Abstract
A new type of magnetic resonance imaging analysis, based on fusion of three-dimensional reconstructions of time-to-peak parametric maps and high-resolution T1-weighted images, is proposed in order to evaluate the perfusion of selected volumes of interest. Because in recent years a wealth of data have suggested the crucial involvement of vascular alterations in mental diseases, we tested our new method on a restricted sample of schizophrenic patients and matched healthy controls. The perfusion of the whole brain was compared with that of the caudate nucleus by means of intrasubject analysis. As expected, owing to the encephalic vascular pattern, a significantly lower time-to-peak was observed in the caudate nucleus than in the whole brain in all healthy controls, indicating that the suggested method has enough sensitivity to detect subtle perfusion changes even in small volumes of interest. Interestingly, a less uniform pattern was observed in the schizophrenic patients. The latter finding needs to be replicated in an adequate number of subjects. In summary, the three-dimensional analysis method we propose has been shown to be a feasible tool for revealing subtle vascular changes both in normal subjects and in pathological conditions.
Keywords: brain perfusion, Gadolinium, schizophrenia, segmentation, time-to-peak
Introduction
Dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) provides a non-invasive method for creating maps of the regional distribution of cerebral perfusion. Most DSC-MRI studies conducted to date have focused on the evaluation of patients with cerebral neoplasms, ischaemia or infarction, or epilepsy (Forsting & Weber, 2004). However, recent work has suggested that DSC-MRI may also provide clinically important information for the evaluation of patients with neuropsychiatric disorders, especially dementia and schizophrenia (Cohen et al. 1995; Levin et al. 1996; Renshaw et al. 1997; Loeber et al. 1999; Brambilla et al. 2006).
DSC-MRI provides measures of cerebral perfusion, allowing the investigation of vascular parameters such as ‘time-to-peak’ (TTP), which is the interval between the time the contrast agent is administered and the time it reaches its highest concentration in specific areas of interest. Owing to variation of the time between intravenous injection and the arrival of the bolus in the cerebral arteries, absolute TTP values are difficult to compare. To enable direct comparisons between TTP in different individuals or different examinations, standardized TTP maps can be used (Nasel et al. 2000a), or alternatively normalization with contralateral healthy tissue (Neumann-Haefelin et al. 1999).
DSC-MRI data are rarely merged with morphological acquisitions, where, owing to higher spatial resolution, subtle anatomical structure can be identified and segmented. However, due to the rapid development of image registration techniques (Maes et al. 1997; Vreugdenhil et al. 2004), correlation of the altered cerebral areas detected by structural MRI with perfusion images is feasible. This is essential to determine any causal relationship existing between morphological vs. perfusion alterations, and could supply a new key for reading neurodegeneration mechanisms and/or neuroplasticity in neurological or mental disorders. It thus becomes crucial to determine possible feature markers indicated by cerebral perfusion parameters in relation to structural parameters. A wealth of data now indicate a key role of vascular alterations in neurodegenerative mechanisms (Fabene et al. 2003), by means of neuroinflammatory cascades.
Some recent evidence (Taylor et al. 1999; Brambilla et al. 2006) suggests that vascular components could play a major role in the pathophysiology of mental diseases such as schizophrenia. The principal aim of the present study was thus to describe a new three-dimensional (3D) method based on TTP maps matched with high-resolution T1-images, and to demonstrate that it can be a useful tool for evaluating subtle perfusion changes even in small volumes of interest (VOIs). To test this kind of analysis, we compared TTP values obtained in the caudate nucleus with those measured in the whole brain, both in schizophrenic patients and in healthy controls. Such intrasubject analysis presents little if any dependence on bolus arrival time, resulting in a more direct interpretation of the data.
This tool could be helpful in the diagnosis of mental disorders such as schizophrenia and other psychoses, and may constitute a new approach for the study of these diseases.
Materials and methods
The new method was first applied on a sample of 12 subjects; our analysis uses the data available from a previous study (Brambilla et al. 2006).
MRI acquisition
MRI scans were acquired with a 1.5-T system (Siemens Medical Solutions, Erlangen, Germany) using a standard head coil. Structural MRI analysis was based on 3D MPR T1 coronal acquisitions, in Charcot plane (N sections = 144, TR = 2060 ms, TE = 3.9 ms, flip angle = 15°, FOV = 176 × 235 mm, slice thickness = 1.25 mm, matrix size = 270 × 512, TI = 1100, NEX = 1, t. acquisition = 5 min 23 s). EPI T2*-weighted acquisitions were acquired immediately before, during and after injection of a bolus of gadopentetate dimeglumine (GdDTPA) (20 sequential images for 60 repetitions, TR = 2200 ms, TE = 47 ms, FOV = 230 × 230 mm, slice thickness = 5 mm, matrix size = 256 × 256, NEX = 1, EPI factor = 128). TTPs were calculated using the Siemens inline software package.
Subjects
The clinical diagnosis of schizophrenia was confirmed using the IGC-SCAN Item Group Checklist of the Schedule for Clinical Assessment in Neuropsychiatry (IGC-SCAN) (World Health Organization, 1992) for six patients selected from the South Verona Psychiatric Register (PCR) (Tansella & Burti, 2003). We excluded patients with evidence of co-morbid psychiatric disorders, alcohol or substance misuse in the 6 months preceding the study, a history of traumatic head injury with loss of consciousness, epilepsy, or other neurological diseases. All patients were receiving anti-psychotic (AP) medication at the time of imaging.
Six right-handed healthy controls [mean (± SD) age 33.8 ± 6.2 years] were studied who had no psychiatric disorders themselves or in first-degree relatives, and no history of alcohol or substance misuse. They were compared with the six right-handed patients with schizophrenia (aged 33.5 ± 11.7 years; length of illness: 12.8 ± 12.3 years; duration of AP therapy: 10.3 ± 10.1 years; age at onset: 24.0 ± 8.1 years).
Post-processing
Structural and TTP data were transferred to a PC equipped with Amira® software 3.1 (TGS Inc., San Diego, CA, USA). High-resolution 3D T1 images of the whole brain and the caudate nuclei were segmented semi-automatically by thresholding at several segment levels chosen by visual inspection. The validation procedure was conducted following the method given by Brambilla et al. (2006). Briefly, VOIs corresponding to the whole brain and the caudate nucleus were semi-automatically segmented (see Fig. 1). All measurements were made manually by two independent researchers (P.F.F. and N.A.) who were blind to the diagnosis of schizophrenia and to the subjects’ identity. The intraclass correlation coefficients (ICCs) calculated from the results given by the two independent raters (P.F.F. and N.A.) were higher than 0.93 for both caudate nuclei and whole brain values. Registration of high-resolution and perfusion data was achieved by pre-alignment on anatomical landmarks, followed by non-rigid registration using maximization of mutual information available on a specific module of Amira.
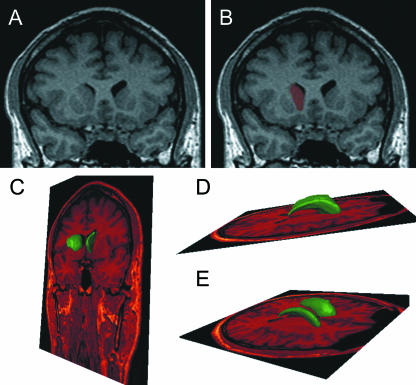
Fig. 1.
(A) Coronal image obtained by the 3D T1 sequence, where the caudate nucleus is clearly delineated and coloured. (B)after semi-automatic segmentation. (C–E) The resulting 3D segmentation of the caudate nucleus is superimposed on the high-resolution dataset in different spatial planes.
After warping of the segmented 3D structures on perfusion data, TTPs were calculated on the corresponding voxels. The TTP values were divided into six clusters. In fact, due to the temporal resolution of the sequence applied (TR = 2.2 s), in all subjects the TTP values of the whole brain voxels resulted in a range of six acquisitions. Owing to possible shifts of contrast medium arrival, the time-point when the first voxels had reached their peak was taken as an intrinsic offset (Nasel et al. 2000b), and all TTP values were presented relative to this offset to obtain cluster distributions for each subject. The TTP distributions in each subject for the caudate nucleus and the whole brain were compared using the t-test. This intrasubject comparison avoided any effect of bolus arrival time. Finally, in order to represent our findings better, a normalized TTP (nTTP) distribution for the caudate nucleus was produced by subtracting the mean TTP value obtained for the whole brain.
Results
The same structures segmented on high-resolution T1 images were used after image registration to identify VOIs for perfusion data analysis. As an example, in Fig, 1(A,B), we show a coronal plane obtained by the 3D T1 sequence, where the caudate nucleus is clearly delineated. The result of the segmentation is also indicated. In Fig. 1(C,D), a 3D view shows the final results of the segmentation of the caudate nucleus as it appears in the high-resolution dataset. TTP map and high-resolution T1 images were registered by mutual information maximization.
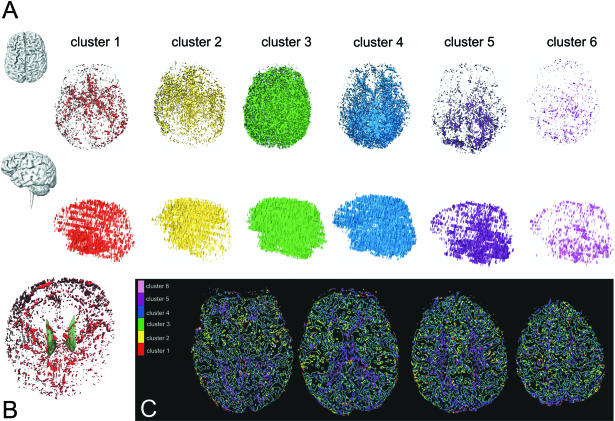
For the perfusion analysis, the values of TTP in each voxel were clusterized with the temporal resolution of the sequence applied (2.2 s), as described in Methods section above. In Fig. 2(A), the lateral view (top) and superior view (bottom) of an example of whole brain perfusion and how TTP is distributed in each cluster is shown in one subject. In Fig. 2(B), the 3D TTP visualization of the first cluster and the caudate nuclei is shown as obtained by warping T1 images and TTP maps. In Fig. 2(C), the bi-dimensional cluster segmentation is shown.
Fig. 2.
(A) Superior (top) and lateral (bottom) views of a representative case of the six (every 2.2 s each) TTP clusters. (B) 3D TTP visualization of the first cluster in red and caudate nuclei (green), obtained by warping T1 images and TTP maps. (C) Cluster segmentation as in bi-dimensional representations.
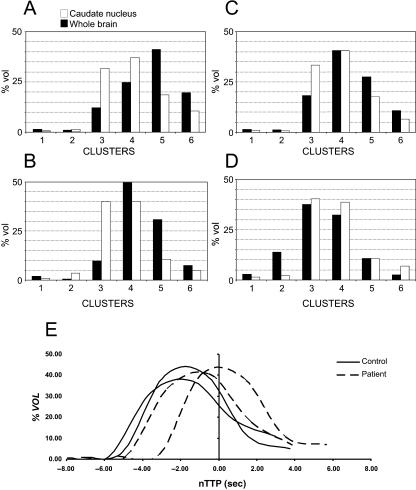
After the TTP values on the voxels assigned as caudate nucleus (CN) and whole brain (WB) were calculated, the percentage of voxels assigned to each cluster was evaluated for every subject. Two representative cases of the six normal controls were plotted in Fig. 3(A,B), and two of the six patients in Fig. 3(C,D): the percentage distribution inside the caudate nucleus is shown together with the distribution in the whole brain. In normal subjects it appears that in both cases there is an increment in the percentage of voxels in the third cluster, paralleled by a decrement in the fifth cluster, when the caudate nucleus is compared with the whole brain. It follows that the caudate nuclei were perfused faster than the whole brain. This finding was obtained in all the control subjects: the mean TTP values in caudate nuclei distribution were always lower in the six normal subjects than the mean TTP values in the whole brain distribution, and this difference was significant (P < 0.01). Despite the small number of controls analysed, this result seems to suggest that a faster perfusion of the caudate nucleus vs. the whole brain is highly specific for healthy subjects, and could be identified as a ‘normal’ pattern.
Fig. 3.
Two representative cases of the six normal controls (A,B) and two of the six patients (C,D); the percentage distribution inside the caudate nucleus is shown, together with the distribution in the whole brain. (E) nTTP distribution for the caudate nucleus of the controls and patients shown in A–D: a zero value of nTTP means a null shift between TTP of the caudate nucleus and the mean value for the whole brain.
By contrast, variable results were observed in the six schizophrenic patients, with the mean TTPs in the caudate nucleus ranging from values lower to higher than the mean value of the whole brain distribution. A faster (although less evident) perfusion was observed in the case shown in Fig. 3(C), where in the third cluster there is a higher percentage of voxels for the caudate nucleus than the whole brain, whereas a different pattern was observed in the case shown in Fig. 3(D), where in the second cluster the percentage of the whole brain is clearly higher.
To clarify this finding better we calculated an nTTP by subtracting the distribution of the TTPs obtained in the caudate nucleus from the mean value of the TTPs obtained on the whole brain of the same subject. The nTTP distributions for the caudate nucleus of the controls and the patients shown in the Fig. 3(A–D) are shown together in Fig. 3(E). The zero value of nTTP means a null shift between TTP of the caudate nucleus and the mean value for the whole brain. It confirms what seems to be a normal healthy pattern, as the caudate nucleus was perfused before the whole brain in control subjects, whereas a probably pathological alteration, which requires further investigation, was found in some patients.
Discussion
We have applied a new MRI approach based on 3D perfusion imaging to investigate perfusion pattern alteration in brain disorders, such as schizophrenia, not characterized by evident and unambiguous morphological or anatomical signs.
Schizophrenia has been studied using various neuroimaging techniques, which have supplied information regarding structural and/or functional alterations in the ventricular system, the temporal and frontal lobes, the thalamus, the cerebellum and the corpus callosum (for a complete review see Shenton et al. 2001). Numerous morphometric studies in vivo have provided valuable insights into the pathogenesis of various types of schizophrenia (DeQuardo et al. 1996; Jacobsen et al. 1997; Gaser et al. 1999; Loeber et al. 2001; Cahn et al. 2002; Hulshoff Pol et al. 2002; McDonald et al. 2002), although the variability of neuromorphological alterations found has not allowed the localization of specific morphological alterations. Most studies have used structural MRI (Henn & Braus, 1999), whereas nearly all studies of cerebral perfusion in major psychosis have been made using Tc99m HMPAO SPECT (Bajc et al. 1989; Abdel-Dayem et al. 1990; Sachdev et al. 1997; Gonul et al. 2003a, b).
To study single deep brain structures which are difficult to detect on source perfusion images, we warped them to high-resolution T1 images, resulting in a potentially more informative analysis that could also match volumetric (not reported) and perfusion data.
In this preliminary study of a limited number of subjects, our analysis focused on the perfusion of the caudate nucleus. We observed that, as expected given vascular anatomy, the caudate nucleus was always perfused faster than the whole brain in all the normal subjects who were analysed, thus identifying a ‘normal pattern’; final confirmation from a larger number of controls would be opportune. On the other hand, a variable pattern was observed in the small number of patients we studied. It will be necessary to study a suitable number of schizophrenic patients to evaluate whether there is some particular pattern specific to this pathology.
Even though the limited number of subjects makes any conclusion tentative, these data seem to suggest that perfusion pattern alteration could be informative in brain disorders not characterized by evident and unambiguous morphological or anatomical signs, and that a 3D approach could provide new insights into less evident alterations.
The method we describe allows both segmentation of small structures (due to image registration of high-resolution T1 images with low-resolution perfusion maps) and an analysis of perfusion by the first passage of contrast medium, which is independent of intersubject contrast arrival variability (due to normalization of the TTP with respect to the whole brain). If extensively applied, this method could potentially be very fruitful in the evaluation of mental disorders, making it possible to analyse cerebral volume and perfusion in baseline conditions, and also the possible relationship between alterations in perfusion and the atrophies which have been reported in psychotic patients.
References
- Abdel-Dayem HM, el Hilu S, Sehweil A, et al. Cerebral perfusion changes in schizophrenic patients using Tc-99m hexamethylpropylene amineoxime (HMPAO) Clin Nucl Med. 1990;15:468–472. doi: 10.1097/00003072-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Bajc M, Medved V, Basic M, Topuzovic N, Babic D. Cerebral perfusion inhomogeneities in schizophrenia demonstrated with single photon emission computed tomography and Tc99m-hexamethylpropyleneamineoxim. Acta Psychiatr Scand. 1989;80:427–433. doi: 10.1111/j.1600-0447.1989.tb03001.x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Cerini R, Fabene PF, et al. Assessment of cerebral blood volume in schizophrenia: a magnetic resonance imaging study. J Psychiatr Res. 2006 doi: 10.1016/j.jpsychires.2006.03.002. doi: 10.1016/j. psychires.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Cahn W, Pol HE, Bongers M, et al. Brain morphology in antipsychotic-naive schizophrenia: a study of multiple brain structures. Br J Psychiatry Suppl. 2002;43:s66–s72. doi: 10.1192/bjp.181.43.s66. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Yurgelun-Todd D, English CD, Renshaw PF. Abnormalities of regional distribution of cerebral vasculature in schizophrenia detected by dynamic susceptibility contrast MRI. Am J Psychiatry. 1995;152:1801–1803. doi: 10.1176/ajp.152.12.1801. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR, Bookstein FL, Green WD, Brunberg JA, Tandon R. Spatial relationships of neuroanatomic landmarks in schizophrenia. Psychiatry Res. 1996;67:81–95. [PubMed] [Google Scholar]
- Fabene PF, Marzola P, Sbarbati A, Bentivoglio M. Magnetic resonance imaging of changes elicited by status epilepticus in the rat brain: diffusion-weighted and T2-weighted images, regional blood volume maps, and direct correlation with tissue and cell damage. Neuroimage. 2003;18:375–389. doi: 10.1016/s1053-8119(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Forsting M, Weber J. MR perfusion imaging: a tool for more than stroke. Eur Radiol. 2004;14(Suppl. 5):M2–M7. doi: 10.1007/s10406-004-0046-9. [DOI] [PubMed] [Google Scholar]
- Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations – application to schizophrenia research. Neuroimage. 1999;10:107–113. doi: 10.1006/nimg.1999.0458. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Kula M, Esel E, Tutus A, Sofuoglu S. A Tc-99m HMPAO SPECT study of regional cerebral blood flow in drug-free schizophrenic patients with deficit and non-deficit syndrome. Psychiatry Res. 2003a;123:199–205. doi: 10.1016/s0925-4927(03)00067-2. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Kula M, Sofuoglu S, Tutus A, Esel E. Tc-99 HMPAO SPECT study of regional cerebral blood flow in olanzapine-treated schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2003b;253:29–33. doi: 10.1007/s00406-003-0401-1. [DOI] [PubMed] [Google Scholar]
- Henn FA, Braus DF. Structural neuroimaging in schizophrenia. An integrative view of neuromorphology. Eur Arch Psychiatry Clin Neurosci. 1999;249(Suppl. 4):48–56. doi: 10.1007/pl00014185. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MG, et al. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Berquin PC, et al. Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am J Psychiatry. 1997;154:1663–1669. doi: 10.1176/ajp.154.12.1663. [DOI] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Harris G, Renshaw PF. Applications of dynamic susceptibility contrast magnetic resonance imaging in neuropsychiatry. Neuroimage. 1996;4:S147–S162. doi: 10.1006/nimg.1996.0065. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Differences in cerebellar blood Volume in schizophrenia and bipolar disorder. Schizophr Res. 1999;37:81–89. doi: 10.1016/s0920-9964(98)00137-6. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Cintron CM, Yurgelun-Todd DA. Morphometry of individual cerebellar lobules in schizophrenia. Am J Psychiatry. 2001;158:952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- McDonald C, Grech A, Toulopoulou T, et al. Brain volumes in familial and non-familial schizophrenic probands and their unaffected relatives. Am J Med Genet. 2002;114:616–625. doi: 10.1002/ajmg.10604. [DOI] [PubMed] [Google Scholar]
- Nasel C, Azizi A, Veintimilla A, Mallek R, Schindler E. A standardized method of generating time-to-peak perfusion maps in dynamic-susceptibility contrast-enhanced MR imaging. Am J Neuroradiol. 2000a;21:1195–1198. [PMC free article] [PubMed] [Google Scholar]
- Nasel C, Azizi A, Veintimilla A, Mallek R, Schindler E. A standardized method of generating time-to-peak perfusion maps in dynamic-susceptibility contrast-enhanced MR imaging. Am J Neuroradiol. 2000b;21:1195–1198. [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Levin JM, Kaufman MJ, Ross MH, Lewis RF, Harris GJ. Dynamic susceptibility contrast magnetic resonance imaging in neuropsychiatry: present utility and future promise. Eur Radiol. 1997;7(Suppl. 5):216–221. doi: 10.1007/pl00006895. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Brodaty H, Rose N, Haindl W. Regional cerebral blood flow in late-onset schizophrenia: a SPECT study using 99mTc-HMPAO. Schizophr Res. 1997;27:105–117. doi: 10.1016/s0920-9964(97)00088-1. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansella M, Burti L. Integrating evaluative research and community-based mental health care in Verona, Italy. Br J Psychiatry. 2003;183:167–169. doi: 10.1192/bjp.183.2.167. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Tandon R, Koeppe RA. Global cerebral blood flow increase reveals focal hypoperfusion in schizophrenia. Neuropsychopharmacology. 1999;21:368–371. doi: 10.1016/S0893-133X(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil C, Vermeiren R, Wouters LF, Doreleijers TA, van den Brink W. Psychotic symptoms among male adolescent detainees in The Netherlands. Schizophr Bull. 2004;30:73–86. doi: 10.1093/oxfordjournals.schbul.a007069. [DOI] [PubMed] [Google Scholar]
- Wing JK, editor. World Health Organization. Schedules for Clinical Assessment in Neuropsychiatry. Geneva: WHO; 1992. [Google Scholar]