Abstract
The intracranial carotid rete (or rete mirabile epidurale) is a unique blood vascular system supplying the brain of artiodactyls, which have either an involuted or no internal carotid artery. Although the lesser and greater mouse deer (Tragulus javanicus and T. napu, respectively) are ruminants, the rete mirabile epidurale is absent. In these animals, as in non-artiodactyls, such as canines, equines and humans, the complete internal carotid artery supplies the brain. It is currently uncertain whether the absence of the rete is confined to mouse deer among ruminants. The absence of the rete in mouse deer provides new insights into the evolution of the arterial system in artiodactyls.
Keywords: artiodactyls, blood supply to brain, carotid rete mirabile, mouse deer
Introduction
The rete mirabile is an arterial or arteriolar meshwork formed by branches from one or a few arteries that then converge on a single artery. The glomerular arteriole in the kidney is a rete and is found in most mammals. The most striking rete is the intracranial carotid rete, the rete mirabile epidurale, found in artiodactyls (Daniel et al. 1952; Ghoshal & Nanda, 1975; Ghoshal, 1975). The carotid rete is present within the cranium and carries arterial blood supplying the brain, replacing the absent internal carotid artery or the involuted one in these animals. Felids have a similar extracranial arterial system (Daniel et al. 1952; Hsieh & Takemura, 1994). Generally, in ruminants, a few branches from the maxillary artery enter the cranium through the foramen oval and foramen orbitorotundum, form a complex arterial mesh on the floor of the cranium, converge to a single artery called the distal segment of the internal carotid artery or cerebral carotid artery, and penetrate the dura mater to enter the brain (Fig. 1). The converged artery from the rete supplies blood to the brain on both sides as well as the internal carotid artery in non-artiodactyl animals.
Fig. 1.
Intracranial carotid rete (CR) of a miniature Shiba goat injected vascularly with red latex. A paired arterial meshwork is formed at both sides and a single converged artery emerges from each rete (arrows) toward the brain. Dorsal view of the floor of the cranium with the dura mater removed. HF, hypophyseal fossa; OC, optic chiasm.
Mouse deer, Tragulus spp., are the smallest living ruminants. They are called primitive artiodactyls, because they diverged from the stem of the phylogenic tree of the artiodactyls in the Miocene and have survived without remarkable changes. The family Tragulidae consists of three genera, including four species (Nowak, 1999). Of these, the lesser mouse deer, T. javanicus, and greater mouse deer, T. napu, inhabit south-east Asia. Using a latex injection method and resin corrosion casts of arterial blood vessels, we examined the blood supply from the common carotid arteries to the brain in lesser and greater mouse deer from Malaysia, with the permission of the Department of Wildlife, Sabah, and the Malaysian Government.
Materials and methods
Five lesser mouse deer of both sexes and two female greater mouse deer were used to observe the arterial system by injecting latex and resin into arterial vessels. All the animals were originally from the wild in Malaysia and were maintained in the National Institute of Animal Health, Tsukuba, Japan, and a mouse deer farm in Sabah, Malaysia. Lesser mouse deer were between 3 months and 4 years of age and weighed 950–2300 g. We did not know the age of the greater mouse deer, but both seemed to be adult (judging by their pregnancy) and weighed 3450 and 4100 g.
Soon after the death of mouse deer as a result of accident or exsanguination under ketamine anaesthesia, red-coloured Neoprene latex (Du Pont 601A) or acrylic resin (Mercox CL-2R, Dai Nippon Ink Ltd) was injected through the common carotid artery or the heart. In the specimens injected with latex, the arterial vascular system was observed macroscopically and fixed in 5–10% neutral formalin for detailed observation. The specimens injected with resin were soaked in 10% NaOH solution after resin hardening and the tissues were dissolved to make a corrosion cast of the blood vessels. The corrosion casts were observed by the naked eye and under a stereomicroscope.
As other artiodactyls for comparison, two miniature Shiba goats and two miniature pigs were purchased from breeders and killed to examine carotid rete mirabile, and two Reeves's muntjacs were also used as small wild ruminants. The male Shiba goat was 3 years of age, with a body weight of 17 kg, and the female was 9 years old and weighed 25 kg. The pigs were 11 months old and weighed 28.2 kg (male) and 29.8 (female). The muntjacs were supplied as exterminated animals from the Chiba prefectural government, Japan. The two males weighed 11.9 and 6.0 kg, but their ages were unknown.
Results
In the lesser and greater mouse deer, the right and left common carotid arteries arise from the brachiocephalic trunk and run cranially along both sides of the trachea. The common carotid artery divides into the external carotid artery and the internal carotid artery at the level of the caudal end of the mandible (Fig. 2). The internal carotid artery is slightly thinner than the external carotid and runs upward toward the cranium without branching and enters the cranium through the foramen lacerum. Within the cranium, the internal carotid artery runs rostrally on the floor of the cranium, turns upward at the sides of the hypophysis, penetrates the dura mater, and divides into rostral and caudal branches of almost equal diameter (Figs 3 and 4). The rostral branch runs forward and gives off a middle cerebral artery, and the remainder continues as an anterior cerebral artery. In the intercerebral fissure, a fine ramus from the anterior cerebral artery fuses with that from the opposite site. The caudal branch extends backward and gives off a caudal cerebral artery and arteries supplying the cerebellum, and the remainders of both sides unite at midline, forming a single basilar artery. Thus, the rostral and caudal branches of the internal carotid artery contribute to form the circle of Willis, which supplies the cerebrum, cerebellum and rostral part of the spinal cord. In the greater mouse deer, branches of the internal carotid arteries on both sides may form an anastomosis around the hypophysis. However, no rete mirabile epidurale was present in any of the lesser or greater mouse deer examined (Figs 3 and 4).
Fig. 2.
Cervico-mandibular region of a lesser mouse deer in which the arterial vessels were injected with red latex. From the common carotid artery (CCA), a thinner internal carotid artery (IC) and a thick external carotid artery (EC) diverge. OA, occipital artery.
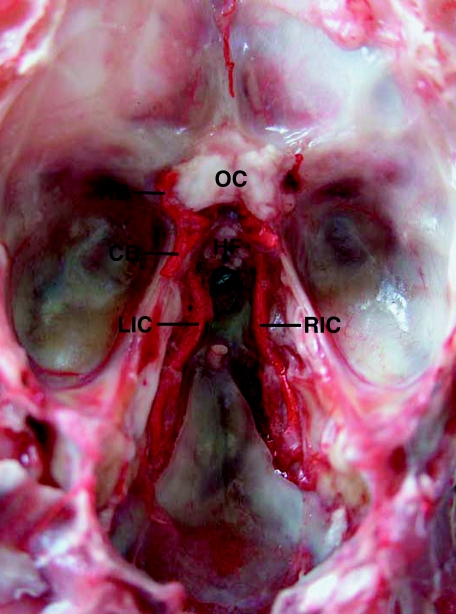
Fig. 3.
Left and right internal carotid arteries (LIC and RIC) running rostrally on the floor of the cranium in a lesser mouse deer. After penetrating the dura mater, the artery diverges into rostral and caudal branches (RB and CB), which form the circle of Willis. No carotid rete is present. Dorsal view of the floor of the cranium with the dura mater removed. HF, hypophyseal fossa; OC, optic chiasm.
Fig. 4.
Corrosion cast of arterial vessels in the brain of a lesser mouse deer. The internal carotid artery (IC) runs rostrally (left side) and diverges into rostral and caudal branches (RB and CB). In its course on the floor of the cranium, the internal carotid artery thins (arrowhead) and is surrounded by the cavernous sinus (CS).
The miniature Shiba goats, miniature pigs and Reeves's muntjacs have complete carotid rete mirabile as in other ruminants (Fig. 1).
Discussion
The intracranial carotid rete or rete mirabile epidurale is a striking arterial system that is observed in most artiodactyls, including cattle (Daniel et al. 1952; Baldwin, 1964; Gillian, 1974; Uehara et al. 1978; Ocal & Aslan, 1994), sheep (Daniel et al. 1952; Baldwin, 1964; Baker & Hayward, 1968; Khamas & Ghoshal, 1985), goats (Daniel et al. 1952; Edelman et al. 1972), buffalo (Bamel et al. 1975), camels (Zguigal & Ghoshal, 1991; Ocal et al. 1998), dik-diks (Kamau et al. 1984), giraffes (Lawrence & Rewell, 1948), okapis (Lawrence & Rewell, 1948) and pigs (Daniel et al. 1952; Ghoshal & Khamas, 1985). In these animals, the internal carotid arteries degenerate or vanish during embryonic development, so that the branches of the maxillary and ascending pharyngeal arteries enter the complex arterial meshwork, carotid rete, instead of an arterial supply to the brain via the internal carotid artery. This unique arterial system is thought to regulate blood temperature principally as the blood passes through the arterial meshwork of the rete in close association with the cavernous sinus, thereby protecting the brain (Baker & Hayward, 1968; Kamau et al. 1984; Hsieh & Takemura, 1994; Jessen, 1998, 2001). In the current study, the corrosion casts showed that the internal carotid artery thins and is surrounded by the cavernous sinus in its course on the floor of the cranium (Fig. 4). This may represent a blood cooling system in the mouse deer.
Artiodactyls are grouped taxonomically into 80 genera and about 180 species. The absence of the rete mirabile in a ruminant has not previously been reported. It is not yet clear whether the absence of the rete is confined to lesser and greater mouse deer among ruminants. The lesser and greater mouse deer are interesting animals that exhibit some unique characteristics (Fukuta et al. 1996; Kimura et al. 2004). They have previously been recognized as nocturnal animals, but recently Matsubayashi et al. (2003) have reported that the mouse deer is diurnal. In captured animals, we also observed that the mouse deer is more active around dawn and sunset than during the night. The considerable phylogenetic divergence between mouse deer and other, more recently evolved, ruminants has been established by means of DNA sequence analysis (Hassanin & Douzery, 2003).
We can provide two hypotheses for evolutionary absence of the carotid rete in the mouse deer. One is that the internal carotid artery is retained completely and no rete has been formed, i.e. the absence is a plesiomorphic feature of the mouse deer among ruminants. The second hypothesis is that the formed carotid rete has been lost via adaptation to their lifestyle, i.e. the absence is an apomorphic feature. We are unable to say which of these is true at present. Further study on the embryological development of the mouse deer might contribute to explaining the absence of the carotid rete. The vascular system of other species of the family Tragulidae, such as the African water chevrotain and Indian spotted mouse deer, and other small wild ruminants, such as the royal antelope, should be examined from the standpoint of the arterial supply to the brain.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research (nos 13575027 and 15405037) from the Japan Society for the Promotion of Science.
References
- Baker MA, Hayward JN. The influence of the nasal mucosa and the carotid rete upon hypothalamic temperature in sheep. J Physiol. 1968;198:561–579. doi: 10.1113/jphysiol.1968.sp008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin BA. The anatomy of the arterial supply to the cranial regions of the sheep and ox. Am J Anat. 1964;115:110–117. doi: 10.1002/aja.1001150107. [DOI] [PubMed] [Google Scholar]
- Bamel SS, Dhingra LD, Sharma DN. Anatomical studies on the arteries of the brain of buffalo (Bubalus bubalus). I. The rete mirabile cerebri. Anat Anz. 1975;137:440–446. [PubMed] [Google Scholar]
- Daniel PM, Dawes JDK, Prichard MML. Studies of the carotid rete and its associated arteries. Phil Trans R Soc Lond Ser B. 1952;237:173–208. [Google Scholar]
- Edelman NH, Epstein P, Cherniak NS, Fishman AP. Control of cerebral blood flow in goat: role of the carotid rete. Am J Physiol. 1972;223:615–619. doi: 10.1152/ajplegacy.1972.223.3.615. [DOI] [PubMed] [Google Scholar]
- Fukuta K, Kudo H, Jalaludin S. Unique pits on the erythrocytes of the lesser mouse-deer, Tragulus javanicus. J Anat. 1996;189:211–213. [PMC free article] [PubMed] [Google Scholar]
- Ghoshal NG. Ruminant heart and arteries. In: Getty R, editor. Sisson and Grossman's the Anatomy of the Domestic Animals. 5. Vol. 1. Philadelphia: W.B. Saunders; 1975. pp. 960–976. [Google Scholar]
- Ghoshal NG, Nanda BS. Porcine heart and arteries. In: Getty R, editor. Sisson and Grossman's the Anatomy of the Domestic Animals. 5. Vol. 2. Philadelphia: W.B. Saunders; 1975. pp. 1306–1320. [Google Scholar]
- Ghoshal NG, Khamas WAG. Gross and histomorphological study on the rostral epidural rete mirabile of the pig. Indian J Anim Sci. 1985;55:304–310. [Google Scholar]
- Gillian LA. Blood supply to brains of ungulates with and without a rete mirable caroticum. J Comp Neurol. 1974;153:275–290. doi: 10.1002/cne.901530305. [DOI] [PubMed] [Google Scholar]
- Hassanin A, Douzery EJP. Molecular and morphological phylogenetical of ruminantia and the alternative position of the moschidae. Syst Biol. 2003;52:206–228. doi: 10.1080/10635150390192726. [DOI] [PubMed] [Google Scholar]
- Hsieh H-M, Takemura A. The rete mirabile of the maxillary artery in the lion (Panthera leo) Okajimas Folia Anat Jpn. 1994;71:1–12. doi: 10.2535/ofaj1936.71.1_1. [DOI] [PubMed] [Google Scholar]
- Jessen C. Brain cooling: an economy mode of temperature regulation in artiodactyls. News Physiol Sci. 1998;13:281–286. doi: 10.1152/physiologyonline.1998.13.6.281. [DOI] [PubMed] [Google Scholar]
- Jessen C. Selective brain cooling in mammals and birds. Jpn J Physiol. 2001;51:291–301. doi: 10.2170/jjphysiol.51.291. [DOI] [PubMed] [Google Scholar]
- Kamau JM, Maina JN, Maloiy GMO. The design and the role of the nasal passages in temperature regulation in the dik-dik antelope (Rhynchotragus kirkii) with observations on the carotid rete. Resp Physiol. 1984;56:183–194. doi: 10.1016/0034-5687(84)90102-6. [DOI] [PubMed] [Google Scholar]
- Khamas WA, Ghoshal NG. Gross and scanning electron microscopy of the carotid rete-cavernous sinus complex of the sheep (Ovis aries) Anat Anz. 1985;159:173–179. [PubMed] [Google Scholar]
- Kimura J, Sasaki M, Endo H, Fukuta K. Anatomical and histological characterization of the female reproductive organs of mouse deer (Tragulidae) Placenta. 2004;25:705–711. doi: 10.1016/j.placenta.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Lawrence WE, Rewell RE. The cerebral blood supply in the Giraffidae. Proc Zool Soc Lond. 1948;118:202–212. [Google Scholar]
- Matsubayashi H, Bosi E, Kohshima S. Activity and habitat use of lesser mouse-deer (Tragulus javanicus) J Mammal. 2003;84:234–242. [Google Scholar]
- Nowak RM. Walker's Mammals of the World. 6. Vol. 2. Baltimore: Johns Hopkins University Press; 1999. Chevrotains or mouse deer; pp. 1081–1084. [Google Scholar]
- Ocal MK, Aslan K. A putative study on the retinal arteries in the bovine fetus. Ann Anat. 1994;176:151–153. doi: 10.1016/s0940-9602(11)80438-3. [DOI] [PubMed] [Google Scholar]
- Ocal MK, Erden H, Ogut I, Kara ME. A quantitative study on the retinal arteries in one-humped camels. Ann Anat. 1998;180:369–371. doi: 10.1016/S0940-9602(98)80046-0. [DOI] [PubMed] [Google Scholar]
- Uehara M, Kudo N, Sugimura M. Morphological studies on the rete mirabile epidurale in the calf. Jpn J Vet Res. 1978;26:11–18. [PubMed] [Google Scholar]
- Zguigal H, Ghoshal NG. Gross and histologic study of the rostral epidural rete mirabile and cavernous sinus in one-humped camels. Am J Vet Res. 1991;52:1173–1177. [PubMed] [Google Scholar]