn (sternum). Sources for images: (A,B) modified from Parker (1868); (C) reprinted from Functional Morphology in Vertebrates, vol. 1, M. Klima, ‘Development of the shoulder girdle and sternum in mammals’, pp. 81–83 (1985).
Developmentally the interclavicle is exceptional, receiving contributions from two skeletogenetic sources: an unpaired endochondral condensation (the pars chondralis interclaviculae) that combines with paired intramembranous condensations (the pars desmalis interclaviculae) to form a composite anlagen (Fig. 5C; Klima, 1973, 1987). A pair of slender clavicles form along the cranial margin of the interclavicle, each derived from a single intramembranous condensation. The skeletally mature sternum includes a cranialmost manubrium sterni, followed by a series of variably co-ossified block-like sternebrae (and in tachyglossids, but not Ornithorhynchus anatinus, a terminal xiphisternum), all of which develop from a longitudinal pair of condensations, the sternal bands (Klima, 1973).
In contrast to adult monotremes, the pectoral apparatus of therians is composed of fewer osteological components, with only the scapula and sternum present in all forms. Adult therians lack a discrete interclavicle (although see below), the clavicles may be reduced or completely absent (e.g. ungulates, peramelid marsupials; Flower, 1876; see also Hall, 2001), and the coracoid elements are either rudimentary or vestigial (Klima, 1973, 1985, 1987).
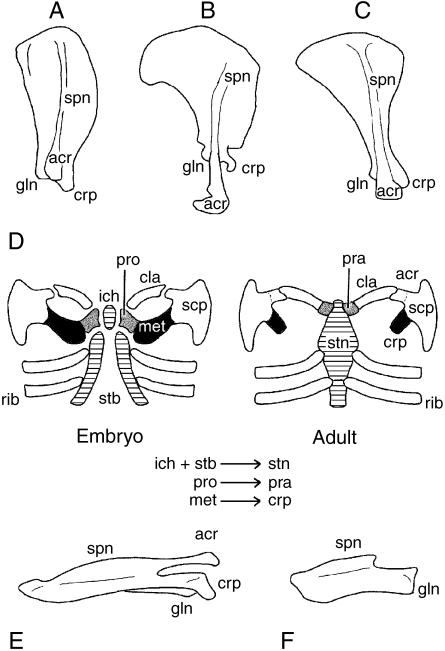
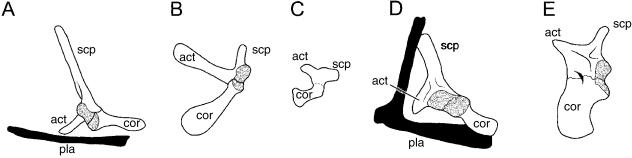
The scapula is the only therian pectoral apparatus element to articulate with the forelimbs. Although morphologically variable in profile (Fig. 6A–C), virtually all therian scapulae have a coracoid process adjacent to the glenoid and at least one conspicuous scapular spine that divides the lateral surface into two relatively large, shallow fossae (Sánchez-Villagra & Maier, 2002). The distal terminus of the scapular spine gives rise to a second process, the acromion, that comes into close proximity or articulates with the clavicle, when present.
Fig. 6.
Therian scapula morphology and pectoral apparatus development. Adult (right) scapulae of (A) Canis, (B) Priodontes and (C) Mus in lateral view, demonstrating the morphological variation of this element. (D) Early during skeletogenesis the cellular condensations (membranous skeleton) giving rise to the pectoral apparatus include a median unpaired pars chondralis interclaviculae and bilateral coracoid–scapular plates, clavicular condensations, sternal bands and rib primordia. The bulk of the coracoid–scapular plate gives rise to the scapula, although a rudiment homologous with the procoracoid joins with the cranialmost portion of the coalescing sternal bands and pars chondralis interclaviculae to form the manubrium sterni. In addition, procoracoid may give rise to vestiges adjacent to the clavicular–sternal contact (the praeclavia). The portion of the coracoid–scapular plate homologous with the metacoracoid gives rise to the coracoid process and, at least in some eutherians, the acromion and part of the glenoid. Dorsal view of adult (left) scapula in Mus (E) wildtype and (F) undulated (Pax1 deficient) mutant (see text for details). Abbreviations: acr (acromion), cla (clavicle), cpr (coracoid process), gln (glenoid), ich (pars chondralis interclaviculae), met (metacoracoid), pra (praeclavium/suprasternal), pro (procoracoid), rib (rib), scp (scapula), spn (scapular spine), stb (sternal band), stn (sternum). Source for images: (A,B) modified from Flower (1876); (C,F,G) modified from Timmons et al. (1994), reproduced with permission from The Company of Biologists Ltd; (D) reprinted from Functional Morphology in Vertebrates, vol. 1, M. Klima, ‘Development of the shoulder girdle and sternum in mammals’, pp. 81–83 (1985).
Like monotremes, therians develop a coracoid–scapular plate early during morphogenesis that becomes dominated by the presumptive scapula. Although the procoracoid precursor is initially present, it does not develop beyond a rudiment, merging with the sternal bands and a median (unpaired) endochondral condensation (Fig. 6D; discussed below), or a vestige nested between the clavicle and manubrium sterni. Among various marsupial lineages (e.g. dasyurids) these vestiges, the praeclavia (Klima, 1987), are relatively common, and may remain cartilaginous or ossify with age. For eutherians such vestiges (termed suprasternals; Hanson, 1919; Klima, 1968, 1972, 1987) are rare and considered to be atavisms.
Similar to the procoracoid, the metacoracoid primordium develops only as a rudiment. Initially it joins the procoracoid in contacting the manubrium sterni. This connection is transient, however, and eventually disappears, with the metacoracoid remaining firmly affixed to the scapular condensation (Fig. 6A). Ultimately, the metacoracoid gives rise to the coracoid process. In some taxa (e.g. the marsupial Monodelphis domestica, the insectivores Crocidura russula and Suncus etruscus) the metacoracoid condensation also contributes to the glenoid (Großman et al. 2002; Sánchez-Villagra & Maier, 2002).
The acromion develops prior to the scapular spine, arising from a cartilaginous primordium that splits off the scapula (Großmann et al. 2002; Sánchez-Villagra & Maier, 2002, 2003). In most taxa, the scapular spine subsequently forms by appositional ossification of the scapular perichondrium (e.g. Monodelphis, Crocidura; Sánchez-Villagra & Maier, 2002; Großmann et al. 2002). However, in the rodent Mesocricetus auratus, the spine reportedly develops from a cartilage precursor stemming from the acromion (Großman et al. 2002).
Experimental work on the laboratory rodent, Mus musculus, illustrates that normal development of the mature scapula is dependent on expression of Pax1, Hoxc6 and Emx2 genes. The acromion is greatly affected in mutants deficient for Pax1 (undulated mutants), and may be completely absent, present and grossly deformed, or present and fused with the clavicle (compare Fig. 6E and F; Timmons et al. 1994; Dietrich & Gruss, 1995). By contrast, defects of the rest of the scapula including the scapular spine involve a relatively minor reduction in size. Similarly, the scapular blade remains virtually unchanged in mice with a mutation in the platelet-derived growth factor α gene receptor (PDGFαR; required for normal patterning of the somites) or Hoxc6, whereas the acromion (PDGFαR; Soriano, 1997), or acromion and glenoid (Hoxc6; Pellegrini et al. 2001) are altered. In another dramatic deviation, the scapular blade fails to form in Emx2 homozygous knockout mutants, with only the coracoid process, acromion and glenoid of the scapula developing (Pellegrini et al. 2001). Data from the mouse mutants are consistent with the individuation of pectoral elements, which is consistent with an independent evolutionary origin.
The bulk of the sternum is derived exclusively from condensations of lateral plate mesoderm, the sternal bands. As noted earlier, the cranial end of the sternum, the manubrium sterni, receives contributions from three distinct sources: the procoracoid rudiments, the sternal bands and an unpaired cartilaginous condensation (Fig. 6D). Based on location, morphology and histology of the unpaired condensation, Klima (1987) identified this unmatched rudiment as the homologue of the pars chondralis interclaviculae of monotremes. As for the scapula, mutations in Pax1 lead to defects of the sternum (Timmons et al. 1994).
Unique to mysticete (toothless) cetaceans, the sternal bands fail to form during embryogenesis. Among adults, however, the sternum is present, derived exclusively from the procoracoid rudiments and the pars chondralis interclaviculae (Klima, 1978, 1985, 1987, 1990).
Fossil evidence
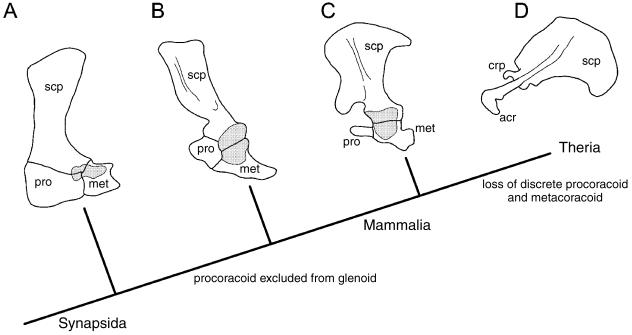
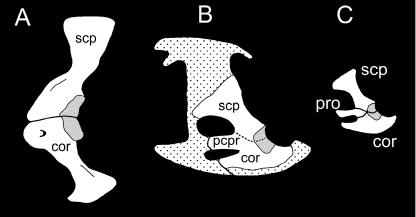
Among outgroups to modern mammals (Monotremata + Theria) the pectoral apparatus includes both the procoracoid and the metacoracoid (Fig. 7; Luo et al. 2002). Whereas the glenoid is restricted to the metacoracoid and scapula among monotremes and various closely related lineages (e.g. †Morganucodon spp., †Sinoconodon rigneyi; Jenkins & Parrington, 1976; Luo et al. 2002), it receives a minor contribution from the procoracoid in more basal synapsids (see Romer, 1956; Jenkins & Parrington, 1976). In the basalmost forms, the paraphyletic †‘pelycosaurian-grade synapsids’ or ‘pelycosaurs’ (e.g. Dimetrodon spp.), each of the procoracoid and metacoracoid gain prominence as broad, plate-like elements. Among †’pelycosaurs’ it has frequently been observed that the scapula and procoracoid are co-ossified (or at least tightly sutured), whereas the junction between the metacoracoid and the other elements is rarely fused or well sutured (e.g. Williston, 1911; Case & Williston, 1913; Romer & Price, 1940).
Fig. 7.
Evolution of the synapsid primary girdle. Among basal synapsids (A), the primary girdle consists of the scapula and the large, plate-like procoracoid and metacoracoid. All three elements contribute to the glenoid. Among more deeply nested non-mammalian synapsids (B), the procoracoid contribution to the glenoid is relatively reduced. For modern mammals, only monotremes (C) retain the procoracoid and metacoracoid as discrete elements. However, the procoracoid is excluded from the glenoid. In therians the scapula dominates the primary girdle, with the metacoracoid rudiment contributing to the coracoid process and (at least in some eutherians) the acromion and part of the glenoid. Abbreviations: acr (acromion), cpr (coracoid process), gln (glenoid), met (metacoracoid), pro (procoracoid), scp (scapula). Glenoid is dark grey stippled. Phylogeny based on Luo et al. (2002). Sources for images: (A) †Dimetrodon modified from Romer & Price (1940) and reproduced with fair use permission from the Geological Society of America; (B) unidentified †‘cynodont’ and (C) Tachyglossus reproduced by permission of F. A. Jenkins, Jr. from The postcranial remains of Eozostrodon, Megazostrodon and Erythrotherium, fig. 19 (p. 419), 1976, published by the Royal Society; (D) Priodontes modified from Flower (1876). Not to scale.
Discussion
Developmental and palaeontological evidence clearly demonstrates that whereas the modern adult therian pectoral apparatus is composed of few elements, ontogenetically and historically it is quantitatively more complex. Embryological data suggest that the majority of the membranous skeleton initiating the multipartite pectoral pattern of monotremes (Fig. 5C) is retained by virtually all therians, regardless of the adult phenotype (Fig. 6D). Hence, the sequential non-appearance of discrete pectoral ossifications among modern adult therians (both developmentally and phylogenetically) may be correlated with reorganization of skeletogenetic condensations. The presence of a discrete procoracoid and metacoracoid is primitive for mammals but lost in therians. The therian procoracoid unites with the pars chondralis interclaviculae and sternal bands to form the manubrium sterni, although in some taxa a discrete portion may be retained as a separate element (the praeclavium or suprasternal). Uniquely therian features of the pectoral apparatus include derivatives of the metacoracoid (the coracoid process and, at least among some eutherians, the acromion and multipartite glenoid) and the combined procoracoid and interclavicle (the composite manubrium sterni).
The reptilian pectoral apparatus
Among reptiles the morphological diversity of the pectoral apparatus is immense. In the interest of clarity, this review recognizes three structurally similar groups: ornithodirans (birds and their ancestors), non-avian diapsids (a paraphyletic group consisting of lepidosauromorphs, crocodylians and their fossil relatives) and Testudines (turtles).
Reptilia: Diapsida: Ornithodira
Extant development and morphology
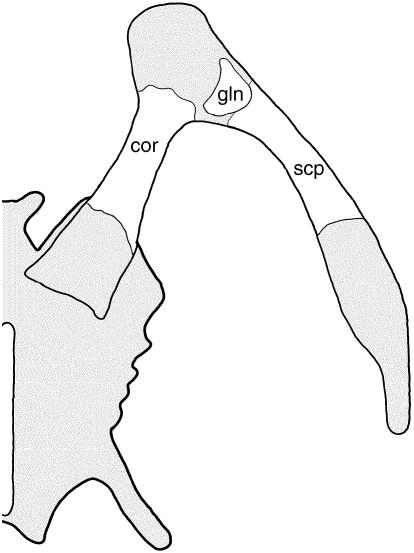
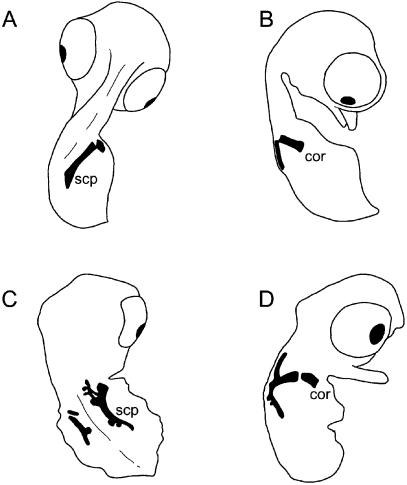
The avian pectoral apparatus consists of a narrow blade-like scapula, tubular/strut-like coracoid and a large unsegmented (and usually keeled) sternum (Fig. 8). Most (but not all) modern avians also demonstrate a single chevron-shaped furcula (Parker, 1868; Hall, 2001). As in mammals the scapula and coracoid develop from a unified homogeneous condensation at the base of the limb bud (Romanoff, 1960; Hamilton, 1965). During chondrogenesis, the prospective elements detach from one another, and typically remain separated prior to and following ossification (Fig. 8; Romanoff, 1960), although in some large flightless birds the scapula and coracoid may secondarily rejoin post-hatching (Glutz von Blotzheim, 1958; see also Elzanowski, 1988). In many taxa the coracoid develops a bony medial projection, the procoracoid process.
Fig. 8.
The avian primary girdle and sternum. Although the scapula and coracoid initially begin as a common condensation, the two elements separate from one another and ossify independently. Furcula not depicted. Cartilage shaded. Abbreviations: cor (coracoid), gln (glenoid), scp (scapula), stn (sternum). Source for image: embryonic Linota cannabina, left primary girdle and left half of sternum in ventral view, modified from Parker (1868).
Using the domestic fowl Gallus gallus, it has been established experimentally that the scapula is derived, at least in part, from an embryological source distinct from the rest of the pectoral apparatus. Whereas the coracoid and sternum are derivatives of lateral plate mesoderm, chick-quail transplantation experiments by Chevallier (1975, 1977; see also Beresford, 1983) have shown that most of the scapula arises from somites. More recently, Huang et al. (2000) demonstrated that the proximal-most portion of the scapula (the area around the glenoid), like the coracoid, is derived from lateral plate mesoderm. Other experimental observations further reinforce this idea. While Talpid3 chick mutants demonstrate several primary girdle defects, the deviant scapula morphology (resembling an open chevron with tooth-like projections) is considerably more severe than the modestly foreshortened coracoid (Fig. 9; Ede & Kelly, 1964). Work examining the role of Pax1 found that repression of this gene by application of BMP-2- and -4 soaked beads resulted in defects of the proximal part of the scapula and (in some instances) the coracoid, including coracoid aplasia (Hofmann et al. 1998). Localized application of retinoic acid (via bead implants) or tissue grafts of the polarizing region of an exogenous limb bud to the developing wing buds of Gallus enlarges the Hoxc6 (formerly X1Hbox 1) gradient and may result in fusion of the scapula with the coracoid, as well as duplication or foreshortening of the coracoid and absence of the procoracoid process (Oliver et al. 1990; see also Williams, 2003). Thus, patterning of the procoracoid process appears to be at least partially independent from the rest of the coracoid.
Fig. 9.
Normal and abnormal development of the scapula and coracoid in 11-day-old Gallus embryos stained for cartilage, with all other elements omitted and limbs removed. Normal embryos, in (A) dorsal and (B) lateral views. Talpid3 mutant embryos, in (C) dorsal and (D) lateral views. Note the extraordinary morphology of the scapula in talpid3 mutant embryos compared with somewhat abbreviated coracoid. Abbreviations: cor (coracoid), scp (scapula). Source of images: modified from Ede & Kelly (1964) and reproduced with permission from the Company of Biologists Ltd. Not to scale.
Whereas modern researchers have largely overlooked the development of the procoracoid process, early anatomists recorded that it developed independent of the rest of the avian coracoid (Fig. 10A; Parker, 1868; Newton & Gadow, 1893–1896). Indeed, Parker remarked that ‘the præ-coracoid (procoracoid) is always segmented from the head of the coracoid’ (p. 143), and noted that it may become mineralized independent of the latter. Lindsay (1885) and Broom (1906b) later examined the development of the procoracoid process in Struthio camelus (the ostrich; Fig. 10B) but arrived at conflicting interpretations. Whereas Lindsay argued that the procoracoid process developed independent of the coracoid, according to Broom the procoracoid process represents a ‘descending process of the scapula’ (p. 357). Broom further argued that the procoracoid process could not be homologous with the procoracoid of basal amniotes as such a bone was not present in any immediate ancesstors of modern avians (although see below).
Fig. 10.
Development of the avian procoracoid process. Many avian taxa have been observed to develop a discrete skeletal centre for the procoracoid process (A), a medial projection that contacts the furcula. (B) In Struthio the procoracoid process is hypertrophied and reorientated ventrally to contact the sternum. It remains unclear if the procoracoid process of Struthio develops independent of the scapula or as a ventrally orientated projection (see text for details). Cartilage shaded. Abbreviations: cor (coracoid), pcpr (procoracoid process), scp (scapula). Source for images: (A) embryonic Linota cannabina in lateral view and (B) embryonic Struthio camelus in cranial view modified from Parker (1868).
Fossil evidence
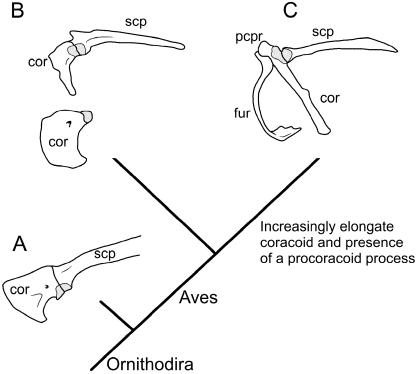
The morphology of the pectoral apparatus in most fossil avians is similar to that of modern birds including the ‘U’- or ‘Y’-shaped furcula, although the procoracoid process often appears to be absent (Chiappe et al. 1999). In the basalmost avian taxon (†Archaeopteryx lithographica) the coracoid no longer exhibits the derived strut-like morphology of modern forms, but instead is almost rectangular when viewed cranially (Fig. 11; Ostrom, 1976; Elzanowski, 2002). The morphology of the coracoid among non-avian dinosaurs (e.g. †non-avian theropods, †sauropodomorphs, †ornithischians) is structurally conserved as a broad and plate-like element.
Fig. 11.
Evolution of the ornithodiran primary girdle. (A) Among non-avian ornithodirans (e.g. †Deinonychus) the primary girdle consists of a strap-like scapula and a large, plate-like coracoid. (B) The coracoid of the basal avian †Archaeopteryx remains broad, but has become reorientated with respect to the rest of the pectoral apparatus. (C) For modern avians (e.g. Sturnus) the coracoid is elongate and somewhat tubular. The furcula is unknown (and thus not illustrated) but presumed to be present in †Deinonychus and has been omitted for †Archaeopteryx. Abbreviations: cor (coracoid), fur (furcula), pcpr (procoracoid process), scp (scapula). Phylogeny based on Brochu (2001). Glenoid shaded. Sources for images: (A–C) modified from Jenkins (1993) ‘The evolution of the avian shoulder joint’ reprinted by permission of the American Journal of Science; (B) †Archaeopteryx coracoid modified from Ostrom (1976). Not to scale.
Reptilia: Diapsida: Non-avian diapsids
Extant development and morphology
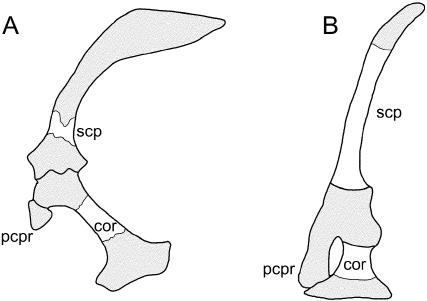
With the exception of ophidians (among which the forelimbs and pectoral apparatus are always absent, even as rudiments; Cohn & Tickle, 1999), modern pseudosuchians (crocodylians) and lepidosauromorphs (sphenodontids + squamates), including virtually all limbless ‘lizards’ and some amphisbaenians, develop a scapula and coracoid (Fig. 12; Parker, 1868; Cope, 1892; Camp, 1923; Zangerl, 1945; Stokely, 1947; Montero et al. 1999; Kearney, 2002). Each element is capped distal to the glenoid by a cartilaginous extension (the suprascapular and epicoracoidal cartilages). The morphology of the lepidosauromorph scapula and coracoid (including cartilaginous extensions) is highly variable and frequently includes multiple deep emarginations, fenestrations or both along the cranioventral borders (Fig. 12B; Romer, 1956; Lécuru, 1968). Among various authors it has been suggested that there is an additional pectoral element, the pre- or procoracoid, that either remains cartilaginous (and has been suggested as part of the epicoracoidal cartilage; Broom, 1906a; Romer, 1956; Skinner, 1959) or forms an ossified strut-like process between two emarginations/fenestrations (e.g. Parker, 1868; Cope, 1892). Conrad (2006) observed an independent ossification (his precoracoid) separated from the coracoid by a suture in subadult specimens of Shinisaurus crocodilurus (Fig. 12C). Regrettably, the situation for most taxa remains unresolved. A comparable procoracoid element has not been reported for crocodylians.
Fig. 12.
Non-avian reptile scapulae and coracoids. (A) The pseudosuchian (crocodylian) Alligator and (B,C) the lepidosauromorphan (squamate) Shinisaurus. (C) Evidence from an osteological study of Shinisaurus indicates that the procoracoid process of the scapula is derived from a discrete skeletal element, herein considered to be the homologue of the procoracoid (see text for details). Abbreviations: cor (coracoid), pcpr (procoracoid process), pro (procoracoid), scp (scapula). Glenoid shaded, cartilage stippled. Sources for images: (A) Alligator modified from Mook (1921) and reproduced with permission from the American Museum of Natural History; (B) adult and (C) subadult Shinisaurus modified from Postcranial anatomy of Shinisaurus crocodilurus (Squamata: Anguimorpha), J. L. Conrad (in press, ©Journal of Morphology); reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. Not to scale.
Fossil evidence
The pectoral apparatus of fossil non-avian diapsids such as pseudosuchians, lepidosauromorphs and basal archosauromorphs falls within the morphological range revealed by modern forms. As in extant (skeletally mature) taxa there is only one discrete coracoid ossification, although numerous specimens preserve evidence of the cartilaginous epicoracoidal and suprascapular cartilages (Osborn, 1899; Müller, 2001). Notwithstanding their absence among non-avian diapsids, both the procoracoid and the metacoracoid are present among immature and some adult specimens of the diapsid outgroup †Captorhinidae (Fig. 13). Conspicuously, in larger (adult-sized) material all elements of the †captorhinid primary girdle are fused, often with the sutures obliterated. As in synapsids (and proganosaurs; see below), the scapula and procoracoid are the first primary girdle elements to coalesce (Holmes, 1977; Sumida, 1989).
Fig. 13.
Basal reptilian pectoral apparatus as exemplified by †Captorhinus. Adult in (A) lateral and (B) ventral views (C) subadult in ventral view. The dashed line separating the procoracoid from the scapula denotes that these elements are often fused or tightly sutured, even in subadult specimens. Abbreviations: cla (clavicle), int (interclavicle), met (metacoracoid), pro (procoracoid), scp (scapula). Glenoid is dark grey stippled. Sources for images: (A,B) modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History; (C) modified from The Osteology and Musculature of Small Captorhinids, R. Holmes (1977, Journal of Morphology); reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. Not to scale.
Reptilia: ? Proganosauria/?Diapsida: Testudines
Extant development and morphology
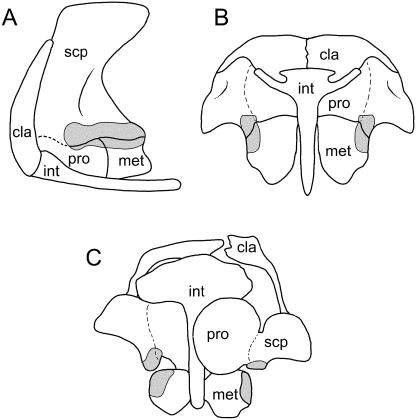
The phylogenetic position of Testudines remains contested, oscillating between the traditional assignment as a proganosaur [e.g. Romer, 1956; Lee, 1996, 1997; proganosaurs include all members of the group formerly identified as Anapsida (Modesto & Anderson, 2004)], and the more recent inclusion as a member of Diapsida (e.g. Rieppel & deBraga, 1996). Irrespective of the genealogical scenario, the morphology of the pectoral apparatus of turtles is unique among amniotes, and (at least among adults) bears little resemblance to that of other taxa. Of particular note is the radical emplacement of the replacement bones of the pectoral apparatus within the rib cage, integration of clavicular and interclavicular homologues (the paired epiplastra and median entoplastron, respectively, e.g. Parker, 1868; Romer, 1956; Rieppel, 1993; Gilbert et al. 2001) into the plastron, and the apparent absence of a sternum (although see below). Whereas developmental studies have not specifically targeted the turtle pectoral apparatus, a variety of incidental observations have been made during investigations of gross embryology and ontogeny of the carapace/plastron.
In adults, the primary girdle forms a triradiate structure with slender dorsal, caudal and ventromedial processes (Fig. 14A,B). The glenoid is positioned at the crux. Whereas the column-like dorsal ramus is widely accepted as the scapula, the identity of the remaining processes has been the subject of debate. The ventromedial or acromial process has been identified as the homologue of the procoracoid (e.g. Parker, 1868; Korringa, 1938; Gaffney, 1990; deBraga & Rieppel, 1997) or alternatively as an outgrowth of the scapula (e.g. Romer, 1956; Walker, 1947, 1973; Lee, 1997). The caudal ramus (the coracoid) has been presumed to represent either the metacoracoid (e.g. Parker, 1868; Gaffney, 1990; deBraga & Rieppel, 1997; Lee, 1997) or the procoracoid (Walker, 1947, 1973).
Fig. 14.
Testudines pectoral apparatus. Macroclemys (=Macrochelys), adult in (A) lateral and (B) ventral views; (C) subadult in ventral view. †Proganochelys, adult in (D) lateral and (E) ventral views. Abbreviations: act (turtle acromial process), cor (coracoid), pla (plastron), scp (scapula). Glenoid shaded, cartilage stippled, plastron black. Sources for images: (A,B,D,E) modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History; (C) modified from Skeletal Development of Macrochelys temminckii (Reptilia: Testudines: Chelydridae), C. A. Sheil 2005 (©Journal of Morphology); reprinted with permission of Wiley-Liss, a subsidiary of John Wiley & Sons. Not to scale.
During the earliest stages of chondrogenesis, the presumptive scapula, acromial process and coracoid of each side develop from either a unified trifurcate condensation or a trifurcate condensation in which the caudal ramus is initially isolated (Fig. 14C; Walker, 1947; Rieppel, 1993; Sheil, 2003, 2005). In at least one taxon (Chrysemys picta marginata), a supracoracoid foramen transmitting a neurovascular bundle is present early during development, nested between the presumptive scapula and coracoid (Walker, 1947). As the scapula and coracoid continue to differentiate, the neurovascular bundle is liberated from the now cartilaginous complex and the foramen disappears. In all taxa studied thus far (e.g. Apalone spinifera, Chelydra serpentina) each ramus begins to ossify from a separate centre within the condensation. The notion of the scapula developing independent of the other replacement bones is supported by the extirpation experiments of Burke (1991b). The majority of Chelydra embryos that had cervical somites 8–12 removed demonstrated scapular aplasia or incomplete scapula development, whereas the acromial process, coracoid and elements of the limb appear to have been minimally affected.
Fossil evidence and discussion, hypothesis 1 – Testudines as proganosaurs
The remarkable osteology of turtles dates back over 200 millions years (Gaffney, 1990). Similar to modern forms, the oldest well-preserved fossil taxon, †Proganochelys quenstedti, has a bony shell (plastron/carapace) and an internalized primary girdle. Although lacking a strictly tripodal morphology, the primary girdle does consist of a scapula with a prominent acromial process and a coracoid (Fig. 14E,F). A supracoracoid foramen is present nested in the suture between the two elements, similar to the aforementioned (albeit transient) aperture of modern turtle embryos.
In view of their unusual morphology, the genealogical relationship of turtles to other amniotes is difficult to resolve unequivocally. Historically, however, they have long been considered members of Proganosauria (demonstrating the anapsid condition, a skull without temporal fenestrations; Fig. 15A). Among the most deeply nested non-turtle fossil proganosaurs (e.g. †pareiasaurs and †procolophonids), the pectoral apparatus closely resembles the morphology previously noted for basal synapsids (namely †′pelycosaurian grade synapsids′), with a robust, plate-like procoracoid and metacoracoid (Lee, 1996, 1997; deBraga, 2003). As in basal synapsids, the glenoid may receive contributions from the metacoracoid and scapula alone, or include minor involvement of the procoracoid. Among basal proganosaurs (e.g. †mesosaurids, †millerettids), the scapula and coracoid element(s) are typically fused together into a single element (Gow, 1972; Modesto, 1999). Unfortunately, few data are available on the ontogeny of fossil proganosaurs and it remains unclear if both coracoids are present and fuse together during early skeletogenesis or if one element fails to form.
Fig. 15.
Evolution of the turtle primary girdle. (A) The proganosaur hypothesis as proposed by Lee (1996), wherein Testudines share a close common ancestry with †pareisaurs. According to this hypothesis, the evolution of the acromial process of turtles precedes the loss of the procoracoid (see text for details). (B) The diapsid hypothesis as proposed by Rieppel and others (Rieppel & deBraga, 1996; deBraga & Rieppel, 1997; Rieppel & Reisz, 1999), wherein turtles share a close common ancestry with lepidosauromorphs. According to this hypothesis, the evolution of the acromial process of turtles is linked with loss of the procoracoid. Glenoid shaded. Abbreviations: act (turtle acromial process), pro (procoracoid), pcpr (procoracoid process). Sources for images, †Captorhinus, †Proganochelys, Macroclemys, modified from Gaffney (1990) and reproduced with permission from the American Museum of Natural History; †Scutosaurus reproduced by permission of M. S. Y. Lee from The homologies and early evolution of the shoulder girdle in turtles, fig. 2 (p. 113), 1996, published by the Royal Society; Shinisaurus modified from Postcranial anatomy of Shinisaurus crocodilurus (Squamata: Anguimorpha), J. L. Conrad (in press, ©Journal of Morphology); reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Corresponding to the burgeoning interest in turtle evolution, several efforts have been made to resolve the identification of the acromial process. At present, two competing views are held: the acromial process as an outgrowth of the scapula or as the homologue of the procoracoid (see below). Assuming Testudines as proganosaurs, it has recently been proposed that the evolution of the acromial process may be traced phylogenetically from †procolophonids – which lack any conspicuous scapular processes – to †pareiasaurs – which demonstrate an incipient ridge-like projection – to modern turtles and allies (Fig. 15A; Lee, 1996, 1997; although see Rieppel, 1996, for a contradictory interpretation of the same data). According to this hypothesis, the advent of the acromial process (in †pareiasaurs) precedes loss of the procoracoid.
Fossil evidence and discussion, hypothesis 2 – Testudines as diapsids
Recently, a number of studies have suggested that testudines represent derived diapsids more closely related to lepidosauromorphs than archosauromorphs (Fig. 15B; Rieppel and deBraga, 1996; deBraga & Rieppel, 1997; Rieppel & Reisz, 1999; see also Wilkinson et al. 1997). Although it is widely acknowledged that this relationship seems counterintuitive (conventional diapsid skulls are fenestrated, turtle skulls are not), the hypothesis gleans support from its global or broad-based perspective (Rieppel & Reisz, 1999); the removal of certain groups not normally considered in turtle phylogenetics (namely sauropterygians, a sister group to lepidosauromorphs not discussed here) repositions Testudines within Proganosauria. Among the anatomical details used to underline the diapsid theory is a reappraisal of the acromial process of the scapula. Various authors (Gaffney, 1990; Rieppel, 1996; deBraga & Rieppel, 1997) have rejected the homology of the acromial process as an outgrowth of the scapula and alternatively suggested that it is homologous with the procoracoid (Fig. 15B). Indeed, the acromial process shares with the procoracoid a similar topology and is likewise connected with the interclavicle/clavicle homologues. Accordingly, Gaffney (1990) has used this evidence to hypothesize that the coracoid element of Testudines represents the metacoracoid and not the procoracoid (see discussion below).
Reptilia discussion – evolution of the amniote coracoid(s)
Previous work suggested that the procoracoid is maintained by modern reptiles whereas the metacoracoid was either independently derived (homoplasious) and lost in some basal members, or was only transiently present. This investigation finds the procoracoid-equals-coracoid proposal to be inconsistent with current data, and indicates (following the work of Gaffney, 1990) that the reptilian coracoid is the homologue of the metacoracoid. For the sake of convenience this evidence may be summarized in three main categories, although none is mutually exclusive.
1. Palaeontology and genealogy: Regardless of genealogical scenario, a three-element primary girdle is plesiomorphic for all amniotes. Within each of Synapsida and Reptilia one coracoid element appears to be independently lost. However, fossil material representing various ontogenetic stages of both basal synapsids (e.g. specimens of †Dimetrodon; Romer & Price, 1940) and reptiles (†Captorhinus; Holmes, 1977) demonstrates that this apparent loss is an example of two skeletal centres coalescing to form a single mature morphology. Thus, while the procoracoid is present and discrete among subadults, it generally fuses with the scapula (and the sutures disappear) in larger ‘adult-sized’ individuals (see Fig. 13). Consequently the scapula element of skeletally mature specimens in effect represents the scapula + procoracoid. By contrast, the metacoracoid frequently remains independent and discrete (Williston, 1925; Romer & Price, 1940; see also Gaffney, 1990).
2. Topography and connectivity: Several arguments can be made on the basis of the position and association of the various elements. Among basal amniotes the glenoid is dominated by the metacoracoid, with a decreasing contribution from the scapula and (when apparent) the procoracoid. According to the theory of Williston (1925) and Romer (1956), the glenoid of modern reptiles is taken over by the formerly minimally contributing procoracoid. By contrast, the revised hypothesis presented herein proposes that the glenoid of extant reptiles, similar to basal forms, remains dominated by the metacoracoid.
Of the two coracoid elements of basal amniotes, only the procoracoid contacts both the clavicle and the interclavicle. In Testudines (including †Proganochelys), the acromial process shares a common topology with the procoracoid of basal amniotes and retains a comparable contact with the clavicle–interclavicle homologues (namely the epiplastron and entoplastron; Gaffney, 1990; Fig. 14A). Among avians, the procoracoid process also demonstrates a similar connection with the intramembranously derived furcula. In both these representative amniotes, the process contacting the dermal element(s) is held to be the procoracoid, secondarily joined with either the scapula (Testudines) or coracoid (Aves). Gaffney (1990) also observed that the interpreted position of supracoracoideus muscle attachment on the procoracoid of basal amniotes (sensuRomer, 1922; Holmes, 1977) corresponds to the same muscle origin on the acromial process of the turtle coracoid.
3. Development and experimental embryology: Among therians, the sternum and primary girdle elements receive contributions from multiple embryonic condensations. For instance, the skeletally mature scapula element minimally represents a coalescence of the metacoracoid rudiment with the scapular condensation, and the manubrium sterni integrates components of the procoracoid, pars chondralis interclaviculae rudiment and sternal band. These developmental data are supplemented by evidence gleaned from the study of mutant mouse strains. In Emx2 mutants, the coracoid process, acromion and glenoid develop, whereas the blade of the scapula fails to form (Pellegrini et al. 2001). This indicates that Emx2 plays a role in patterning the scapular condensation but not the metacoracoid rudiment. By contrast, normal development for derivatives of the metacoracoid (e.g. coracoid process, glenoid and acromion) is dependent on Pax1, PDGFαR and Hoxc6 (Timmons et al. 1994; Soriano, 1997; Pellegrini et al. 2001; Fig. 6E,F). At the present time, however, the embryological source of the mammalian scapula (somites and/or lateral plate mesoderm) remains to be determined.
Among lepidosauromorphs the presence of a discrete procoracoid has been reported (Fig. 12C; Conrad, 2006), although in most instances it is unclear if the element in question is different from the coracoid element proper or the epicoracoidal cartilage. In the reptilian lineage Pseudosuchia there is (presently) no evidence for the development of more than one coracoid element, even transiently. However, neither group has been explored experimentally so future efforts will undoubtedly yield important data.
Among avians, the procoracoid process of the coracoid, as noted above, occupies a comparable position and set of articulations with the procoracoid of basal amniotes. Furthermore, it develops independent of the coracoid, and fuses with the latter at some later point of skeletogenesis (Fig. 10A; Parker, 1868). Experiments on the avian Gallus that enlarge the Hoxc6 gradient result in a reduction or complete absence of the procoracoid process (a derivative of the procoracoid), along with defects of the glenoid region of the scapula (possible procoracoid or metacoracoid origin) and (meta)coracoid malformations (Oliver et al. 1990). Other experimental work (including somite extirpation, chick-quail grafting and gene expression labelling) also demonstrates that the avian scapula receives contributions from more than one embryological source, namely somites and lateral plate mesoderm (see Chevallier, 1977; Timmons et al. 1994; Hofmann et al. 1998; Huang et al. 2000; Pellegrini et al. 2001). By contrast, the (meta)coracoid is exclusively lateral plate. Huang et al. (2000) suggested that the non-somitic source of the scapula was homologous with the metacoracoid. It should be noted, however, that the procoracoid is a comparable candidate. Similar to scapula development in mice, the gene Pax1 has been observed to play a role in patterning of the normal avian scapula element (Hofmann et al. 1998; Huang et al. 2000), whereas Hoxc6 is involved in regulation of the procoracoid and metacoracoid derivatives. Indeed, mutations of Pax1 and Hoxc6 in Gallus embryos result in primary girdle defects, minimally including the proximal part of the scapula, and in more extreme cases, the coracoid (Oliver et al. 1990; Hofmann et al. 1998). This indicates that similar to mice, Pax1 and Hoxc6 play a role in specifically patterning derivatives of the metacoracoid and (at least in avians) the procoracoid.
As with the avian procoracoid process, the acromial process of Testudines is topographically, connectively and developmentally comparable with the procoracoid. During skeletogenesis each of the turtle scapula, acromial process and coracoid arises from separate centres of ossification (Parker, 1868; Rieppel, 1993; Sheil, 2003). Removal (extirpation) of cervical–thoracic somites results in aplasia of the scapular blade but has little effect on the development of the acromial process, glenoid region or coracoid (Burke, 1991b). The data clearly imply a separate origin for each of the acromial process and scapula blade, one that may readily be explained by integration of the procoracoid rudiment into the scapula. This parallels the fusion/integration of the procoracoid and scapula in basal diapsids and synapsids.
Remaining issues
Two remaining issues deserving mention necessitate a brief departure before assembling a new scenario of coracoid evolution. The first concerns the position of the supracoracoid foramen. Among basal amniotes the procoracoid is pierced by a neurovascular passage, the supracoracoid foramen, proximal to the procoracoid–metacoracoid contact. Acceptance of the metacoracoid-equals-coracoid hypothesis for non-basal reptiles requires that the supracoracoid foramen relocate. As observed by Gaffney (1990), in the basal testudine †Proganochelys, and Walker (1947), in developing Chrysemys, this aperture has shifted position to be found within the suture connecting the remaining coracoid with the scapula (Fig. 14F). Among extant reptiles (exclusive of Testudines) the supracoracoid opening for the neurovascular bundle only pierces the coracoid, the modern homologue of the basal amniote metacoracoid. It is the contention here that, following the condition of †Proganochelys and embryonic Chrysemys, the supracoracoid foramen has further migrated to become bounded by the metacoracoid proper.
The second topic relates to the apparent absence of the sternum in turtles. The view that turtles lack this pectoral element is widely held (e.g. Parker, 1868; Romer, 1956) inasmuch as the ventrally positioned plastron develops entirely via intramembranous ossification (Parker, 1868; Gilbert et al. 2001). Nevertheless, Walker (1947) suggested that a connective tissue band (the acromialcoracoid ligament; Wyneken, 2001) linking each ipsilateral acromial process and coracoid might be homologous with the sternum. Walker offered four observations to support his hypothesis: (1) ligaments may represent vestiges of cartilage (e.g. in undulated mutant mice a ligament may replace the acromion [Timmons et al. 1994; see also Hall (1970) for a discussion of the relationship between fibroblasts (producing ligaments) and chondroblasts (producing cartilage)]; (2) the acromialcoracoid ligament and sternum are both fundamentally paired structures; (3) the acromialcoracoid ligament and sternum arise in association with the coracoid; and (4) the acromialcoracoid ligament and sternum share a general topography with the rest of the pectoral apparatus. However unlike the sternum, acromialcoracoid ligaments do not contact the ribs and do not extend cranially to the clavicular homologues (the epiplastra) or caudally past the coracoids. Walker countered these differences by remarking that acromialcoracoid ligaments represent vestiges (and therefore are not developed to the same extent as a sternum) and that turtle ribs are fully integrated into the carapace (having become ensnared by the carapacial ridge; see Ruckes, 1929; Burke, 1991a; Gilbert et al. 2001). Moreover, as has been demonstrated for other parts of the pectoral apparatus (see above), the mere absence of a skeletal counterpart does not necessarily speak to definitive non-existence. This notion arguably receives indirect support from a study conducted on the marine reptile clade †Plesiosauria.
Similar to Testudines, among †plesiosaurs a discrete sternum is both unreported and architecturally problematic. Whereas turtles demonstrate a well-ossified plastron, in †plesiosaurs the presence of a sternum seems to be precluded by the enlarged and ventrally displaced coracoids. However, unlike turtles, in which the ribs are integrated into the carapace, in †plesiosaurs the ribs make no obvious connections with any preserved skeletal elements. Nicholls & Russell (1991) reviewed the issue of the sternum in †plesiosaurs and, based on data gleaned from sternal development, function and phylogeny, found that the suggestion of such an element was anatomically justified and functionally required. Without a sternum, the †pleisosaur girdle would probably be unable to withstand tensile forces associated with cranially directed movements of the forelimbs (Nicholls & Russell, 1991). Nicholls and Russell postulated that the position of the unmineralized (and thus unpreserved) sternum was dorsal (visceral) to the coracoid. Whereas these data do not speak directly to the presence or existence of a sternum in Testudines (indeed the architecture of the turtle pectoral apparatus fundamentally differs from that of †plesiosaurs in the internalization of these elements with respect to the ribcage), they suggest that connective tissues in this region (e.g. the acromialcoracoid ligament) might represent a latent homologue (sensuStone & Hall, 2004). Finally, it is worth observing that regardless of genealogical affinity (proganosaur or diapsid), the ancestors of modern turtles did demonstrate ossified rib cages requiring a sternal underpinning.
A new scenario
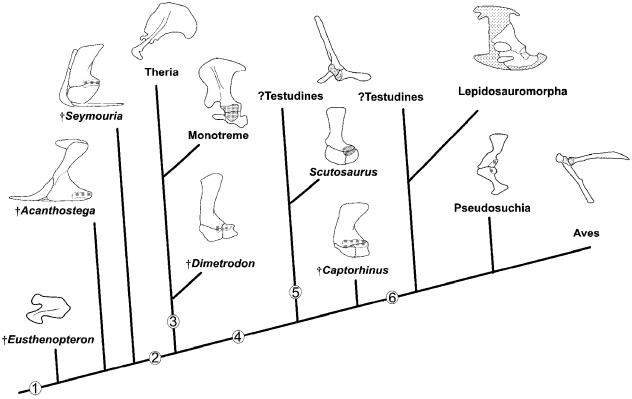
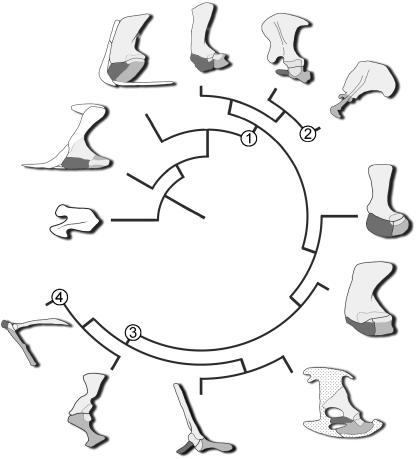
A revised scenario for the evolution of the amniote primary girdle is offered (Fig. 16), drawing on a global review of available data (documented above) and taking into consideration the role of cell condensations in skeletogenesis. It is argued that disruptions in preskeletogenic cell aggregation, proliferation and differentiation, and the acceleration (or retardation) of osteogenesis play vital roles in the evolution of the amniote primary girdle. Plesiomorphically, basal tetrapods have a unified scapulocoracoid (e.g. †Acanthostega) or a scapula and a single coracoid (e.g. †Seymouria). Among basal amniotes, the primary girdle consists of a scapula, procoracoid and metacoracoid. This transformation may be achieved as the normal osteogenic pattern of the condensation forming the membrane skeleton of the primary girdle elements (the coracoid–scapular plate, sensuKlima, 1973, 1987) is delayed and/or the failure (and delay) of preskeletogenic cells to differentiate, resulting in the formation of sutural contacts. The metacoracoid is more strongly influenced by osteogenic retardation, and unlike the procoracoid will often appear discrete even late in ontogeny.
Fig. 16.
Evolution of the amniote scapula, procoracoid and metacoracoid; a new scenario. Amniote phylogeny based on the work of Brochu (2001), Lee (1996), Luo et al. (2002), Rieppel & Reisz (1999) and Ruta et al. (2003). Beginning with the unified scapulocoracoid of †Eusthenopteron (far left, white) and continuing in a clockwise progression; †Acanthostega; †Seymouria; †Dimetrodon; Tachyglossus; Priodontes; †Scutosaurus; †Captorhinus; Shinisaurus; Macroclemys; Alligator; Sturnus. The phylogenetic arrangement follows the hypothesis of Testudines as diapsids; alternatively, Macroclemys could be positioned as the sister group to †Scutosaurus in accordance with the turtles as proganosaurs hypothesis (see text for details). Nodes: 1, delay in osteogenesis and/or the differentiation of skeletogenic cells results in the formation of sutures and the discrete development of each of the scapula, procoracoid and metacoracoid; 2, integration of the procoracoid rudiment into the sternum combined with reduction in preskeletogenic cell proliferation of each of the procoracoid and metacoracoid rudiments results in the scapula dominating the primary girdle; 3, reduction of preskeletogenic cell mitosis and accelerated osteogenesis of the procoracoid condensation results in this element remaining linked with the scapula, albeit as a vestige; 4, delay in procoracoid osteogenesis and/or reaggregation of the procoracoid condensation alongside the presumptive metacoracoid results in coalescence of the two elements. See text for details. Glenoid shaded, cartilage stippled, scapula yellow, procoracoid red, metacoracoid blue. For sources of images please see Fig. 4. Not to scale.
Within Synapsida, the various outgroups leading to Theria (e.g. †Dimetrodon, monotremes) retain the three-part primary girdle. Among therians, phenotypic expression of both the procoracoid and the metacoracoid becomes dramatically diminished. This reduction reflects a decrease in preskeletogenic cell proliferation. The procoracoid rudiment detaches from the coracoid–scapular plate and either forms a vestigial element (the praeclavium of marsupials) or becomes subsumed into the manubrium sterni (eutherians). By contrast, the therian metacoracoid returns to the primitive condition of remaining joined with the scapula, giving rise to the coracoid process, acromion and probably contributes to the glenoid. At least among some eutherians, different genes appear to play roles in patterning the metacoracoid rudiment (e.g. Pax1, Hoxc6) compared with the scapula condensation (Emx2), consistent with the aforementioned homology argument.
Among reptiles, most skeletally mature individuals demonstrate only a single coracoid element, herein considered to be the metacoracoid. The procoracoid is not lost per se, but is retained as a rudiment that does not detach from the scapular portion of the coracoid–scapular plate. Retention of the combined scapula + procoracoid is in part achieved by a reduction in the duration of preskeletogenic cell mitosis (resulting in a decrease in size of the presumptive procoracoid) and an acceleration of osteogenesis (resulting in fusion of the procoracoid and scapula). Observations gleaned from experimental work on turtles and avians suggest that the reptilian scapula is a derivative of both the somites (forming the blade) and lateral plate mesoderm (forming the region around the glenoid).
Unique to avians the procoracoid rudiment may subdivide to join with both the metacoracoid (as the coracoid process) and possibly the scapula (at the glenoid). This partitioning of the procoracoid reflects either a further delay in osteogenesis or a delay in aggregation of the condensation or both. As in eutherians there is evidence to imply a patterning role of the procoracoid and metacoracoid components by Pax1 and Hoxc6. In the absence of data it remains unclear if crocodylians and most lepidosauromorphs retain the procoracoid rudiment (integrated into the scapula) or if this component is lost (i.e. a failure of the presumptive skeletogenic cells to proliferate and/or differentiate).
Future directions
Whereas the newly conceived scenario of amniote pectoral evolution is well supported, important data are lacking in several key areas. As demonstrated, a comprehensive understanding of development, in particular the role of cell condensations, forms the critical underpinning to understanding the evolution of morphologically complex structures. Regrettably, our knowledge of the membranous skeleton in many reptilian taxa, especially lepidosauromorphs and crocodylians, remains incomplete. Another important area for consideration is the use of experimental embryology. With the exception of turtles, most non-avian reptiles are infrequent experimental subjects. Even among avians, the diversity of taxa commonly employed is overwhelmingly dominated by a single species, Gallus gallus. This is despite previous (and in the case of turtles, ongoing) successes with the use of tissue grafts and culturing, gene expression and extirpation experiments, and chimeric surgeries in various non-model taxa (Gans, 1985 and references therein; see also Burke, 1991b; Vincent et al. 2003; Nagashima et al. 2005). Increased application of such experimental methods holds great promise as a virtually untapped resource of developmental and evolutionary data.
Acknowledgments
We would like to express their sincerest thanks to Tim Fedak (http://www.figs.ca and Dalhousie University) for his excellent work on all 16 figures. T. Fedak and Dr A. P. Russell (University of Calgary) reviewed early drafts of this work and their input is greatly appreciated. Special thanks to Dr S. S. Sumida (California State University, San Bernardino) and an anonymous reviewer for their useful comments that significantly enhanced the text. This work was supported by an NSERC operating grant to B.K.H.
References
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Belon P. L'Histoire de la Nature Des Oyseaux. Paris: Guillaume Cavellat; 1555. [Google Scholar]
- Beresford B. Brachial muscles in the chick embryo: the fate of individual somites. J Embryol Exp Morph. 1983;77:99–116. [PubMed] [Google Scholar]
- de Braga M, Rieppel O. Reptile phylogeny and the interrelationships of turtles. Zoological Journal of the Linnean Society. 1997;120:281–354. [Google Scholar]
- deBraga M. The postcranial skeleton, phylogenetic position, and probable lifestyle of the Early Triassic reptile Procolophon trigoniceps. Can J Earth Sci. 2003;40:527–556. [Google Scholar]
- Brochu CA. Progress and future directions in archosaur phylogenetics. J Paleontology. 2001;75:1185–1201. [Google Scholar]
- Broom R. On the development and morphology of the marsupial shoulder-girdle. Trans Royal Soc Edinburgh. 1899;39:749–770. [Google Scholar]
- Broom R. Note on the lacertilian shoulder girdle. Trans South African Philosoph Soc. 1906a;16:373–375. [Google Scholar]
- Broom R. On the early development of the appendicular skeleton of the ostrich, with remarks on the origin of birds. Trans South African Philosoph Soc. 1906b;16:355–368. [Google Scholar]
- Broom R. The morphology of the coracoid. Anat Anz. 1912;41:625–631. [Google Scholar]
- Burke AC. The development and evolution of the turtle body plan: Inferring intrinsic aspects of the evolutionary process from experimental process from experimental embryology. Am Zool. 1991a;31:616–627. [Google Scholar]
- Burke AC. Proximal elements in the vertebrate limb: Evolution and developmental origin of the pectoral girdle. In: Hinchliffe JR, Hurle J, Summerbell D, editors. Developmental Patterning of the Vertebrate Limb. Vol. 205. London: Plenum Press; 1991b. pp. 385–394. [Google Scholar]
- Camp CL. Classification of the lizards. Bull Am Museum Natural History. 1923;48:289–482. [Google Scholar]
- Case EC, Williston SW. A description of certain collections of bones referred to Sphenacodon Marsh. Carnegie Inst Washington Publ. 1913;181:61–70. [Google Scholar]
- Chevallier A. Role du mesoderme somitique dans le developpwment de la cage thoracique de l'embryon d'oiseau. I. Origine du segment sternal et mecanismes de la differenciation des cotes. J Embryol Exp Morph. 1975;33:291–311. [PubMed] [Google Scholar]
- Chevallier A. Origine des ceintures scapulaires et pelviennes chez l'embryon d'oiseau. J Embryol Exp Morph. 1977;42:275–292. [Google Scholar]
- Chiappe LM, Shu'an J, Qiang J, Norell MA. Anatomy and systematics of the Confusciusornithidae (Theropoda: Aves) from the Late Mesozoic of Northeastern China. Bull Am Museum Natural History. 1999;242:1–89. [Google Scholar]
- Clack JA. Gaining Ground. Bloomington: Indiana University Press; 2002. [Google Scholar]
- Cohn MJ, Tickle C. Developmental basis of limblessness and axial patterning in snakes. Nature. 1999;399:474–479. doi: 10.1038/20944. [DOI] [PubMed] [Google Scholar]
- Conrad JL. Postcranial skeleton of Shinisaurus crocodilurus (Squamata: Anguimorpha) J Morphol. 2006 doi: 10.1002/jmor.10291. in press. [DOI] [PubMed] [Google Scholar]
- Cope ED. On degenerate types of scapular and pelvic arch in the Lacertilia. J Morphol. 1892;7:223–244. [Google Scholar]
- Dietrich S, Gruss P. Undulated phenotypes suggest a role for Pax1 in the development of vertebral and extravertebral structures. Dev Biol. 1995;167:529–548. doi: 10.1006/dbio.1995.1047. [DOI] [PubMed] [Google Scholar]
- Dollé P, Dierich A, LeMeur M, et al. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell. 1993;75:431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- Eames BF, de la Fuente L, Helms JA. Molecular ontogeny of the skeleton. Birth Defects Res (Part C) 2003;69:93–101. doi: 10.1002/bdrc.10016. [DOI] [PubMed] [Google Scholar]
- Ede DA, Kelly WA. Developmental abnormalities in the trunk and limbs of the talpid3 mutant of the fowl. J Embryol Exp Morph. 1964;12:339–356. [PubMed] [Google Scholar]
- Elzanowski A. Ontogeny and evolution of the ratites. In: Ouellet H, editor. Acta XIX Congressus Internationalis Ornithologici. II. Ottawa: University of Ottawa Press; 1988. pp. 2037–2046. [Google Scholar]
- Elzanowski A. Archaeopterygidae. In: Chiappe LM, Witmer LM, editors. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley: University of California Press; 2002. pp. 129–159. [Google Scholar]
- Flower WH. An Introduction to the Osteology of the Mammalia: Being the Substance of the Course of Lectures Delivered at the Royal College of Surgeons of England in 1870. London: MacMillan; 1876. [Google Scholar]
- Gaffney ES. The comparative osteology of the Triassic turtle Proganochelys. Bull Am Museum Natural History. 1990;194:1–263. [Google Scholar]
- Gans C. Biology of the Reptilia, Development A. New York: Academic Press; 1985. [Google Scholar]
- Gilbert SF, Loredo GA, Brukman A, Burke AC. Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev. 2001;3:47–58. doi: 10.1046/j.1525-142x.2001.003002047.x. [DOI] [PubMed] [Google Scholar]
- Glutz von Blotzheim U. Zur Morphologie und Ontogenese von Schultergurtel, Sternum und Becken von Struthio, Rhea, und Dromiceius. Rev Suisse Zool. 1958;65:609–772. [Google Scholar]
- Gow CE. The osteology and relationships of the Millerettidae (Reptilia: Cotylosauria) J Zool. 1972;167:219–264. [Google Scholar]
- Gregory WK, Camp CL. Studies in comparative myology and osteology, Part IV. Second note on the evolution of the coracoid elements in reptiles and mammals. Bull Am Museum Natural History. 1918;38:545–552. [Google Scholar]
- Grossman M, Sanchez-Villagra MR, Maier W. On the development of the shoulder girdle in Crocidura russula (Soricidae) and other placental mammals: evolutionary and functional aspects. J Anat. 2002;201:371–381. doi: 10.1046/j.0021-8782.2002.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüneberg H. The Pathology of Development: a Study of Inherited Skeletal Disorders in Animals. Oxford: Blackwell; 1963. [Google Scholar]
- Hall BK. Cellular differentiation in skeletal tissues. Biol Rev. 1970;45:455–484. doi: 10.1111/j.1469-185x.1970.tb01174.x. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Hall BK. Development of the clavicles in birds and mammals. J Exp Zool. 2001;289:153–161. doi: 10.1002/1097-010x(20010215)289:3<153::aid-jez1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hamilton HL. Lillie's Development of the Chick; An Introduction to Embryology. New York: Holt, Rinehart and Winston; 1965. [Google Scholar]
- Hanson FB. The ontogeny and phylogeny of the sternum. Am J Anat. 1919;26:41–115. [Google Scholar]
- Hanson FB. The problem of the coracoid. Anat Rec. 1920;19:327–345. [Google Scholar]
- Hofmann C, Drossopoulou G, McMahon A, Balling R, Tickle C. Inhibitory action of BMPs on Pax1 expression and on shoulder girdle formation during limb development. Dev Dynamics. 1998;213:199–206. doi: 10.1002/(SICI)1097-0177(199810)213:2<199::AID-AJA5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Holmes R. The osteology and musculature of the pectoral limb of small captorhinids. J Morphol. 1977;152:101–140. doi: 10.1002/jmor.1051520107. [DOI] [PubMed] [Google Scholar]
- Howes GB. The morphology of the mammalian coracoid. J Anat Physiol. 1887;21:190–198. [PMC free article] [PubMed] [Google Scholar]
- Howes GB. On the coracoid of the terrestrial Vertebrata. Proc Zool Soc Lond. 1893;1893:585–592. [Google Scholar]
- Huang R, Zhi Q, Patel K, Wilting J, Christ B. Dual origin and segmental organisation of the avian scapula. Development. 2000;127:3789–3794. doi: 10.1242/dev.127.17.3789. [DOI] [PubMed] [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates. New York: Academic Press; 1980. [Google Scholar]
- Jenkins FA, Jr, Parrington FR. The postcranial skeletons of the Triassic mammals Eozostrodon, Megazostrodon and Erythrotherium. Phil Trans Royal Soc Ser B, Biol Sci. 1976;273:387–431. doi: 10.1098/rstb.1976.0022. [DOI] [PubMed] [Google Scholar]
- Jenkins FA., Jr The evolution of the avian shoulder joint. Am J Sci. 1993;293-A:253–267. [Google Scholar]
- Kearney M. The appendicular skeleton in amphisbaenians. Copeia. 2002;2002:719–738. [Google Scholar]
- Klima M. Early development of the human sternum and the problem of homologization of the so-called suprasternal structures. Acta Anat. 1968;69:473–484. doi: 10.1159/000143096. [DOI] [PubMed] [Google Scholar]
- Klima M. Ossa suprasternalia der Primaten und die Spezialanpassungen des Manubrium sterni bei den Brullaffen (Alouatta) Folia Primatologia. 1972;17:421–433. doi: 10.1159/000155459. [DOI] [PubMed] [Google Scholar]
- Klima M. Die Fruhentwicklung des Schultergurtels und des Brustbeins bei den Monotremen (Mammalia: Prototheria) Adv Anat, Embryol Cell Biol. 1973;47:1–80. [Google Scholar]
- Klima M. Comparison of the early development of the sternum and clavicle in striped dolphin and in humpback whale. Scientific Reports Whales Res Instit, Tokyo. 1978;30:253–269. [Google Scholar]
- Klima M. Development of the shoulder girdle and sternum in mammals. In: Duncker H-R, Fleischer G, editors. Functional Morphology in Vertebrates; Proceedings of the 1st International Symposium on Vertebrate Morphology. New York: Gustav Fischer; 1985. pp. 81–83. [Google Scholar]
- Klima M. Early development of the shoulder girdle and sternum in marsupials (Mammalia: Metatheria) Adv Anat, Embryol Cell Biol. 1987;109:1–91. doi: 10.1007/978-3-642-72994-2. [DOI] [PubMed] [Google Scholar]
- Klima M. Rudiments of the clavicle in the embryos of whales (Cetacea) Z Saugetierkunde. 1990;55:202–212. [Google Scholar]
- Korringa P. Uber den Schultergurtel der Schildkroten. Anat Anz. 1938;86:259–268. [Google Scholar]
- Lécuru S. Remarques sur le scapulo-coracoide des lacertiliens. Annales Sci Naturelles, Zool. 1968;10:475–510. [Google Scholar]
- Lee MSY. The homologies and early evolution of the shoulder girdle in turtles. Proc Royal Soc Lond Ser B. 1996;263:111–117. [Google Scholar]
- Lee MSY. Pareiasaur phylogeny and the origin of turtles. Zool J Linnean Soc. 1997;120:197–280. [Google Scholar]
- Lindsay B. On the avian sternum. Proc Zool Soc L. 1885;53:684–716. [Google Scholar]
- Luo Z-X, Kielan-Jaworowska Z, Cifelli RL. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontol Polonica. 2002;47:1–78. [Google Scholar]
- Lydekker R. Note on the coracoidal element in adult sloths, with remarks on its homology. Proc Zool Soc Lond. 1893;1893:172–174. [Google Scholar]
- McGonnell IM. The evolution of the pectoral girdle. J Anat. 2001;199:189–194. doi: 10.1046/j.1469-7580.2001.19910189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto SP. Observations on the structure of the Early Permian reptile Stereosternum tumidum Cope. Palaeontol Africana. 1999;35:7–19. [Google Scholar]
- Modesto SP, Anderson JS. The phylogenetic definition of Reptilia. Syst Biol. 2004;53:815–821. doi: 10.1080/10635150490503026. [DOI] [PubMed] [Google Scholar]
- Mook CC. Notes on the postcranial skeleton in the Crocodilia. Bull Am Mus Nat His. 1921;44:67–100. [Google Scholar]
- Montero R, Gans C, Lions ML. Embryonic development of the skeleton of Amphisbaenia darwini heterozontata (Squamata: Amphisbaenidae) J Morphol. 1999;239:1–25. doi: 10.1002/(SICI)1097-4687(199901)239:1<1::AID-JMOR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Müller J. Osteology and relationships of Eolacerta robusta, a lizard from the Middle Eocene of Germany (Reptilia, Squamata) J Vertebrate Paleontol. 2001;21:261–278. [Google Scholar]
- Nagashima H, Uchida K, Yamamoto K, Kuraku S, Usuda R, Kuratani S. Turtle–chicken chimera: an experimental approach to understanding evolutionary innovation in the turtle. Dev Dynamics. 2005;232:149–161. doi: 10.1002/dvdy.20235. [DOI] [PubMed] [Google Scholar]
- Newton A, Gadow H. A Dictionary of Birds. London: Black; 1893–1896. [Google Scholar]
- Nicholls EL, Russell AP. The plesiosaur pectoral girdle: the case for a sternum. NJ Geol Palaontol Abhd. 1991;182:161–185. [Google Scholar]
- Oliver G, DeRoberts EM, Wolpert L, Tickle C. Expression of a homeobox gene in the chick wing bud following application of retinoic acid and grafts of polarizing region tissue. EMBO J. 1990;9:3093–3099. doi: 10.1002/j.1460-2075.1990.tb07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn HF. A complete mosasaur skeleton, osseous and cartilaginous. Mem Am Museum Natural History. 1899;1:167–188. doi: 10.1126/science.10.260.919. [DOI] [PubMed] [Google Scholar]
- Ostrom JH. Some hypothetical anatomical stages in the evolution of avian flight. Smithson Contrib Paleobiol. 1976;27:1–21. [Google Scholar]
- Parker WK. A monograph on the structure and development of the shoulder-girdle and sternum in the Vertebrata. The Ray Society; 1868. [Google Scholar]
- Pellegrini M, Pantano S, Fumi MP, Lucchini F, Forabosco A. Agenesis of the scapula in Emx2 homozygous mutants. Dev Biol. 2001;232:149–156. doi: 10.1006/dbio.2001.0159. [DOI] [PubMed] [Google Scholar]
- Rieppel O. Studies on skeleton formation in reptiles: patterns of ossification in the skeleton of Chelydra serpentina (Reptilia, Testudines) J Zool. 1993;231:487–509. [Google Scholar]
- Rieppel O. Testing the homology by congruence: the pectoral girdle of turtles. Proc R Soc Lond Ser B, Biol Sci. 1996;263:1395–1398. doi: 10.1098/rspb.1996.0204. [DOI] [PubMed] [Google Scholar]
- Rieppel O, deBraga M. Turtles as diapsids. Nature. 1996;384:453–455. [Google Scholar]
- Rieppel O, Reisz R. The origin and early evolution of turtles. Annu Rev Ecol Syst. 1999;30:1–22. [Google Scholar]
- Romanoff AL. The Avian Embryo; Structural and Functional Development. New York: MacMillan Co; 1960. [Google Scholar]
- Romer AS. The comparison of mammalian and reptilian coracoids. Anat Rec. 1922;24:39–47. [Google Scholar]
- Romer AS. Osteology of the Reptiles. Chicago: University of Chicago Press; 1956. [Google Scholar]
- Romer AS, Price LW. Review of the Pelycosauria. Geol Soc Am Special Papers. 1940;28:1–538. [Google Scholar]
- Ruckes H. Studies in chelonian osteology, part II; the morphological relationships between the girdles, ribs and carapace. Ann New York Acad Sci. 1929;31:81–120. [Google Scholar]
- Ruta M, Coates M, Quicke DLJ. Early tetrapod relationships revisited. Biol Rev. 2003;78:251–345. doi: 10.1017/s1464793102006103. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villagra MR, Maier W. Ontogenetic data and the evolutionary origin of the mammalian scapula. Naturwissenschaften. 2002;89:459–461. doi: 10.1007/s00114-002-0362-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villagra MR, Maier W. Ontogenesis of the scapula in marsupial mammals, with special emphasis on perinatal stages of didelphids and remarks on the origin of the therian scapula. J Morphol. 2003;258:115–129. doi: 10.1002/jmor.10096. [DOI] [PubMed] [Google Scholar]
- Sheil CA. Osteology and skeletal development of Apalone spinifera (Reptilia: Testudines: Trionychidae) J Morphol. 2003;256:42–78. doi: 10.1002/jmor.10074. [DOI] [PubMed] [Google Scholar]
- Sheil CA. Skeletal development of Macrochelys temminckii (Reptilia: Testudines: Chelydridae) J Morphol. 2005;263:71–106. doi: 10.1002/jmor.10290. [DOI] [PubMed] [Google Scholar]
- Skinner JH. Ontogeny of the breast-shoulder apparatus of the South African lacertilian, Microsauria pumila pumila (Daudin) Ann Univ Stellenbosch. 1959;35:5–66. [Google Scholar]
- Soriano P. The PDGFalpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- Stokely PS. Limblessness and correlated changes in the girdles of a comparative morphological series of lizards. Am Natur. 1947;38:725–754. [Google Scholar]
- Stone JR, Hall BK. Latent homologues for the neural crest as an evolutionary novelty. Evol Dev. 2004;6:123–129. doi: 10.1111/j.1525-142x.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- Sumida SS. The appendicular skeleton of the early Permian genus Labidosaurus (Reptilia, Captorhinomorpha, Captorhinidae) and the hind limb musculature of captorhinid reptiles. J Vertebrate Paleontol. 1989;9:295–313. [Google Scholar]
- Timmons PM, Wallin J, Rigby PWJ, Balling R. Expression and function of Pax1 during development of the pectoral girdle. Development. 1994;120:2773–2785. doi: 10.1242/dev.120.10.2773. [DOI] [PubMed] [Google Scholar]
- Vaughn PP. The Permian reptile Araeoscelis restudied. Bull Mus Compar Zool. 1955;113:305–467. [Google Scholar]
- Vincent C, Bontoux M, Le Douarin NM, Pieau C, Monsoro-Burq A-N. Msx genes are expressed in the carapacial ridge of turtle shell: a study of the European pond turtle, Emys orbicularis. Dev Genes Evol. 2003;213:464–469. doi: 10.1007/s00427-003-0347-3. [DOI] [PubMed] [Google Scholar]
- Walker WF. The development of the shoulder region in the turtle, Chrysemys picta marginata, with special reference to the primary musculature. J Morphol. 1947;80:195–249. doi: 10.1002/jmor.1050800204. [DOI] [PubMed] [Google Scholar]
- Walker WF. The locomotor apparatus of Testudines. In: Gans C, Parsons TS, editors. Biology of the Reptilia, Morphology D. Vol. 4. New York: Academic Press; 1973. pp. 1–100. [Google Scholar]
- Watson DMS. The evolution of the tetrapod shoulder-girdle and forelimb. J Anat. 1917;52:1–63. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M, Thorley J, Benton MJ. Uncertain turtle relationships. Nature. 1997;387:466. [Google Scholar]
- Williams MS. Developmental anomalies of the scapula – the ‘omo'st forgotten bone. Am J Med Genet. 2003;120A:583–587. doi: 10.1002/ajmg.a.20091. [DOI] [PubMed] [Google Scholar]
- Williston SW. American Permian Vertebrates. Chicago: University of Chicago Press; 1911. [Google Scholar]
- Williston SW. The Osteology of the Reptiles. Cambridge, MA: Harvard University Press; 1925. [Google Scholar]
- Wyneken J. The Anatomy of Sea Turtles. 2001. pp. 1–172. US Department of Commerce NOAA Technical Memorandum NMFS-SEFSC-470.
- Zangerl R. Contributions to the osteology of the post-cranial skeleton of the Amphisbaenidae. Am Midland Natur. 1945;33:764–780. [Google Scholar]