Abstract
Fibrous extracellular matrix of tendon is considered to be an inextensible anatomical structure consisting of type I collagen fibrils arranged in parallel bundles. Under polarized light microscopy the collagen fibre bundles appear crimped with alternating dark and light transverse bands. This study describes the ultrastructure of the collagen fibrils in crimps of both relaxed and in vivo stretched rat Achilles tendon. Under polarized light microscopy crimps of relaxed Achilles tendons appear as isosceles or scalene triangles of different size. Tendon crimps observed via SEM and TEM show the single collagen fibrils that suddenly change their direction containing knots. The fibrils appear partially squeezed in the knots, bent on the same plane like bayonets, or twisted and bent. Moreover some of them lose their D-period, revealing their microfibrillar component. These particular aspects of collagen fibrils inside each tendon crimp have been termed ‘fibrillar crimps’ and may fulfil the same functional role. When tendon is physiologically stretched in vivo the tendon crimps decrease in number (46.7%) (P < 0.01) and appear more flattened with an increase in the crimp top angle (165° in stretched tendons vs. 148° in relaxed tendons, P < 0.005). Under SEM and TEM, the ‘fibrillar crimps’ are still present, never losing their structural identity in straightened collagen fibril bundles of stretched tendons even where tendon crimps are not detectable. These data suggest that the ‘fibrillar crimp’ may be the true structural component of the tendon crimp acting as a shock absorber during physiological stretching of Achilles tendon.
Keywords: crimp, SEM, stretching, TEM, tendon
Introduction
Tendon is mainly composed of a dense connective tissue basically consisting of type I collagen fibres containing collagen fibrils embedded in a non-collagenous hydrophilic matrix of proteoglycans, mainly biglycan and decorin, glycoprotein and glycosaminoglycans (Scott et al. 1981; Kannus, 2000; Derwin et al. 2001; Magnusson et al. 2003a; Scott, 2003). This anatomical structure may be considered inextensible and serves to transmit the forces generated by muscle contraction directly to the skeleton with a minimal dispersion of energy (Jozsa & Kannus, 1997; Screen et al. 2004). The collagen fibres are assembled in highly ordered parallel bundles to respond better to mechanical loading and are aligned along the long axis of the tendon. However, when observed via polarized light microscopy, the collagen fibres show a periodic waveform configuration (Viidik & Ekholm, 1968; Viidik, 1972), which has been widely investigated and is commonly known as a crimp (Diamant et al. 1972; Kastelic et al. 1980; Niven et al. 1982; Rowe, 1985a; Gathercole & Keller, 1991; Jozsa et al. 1991; De Campos Vidal, 1995, 2003; Viidik et al. 1996; Hansen et al. 2002).
Most studies on crimps in tendon have been carried out by light microscope analysis and the few ultrastructural reports have failed to explain the morphological change occurring in collagen fibrils of tendon crimp (Rowe, 1985a; 1985b; Gathercole & Keller, 1991; Magnusson et al. 2002; Hurschler et al. 2003).
Biomechanical studies investigating the physiological function of tendon crimps have demonstrated that they disappeared when tendons were slightly stretched (Elliott, 1965; Viidik & Ekholm, 1968; Viidik, 1972; Hess et al. 1989; Jozsa & Kannus, 1997; Hansen et al. 2002). Crimp disappearance was related to the alignment of crimped collagen fibres in the direction of loading (Diamant et al. 1972; Hansen et al. 2002). In stretched tendons crimps began to disappear progressively, i.e. individually rather than simultaneously, from the ends toward the centre of the collagen fascicle (Hansen et al. 2002). It was also suggested that the initial non-linear toe region of the stress–strain curve of the tendon may correspond to straightening of the crimps (Diamant et al. 1972; Atkinson et al. 1999). Moreover, local strains within the collagen fascicle are smaller than the externally applied strains, never exceeding 1.2% of axial strain, even at 8% gross applied strain (Screen et al. 2004).
However, most of these mechanical tests were carried out on tendons explanted from animals and the morphological studies investigated tendons fixed only after explantation (Magnusson et al. 2003a). Hence, these investigations did not consider the physiological tension of tendon in nature, even in the relaxed condition, being connected both to muscle and to bone.
Recently, Raspanti et al. (2005) demonstrated by scanning electron microscopy (SEM) and atomic force microscopy (AFM) that each collagen fibril at the top of the crimps bends with a variable but sharp angle and loses the D-band.
Starting from these recent ultrastructural results, we further investigated the histological and ultrastructural aspects of tendon crimps, and their qualitative and quantitative changes, in the mechanism of Achilles tendon stretching.
Materials and methods
Animals
Twelve female Sprague–Dawley rats (90 days old) were anaesthesized with an intraperitoneal injection of 87 mg kg−1 i.m. ketamine (Ketavet, Farmaceutici Gellini Spa, Italy) and 13 mg kg−1 i.m. xylazine (Rompun, Bayer Italia SPA, Italy). A resin brace, modified to induce foot dorsal flexion, was applied to one posterior leg and the foot was progressively flexed to a final 55° angle. The stretching position was maintained for 10 min. At the end of the stretching session, and still under anaesthesia, the tendon of the gastrocnemius muscle still under tension was surgically exposed, isolated and fixed in situ (still connected to the muscle–tendon junction and to the bone) in Karnovsky's solution (Fig. 1). The tendon of the contralateral leg of each animal was kept relaxed and fixed as with the stretched tendon to be used as a control sample. Finally, the rats were killed via an intracardiac injection of Tanax (Hoechst, Frankfurt am Main, Germany).
Fig. 1.
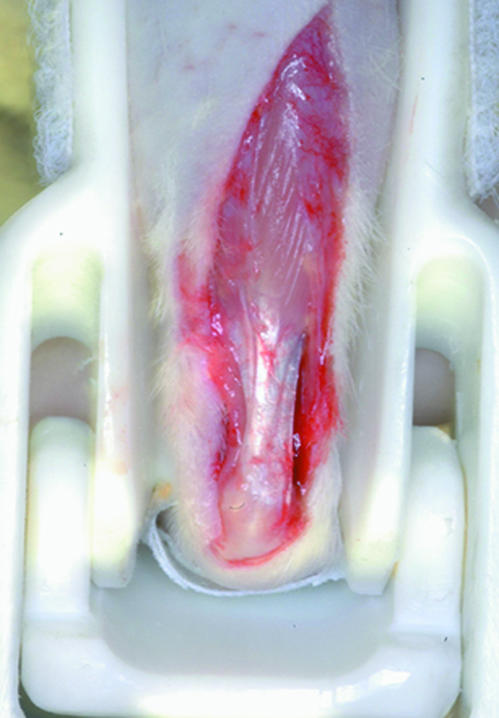
Resin brace used to induce foot dorsal flexion. Gastrocnemius muscle tendon, still under tension, is surgically exposed, isolated and fixed in situ.
All stretched or control tendons were excised. Ten tendons (five stretched and five control) were processed to be analysed under direct and polarized light microscopy. The other eight tendons (four stretched and four controls) were processed for transmission electron microscopy (TEM) and the last six (three stretched and three controls) were longitudinally cut to be analysed by SEM.
All the procedures were performed strictly following Italian and European Law on animal experimentation.
Light microscopy
Specimens were fixed in Karnovsky's solution, dehydrated in a graded series of alcohol, embedded in paraffin and longitudinally sectioned to obtain serial sections of 6 µm. The sections were then stained with eosin–haematoxylin to be observed via direct light microscopy (Leitz Orthoplan, Wetzlar, Germany) or stained with 5% Picrosirius Red to highlight collagen fibre structure and to improve its natural birefringence under the polarized light microscope (Leitz Ortholux 2, Wetzlar, Germany).
Scanning electron microscopy
Specimens for SEM were fixed in 1% Karnovsky's solution, dehydrated in a graded series of alcohol and then in hexamethyldisilazane. Finally they were mounted on metal stubs, coated with 5 nm gold in an Emitech K550 sputter-coater to be observed under SEM (Philips 515 and Philips XL30-FEG, Eindhoven, The Netherlands) operating in secondary-electron mode.
Transmission electron microscopy
Specimens for TEM were fixed in Karnovsky's solution, post-fixed in 1% osmium tetroxide, dehydrated through a graded series of alcohol and then embedded in Araldite resin. The ultrathin sections were stained with uranyl acetate and lead citrate and examined under TEM (Philips CM-10, Eindhoven, The Netherland).
Morphometric analysis
Tendon crimps were shown by rotating the sections between crossed polars in different orientations, which results in a changing pattern of alternating dark and light bands.
Eight polarized light images for each sample at a final magnification of 25× were randomly chosen and within each image the number of crimps was counted. In each image the crimp top angle was measured at four different points. Morphometric analysis was performed using a computerized image analyser (QWin, Leika Microsystem Imaging Solution Ltd, Cambridge, UK). For each photograph at a final magnification of 16× the Leika QWin software provided (1) the total number of crimps and (2) the width angle at the top of four randomly selected crimps.
Results
Light microscopy
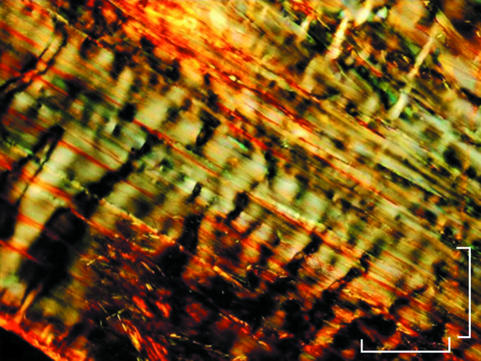
Relaxed tendons observed under light microscopy appeared to consist of straight collagen fibre bundles organized in a parallel arrangement. When observed under polarized light microscopy the fibre bundles showed a zig-zag course with an alternation of dark and light transverse bands, which were expressions of the tendon crimp pattern described in the literature (Fig. 2). In the same tendon, crimps differed in geometry, appearing as isosceles or scalene triangles of different size and variable width of the crimp top angle (Fig. 3).
Fig. 2.
Polarized light micrograph of collagen fibre bundles of relaxed Achilles tendon. Note alternating dark and light transverse bands indicating crimps. Scale bar = 100 µm.
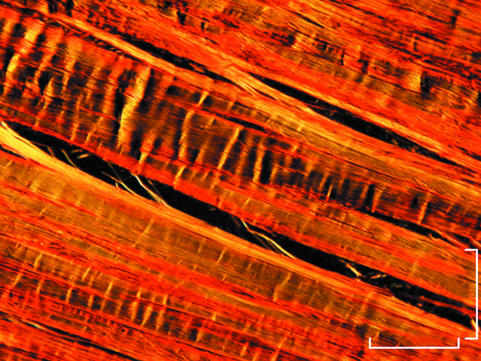
Fig. 3.
Polarized light micrograph of relaxed Achilles tendon. Crimps appear as isosceles or scalene triangles of different length and with variable width of the crimp top angle. Scale bar = 200 µm.
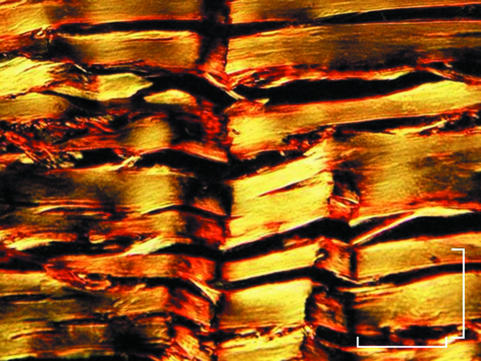
In stretched tendons both light and polarized light microscopy showed some areas with collagen fibre bundles running straight and parallel to the long axis of the tendon with no birefringence, and other areas with collagen fibre bundles showing characteristic birefringence. When present, in these regions, the crimps appeared as more flattened triangles (Fig. 4).
Fig. 4.
Polarized light micrograph of stretched Achilles tendon. Note some areas with collagen fibre bundles running straight and parallel to the long axis of the tendon with no birefringence, and other areas where birefringence is observed and reveals flattened crimps. Scale bar = 100 µm.
Scanning electron microscopy
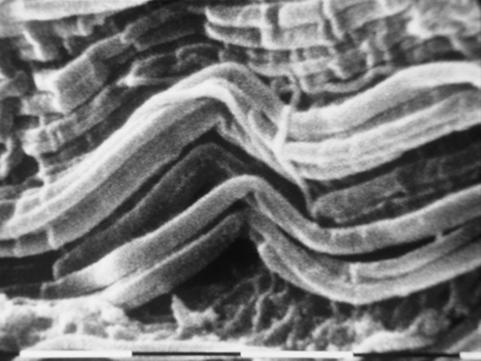
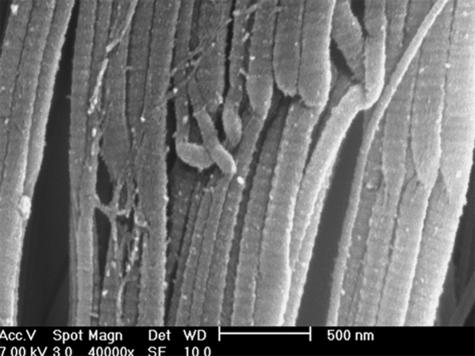
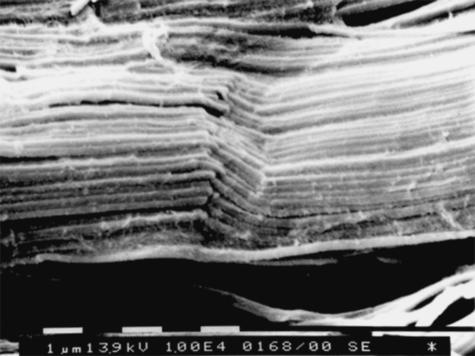
Longitudinally sectioned relaxed tendons showed long, straight and parallel bundles of D-banded collagen fibrils alternating in sequence with short segments I which the fibril bundles suddenly changed their direction showing a crimp with an evident top angle (Figs 5 and 6). In every crimp, single D-banded collagen fibrils drastically changing direction formed individual knots termed ‘fibrillar crimps’. At higher magnification these knots corresponded to irregular aspects of the collagen fibrils, which appeared partially squeezed, or bent on the same plane like bayonets, or twisted and bent (Figs 6 and 7). Each tendon crimp included more than one knot or ‘fibrillar crimp’ and several knots were often visible in each collagen fibril of a tendon crimp (Fig. 6).
Fig. 5.
SEM image of longitudinally sectioned relaxed Achilles tendon. Long, straight and parallel bundles of D-banded collagen fibrils alternate in sequence with short segments where the fibril bundles suddenly change their direction drawing a crimp. Scale bar = 10 µm.
Fig. 6.
SEM image of longitudinally sectioned relaxed Achilles tendons. Collagen fibril bundles arranged in a crimp pattern with a sharp top angle are evident. In the same crimp, single collagen fibrils drastically changing direction form individual knots termed ‘fibrillar crimps’. As in each collagen fibril many knots are often visible in the same tendon crimp. Scale bar = 1 µm.
Fig. 7.
SEM image of relaxed Achilles tendon. Note the presence of crimps where single D-banded collagen fibrils drastically changing direction form individual knots. Collagen fibrils appear partially squeezed, or bent on the same plane like a bayonet, or twisted and bent. Scale bar = 500 nm.
In stretched tendons most of the collagen fibrillar bundles ran straight and parallel to the long axis of the tendon. Flattened and only partially stretched crimped bundles were still recognizable in only a few segments. Even here, the ‘fibrillar crimps’ of the single collagen fibrils were detectable at the top of the crimped bundles (Fig. 8). Residual knots corresponding to ‘fibrillar crimps’ were still present in some segments of straightened fibrils (Fig. 9). At higher magnification they showed the same squeezed, or bent, or twisted and bent morphological aspects.
Fig. 8.
SEM image of stretched Achilles tendon. Note few segments of collagen fibre bundles with flattened and only partially stretched crimps. At the top angle of these crimps, ‘fibrillar crimps’ of the single collagen fibrils are detectable. Scale bar = 1 µm.
Fig. 9.
SEM image of stretched Achilles tendon showing completely straightened collagen fibrils with residual knots corresponding to ‘fibrillar crimps’. Scale bar = 1 µm.
Transmission electron microscopy
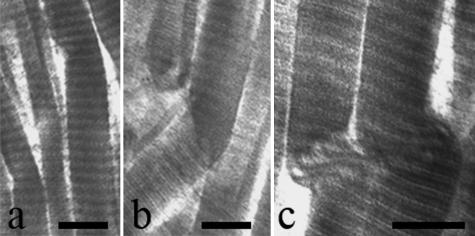
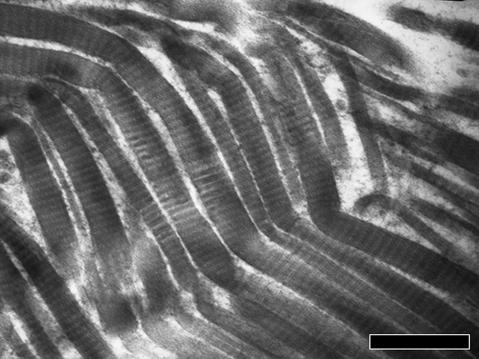
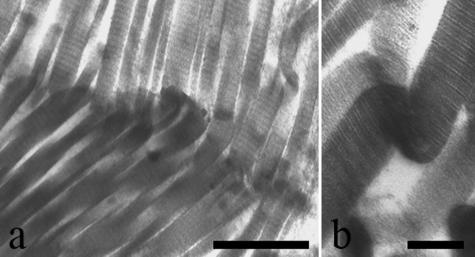
TEM analysis of relaxed tendons revealed wavy collagen fibre bundles containing collagen fibrils that gradually changed their direction without any morphological modification. However, in some fibre bundles the collagen fibrils suddenly changed their direction showing irregularities in their cylindrical shape. They appeared squeezed, or bent with a bayonet shape, or twisted and bent corresponding to the ‘fibrillar crimps’ observed under SEM (Fig. 10a–c). Some collagen fibrils at the ‘fibrillar crimp’ lost their D-banding pattern, with microfibrillar subunits visible (Fig. 10c). As observed in SEM analysis the same tendon crimp was composed of more than one ‘fibrillar crimp’ and frequently each collagen fibril included many ‘fibrillar crimps’ (Fig. 11).
Fig. 10.
Electron micrographs of relaxed Achilles tendons. Collagen fibrils suddenly changing direction at ‘fibrillar crimps’ show irregularities in their cylindrical shape. They appear (a) squeezed, or (b) bent on the same plane like a bayonet, or (c) twisted and bent. Some collagen fibrils at the ‘fibrillar crimp’ (c) lose their D-banding pattern and show their microfibrillar subunits. Scale bar = 250 nm.
Fig. 11.
Electron micrograph of stretched Achilles tendon showing that the same tendon crimp is composed of more than one ‘fibrillar crimp’ and that each collagen fibril includes many ‘fibrillar crimps’. Scale bar = 1 µm.
The stretched tendons showed predominantly collagen fibril bundles running straight and parallel whereas crimped bundles and relative ‘fibrillar crimps’ were seldom detected (Fig. 12a,b).
Fig. 12.
Electron micrographs of stretched Achilles tendon showing few crimped collagen bundles and their relative ‘fibrillar crimps’ (a). Scale bar = 1 µm. See in (b) a twisted and bent collagen fibril. Scale bar = 250 nm.
Morphometric analysis
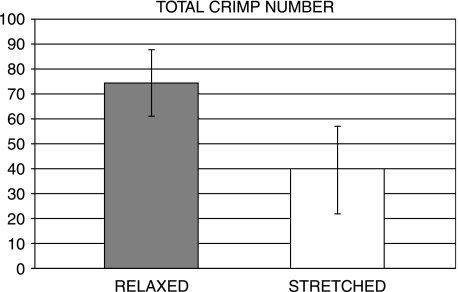
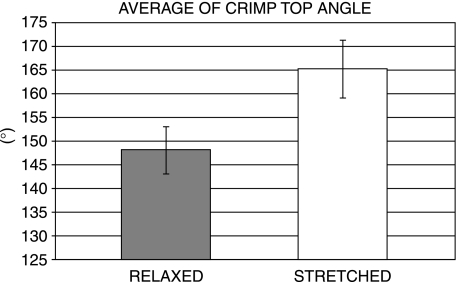
The histomorphometric analysis of both relaxed and stretched tendons observed by polarized light microscopy revealed changes in the number and top angle width of the tendon crimps. The total number of crimps detectable in iamges of relaxed tendons was 74.4 whereas in stretched tendons this was 39.6, indicating a reduction of 46.7% during tendon stretching (P < 0.01) (Fig. 13). In relaxed tendons the average width angle was 148° compared with 165° (P < 0.005) in stretched tendons (Fig. 14).
Fig. 13.
Number of tendon crimps in relaxed and stretched Achilles tendons of rat.
Fig. 14.
Average crimp top angle in relaxed and stretched Achilles tendons of rat.
Discussion
During skeletal muscle contraction the contractile portion represented by the muscle fibres shortens and tendon stretches, transferring muscle forces to the bone with a minimal loss of energy (Huijing et al. 1989; Griffiths, 1991; Scott & Loeb, 1995; Muramatsu et al. 2001). Tendon collagen fibres have alternating straight segments with segments showing a particular triangle–waveform arrangement known as a crimp and which seems to provide a mechanism of protecting the muscle during contraction (Diamant et al. 1972; Jozsa & Kannus, 1997; Magnusson et al. 2003a; Screen et al. 2004). Polarized light microscopy studies have demonstrated that the crimping pattern observed in unstressed explanted tendons disappears when tendons are stretched with increasing strain (Rigby et al. 1959; Viidik & Ekholm, 1968; Stromberg & Wiederhielm, 1969; Hansen et al. 2002). According to the current interpretation, when tendon is stretched crimp acts as a mechanical buffer and it has been suggested that elongation of the tendon corresponds to straightening of the crimps (Diamant et al. 1972; Atkinson et al. 1999; Hansen et al. 2002; Magnusson et al. 2003b; Screen et al. 2004).
All morphological studies carried out to date have examined only tendons explanted from their anatomical site, thus eluding the real physiological tension of tendon in vivo. The current study investigated the morphology of collagen crimps in rat Achilles tendon when examined in physiological conditions of relaxation and stretching.
Our observations show that tendon crimp morphology in relaxed tendon may assume a different length and geometry, corresponding to isosceles and scalene triangles.
Both SEM and TEM observations demonstrate that at the level of the tendon crimp single D-banded collagen fibrils form characteristic knots when they suddenly change their orientation. The collagen fibrils in these knots lose their cylindrical shape appearing squeezed, or bent on the same plane like a bayonet, or twisted and bent. The periodic D-banding in many fibrils is no longer detectable and microfibrillar subunits are readily observed, as shown by AFM studies (Raspanti et al. 2005). We termed these hitherto undescribed knot shapes ‘fibrillar crimps’. Our observations suggest that the different aspects of ‘fibrillar crimps’ observed in our study by SEM and TEM and in a previous study by AFM (Raspanti et al. 2005) should fulfil the same functional role. Similar TEM images (but not commented upon) were shown previously by Gathercole & Keller (1991). It is noteworthy that each tendon crimp observed by polarized light microscopy includes several ‘fibrillar crimps’ distributed along the length of the tendon crimp. Stretched tendons fixed in situ and still under mechanical tension observed under polarized light microscopy show a marked reduction of tendon crimps. Our morphometric data show that during stretching the crimp number decreases by 46.7%, and the top angle width of tendon crimp increases from 148° to 165°, showing that where crimps persist during stretching they appear more flattened. These results are confirmed by SEM observations, which show predominantly straight fibrillar bundles with an apparent partial or even total extinction of tendon crimps. At higher magnification a series of knots are still present even in straight fibrillar bundles and may correspond to residual ‘fibrillar crimps’, which never lose their structural identity even under stretching. It is reasonable to assume that the true structural component of a tendon crimp acting as a shock absorber during stretching is the ‘fibrillar crimp’.
Acknowledgments
We are indebted to Gianfranco Filippini, D.I.S.T.A., University of Bologna, for his technical assistance with SEM. This work was supported by MIUR grant (prot. 2004055533).
References
- Atkinson TS, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- De Campos Vidal B. Crimp as a part of a helical structure. CR Acad Sci III. 1995;318:173–178. [PubMed] [Google Scholar]
- De Campos Vidal B. Image analysis of tendon helical superstructure using interference and polarized light microscopy. Micron. 2003;34:423–432. doi: 10.1016/S0968-4328(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Derwin KA, Soslowsky LJ, Kimura JH, Plaas AH. Proteoglycans and glycosaminoglycan fine structure in the mouse tail tendon fascicle. J Orthopaed Res. 2001;19:269–277. doi: 10.1016/S0736-0266(00)00032-2. [DOI] [PubMed] [Google Scholar]
- Diamant J, Keller A, Baer E, Litt M, Arridge RG. Collagen: ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc B. 1972;180:293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- Elliott DH. Structure and function of mammalian tendon. Biol Rev Camb Philos Soc. 1965;40:392–421. doi: 10.1111/j.1469-185x.1965.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Gathercole LJ, Keller A. Crimp morphology in the fibre-forming collagens. Matrix. 1991;11:214–234. doi: 10.1016/s0934-8832(11)80161-7. [DOI] [PubMed] [Google Scholar]
- Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng. 2002;124:72–77. doi: 10.1115/1.1427698. [DOI] [PubMed] [Google Scholar]
- Hess GP, Cappiello WL, Poole RM, Hunter SC. Prevention and treatment of overuse tendon injuries. Sports Med. 1989;8:371–384. doi: 10.2165/00007256-198908060-00005. [DOI] [PubMed] [Google Scholar]
- Huijing PA, van Lookeren Campagne AA, Koper JF. Muscle architecture and fibre characteristics of rat gastrocnemius and semimembranosus muscles during isometric contractions. Acta Anat. 1989;135:46–52. doi: 10.1159/000146721. [DOI] [PubMed] [Google Scholar]
- Hurschler C, Provenzano PP, Vanderby R., Jr Scanning electron microscopic characterization of healing and normal rat ligament microstructure under slack and loaded conditions. Connect Tissue Res. 2003;44:59–68. [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, Reffy A. Three-dimensional ultrastructure of human tendons. Acta Anat. 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Spor. 1997;7:113–118. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Spor. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Palley I, Baer E. A structural mechanical model for tendon crimping. J Biomech. 1980;13:887–893. doi: 10.1016/0021-9290(80)90177-3. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–377. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Spor. 2003a;13:211–223. doi: 10.1034/j.1600-0838.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, et al. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003b;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muraoka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T. Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J Appl Physiol. 2001;90:1671–1678. doi: 10.1152/jappl.2001.90.5.1671. [DOI] [PubMed] [Google Scholar]
- Niven H, Baer E, Hiltner A. Organization of collagen fibers in rat tail tendon at the optical microscope level. Coll Relat Res. 1982;2:131–142. doi: 10.1016/s0174-173x(82)80029-0. [DOI] [PubMed] [Google Scholar]
- Raspanti M, Manelli A, Franchi M, Ruggeri A. The 3D structure of crimps in the rat Achilles tendon. Matrix Biol. 2005;24:503–507. doi: 10.1016/j.matbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Rigby BJ, Hirai N, Spikes JD, Eyring H. The mechanical properties of rat tail tendon. J Gen Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RW. The structure of rat tail tendon. Connect Tissue Res. 1985a;14:9–20. doi: 10.3109/03008208509089839. [DOI] [PubMed] [Google Scholar]
- Rowe RW. The structure of rat tail tendon fascicles. Connect Tissue Res. 1985b;14:21–30. doi: 10.3109/03008208509089840. [DOI] [PubMed] [Google Scholar]
- Scott JE, Orford CR, Hughes EW. Proteoglycan–collagen arrangements in developing rat tail tendon. An electron microscopical and biochemical investigation. J Biochem. 1981;195:573–581. doi: 10.1042/bj1950573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE. Elasticity in extracellular matrix shape modules of tendon, cartilage, etc. A sliding proteoglycan–filament model. J Physiol. 2003;553:335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH, Loeb GE. Mechanical properties of aponeurosis and tendon of the cat soleus muscle during whole-muscle isometric contractions. J Morph. 1995;224:73–86. doi: 10.1002/jmor.1052240109. [DOI] [PubMed] [Google Scholar]
- Screen HR, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng [H] 2004;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- Stromberg DD, Wiederhielm CA. Viscoelastic description of a collagenous tissue in simple elongation. J Appl Physiol. 1969;26:857–862. doi: 10.1152/jappl.1969.26.6.857. [DOI] [PubMed] [Google Scholar]
- Viidik A, Ekholm R. Light and electron microscopic studies of collagen fibers under strain. Z Anat Entwicklungsgesch. 1968;127:154–164. doi: 10.1007/BF00521981. [DOI] [PubMed] [Google Scholar]
- Viidik A. Simultaneous mechanical and light microscopic studies of collagen fibers. Z Anat Entwicklungsgesch. 1972;136:204–212. doi: 10.1007/BF00519178. [DOI] [PubMed] [Google Scholar]
- Viidik A, Nielsen HM, Skalicky M. Influence of physical exercise on aging rats. II. Life-long exercise delays aging of tail tendon collagen. Mech Ageing Dev. 1996;88:139–148. doi: 10.1016/0047-6374(96)01729-0. [DOI] [PubMed] [Google Scholar]