Abstract
Fluorochrome sequential labelling of mineralizing tissues is commonly used in different fields of clinical and basic research. Recently we improved polychrome fluorescent sequential labelling of bone by applying spectral image analysis to discriminate seven different fluorochromes. Although basic mineralization processes of bone and teeth follow comparable principles, the respective tissues differ in terms of matrix composition and mineral assembly. The aim of this study therefore was to investigate the feasibility of this new technique for polychrome sequential labelling of teeth and to demonstrate the advantages in the field of dentistry. Furthermore, the exact labelled area of each fluorochrome could be measured, even in regions of overlapping fluorochromes. The technique presented may provide a basis for further investigations of mineralization processes of different anatomical dental structures.
Keywords: bone, fluorochrome, polychrome labelling, sequential labelling, spectral image acquisition, teeth
Introduction
The technique of fluorochrome sequential labelling using calcium-binding fluorescent dyes is widely used for addressing different questions concerning the process of mineralization. In teeth it is used to investigate biological rhythms (Smith, 2006), rates of mineralization (Dean, 2000), chronological development and individual age (Smith et al. 2006), or pulpal response on external influences (Springer et al. 2005). The general limitation of this technique, however, lies in the problematic demarcation of overlapping colours as well as in the distinction of more than four different fluorochromes (Harris, 1960; Roldan et al. 2004). In a recent study we were able to overcome several limitations in polychrome sequential labelling of bone by applying spectral image analysis. More detailed information regarding bone accretion could be obtained and a higher number of different fluorochromes could be used in a shorter time interval application scheme as a result of superior colour discrimination (Pautke et al. 2005). Although the mineralization processes and matrix formation of teeth and bone are comparable, these tissues differ in many aspects.
Unlike bone, teeth are composed of three different mineralizing tissues: enamel, dentine and cementum. Whereas enamel formation is controlled by ameloblasts, which are specialized epithelial cells from the ectoderm, dentin and cementum are formed by neural crest-derived ectomesenchymal cells known as odontoblasts and cementoblasts, respectively (Dean, 2000; McCollum & Sharpe, 2001). Owing to its high mineral content, the deposited enamel is the hardest tissue in the body. Cementum and in particular dentine resemble bone in terms of hardness and mineral content, but the molecular composition as well as the protein and apatite assembly of bone and dentine differ significantly (Butler et al. 2002; Smith, 2006). Furthermore, dentine and enamel do not contain cells but only cell processes of the odontoblasts. Consequently, the remodelling of these tissues is not comparable with that of bone and minor mineralization disturbances, such as the application of fluorochromes, lead to structural irregularities in the acellular dental structures that are detectable even years later (Smith, 2006). This is not the case in bone because of its continuous remodelling. Given the above, insights into sequential bone labelling cannot simply be correlated to the labelling of teeth. Therefore, in this technical approach, the first aim was to investigate the feasibility and use of polychrome labelling in combination with spectral image analysis for different dental structures. The second aim was to improve the accuracy of the investigation of mineralization processes to define better the influences of external factors – such as the application of fluorochromes – on mineralization disturbances in particular on dentine.
Materials and methods
Fluorochrome application
Eight different fluorochromes were administered sequentially in 12-week-old male Wistar rats (∼450 g body weight) by subcutaneous injection (3-day intervals between each injection): calcein blue, xylenol orange, calcein, alizarine complexone, doxycycline, rolitetracycline, hematoporphyrin and BAPTA [1,2-Bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid]. The animals exhibited a fully developed dentition (dental formula: one incisor and three molars).
All fluorochromes were purchased from Sigma-Aldrich (Munich, Germany). Details of the fluorochromes are given in Table 1. Before use, all fluorochromes were adjusted to pH values of 7.2 and sterilized by filtration.
Table 1.
Details of fluorochromes including source and dose based on bodyweight
| Fluorochrome | Specification (Sigma-Aldrich, Munich, Germany) | Dose [sc (mg kg−1 bw)] |
|---|---|---|
| Calcein blue | M1255 | 30 |
| Xylenolorange | 227854 | 90 |
| Calcein | C0875 | 15 |
| Alizarin complexone | A3882 | 30 |
| Doxycycline | D9891 | 50 |
| Rolitetracycline | R2253 | 25 |
| Hematoporphyrin | H5518 | 300 |
| BAPTA | A4926 | 75 |
Three days after the last fluorochrome injection rats were killed with an intraperitoneal injection of an overdose of pentobarbitone sodium. The mandible was then exarticulated and fixed in methanol for 4 days at 4 °C, kept in acetone for 2 days and then block-embedded in methylmethacrylate over 12 days. Specimens were cut into 100-µm-thick sections in the sagittal direction using a saw microtome (Leitz, Wetzlar, Germany). For histological orientation sections were additionally stained with haematoxylin–eosin. The region of the open-rooted lower incisor was investigated by fluorescence analysis, because both dentine and enamel are continuously deposited.
Fluorescence microscopy and spectral image analysis
Fluorescence analysis was performed using a microscope (Axiophot 2, Zeiss, Germany) with an XBO 75 xenon short-arc lamp in combination with appropriate fluorescence filter sets: a long-pass emission filter (filter no. 01, Zeiss), a filter for red (no. 15, Zeiss) and green fluorescence (no. 09), and a triple-band pass filter set for green, red and infrared [SKY, Applied Spectral Images (ASI), Israel]. For image acquisition a Sagnac-type interferometer (SpectralCube SD-200, ASI) was used as well as a conventional digital camera with a fluorescence mode as control (Cybershot DSC S 75, Sony, Japan) under identical conditions from identical bone regions.
Details of the principle of the Sagnac-type interferometer have been published elsewhere (Schieker et al. 2004; Pautke et al. 2005). Briefly, the fluorescence emission spectra are split in the interferometer by a dichromatic filter in different directions and recombined by reflecting mirrors at the exit. As a result the different spectra reveal an optical path difference (OPD), which is caused by the different optical pathways for non-zero angles. The visible spectral region is analysed by applying the Fourier technique and by synchronizing the recording of successive charge-coupled device (CCD) frames with the steps of the motor used to rotate the collimated beam, so that the instantaneous OPD is known for every pixel in every recorded frame. By this technique wavelength ranges of 10 nm and less can be distinguished (Malik et al. 1996). SpectraView software (ASI) enabled image analysis with linear unmixing based on decomposition of the image in its pure spectral components. The data acquisition time for each spectral image was dependent on the field of view and ranged between 120 and 150 s.
Results
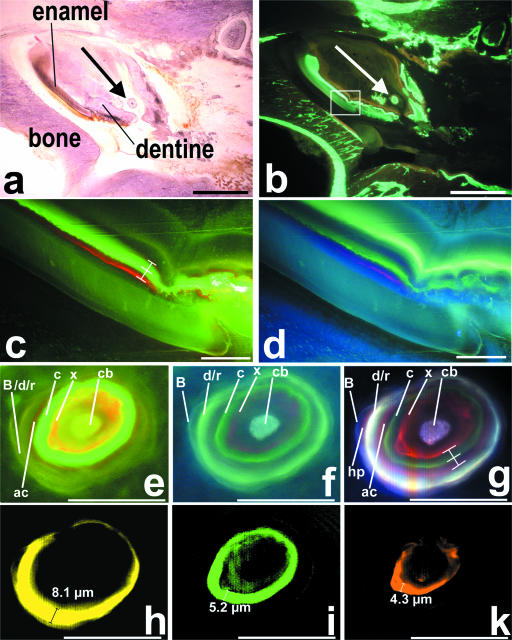
In teeth dentine in particular exhibited a strong fluorescence signal whereas enamel revealed only weak fluorescent signal (Fig. 1c,d). In the region of the investigated open-rooted lower incisor no cementum deposition was detected. In dentine spectral image analysis enabled demarcation of seven of the eight applied fluorochromes: calcein blue, xylenol orange, calcein, alizarine complexone, doxycycline/rolitetracycline, hematoporphyrin and BAPTA (Fig. 1g). Single depiction of each of these seven fluorochromes was feasible after linear unmixing. Using this procedure the width of the fluorescent bands could be measured exactly. We found that the fluorescent bands of the different fluorochromes were of different thickness, although each fluorochrome was given as a single application in identical time intervals. For example, the doxycycline/rolitetracycline band was 8.1 µm, the calcein band 5.2 µm and the xylenol orange band 4.3 µm thick (Fig. 1h,I,k).
Fig. 1.
Region of rat mandibular bone with a lower right incisor. (a) Haematoxylin–eosin staining and (b) fluorescence image using a filter set for green and red fluorescence (SKY, ASI, Israel) (scale bar = 1 mm). The area marked in the rectangle is magnified in (c) and (d). Large arrows mark a calcifying dentine microstructure (denticle) magnified in panels (e)–(k). (c,d) Conventional fluorescence images showing unlabelled enamel and the sequentially labelled dentine using (c) a triple band filter (SKY, ASI) and (d) a long-pass emission filter (no. 01, Zeiss). (e,f) A calcifying dentine microstructure recorded by conventional fluorescence imaging using the same filter sets as in (c) and (d). (g) Identical area recorded by spectral image acquisition using the long-pass emission filter. Note that seven different fluorescent bands are visible simultaneously. In contrast to the conventional images, the hematoporphyrin band was clearly visible by spectral image acquisition. (h–k) Linear unmixing enabled single depiction of each fluorochrome, allowing exact quantification of the labelled area: (h) doxycycline/rolitetracycline (note that the width of the band is caused by two fluorochromes that were not distinguishable), (i) calcein and (k) xylenol orange. Abbreviations: cb, calcein blue; x, xylenol orange; c, calcein; ac, alizarine complexone; d/r, doxycycline/rolitetracycline; hp, hematoporphyrin; B, BAPTA. Scale bars in (c)–(k) = 50 µm.
In different areas of the investigated specimens, the distances between the fluorescent bands were different, but the relative values were constant (Fig. 1c,g). Distances between the xylenol orange and the calcein band or between the calcein and the alizarin complexone band in the displayed region in Fig. 1(c) were 10.7 vs. 9.7 µm (ratio 1.1). The respective values in Fig. 1(f) were 5.4 vs. 4.9 µm (ratio 1.1).
Spectral image acquisition resulted in a higher sensitivity compared with conventional digital images: under identical conditions the weak fluorescent signal of hematoporphyrin in dentine was hardly visible in conventional images but reproducibly detectable using spectral image acquisition (Fig. 1e–g). In addition, single representation of similar colours such as red and orange or yellow and green was not possible by conventional image acquisition even if different filter sets were used (Fig. 1e,f).
Discussion
Hard tissue mineralization processes can be visualized and analysed by the technique of polychrome sequential labelling. By applying calcium-chelating fluorescent dyes, the area of active mineralization is labelled (Frost et al. 1961; Jee, 2005; Smith, 2006). This technique, however, is limited in terms of colour discrimination using conventional image analysis and different filter sets. Consequently, the investigation of microstructures in particular is restricted in areas of overlapping fluorochromes, because different colours cannot be unmixed or depicted without interference. This is a crucial drawback of this method. The sophisticated technique of spectral image analysis contributes greatly to the investigation of mineralizing tissues and the advantages for the sequential labelling of bone have recently been presented (Pautke et al. 2005). In this study, we demonstrate the improvements for the analysis of different dental structures.
In rodents, odontogenesis is different to that in humans as enamel formation in human teeth is terminated after tooth eruption. By contrast, in rats, enamel formation takes place continuously and over the life of the animal in the regions of the open-rooted incisors even after the dentition is completed approximately after 6 weeks. This region was therefore investigated in particular. The weak fluorescence of enamel with signs of autofluorescence observed is in agreement with the findings of other studies (Rahn & Perren, 1972; Smith, 2006). This phenomenon is due to the fact that apatite deposition and assembly are different to that of dentine, so that the increment lines of enamel cannot be properly visualized by sequential fluorescence labelling (Smith, 2006). Unlike enamel, dentine showed strong fluorescence bands.
The mineralization of teeth is altered by different factors such as biological rhythms (Smith, 2006), age (Smith et al. 2006), and external influences like caries or trauma (Springer et al. 2005), hormonal (Kitahara et al. 2002) or vitamin changes (Sakamoto & Takano, 2005). The mineralization of teeth is affected more than bone because bone undergoes permanent remodelling. Consequently, fluorochromes may lead to a permanent discoloration of teeth when administered too early in children. This has to be considered in particular for tetracycline derivates, which are the only fluorochromes approved for human application (Sanchez et al. 2004).
The extent of labelling of the mineralizing region is dependent, among other factors, on the fluorochromes applied (Sun et al. 1992; Smith, 2006) in particular on the fluorochrome metabolization, calcium affinity and half-life in vivo. Fluorochromes that are eliminated more slowly result in a broader fluorescent band, because they are incorporated over a longer time period. Fluorochromes exhibiting a high calcium affinity label a broader fluorescent band. These effects could be shown for calcein, which exhibited the broadest fluorescent band despite the lowest application dose. The binding of calcium by the fluorochromes inhibits the mineralization processes. This is demonstrated in dentine, where the width of the following fluorescent band after a fluorochrome with a high calcium affinity, such as calcein, is reduced.
However, using conventional techniques, e.g. different fluorescent filter sets, these effects cannot be investigated, because no colour discrimination and single depiction of similar colours such as yellow, green or orange is clearly possible.
The superior sensitivity of spectral image analysis in comparison with conventional fluorescence microscopy improved the analysis of mineralizing processes in different ways: the dose of the applied fluorochromes could be reduced (Pautke et al. 2005) so that the negative effects on the mineralization mentioned above may be minimized. Furthermore, toxic side-effects may be reduced; this is of particular interest with regard to the fluorochromes hematoporphyrin (Solheim, 1974) and alizarin complexone (Harris, 1960) in further animal studies. With increasing sensitivity, thinner tissue sections could be investigated and the time period for fluorescence analysis could be extended (Pautke et al. 2005).
Therefore, we conclude that the technique of polychrome sequential labelling in combination with spectral image analysis offers great advantages for the detailed investigation of teeth, in particular for the analysis of the mineralization of dental microstructures as well as influences of external factors such as fluorochromes.
References
- Butler WT, Brunn JC, Qin C, McKee MD. Extracellular matrix proteins and the dynamics of dentin formation. Connect Tissue Res. 2002;43:301–307. doi: 10.1080/03008200290000682. [DOI] [PubMed] [Google Scholar]
- Dean C. Progress in understanding hominoid dental development. J Anat. 2000;197:77–101. doi: 10.1046/j.1469-7580.2000.19710077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM, Villanueva AR, Roth H, Stanislavljevic S. Tetracycline bone labeling. J New Drugs. 1961;1:206–216. doi: 10.1177/009127006100100503. [DOI] [PubMed] [Google Scholar]
- Harris WH. A microscopic method of determining rates of bone growth. Nature. 1960;188:1039. doi: 10.1038/1881038a0. [DOI] [PubMed] [Google Scholar]
- Jee WS. The past, present, and future of bone morphometry: its contribution to an improved understanding of bone biology. J Bone Miner Metab. 2005;23(Suppl.):1–10. doi: 10.1007/BF03026316. [DOI] [PubMed] [Google Scholar]
- Kitahara Y, Suda N, Kuroda T, Beck F, Hammond VE, Takano Y. Disturbed tooth development in parathyroid hormone-related protein (PTHrP)-gene knockout mice. Bone. 2002;30:48–56. doi: 10.1016/s8756-3282(01)00669-x. [DOI] [PubMed] [Google Scholar]
- Malik Z, Cabib D, Buckwald RA, Talmi A, Garini Y, Lipson SG. Fourier transform multipixel spectroscopy for quantitative cytology. J Microsc. 1996;182:133–140. [Google Scholar]
- McCollum M, Sharpe PT. Evolution and development of teeth. J Anat. 2001;199:153–159. doi: 10.1046/j.1469-7580.2001.19910153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautke C, Vogt S, Tischer T, et al. Polychrome labeling of bone with seven different fluorochromes: enhancing fluorochrome discrimination by spectral image analysis. Bone. 2005;37:441–445. doi: 10.1016/j.bone.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Rahn BA, Perren SM. Alizarin complexon-fluorochrome for bone and dentine labeling. Experientia. 1972;28:180. doi: 10.1007/BF01935743. [DOI] [PubMed] [Google Scholar]
- Roldan JC, Jepsen S, Miller J, et al. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone. 2004;34:80–90. doi: 10.1016/j.bone.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Takano Y. Site-specific effect of ascorbic acid deficiency on the structure and function of odontoblasts in the teeth of osteogenic disorder rat in vivo. Tissue Cell. 2005;37:11–23. doi: 10.1016/j.tice.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Sanchez AR, Rogers RS, III, Sheridan PJ. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol. 2004;43:709–715. doi: 10.1111/j.1365-4632.2004.02108.x. [DOI] [PubMed] [Google Scholar]
- Schieker M, Pautke C, Reitz K, et al. The use of four-colour immunofluorescence techniques to identify mesenchymal stem cells. J Anat. 2004;204:133–139. doi: 10.1111/j.1469-7580.2004.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM. Experimental determination of the periodicity of incremental features in enamel. J Anat. 2006;208:99–113. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TM, Reid DJ, Sirianni JE. The accuracy of histological assessments of dental development and age at death. J Anat. 2006;208:125–138. doi: 10.1111/j.1469-7580.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim T. Pluricolor fluorescent labeling of mineralizing tissue. Scand J Dent Res. 1974;82:19–27. doi: 10.1111/j.1600-0722.1974.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Springer IN, Acil Y, Spies C, et al. RhBMP-7 improves survival and eruption in a growing tooth avulsion trauma model. Bone. 2005;37:570–577. doi: 10.1016/j.bone.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Sun TC, Mori S, Roper J, Brown C, Hooser T, Burr DB. Do different fluorochrome labels give equivalent histomorphometric information? Bone. 1992;13:443–446. doi: 10.1016/8756-3282(92)90088-e. [DOI] [PubMed] [Google Scholar]