Abstract
Myosin heavy chain (MHC) isoform content and citrate synthase (CS) activities were measured in the Quadriceps femoris (QF) muscle of 18 female rats. The muscle group was divided into superficial, middle and deep, distal, central and proximal parts. MHC IIb and IIx were more abundant in superficial regions (P < 0.05) with low CS activities compared with deeper parts. The deeper parts expressed all four isoforms (MHC IIb, MHC IIx, MHC IIa and MHC I), with a concomitantly higher CS activity. MHC I, MHC IIa and MHC IIb isoform content varied significantly along the length of the deep regions. Only MHC IIb and CS activity in the proximal middle part correlated (negatively) with each other. This study showed that the QF has regional specialization and that standardization of sampling site is important. Furthermore, CS activity and MHC isoforms are only loosely associated, or not at all.
Keywords: enzyme, fibre type
Introduction
Skeletal muscle is heterogeneous with respect to fibre types and enzyme activities, both of which have been investigated in various muscle groups from various species (Elder et al. 1982; Maltin et al. 1989; Hitomi et al. 2005; Kohn et al. 2005). A large variation exists between some of these muscle groups, e.g. the Soleus vs. the Gastrocnemius muscle. A muscle group itself may also vary substantially in fibre type and metabolic capacity in different areas, an observation researchers have termed muscle regionalization (Kernell, 1998; Punkt, 2002). This variation within a muscle may allow the muscle to function as a slow or a fast contracting muscle, depending on the motor units utilized. Furthermore, a single muscle fibre may, or may not, vary in myosin heavy chain (MHC) isoform expression and metabolic characteristics, which would also affect the contractile properties of that fibre (Edman et al. 1985; Staron & Pette, 1987; Reichmann, 1992).
Contractile speed of fibres is mainly determined by the type and relative quantities of MHC isoforms expressed, but may also be further modulated by other factors such as the myosin light chain isoform content (Larsson & Moss, 1993; Schiaffino & Reggiani, 1994; Moss et al. 1995). Four MHC isoforms, namely MHC I, MHC IIa, MHC IIx and MHC Iib, have been identified in rat skeletal muscle (Talmadge & Roy, 1993) with the first mentioned having the slowest ATPase activity, and the last mentioned the fastest (Schiaffino & Reggiani, 1994). The contractile property of fatigability is influenced by oxidative enzyme capacity (Nemeth et al. 1981). Citrate synthase (CS) activity has been used as an indicator of oxidative potential in skeletal muscle (Gollnick & Saltin, 1982; Bouchard et al. 1992) and is associated with fatigue resistance (Essén-Gustavsson & Henriksson, 1984; Nemeth et al. 1981). These properties seem to be associated with the MHC isoforms expressed in the muscle, as fast contracting fibres have lower oxidative enzyme activities and vice versa (Pette, 1985). However, oxidative capacity can increase or decrease depending on the stimulus, without a change in the MHC isoform content (Gollnick et al. 1985). Hence, not all proteins adapt in concert.
In the rat, the Quadriceps femoris (QF) muscles, which consist of the Vastus lateralis, Vastus medialis, Vastus intermedius and Rectus femoris, play an important role in both sprinting- and endurance-type behaviour, thus serving a dual purpose. Delp & Duan (1996) characterized 76 rat muscle groups according to both fibre type and CS activity and found that deep regions of the Vastus lateralis and Vastus medialis had significantly higher CS activities and type I fibre proportions than superficial regions, thus clearly indicating muscle regionalization. Although clear differences in contractile and metabolic properties exist when comparing fibres far from each other on the fibre-type continuum (e.g. type I vs. IIB), how closely these properties are regulated is not that clear as both fibre types expressing pure MHC I or pure MHC IIa are associated with high oxidative activity (Pette & Staron, 1993), and even fibre types expressing pure MHC IIb from different regions may vary in oxidative capacity (Larsson et al. 1991). A further advantage of sampling the various Vastus muscles and the Rectus femoris is that the QF is a large muscle group allowing for a large variety of subsequent biochemical analyses. However, it is then necessary to understand clearly how regionalization may influence results.
Nakatani et al. (2000) investigated both fibre type distribution and succinate dehydrogenase (SDH) activity in cross-sections of the Plantaris and Tibialis anterior (TA) muscles of the rat at levels ranging from superficial to deep. They concluded that type IIB fibres had much higher SDH activity in deep parts compared with superficial parts. Furthermore, although it is generally accepted that slow-twitch fibres have greater oxidative capacity than fast-twitch fibres (Essén et al. 1975; Chi et al. 1986), Nakatani et al. (2000) found that in rat Soleus muscle, the type IIA fibres had a higher SDH activity than the type I fibres. Therefore, the heterogeneity of skeletal muscle is not as predictable as previously thought (Bass et al. 1969; Pette, 1985).
A number of studies have investigated the distribution of fibre types in a specific muscle group, not only superficial to deep, but also along the length of the muscle. Recently, Wang & Kernell (2000) investigated the proximal to distal organization of fibre types in five muscle groups of the rat hind limb [Extensor digitorum longus (EDL), Flexor digitorum and Hallucis longus, Gastrocnemius medialis (GM), Peroneus longus (PE) and TA] and concluded that there is a significant difference in the distribution of the type of fibres along the length of the muscle. They further concluded that in most of these muscles, type I fibres were predominantly located in the proximal vicinity of the muscles analysed. However, Lexell et al. (1994), in a study investigating the fibre type proportions in rabbit TA and EDL muscles, found that EDL muscle contained significantly more type I fibres in the distal than in the proximal parts. Therefore, muscle regionalization may also be related to species. Wang & Kernell (2001a, b) also concluded that fibre type regionalization in muscle groups follows a general and graded pattern from superficial to deep, and from proximal to distal, but may vary between species, e.g. rat, rabbit and mouse. Furthermore, Torrella et al. (2000) showed that lateral to medial differences in fibre type exist in rat TA muscle, but that a large inter-individual variation exists. None of these studies investigated the regionalization of metabolic properties.
As mentioned earlier, enzyme activities may vary from superficial to deep regions of a muscle, but recent studies also indicate that they may vary along the length of the muscle (Reichmann, 1992; Punkt et al. 1998), although not all studies are in agreement (Pette et al. 1980). In a review, Punkt (2002) discussed how the regions of the EDL and the Soleus muscles differed for both metabolic enzyme profiles and fibre type distribution. For EDL, a decrease in slow oxidative and fast oxidative fibre types was observed, with a concomitant increase in fast glycolytic fibre types from proximal to distal. The opposite was observed for the Soleus muscle. Similarly, Sakuma et al. (1995) compared fibre types in proximal, middle and distal regions of rat Soleus and Plantaris muscles and found that these muscles differed in regionalization. Therefore, it seems necessary to characterize regionalization of each specific muscle or muscle group of interest, as general conclusions may not apply.
Finally, although several studies have been done in rats (Table 1), rat skeletal muscle expresses four MHC isoforms, and in most of the studies, inadequate identification of the isoforms was performed. Of these studies, only one (Delp & Duan, 1996) did not focus exclusively on the lower hind limb muscle, despite the fact that the upper hind limb is frequently used to assess other properties that could be influenced by fibre type. Furthermore, only a few studies have investigated enzyme activities in different regions of the muscle groups, and even fewer have done so comprehensively. Therefore, the aims of the present study were: (1) to characterize the QF in terms of distribution of all four MHC isoforms and CS activity; (2) to determine if, in addition to differences from superficial to deep, there are also differences from proximal to distal regions; and (3) to assess whether or not there was an association between a specific MHC isoform and CS activity within the different regions of the QF.
Table 1.
Summary of selected literature that investigated muscle regionalization in various species and muscle groups
| Authors | Species | Muscles | Regions | Fibre types | Enzymes |
|---|---|---|---|---|---|
| Torrella et al. (2000) | Rat | TA | Proximal, equatorial, distal, anterior, posterior, medial, lateral | SO, FOG, FG | None |
| Punkt et al. (1998) | Rat | Soleus, EDL | Insertion, middle, origin, deep, central, superficial | SO, FOG, FG | SDH, GPDH, ATPase |
| Sakuma et al. (1995) | Rat | Soleus, Plantaris | Proximal, middle, distal | I, IIC, IIA, IIB | None |
| Delp & Duan (1996) | Rat | 76 muscle groups | Various | I, IIA, IIX, IIB | CS |
| Wang & Kernell (2001a) | Rat, rabbit, mouse | Soleus, EDL, FD, GM, PL, TA | Proximal, middle, distal | I | None |
| Wang & Kernell (2000) | Rat | EDL, FD, GM, PL, TA | Proximal, middle, distal | I | None |
| Nakatani et al. (2000) | Rat | Plantaris, TA | Superficial, middle, deep | I, IIA, IIB | SDH |
TA, Tibialis anterior; EDL, Extensor digitorum longus; FD, Flexor digitorum; GM, Gastrocnemius medialis; PL, Peroneus longus; SO, slow oxidative; FOG, fast oxidative glycolytic; FG, fast glycolytic; SDH, succinate dehydrogenase; GPDH, glycerol-3-phosphate dehydrogenase; CS, citrate synthase; ATPase, adenosine triphosphatase.
Materials and methods
Animals
The ethics committee of the Research Administration (subcommittee B) at the University of Stellenbosch approved the study. Eighteen female Sprague–Dawley rats, 4 months of age, were selected randomly from litters and were given normal rat chow and water ad libitum. Rats were killed by decapitation and the QF (Vastus lateralis, Vastus medialis, Vastus intermedius and Rectus femoris) muscles were carefully dissected out as a whole group.
First, the hind limb was cleared of all skin and the Vastus lateralis identified on the lateral side. A dissection needle was positioned between the Vastus lateralis and the Adductor magnus, close to the knee joint, and carefully inserted so that the femur was under the needle. The needle was further inserted until it protruded medially between the Vastus medialis and Adductor magnus. The needle was then used to loosen the muscle group by sliding it from the distal to the proximal end of the femur. Care was taken that no Tensor fascia was included.
A string was tied around the QF group and positioned so that it was distal and the knot superficial. The whole muscle group was further exposed by removal of skin and adjacent tissue near the abdomen from the point of origin of the four muscles of the QF. The whole group was detached from the point of insertion and flipped over to expose the Vastus intermedius. Finally, the patellar tendon was cut. After visual confirmation that all four muscles were included in the QF, it was frozen in liquid nitrogen for 5 min and stored at −87 °C until further analyses.
Division of muscle
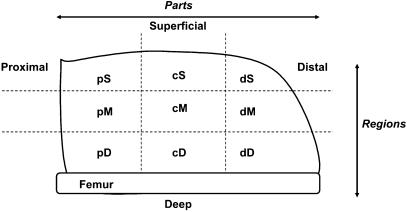
The whole muscle was divided into nine sections according to Fig. 1. The muscle was allowed to thaw briefly on an ice-chilled cutting board, but was still rigid enough to aid in the cutting process and to maintain form of the muscle group during cutting. The muscle was positioned with the Vastus intermedius facing the cutting board and the whole group was divided into two halves. Each half was then further divided into three parts termed superficial, middle and deep. This was done by marking the broadest portion of the muscle group into three equally divided parts and then cutting longitudinally in a parallel and linear fashion. Each of these sections was then similarly marked and divided into thirds giving rise to distal, central and proximal parts. In all cases, care was taken so that one-third of the length or width of the QF was used.
Fig. 1.
Layout of nine sections of quadriceps muscle of rat upper hind limb.
Homogenization of samples
Equivalent parts from the lateral and medial halves of the QF were pooled, chopped finely with a scalpel and thoroughly mixed. Thereafter, a sample of this was weighed and transferred to a glass homogenizer. A 1:19 ratio of a 100 mm phosphate buffer, pH 7.4, containing 0.02% bovine serum albumin was added. The section was thoroughly homogenized with a glass rod on ice and sonicated with an ultrasound disintegrator (Virtis Sonicators, USA) three times for 10 s, also on ice. After sonication, any connective tissue was removed, patted dry, weighed and subtracted from the original muscle weight. Homogenates were stored at −87 °C until analyses were done.
MHC isoform determination
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out according to the method of Talmadge & Roy (1993). Beta-mercaptoethanol was added to the upper running buffer to a final concentration of 0.16% (Blough et al. 1996). The homogenate sample was diluted with a sample buffer containing 10% glycerol, 5% β-mercaptoethanol, 2.3% SDS and 8 mm Tris base. Samples were heated for 10 min at 60 °C and stored at −87 °C until electrophoresis. Before electrophoresis, samples were briefly boiled for 2 min, allowed to cool and loaded onto the gels. Gels were run for 28 h at constant 70 V at 4 °C, stained with Coomassie R250 and scanned using a computer scanner. Band densities were analysed using a software package (CREAM 1-D, KEM-EN-TEC, Denmark). Values are expressed as a percentage of the total number of all bands distinguishable for each sample.
Citrate synthase activity
CS activity was measured using a modified Srere (1969) protocol. Briefly, the assay reagent contained 85 mm Tris buffer, pH 8.3, 0.1 mm 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), 0.2 mm acetyl-coenzyme A, 0.5 mm oxaloacetate and 10 µL of homogenate sample. CS activity was measured for 5 min at 412 nm in a spectrophotometer (Cary-50) at room temperature and expressed as µmol min−1 g−1 wet weight.
Statistical analysis
All values are given as mean ± SD. Data were analysed using a repeated-measures anova with a Bonferroni correction for unequal variance for each MHC isoform in all nine sections. The P < 0.05 confidence level was used to indicate statistical significance. Correlation coefficients between each MHC isoform and CS activity were calculated using the two-tailed Pearson's correlation test.
Results
MHC isoform contents and CS activities of nine sections of the QF muscle of the rat were determined. The nine sections were named S, M or D according to superficial, middle or deep region, and d, c or p from distal through central to proximal parts.
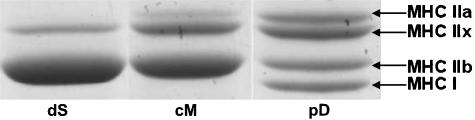
Figure 2 shows an example of the MHC isoforms separated by SDS-PAGE in three different sections (dS, cM and pD). Qualitatively, this figure indicates that superficial regions expressed mostly MHC IIb and IIx, whereas MHC IIa began to appear in middle regions and was more abundant in deep regions. The expression of MHC I was only clearly seen in the deep region, in this example. Where there was no trace found of an isoform, the value was included in the statistical analysis as zero.
Fig. 2.
An example of the MHC isoforms expressed in three different sections of the quadriceps muscle of rat upper hind limb.
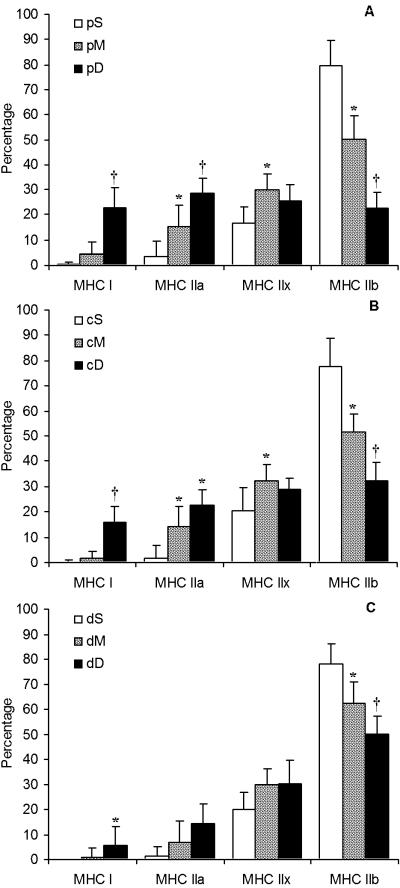
The percentage of each of the MHC isoforms expressed in the different sections is given in Figs 3(A–C) and 4(A–C). In Fig. 3, the comparison is specifically made from superficial to deep in the proximal (panel A), central (panel B) and distal (panel C) regions separately. In all three regions from proximal to distal, the MHC IIb decreased significantly from superficial to middle and more significantly from superficial to deep parts. However, even in the deep portion the quantity of MHC IIb expression was approximately 30%. A concomitant increase was seen not in MHC IIx, but was was seen in MHC IIa and MHC I expression (Fig. 3). The increase in the latter two isoforms was more pronounced in the proximal part and less in the distal part. Although MHC IIx isoform expression was significantly different in some parts, there was no pattern from superficial to deep regions in all three parts.
Fig. 3.
MHC isoform distribution in the nine sections of rat quadriceps muscle. (A) Proximal (p), (B) central (c), and (C) distal (d) regions, each ranging from superficial (S) to middle (M) to deep (D) portions (n = 18). Data are presented as mean ± SD. Statistical analyses were performed using repeated-measures anova with a Bonferroni correction. Different (P < 0.05): * from section S; † from sections S and M.
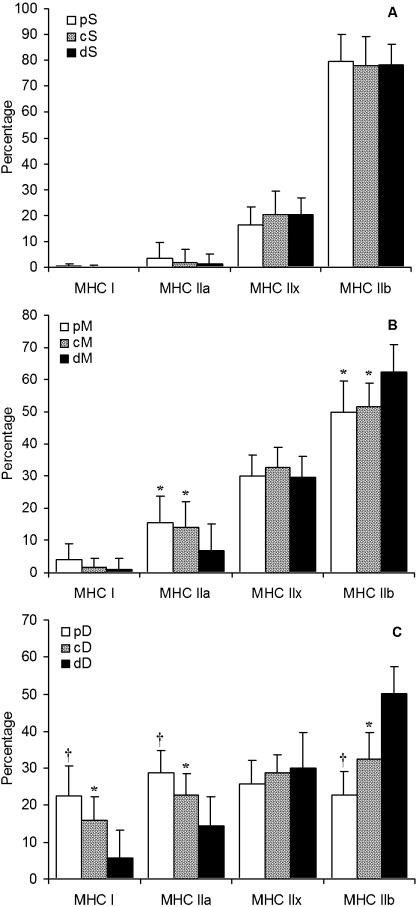
Fig. 4.
MHC isoform distribution in rat quadriceps muscle. (A) Superficial (S), (B) middle (M), and (C) deep (D) regions, each ranging from proximal (p) to central (c) to distal (d) portions (n = 18). Data are presented as mean ± SD. Statistical analyses were performed using repeated-measures anova with a Bonferroni correction. Different (P < 0.05): * from section d; † from sections d and c.
No difference was observed between the percentages of any particular MHC isoform in any part of the superficial region of the QF muscle (proximal, centre or distal) (Fig. 4A). In the middle region of the muscle, section dM expressed significantly more MHC IIb than sections cM and pM (P < 0.05), but again the concomitant lower expression was not MHC IIx but rather MHC IIa (P < 0.05) (Fig. 4B). MHC IIx isoform expression was similar from the proximal through the centre to the distal parts of the superficial, middle and deep regions. However, the deep region showed the most variation in MHC I isoform expression (Fig. 4C). MHC I, MHC IIa and MHC IIb were significantly different between all three parts of the deep region with less MHC I and MHC IIa in the distal-deep part than either the central-deep (P < 0.01) or the proximal-deep (P < 0.001) parts. However, MHC IIb isoform expression had the opposite distribution: higher in the distal-deep part than in the central-deep part (P < 0.001), which in turn was higher than the proximal-deep part (P < 0.05). By contrast, no difference in MHC IIx expression was observed between any of the parts of the deep region.
CS activities were determined as a marker for oxidative capacity in the different regions and parts of the QF. The CS activities were similarly low in the three superficial regions but increased in both middle and deep regions (Table 2). The deep region had approximately twice the activity of the superficial region. Statistical analysis revealed that middle and deep regions, with the exception of the distal-middle section, had significantly higher CS activities than the corresponding part of the superficial region (all P < 0.05), with no differences in activity between the middle and deep regions.
Table 2.
Citrate synthase activities of nine sections in rat Quadriceps muscle (µmol min−1 g−1 wet weight) (n = 18)
| p | c | d | |
|---|---|---|---|
| S | 12.0 ± 3.4 | 12.4 ± 3.0 | 12.2 ± 2.3 |
| M | 23.5 ± 9.6* | 23.1 ± 4.9* | 18.8 ± 4.9 |
| D | 28.0 ± 9.5* | 24.8 ± 8.0* | 22.5 ± 5.2* |
Data are presented as mean ± SD. Statistical analysis was performed using repeated-measures anova with a Bonferroni post-hoc test.
Different from S (P < 0.05).
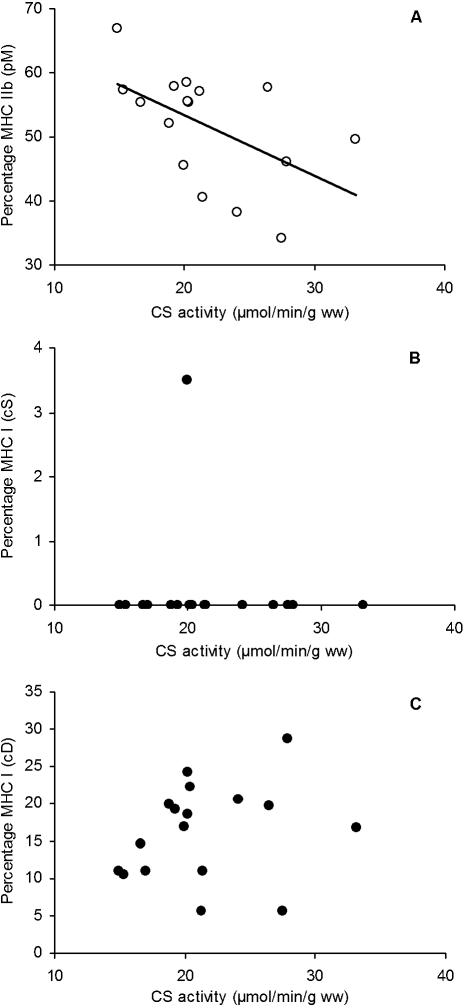
Figure 5(A) illustrates the relationship between MHC IIb proportions and CS activities of the proximal-middle section (r = –0.54, P < 0.05). No relationships were observed between any of the other isoforms and CS activities of the remaining eight sections (e.g. Fig. 5B,C). In some parts this was because of an absence of the isoform (e.g. Fig. 5B) and in other parts there was simply no relationship (e.g. Fig. 5C), despite a range in MHC I expression from 5 to 30% and in CS activity from ∼15 to 30 µmol min−1 g−1 wet weight.
Fig. 5.
Relationship between isoform content and citrate synthase activity in rat quadriceps muscle. (A) MHC IIb of the proximal, mid-portion (section pM). Pearson's r and significance: r = −0.54, P < 0.05. (B) MHC I of the superficial mid-portion (section cS). Pearson's r and significance: r = 0.13, non-significant. (C) MHC IIb from the proximal, mid-portion (section cD). Pearson's r and significance: r = 0.04, non-significant.
Discussion
Comparison of the expression of four MHC isoforms and CS activities in nine sections of the QF in rat skeletal muscle was done by investigating these parameters from superficial to deep regions, and within each region from proximal to distal parts. The main finding was that MHC I, MHC IIa and MHC IIb expression was significantly different across the length of the deep region, with the proximal portion having more slow-twitch MHC and the distal portion more fast-twitch (P < 0.05, Fig. 4C). A similar finding was also apparent in the middle region, but only for MHC IIa and MHC IIb (proximal, central and distal, Fig. 4B). By contrast, no difference in CS activities was observed across the length of the muscle in any region (Table 2). However, from superficial to deep, both MHC isoform expression and CS activities were significantly different (P < 0.05). The change in MHC isoform expression showed a graded pattern: as expected in the superficial region there was high expression of MHC IIb, which decreased gradually with depth, but MHC IIb expression was still apparent at a relatively high level of approximately 30% in deep regions. Also as expected, CS activity was low in superficial regions, but in contrast to MHC expression, which changed gradually with increasing depth, the CS activities had increased markedly by the middle region (Fig. 3, Table 2).
Previous studies indicated that a muscle group could have large differences both in fibre type and in oxidative capacity (Punkt, 2002). In addition, some fibre types may also be absent, such as type IIb in the rat Soleus muscle (Talmadge & Roy, 1993; Delp & Duan, 1996). In the present study, all four MHC isoforms commonly expressed in rat skeletal muscle were found in QF. However, when different sections were investigated it was found that some isoforms were absent. The two most commonly expressed isoforms were MHC IIb and MHC Iix, which were detected in all nine sections, including those in the deep region (Fig. 3).
The absence of certain isoforms, especially MHC I and MHC IIa, from the superficial region of the QF muscle was not surprising and may be attributed to function (i.e. infrequent recruitment of fibres from this specific region for postural control or during most daily activities), motor neuron innervation (i.e. fast motor neuron innervation) or genetic programming (i.e. developmental differentiation), or a combination of these factors (Allen et al. 2001; Eken & Gundersen, 1988; Pette & Vrbova, 1992; Caiozzo et al. 1998; Windisch et al. 1998; Siu et al. 2003).
The most superficial muscle of the QF, namely the Vastus lateralis, is known to be very ‘white’ and exclusively or almost exclusively non-oxidative (Delp & Duan, 1996). It is likely that the largest proportion of the superficial region in the current study of the QF was made up of the Vastus lateralis. However, even in individual muscles (albeit of the lower hind limb), Kernell & Wang (2000) have demonstrated that type I fibres are clustered predominantly in one half of the muscle.
The pattern of MHC isoform expression showed a gradual increase in MHC I and IIa from superficial to deep, with a concomitant decrease in MHC IIb expression. This finding of more type I fibres in deeper sections correlates with the fact that this is a common phenomenon in other muscle groups of the rat (Delp & Duan, 1996; Punkt, 2002). Despite these patterns of decreasing MHC IIb expression, the deep region still had substantial proportions of this fast isoform.
The continued expression of these fast glycolytic fibres in middle and deep regions indicates that full conversion to slower isoforms does not occur in laboratory-housed rats, even in the deepest region. Although upper hind limb muscle samples are frequently described simply as either ‘red’ or ‘white’ (Taylor et al. 2005; Chang et al. 2006; Morozov et al. 2006), our data indicate that this may be a misleading nomenclature. Muscle fibre type conversion between the various fast isoforms is relatively easily achieved by regular exercise (Andersen et al. 1994; Simoneau, 1995), but previous studies showing full conversion of fibre type from fast to slow used extreme protocols such as 24 h of electrical stimulation (Windisch et al. 1998) or exposure to a specific transcription factor. For example, in the Gastrocnemius muscle, considered to be a fast-twitch muscle, transgenic mice over-expressing peroxisome proliferator-activated receptor δ showed almost double the number of type I fibres compared with the same muscle from wild-type mice (Wang et al. 2004).
The current data confirm the conclusion of Wang & Kernell (2000) in terms of the increased expression of type I fibres in the deep region, but that in the middle region, the significant difference was actually seen in the MHC IIa fibres rather than the MHC I fibres. The present study, however, expands on their findings by indicating which of the subdivisions of the type II fibres show the concomitant opposite tendency (i.e. a decrement). This was not distributed between the fast isoform types, but was restricted to the MHC IIb isoform (Figs 3 and 4).
In cardiac muscle it has recently been shown that a natural MHCβ antisense RNA is expressed in quantities that correlate with the protein content of the MHCα isoform (Haddad et al. 2003). It is unknown if a similar post-transcriptional control mechanism might exist between skeletal muscle slow and fast isoforms.
Significant differences in MHC isoform content were also observed from proximal to distal in the QF muscle. Specifically, the regions closer to the hip had more oxidative fibre types whereas closer to the knee, there were more fibres expressing MHC IIb. However, this significance was more pronounced in the deep than the middle region and was not apparent in the superficial region. Although MHC expression of the fastest fibre type differed significantly, the variation along the length of the deep region was not observed for the MHC IIx isoform, the next fastest of the isoforms (Fig. 4).
Sakuma et al. (1995) also compared fibre types in proximal, central and distal regions of rat Soleus and Plantaris muscles and found higher proportions of type I fibres in the proximal region of the Soleus, but higher proportions of type I and IIA in the middle region of the Plantaris. In the present study, the QF muscle followed a similar pattern to that reported by Sakuma et al. However, it may be that these patterns vary between muscle groups of various species and should not be taken as a general phenomenon (Wang & Kernell, 2000, 2001b). For example, two studies have attempted to determine the mechanisms underlying such regional differences in fibre type. Campbell et al. (1996) determined that the proximity of a region to the synapse may influence SDH activity. These authors showed that there are large differences in SDH activities between regions at the motor endplate, subsarcolemmal and intermyofibrillar portions. However, they also showed that the activities of SDH in these regions remained the same in muscle subjected to 6 weeks of muscle overload, despite a significant increase in overall muscle mass and cross-sectional area of the Soleus muscle. Thus, a co-ordinated adaptation of both cell size and oxidative capacity was observed, without the relatively higher expression of oxidative capacity usually seen with endurance training (Sugiura et al. 1992). Maturation may also influence muscle fibre type in muscle groups, as was shown by Maltin et al. (1989), in which nine muscle groups were investigated in rats from ages of 19 (postnatal), 50 (young), 100 (adult) and 360 days (aged). Fibre type in those muscles analysed varied significantly, but most of the variation occurred between the ages of 19 and 100 days, whereafter no further variation was observed. The animals used in the present study were 4 months old (∼120 days) and therefore considered as adult rats. It may therefore be concluded that neuromuscular activity after birth plays an important role in determining muscle fibre type in adulthood.
In a study by Torrella et al. (2000), significant differences in fibre type distribution were found not only for superficial to deep and proximal to distal, but also lateral to medial in rat TA muscle. These authors also reported that large variations in fibre type distribution exist between rats. In the present study, the question of whether such variations are mirrored by variations in CS activities was investigated. The activities of CS did not follow a graded increase from superficial to deep except in the distal region. In the proximal and central parts, there were no differences in CS activity between middle and deep regions. However, no differences in activities were observed from proximal to distal despite the observed differences in fibre type. Only the proximal-middle section showed an inverse relationship between MHC IIb proportions and CS activities (Fig. 5A). No relationships were observed between any of the other isoforms and CS activities in any of the remaining eight sections. This could be explained, only in part, by the lack of expression of, for instance, MHC I in superficial regions (Fig. 5B for central-superficial). In the central-deep region where both CS activity and MHC I expression were high, there was also no correlation despite variation in both parameters between different rats (Fig. 5C).
It is generally accepted that slow-twitch fibres have greater oxidative capacity than fast-twitch fibres. However, Nakatani et al. (2000) found that in rat Soleus muscle, the type IIA fibres had a higher SDH activity than type I fibres. Similarly, the authors also reported higher SDH activity in type IIB fibres from deep regions of the Plantaris and TA muscles compared with more superficial parts. Pette (1985) also claimed that a large variation in enzyme activities exists between and within fibre types. Therefore, the possibility arises that in rodents anatomical position has a stronger influence on fibre type than physical activity, which is reflected in CS activity, and that this contributes to the poor relationships between the MHC isoforms and CS activities of the present study. Our study is also in accordance with previous studies showing that MHC-based fibre types IIa, IIx and IIb are not defined by oxidative enzyme activities, where considerable overlaps between the fibre types have been found (Reichmann, 1992; Punkt et al. 2002). A question that remains unanswered by this study is whether fibre type is more closely related to postural activation (or lack thereof) or a preprogrammed anatomical position or an unequal mechanical stimulus along the longitudinal axis of a muscle belly. Molecular mechanisms for control of the expression of contractile proteins and mitochondrial enzymes are not yet fully understood and are seldom investigated in the same studies. Although Bigard et al. (2000) have suggested that calcineurin co-regulates fibre type and metabolic characteristics in muscle, Meissner et al. (2001) have illustrated that this regulation is not always co-ordinated and is very complex.
In summary, the current investigation revealed that there is high diversity in MHC isoform expression across the QF muscle group of the rat. The QF is an important muscle group for mobility of mammals. In the case of the rat, it seems that the QF muscle has a much greater variety of uses than, for example, the Soleus because of the diversity of fibre type distribution and oxidative capacity. It was observed that relative to the femur, vertical levels of the muscle show the most differences in MHC isoform expression and oxidative potential, but a novel finding is that these differences also appear horizontally along the axis parallel to the femur.
The biochemical data also seem to imply that the superficial part is the centre for short exploding bursts, using anaerobic metabolism as the main fuel source. Moving deeper and more proximal, it appears that these regions would be the most active in endurance activities, with high oxidative capacity and the expression of slow MHC I. It is not clear whether these activities are related to posture (frequent, low-power activation) or physical activity (less frequent, but more powerful). Possibly, the deep region closest to the hip may be involved in postural activities (having both the highest CS activity and high MHC I content). Differences in exercise habits between these laboratory rats only seemed to co-influence the MHC IIb content and CS activities in the mid-region closest to the knee and hip. In other regions it is not clear whether CS activities were related to exercise and fibre type to posture or preprogrammed anatomical position.
The findings of this study stress the concern for accurate reporting of exact sample site when investigating muscle characteristics, or adaptations to stimuli. Many researchers do not report exact sampling site, or merely distinguish between ‘red’ or ‘white’ Vastus or ‘superficial’ or ‘deep’, where there may be, in fact, a significant variation between adjacent regions. The present study focused on the whole QF muscle group, but highlights the need to perform similar analyses on the four individual muscles in this group to ascertain further the relationships between MHC isoform expression and oxidative capacity, in particularly in the proximal and distal regions which are neglected in the literature.
Acknowledgments
This research was institutionally supported by the Department of Physiological Sciences, Stellenbosch University, South Africa. We would like to thank D. Bekker, R. Lawrence and J. Isaacs for technical assistance with the rats, and Dr M. Kidd for aiding with the statistical analyses.
References
- Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Klitgaard H, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Acta Physiol Scand. 1994;151:135–142. doi: 10.1111/j.1748-1716.1994.tb09730.x. [DOI] [PubMed] [Google Scholar]
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Bigard X, Sanchez H, Zoll J, et al. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J Biol Chem. 2000;275:19653–19660. doi: 10.1074/jbc.M000430200. [DOI] [PubMed] [Google Scholar]
- Blough ER, Rennie ER, Zhang F, Reiser PJ. Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem. 1996;233:31–35. doi: 10.1006/abio.1996.0003. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Dionne FT, Simoneau JA, Boulay MR. Genetics of aerobic and anaerobic performance. Exerc Sport Sci Rev. 1992;20:27–58. [PubMed] [Google Scholar]
- Caiozzo VJ, Baker MJ, Baldwin KM. Novel transitions in MHC isoforms: separate and combined effects of thyroid hormone and mechanical unloading. J Appl Physiol. 1998;85:2237–2248. doi: 10.1152/jappl.1998.85.6.2237. [DOI] [PubMed] [Google Scholar]
- Campbell RJ, Jasmin BJ, Michel RN. Succinate dehydrogenase activity within synaptic and extrasynaptic compartments of functionally-overloaded rat skeletal muscle fibers. Pflugers Arch. 1996;431:797–799. doi: 10.1007/BF02253847. [DOI] [PubMed] [Google Scholar]
- Chang CK, Huang HY, Tseng HF, Hsuuw YD, Tso TK. Interaction of vitamin E and exercise training on oxidative stress and antioxidant enzyme activities in rat skeletal muscles. J. Nutr. Biochem. 2006 doi: 10.1016/j.jnutbio.2006.02.007. doi: 10.1016/j.jnutbio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Chi MM, Hintz CS, Henriksson J, et al. Chronic stimulation of mammalian muscle: enzyme changes in individual fibers. Am J Physiol. 1986;251:C633–C642. doi: 10.1152/ajpcell.1986.251.4.C633. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Edman KA, Reggiani C, Te Kronnie G. Differences in maximum velocity of shortening along single muscle fibres of the frog. J Physiol. 1985;365:147–163. doi: 10.1113/jphysiol.1985.sp015764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken T, Gundersen K. Electrical stimulation resembling normal motor-unit activity: effects on denervated fast and slow rat muscles. J Physiol. 1988;402:651–669. doi: 10.1113/jphysiol.1988.sp017227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GC, Bradbury K, Roberts R. Variability of fiber type distributions within human muscles. J Appl Physiol. 1982;53:1473–1480. doi: 10.1152/jappl.1982.53.6.1473. [DOI] [PubMed] [Google Scholar]
- Essén B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Essén-Gustavsson B, Henriksson J. Enzyme levels in pools of microdissected human muscle fibres of identified type. Adaptive response to exercise. Acta Physiol Scand. 1984;120:505–515. doi: 10.1111/j.1748-1716.1984.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol. 1982;2:1–12. doi: 10.1111/j.1475-097x.1982.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Riedy M, Quintinskie JJ, Bertocci LA. Differences in metabolic potential of skeletal muscle fibres and their significance for metabolic control. J Exp Biol. 1985;115:191–199. doi: 10.1242/jeb.115.1.191. [DOI] [PubMed] [Google Scholar]
- Haddad F, Bodell PW, Qin AX, Giger JM, Baldwin KM. Role of antisense RNA in coordinating cardiac myosin heavy chain gene switching. J Biol Chem. 2003;278:37132–37138. doi: 10.1074/jbc.M305911200. [DOI] [PubMed] [Google Scholar]
- Hitomi Y, Kizaki T, Watanabe S, et al. Seven skeletal muscles rich in slow muscle fibers may function to sustain neutral position in the rodent hindlimb. Comp Biochem Physiol B. 2005;140:45–50. doi: 10.1016/j.cbpc.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Kernell D. Muscle regionalization. Can J Appl Physiol. 1998;23:1–22. doi: 10.1139/h98-001. [DOI] [PubMed] [Google Scholar]
- Kernell D, Wang LC. Simple methods for quantifying the spatial distribution of different categories of motoneuronal nerve endings, using measurements of muscle regionalization. J Neurosci Meth. 2000;100:79–83. doi: 10.1016/s0165-0270(00)00236-3. [DOI] [PubMed] [Google Scholar]
- Kohn TA, Kritzinger B, Hoffman LC, Myburgh KH. Characteristics of impala (Aepyceros melampus) skeletal muscles. Meat Sci. 2005;69:277–282. doi: 10.1016/j.meatsci.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Larsson L, Edstrom L, Lindegren B, Gorza L, Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991;261:C93–C101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Jarvis JC, Currie J, Downham DY, Salmons S. Fibre type composition of rabbit tibialis anterior and extensor digitorum longus muscles. J Anat. 1994;185:95–101. [PMC free article] [PubMed] [Google Scholar]
- Maltin CA, Delday MI, Baillie AG, Grubb DA, Garlick PJ. Fiber-type composition of nine rat muscles. I. Changes during the first year of life. Am J Physiol. 1989;257:E823–E827. doi: 10.1152/ajpendo.1989.257.6.E823. [DOI] [PubMed] [Google Scholar]
- Meissner JD, Gros G, Scheibe RJ, Kubis HP. Calcineurin regulates slow myosin, but not fast myosin or metabolic enzymes, during fast-to-slow transformation in rabbit skeletal muscle cell culture. J Physiol. 2001;533:215–226. doi: 10.1111/j.1469-7793.2001.0215b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov VI, Tsyplenkov PV, Golberg ND, Kalinski MI. The effects of high-intensity exercise on skeletal muscle neutrophil myeloperoxidase in untrained and trained rats. Eur. J. Appl. Physiol. 2006 doi: 10.1007/s00421-006-0193-x. doi: 10.1007/s00421-006-0193-x. [DOI] [PubMed] [Google Scholar]
- Moss RL, Diffee GM, Greaser ML. Contractile properties of skeletal muscle fibers in relation to myofibrillar protein isoforms. Rev Physiol Biochem Pharmacol. 1995;126:1–63. doi: 10.1007/BFb0049775. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Nakashima T, Kita T, et al. Cell size and oxidative enzyme activity of different types of fibers in different regions of the rat plantaris and tibialis anterior muscles. Jpn J Physiol. 2000;50:413–418. doi: 10.2170/jjphysiol.50.413. [DOI] [PubMed] [Google Scholar]
- Nemeth PM, Pette D, Vrbova G. Comparison of enzyme activities among single muscle fibres within defined motor units. J Physiol. 1981;311:489–495. doi: 10.1113/jphysiol.1981.sp013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Wimmer M, Nemeth P. Do enzyme activities vary along muscle fibres? Histochemistry. 1980;67:225–231. doi: 10.1007/BF00692756. [DOI] [PubMed] [Google Scholar]
- Pette D. Metabolic heterogeneity of muscle fibres. J Exp Biol. 1985;115:179–189. doi: 10.1242/jeb.115.1.179. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Biochem Pharmacol. 1992;120:115–202. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. The molecular diversity of mammalian muscle fibres. NIPS. 1993;8:153–157. [Google Scholar]
- Punkt K, Mehlhorn H, Hilbig H. Region- and age-dependent variations of muscle fibre properties. Acta Histochem. 1998;100:37–58. doi: 10.1016/S0065-1281(98)80005-6. [DOI] [PubMed] [Google Scholar]
- Punkt K. Fibre types in skeletal muscles. Adv Anat Embryol Cell Biol. 2002;162:1–109. doi: 10.1007/978-3-642-59399-4. [DOI] [PubMed] [Google Scholar]
- Punkt K, Naupert A, Wellner M, Asmussen G, Schmidt C, Buchwalow IB. Nitric oxide synthase II in rat skeletal muscles. Histochem Cell Biol. 2002;118:371–379. doi: 10.1007/s00418-002-0465-4. [DOI] [PubMed] [Google Scholar]
- Reichmann H. Enzyme activity analyses along ragged-red and normal single muscle fibres. Histochemistry. 1992;98:131–134. doi: 10.1007/BF00717004. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A, Katsuta S. Are region-specific changes in fibre types attributable to nonuniform muscle hypertrophy by overloading? Eur J Appl Physiol. 1995;71:499–504. doi: 10.1007/BF00238551. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- Simoneau JA. Adaptation of human skeletal-muscle to exercise-training. Int J Obes. 1995;19:S9–S13. [PubMed] [Google Scholar]
- Siu PM, Donley DA, Bryner RW, Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol. 2003;94:555–560. doi: 10.1152/japplphysiol.00821.2002. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate Synthase. In: Lowenstein JM, editor. Methods in Enzymology. New York: Academic Press; 1969. pp. 3–11. [Google Scholar]
- Staron RS, Pette D. Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflugers Arch. 1987;409:67–73. doi: 10.1007/BF00584751. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Morimoto A, Murakami N. Effects of endurance training on myosin heavy-chain isoforms and enzyme activity in the rat diaphragm. Pflugers Arch. 1992;421:77–81. doi: 10.1007/BF00374736. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Lamb JD, Hurst RW, et al. Endurance training increases skeletal muscle LKB1 and PGC-1alpha protein abundance: effects of time and intensity. Am J Physiol. 2005;289:E960–E968. doi: 10.1152/ajpendo.00237.2005. [DOI] [PubMed] [Google Scholar]
- Torrella JR, Whitmore JM, Casas M, Fouces V, Viscor G. Capillarity, fibre types and fibre morphometry in different sampling sites across and along the tibialis anterior muscle of the rat. Cells Tissues Organs. 2000;167:153–162. doi: 10.1159/000016778. [DOI] [PubMed] [Google Scholar]
- Wang LC, Kernell D. Proximo-distal organization and fibre type regionalization in rat hindlimb muscles. J Muscle Res Cell Motil. 2000;21:587–598. doi: 10.1023/a:1026584307999. [DOI] [PubMed] [Google Scholar]
- Wang LC, Kernell D. Fibre type regionalisation in lower hindlimb muscles of rabbit, rat and mouse: a comparative study. J Anat. 2001a;199:631–643. doi: 10.1046/j.1469-7580.2001.19960631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Kernell D. Quantification of fibre type regionalisation: an analysis of lower hindlimb muscles in the rat. J Anat. 2001b;198:295–308. doi: 10.1046/j.1469-7580.2001.19830295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLOS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch A, Gundersen K, Szabolcs MJ, Gruber H, Lomo T. Fast to slow transformation of denervated and electrically stimulated rat muscle. J Physiol. 1998;510:623–632. doi: 10.1111/j.1469-7793.1998.623bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]