Abstract
The urine collecting duct system of the metanephric kidney develops by growth and branching morphogenesis of an unbranched progenitor tubule, the ureteric bud. Bud branching is mainly dichotomous and new branches form from existing branch tips, which are also the main sites of cell proliferation in the system. This behaviour, and the fact that some genes (e.g. Wnt11, Sox9) are expressed only in tips, suggests that tip cells are in a specific state of differentiation. In this report, we show that the lectin Dolichos biflorus agglutinin (DBA), hitherto regarded and used as a general marker of developing renal collecting ducts, binds to most of the duct system but does not bind to the very tips of growing branches. The zone avoided by DBA corresponds to the zone that expresses Wnt11, and the zone that shows enhanced cell proliferation. If branching of the ureteric bud of cultured embryonic kidneys is inhibited in organ culture, by blocking the kidney's endogenous glial cell-derived neurothrophic factor (GDNF)-based branch-promoting signals, the DBA-binding zone extends to the very end of the tip but is lost from there when branching is re-activated. Similarly, if excess GDNF is provided to growing kidneys, the DBA-free zone expands. DBA-staining status therefore appears to be a sensitive indicator of the morphogenetic activity of the collecting duct system.
Keywords: branching morphogenesis, DBA, Dolichos, kidney, lectin, organogenesis, ureteric bud
Introduction
Branching morphogenesis of epithelia is a key process in mammalian development and is central to the formation of many organs including kidneys, lungs, and prostate, salivary and mammary glands. In recent years, branching morphogenesis has received much attention and studies using transgenic mice and using organ culture have identified many matrix components and growth factors that regulate epithelial growth and branching (Davies, 2002, 2005). Mere identification of such molecules is not enough to explain branching morphogenesis, however, and an understanding of how cells respond to them is urgently required. It is not even clear whether cells that initiate branching are in a specialized cell state, or whether branching can be caused by the activities of a population of completely equivalent cells, as has been suggested on biophysical grounds (Fleury et al. 2004).
The urine collecting duct system of the metanephric kidney is an extremely useful, and also clinically relevant, example of branching morphogenesis. Like the branched epithelia of other organs, it arises by arborization of an initially unbranched epithelium, in this case an outgrowth of the Wolffian duct called the ureteric bud. Growth and branching of the ureteric bud depends on, and is controlled by, various paracrine signals that emanate from the tissues that surround it. Metanephric mesenchyme that has not yet been penetrated by the bud secretes ramogenic growth factors such as glial cell-derived neurothrophic factor (GDNF), neurturin and hepatocyte growth factor (HGF), which act via the Ret and Met tyrosine kinases borne by bud cells to activate the branching programme (Sariola & Saarma, 1999). Once penetrated by the ureteric bud, the mesenchyme responds to bud-derived signals by condensing into clumps and differentiating into nephrons, endothelium and stroma (Davies & Fisher, 2002). Diffusible molecules from these tissues also act on the ureteric bud, preventing it from sending out any more branches in that vicinity but allowing its elongation (more details of this complicated subject can be found in Davies & Fisher, 2002; Vize et al. 2003). Various mutations in the signalling systems mentioned above, and in other genes expressed by ureteric bud cells, are responsible for serious congenital diseases in mice and in humans (Schuchardt et al. 1994; Pichel et al. 1996; Sanchez et al. 1996; Bates, 2000; Harris & Rossetti, 2004). The knowledge base developed over years of research into ureteric bud branching, coupled with the ease with which embryonic kidneys can be made to develop in culture, makes the ureteric bud an ideal system with which to examine basic mechanisms of branching morphogenesis.
In the course of our own studies on the ureteric bud, we have used Dolichos biflorus agglutinin (DBA), an established stain for this tissue, to reveal its morphology as it develops in culture. DBA is known to be an α-N-acetylgalactosamine-binding lectin (Hammarstrom et al. 1977; Imberty et al. 1994) that presumably binds to an α-N-acetylgalactosamine-bearing glycoconjugate carried on the surface of, or in the matrix surrounding, bud cells. The glycoconjugate remains unidentified (and suggestions made for its identity, which include the N-acetylgalactosamine-bearing glycoprotein embigin, are made on evidence no stronger than a similar pattern of expression; see Stuart et al. 2003). Our high-power observations of DBA-stained embryonic kidneys have revealed an unexpected feature of the DBA staining pattern; whereas it stains most of the ureteric bud very strongly, it is excluded from the very tips of the branches where active branching morphogenesis is thought to take place. We have therefore studied the expression of DBA in kidneys that are growing in a variety of culture conditions. We find that DBA binding activity is a sensitive, and inverse, indicator of morphogenetic activity, which supports the idea that cells engaged in branching morphogenesis are in a specialized state of differentiation.
Methods
Organ culture
Kidney rudiments were removed from embryonic day (E)11.5 mouse embryos (strains MF1 and CD1) by microdissection in Earle's minimal essential medium (MEM) (Sigma, Poole, UK, cat. no. M5650), and were cultured on isopore track-etched polycarbonate filters (Millipore, 5 µm pore size) supported at the surface of culture medium by a stainless steel grid. At the time of their isolation, the kidneys had T-shaped ureteric buds. Culture medium consisted of Eagle's MEM with Earle's salts (Sigma M5650) supplemented with 10% heat-inactivated newborn calf serum (NCS/Labtech), penicillin and streptomycin (Sigma). Culture was at 37 °C in 5% CO2. The medium of some kidneys was supplemented with 30 mm sodium chlorate (BDH AnALaR), 100 ng mL−1 GDNF (R&D systems) or 10 µm function-blocking anti-GDNF antibody (R&D systems). Wolffian ducts were removed, with their surrounding mesenchyme, from E10.5 embryos and were cultured using the same media and apparatus as was used for kidney rudiments.
RNA interference
The RNA interference method was that described in Davies et al. (2004). Short interfering RNA (siRNA) duplex sequences were as follows: GDNF 5′-UGUCUCUUCCUGUCCAUCUUUCUCCUU-3′ and 5′-pGGAGAAAGAUGGACAGGAAGAGACA-3′ and Luciferase 5′-CGUACGCGGAAUACUUCGATT-3′ and 5′-UCGAAGUAUUCCGCGUACGTT-3′ this is the same duplex that has been used in Davies et al. (2004) and Elbashir et al. (2001). siRNA was purchased as pre-annealed duplexes from IDT and Ambion. siRNA was complexed to oligofectamine (Invitrogen) and applied to kidney rudiments using the method of Davies et al. (2004). In some cultures, 100 nm exogenous GDNF was added to ‘rescue’ ureteric bud development in kidneys treated with GDNF siRNA, and therefore to prove that the siRNA was having no deleterious effect beyond interference with GDNF synthesis.
Immunofluorescence
After culture, kidney rudiments were fixed on their filters in methanol, initially at −20 °C, and allowed to warm towards room temperature during a 5–15-min period of fixation. They were then rehydrated in phosphate-buffered saline (PBS) and stained with 1 : 100 anti-laminin (Sigma L9393) in PBS with 1% skimmed milk powder. After washing and incubation in a mix of and 10 µg mL−1 FITC DBA (Sigma) and TRITC anti-rabbit secondary antibody (Sigma), specimens were examined using a Leitz epifluorescence microscope or a Leica confocal microscope.
In situ hybridization
The wnt11 plasmid used to generate probes was the same as that used by Kispert et al. (1996), and consisted of 2.1-kb wnt11 cDNA in a pSKII plasmid. Antisense probes were generated by cutting the plasmid with XhoI and using T3 RNA polymerase, while sense ‘probes’ were generated by cutting the plasmid with XbaI and using T7 polymerase, and performing RNA synthesis in the presence of digoxygenin-Uridine Triphosphate. Cultures were fixed first in cold methanol, as above, to enhance their adhesion to their filters, and then fixed overnight in 4% formaldehyde in PBS (made freshly from paraformaldehyde). They were then incubated in 0.1% Tween 20 in PBS (‘PBT’) for 10 min, treated with 10 µg mL−1 proteinase K in PBT for 15 min at room temperature, washed three times each for 5 min in PBT and post-fixed for 40 min in 4% formaldehyde in PBT (made freshly from paraformaldehyde). They were then incubated for 2–4 h at 65 °C in prehybridization solution (50% deionized formamide, 25% 20× SSC, 2% Roche blocking powder, 0.1% Tween 20, 0.5% CHAPS, 1 mg mL−1 yeast tRNA, 0.5 m EDTA and 0.05% heparin). Sense or antisense probe, preheated to 80 °C for 3 min, was then added at 250 ng mL−1 and left overnight at 60 °C. Samples were then washed in post-hybridization solution (50% formamide, 25% 20× SSC, 0.1% Tween 20, 0.5% CHAPS) for twice for 10 min, then in 75% post-hybridization solution/2× SSC, then 50%, then 25%, each for 10 min. They were then washed in 2× SSC, 0.1% CHAPS twice for 30 min, and 0.2× SSC, 0.1% CHAPS for the same time. They were then blocked in TBST with 10% sheep serum, incubated overnight in 1 : 200 alkaline phosphatase-conjugated anti-DIG (Roche) and developed the next day with NBT/BCIP solution. All buffer solutions used for in situ hybridization were treated with diethyl pyrocarbonate, and ProtectRNA (Sigma) was used in all solutions after proteinse K digestion. Sense controls were performed to support antisense experiments, and were negative.
Results
DBA binding is excluded from growing tips of branching ureteric buds
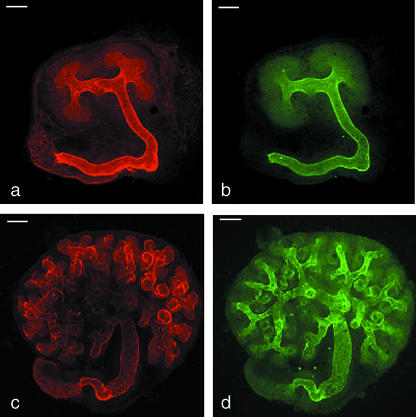
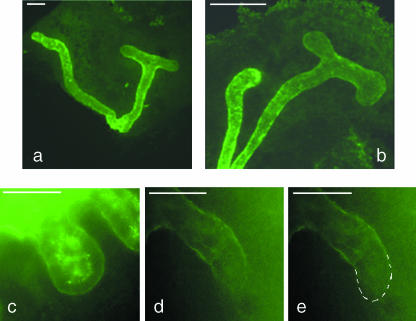
DBA has been described as a marker of the collecting duct in the developing kidneys of several mammalian species, including mouse, rat and rabbit (Watanabe et al. 1981; Holthofer, 1983, 1988b; Holthofer et al. 1987; Laitinen et al. 1987; Plendl et al. 1992; Kovacs et al. 1997; Grupp et al. 1998; Schumacher et al. 2002). We intended to use it as such in our experiments on murine kidneys growing in organ culture, but noticed that the pattern of staining on fixed kidneys was more complex than previous descriptions have implied. In particular, whereas DBA staining was strong along the shafts of the branching ureteric bud, it failed to extend to the tips of growing branches (Fig. 1a,b). This was true of all of the tips, and was true from the earliest stages examined (the ‘T’-bud of E11 kidneys) to 5 days of culture, when the ureteric bud was still actively growing (Fig. 1c,d). The pattern was also visible on kidneys fixed straight from the embryo at E11 and at E14.5; an example of an E14.5 kidney is shown in Fig. 2.
Fig. 1.
Exclusion of DBA staining from the tips of the growing ureteric bud/collecting duct system. (a) Expression of laminin by the ureteric bud of an E11 kidney cultured for 3.5 days, showing the basement membrane in both the stalk and the tip regions; (b) the same kidney stained with DBA, which binds to the stalk but not to the tips. (c,d) The same exclusion of DBA from the tips is seen at 5 days of culture. Scale bar = 100 µm.
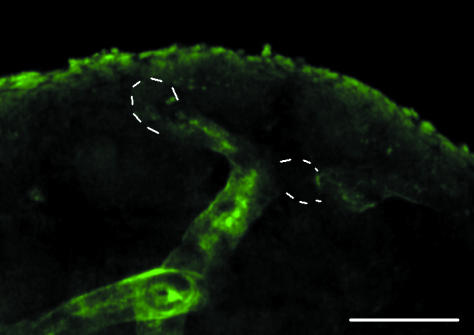
Fig. 2.
DBA staining of the cortical region of a developing kidney removed directly from an E14.5 embryo. As in Fig. 1(d), the DBA staining is strong in the shafts of the ureteric bud/developing collecting duct system but not at its tips (indicated with dotted white lines). Scale bar = 100 µm.
The DBA-negative region is similar in size to the Wnt11-expressing, proliferative tip zone
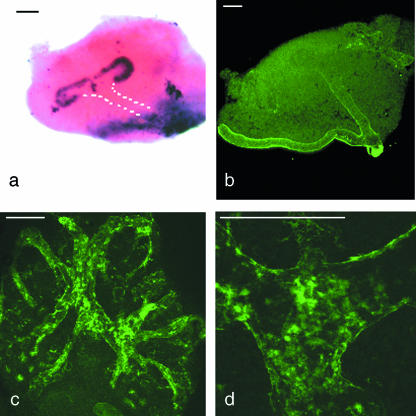
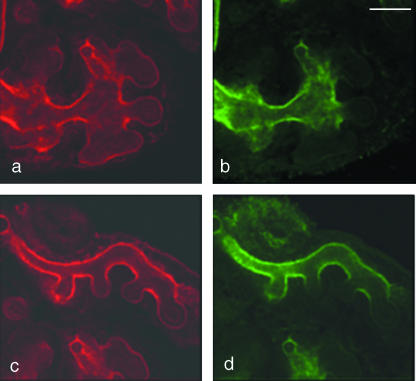
Previous studies of the ureteric bud tip, from this and from other laboratories, have identified other features of tip cells that mark them out from the stalk. One is expression of the wnt11 and sox9 genes, which are transcribed only in a small zone at the very ends of the bud branches (Kispert et al. 1996; Kent et al. 1996). Because the size of the DBA-negative tip region detected by us is reminiscent of the size of the wnt11-expressing domain (Fig. 3a,b), we investigated whether the two markers respected the same boundary; a common boundary would be compatible with the notion that there may be just two states of cell differentiation along the axis of a ureteric bud branch (‘tip’ and ‘stalk’), but a region with neither marker, or a region of overlap, would imply the existence of a succession of differentiation states.
Fig. 3.
(a) The domain of wnt11 mRNA expression in an E11.5 ureteric bud is restricted to the tips. The position of the stalk is shown with a white dotted line. (b) The boundary of DBA expression in a kidney rudiment of the same age is in a similar location to the boundary of wnt11 expression in (b). (c) By 8 days of culture, when branching morphogenesis has ceased, DBA staining extends throughout the ureteric bud/developing collecting duct epithelium. In more mature, medullary regions, it takes on a speckled appearance (d). Scale bar = 100 µm.
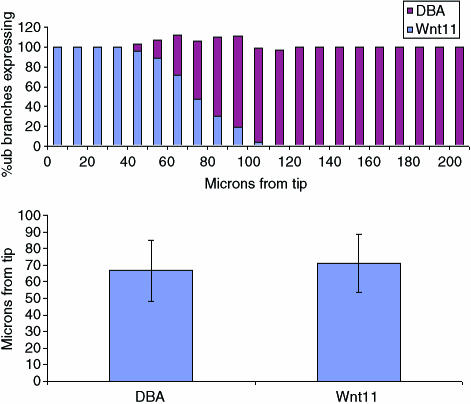
Double-staining of the same specimen for both Wnt11 and DBA proved to be impossible; there are no suitable antibodies to Wnt11 protein, and the in situ hybridization protocol for detection of wnt11 mRNA eliminated DBA staining (probably because the proteinase K used in that protocol destroyed the glycoprotein that bears DBA-binding carbohydrates). We therefore made a series of independent measurements of the distance from a branch tip at which wnt11 mRNA expression ceased, or at which DBA expression began, in E11.5 kidneys cultured for 48 h. The results, shown as a histogram of raw measurements in Fig. 4(a), suggest that the two markers do indeed respect a common boundary. None of the 113 branches examined showed a boundary of expression of either molecule less than 50 µm from the tip or more than 120 µm from it. Significantly, in each 10-µm length of duct in the region between these limits, the sum of the percentage of samples expressing wnt11 mRNA and the percentage binding DBA was maintained remarkably close to 100%; this suggests that, although there might be variation between branches in the exact position of the expression boundary, that same boundary is respected equally by wnt11 expression and DBA staining. The mean position of the boundary of DBA staining was 67 µm (SD = 18 µm) and that of wnt11 mRNA expression was 71 µm (SD = 18 µm; Fig. 4b). An unpaired Student's t-test revealed no evidence of a significant difference.
Fig. 4.
(a) Comparison of the boundaries at which DBA expression commences and Wnt11 expression ceases in E11.5 kidneys cultured for 48 h. The figure shows the data as a histogram of the percentage of samples showing expression of wnt11 (blue) or DBA (red), in each successive 10-µm length of duct, zero being set at the very tip of the branch. DBA data came from 60 measurements, and wnt11 from 53. (b) Bar chart of the mean distance of the DBA binding and wnt11 expression boundaries from the tip. Error bars show the standard deviation.
In an earlier paper in an earlier volume of this journal, we examined the pattern of cell proliferation during ureteric bud development (Michael & Davies, 2004). For quantitative analysis, bud branches of kidneys cultured exactly as in this paper were divided (on the image) into 100-µm lengths and the number of BrdU-positive cells in each length was totalled (100-µm lengths represented the highest feasible resolution, because smaller lengths had too few BrdU-positive cells for statistical analysis). The first 100 µm from the tip contained the main proliferative zone, in which the fraction of cells that were labelled by a pulse of BrdU was over three times that in all other zones. Within the resolution of feasible BrdU incorporation studies, the DBA-negative, Wnt11-positive and the proliferative zones appear to coincide.
DBA binding correlates inversely with morphogenetic activity of ureteric bud tips
By 8 days of culture, when development of the ureteric bud system in our culture conditions was almost complete, DBA staining now extended to the bud tips (Fig. 3c). Its expression along the shafts also changed, acquiring a more punctate pattern as particular cells stained strongly (Fig. 3d). The appearance of strongly staining cells along the collecting duct was most probably a result of differentiation of the primitive ureteric bud into maturing principal cells of the collecting duct, which are known to stain strongly with DBA (Holthofer et al. 1987, 1988; Holthofer, 1988a).
The correlation of the time of culture at which DBA staining begins to extend to the tips of the ureteric bud/collecting duct system, and the time at which that system ceases to grow, suggests that DBA binding activity may reflect the growth status of each tip. To test this, we blocked tip growth in younger cultured kidneys by interfering with a signalling system that is particularly critical to ureteric bud development. In this system, the metanephric mesenchyme produces the diffusible growth-factor GDNF, and this acts on a receptor complex borne by the ureteric bud. The receptor complex consists of the Ret receptor tyrosine kinase, a GFRα1 protein co-receptor and heparan sulfate glycosaminoglycan carbohydrate co-receptors (Sariola & Saarma, 1999; Barnett et al. 2002; Davies et al. 2003). We used three alternative methods to interfere with the GDNF-Ret system. In the first method, which we have used many times before (Davies et al. 1995, 1999; Fisher et al. 2001; Michael & Davies, 2004), 30 mm chlorate ions were used to interfere with the synthesis of the heparin sulfate glycosaminoglycan co-receptor that is required for physiological concentrations of GDNF to activate Ret (Barnett et al. 2002; Davies et al. 2003). In the second method, siRNAs, designed to block GDNF synthesis, were applied to cultured kidney rudiments using the method of Davies et al. (2004). Exogenous GDNF was applied to some siRNA-treated cultures as a ‘rescue’ control, to demonstrate that the siRNA treatment has no deleterious effect on renal development other than inhibiting the synthesis of endogenous GDNF. In the third method (Michael & Davies, 2004), function-blocking anti-GDNF antibodies were used to prevent GDNF from binding to Ret.
All three methods resulted in a severe but reversible inhibition of ureteric bud branching and, in all three cases, DBA staining now extended to the ends of the ureteric bud tips (Fig. 5a–c). Culture of controls in standard medium, or ‘rescue’ of the GDNF siRNA-treated kidneys with exogenous 100 nm GDNF, present throughout, resulted in the development of a normally branched ureteric bud system and normal DBA staining at all times (this ‘rescue’ control was performed to confirm the siRNA effect in non-rescued experiments was specific rather than toxic), and the tips of the bud were then free from DBA binding activity (Fig. 5e,f). Luciferase siRNA had no effect.
Fig. 5.
When branching of the ureteric bud is inhibited by culture in 30 mm chlorate (a) or 10 µm function-blocking anti-GDNF antibody (b), DBA binds even in the tips of the (now inactive) ureteric bud. Compare with Fig. 2(b). Similarly, when GDNF production is inhibited by siRNA that targets GDNF mRNA, DBA stains to the tip of the now almost inactive ureteric bud branches (c), unless exogenous GDNF is used to rescue GDNF activity (d,e: dotted lines in e show the bud outline). Scale bar = 100 µm; all images show 72-h cultures.
The addition of exogenous GDNF to kidneys growing in culture results in an expansion of the ureteric bud tips and an enhanced branching activity (Pichel et al. 1996; Vega et al. 1996; Pepicelli et al. 1997; Sainio et al. 1997). When kidneys grown in the presence of 100 nm GDNF were stained with DBA, staining was absent from the whole of the enlarged tip region, supporting the inverse correlation of branching activity and DBA staining (Fig. 6a,b).
Fig. 6.
(a) Exogenous GDNF causes the tips of ureteric buds, shown here by staining for laminin, to expand. The unusually large tips remain free of DBA staining (b). Treatment of the Wolffian duct with exogenous GDNF causes the emergence of supernumerary ureteric buds (c); these too lack DBA staining at their tips (d). Scale bar = 100 µm.
Ectopic ureteric bud tips induced from the Wolffian duct by GDNF are also DBA negative
In normal development, only one ureteric bud emerges from each Wolffian duct, its emergence being controlled by GDNF released from the caudal end of the intermediate mesoderm (Sainio et al. 1997). Exogenous excess GDNF applied on beads in culture can, however, induce the production of extra ‘ureteric buds’ from the part of the Wolffian duct that lies between mesonephros and metanephros. Because the Wolffian duct, like the shaft of the real ureteric bud, expresses DBA-binding glycoconjugates, we tested whether the emerging tips of GDNF-induced ectopic ‘ureteric buds’ retain the ability of the duct to bind DBA or whether, like the tip of the real ureteric buds, they remain free of DBA-binding glycoconjugates. Control cultures, with no GDNF beads, did not produce ectopic ureteric buds but these buds emerged from most cultures treated with GDNF beads. As shown in Fig. 6(c,d), the tips of the ectopic buds were completely free of DBA binding although the Wolffian duct from which they emerge binds the lectin strongly. Again, the tips are in a state of differentiation distinct from the rest of the epithelial tubule that lies behind them.
Discussion
Using lectin histochemistry, we have shown that the actively growing tips of ureteric buds are in a distinct state of differentiation, with respect to expression of glycoconjugates, compared with the stalk behind them. In particular, they fail to express DBA-binding glycoconjugates that are expressed by stalk cells. We have further shown that this state correlates with morphogenetic activity (when morphogenesis is blocked, the cells express DBA-binding glycoconjugates). Furthermore, we have shown that the transition from the tip state to the stalk state is reversible.
The staining pattern of DBA adds weight to a slowly growing body of evidence that the very tip of the ureteric bud is in a state of differentiation quite distinct from that of its stalk. Other pieces of evidence include restriction of wnt-11 and sox9 gene expression to the ureteric bud tip (Kispert et al. 1996; Pepicelli et al. 1997) and the exclusion of collagen XVIII from it (Lin et al. 2001). It is important to note, however, that some other alleged markers of ‘tips’, such as c-Ret and c-Ros, in fact mark much larger domains of the cortical developing collecting duct tree than the very restricted definition of ‘tip’ used by us – this point is illustrated in Fisher et al. (2001). We have earlier remarked on the concentration of cell proliferation in the area of the ureteric bud tip, and have measured it quantitatively in successive 100-µm lengths of ureteric bud branches (Fisher et al. 2001; Michael & Davies, 2004). Our finding that proliferation is concentrated in the first 100 µm (the smallest resolution at which it was practical to analyse rare and discrete events) is at least compatible with the idea that proliferation, too, respects the same boundary (mean position about 70 µm from the tip) that is respected by wnt11 and DBA.
That the tips might be specialized is perhaps not surprising, for this is the region of the ureteric bud that has to forge a path through mesenchyme and matrix, and that has to undergo periodic branching. By contrast, the ability of cultures of apparently homogeneous cells from cell lines to generate cysts that undergo branching morphogenesis in three-dimensional matrices (Santos & Nigam, 1993) suggests either that specialization of the tip is not a prerequisite for morphogenesis or that even these simple systems self-organize specialized tip domains. Determining whether they too have specialized tips may be a fruitful line of enquiry, the results of which may be of relevance to cancer metastasis as well as basic developmental biology.
Treatment of cultured kidney rudiments with reagents that blocked the branch-promoting GDNF signalling system resulted in cells at the now-inactive branch tips taking on the DBA binding characteristics of stalk cells. Treatment of DBA-expressing Wolffian duct with high concentrations of GDNF, by contrast, caused it to sprout extra ureteric buds, the cells of which ceased to bind to DBA. Taken together, these results imply that the cell states that correspond to branching tip and non-branching stalk are somewhat fluid and interconvertible in both directions. Differentiation in this system may not therefore be a once-and-for-all event that takes place as cells leave the tip area to become new stalk, but rather a reversible choice that is under continuous environmental control. This fact, which is supported by the fact that so many cell lines obtained from mature collecting ducts can undergo branching in three-dimensional culture, may be of great use in the fields of regeneration and of tissue engineering.
The obvious remaining question concerns the molecular identity of the DBA-binding glycoconjugate about which, alas, nothing is known. The immediate carbohydrate ligand of DBA, α-N-acetylgalactosamine, has been known for a long time (Hammarstrom et al. 1977; Imberty et al. 1994) but the renal glycoconjugate that bears it has not been identified even in adult kidneys, and even if it had there is no reason to suppose that this will be the same glycoconjugate that binds DBA in very early embryonic kidneys. Unfortunately, the small size of embryonic kidneys precludes direct isolation of the DBA-binding glycoconjugate in quantities large enough for peptide sequencing.
Acknowledgments
We would like to thank Seppo Vainio for the Wnt11 probe sequence, and Linda Wilson for her help with confocal microscopy. D.E.S. is supported by a studentship from the Anatomical Society. This work was funded by the BBSRC, EuRoGene and the Leverhulme Trust.
References
- Barnett MW, Fisher CE, Perona-Wright G, Davies JA. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci. 2002;115:4495–4503. doi: 10.1242/jcs.00114. [DOI] [PubMed] [Google Scholar]
- Bates CM. Kidney development: regulatory molecules crucial to both mice and men. Mol Genet Metab. 2000;71:391–396. doi: 10.1006/mgme.2000.3072. [DOI] [PubMed] [Google Scholar]
- Davies J, Lyon M, Gallagher J, Garrod D. Sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development. 1995;121:1507–1517. doi: 10.1242/dev.121.5.1507. [DOI] [PubMed] [Google Scholar]
- Davies JA, Millar CB, Johnson EM, Jr, Milbrandt J. Neurturin: an autocrine regulator of renal collecting duct development. Dev Genet. 1999;24:284–292. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<284::AID-DVG11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Davies JA. Do different branching epithelia use a conserved developmental mechanism? Bioessays. 2002;24:937–948. doi: 10.1002/bies.10161. [DOI] [PubMed] [Google Scholar]
- Davies JA, Fisher CE. Genes and proteins in renal development. Exp Nephrol. 2002;10:102–113. doi: 10.1159/000049905. [DOI] [PubMed] [Google Scholar]
- Davies JA, Yates EA, Turnbull JE. Structural determinants of heparan sulphate modulation of GDNF signalling. Growth Factors. 2003;21:109–119. doi: 10.1080/08977190310001621005. [DOI] [PubMed] [Google Scholar]
- Davies JA, Ladomery M, Hohenstein P, et al. Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum Mol Genet. 2004;13:235–246. doi: 10.1093/hmg/ddh015. [DOI] [PubMed] [Google Scholar]
- Davies J. Branching Morphogenesis. Austin, TX: Landes Biomedical; 2005. [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Fisher CE, Michael L, Barnett MW, Davies JA. Erk MAP kinase regulates branching morphogenesis in the developing mouse kidney. Development. 2001;128:4329–4338. doi: 10.1242/dev.128.21.4329. [DOI] [PubMed] [Google Scholar]
- Fleury V, Watanabe W, Nguyen T-H, et al. Physical mechanisms of branching morphogenesis in animals. In: Davies J, editor. Branching Morphogenesis. Austin, TX: Landes Bioscience; 2004. pp. 202–234. [Google Scholar]
- Grupp C, Troche I, Steffgen J, et al. Highly specific separation of heterogeneous cell populations by lectin-coated beads: application for the isolation of inner medullary collecting duct cells. Exp Nephrol. 1998;6:542–550. doi: 10.1159/000020569. [DOI] [PubMed] [Google Scholar]
- Hammarstrom S, Murphy LA, Goldstein IJ, Etzler ME. Carbohydrate binding specificity of four N-acetyl-D-galactosamine-‘specific’ lectins: Helix pomatia A hemagglutinin, soy bean agglutinin, lima bean lectin, and Dolichos biflorus lectin. Biochemistry. 1977;16:2750–2755. doi: 10.1021/bi00631a025. [DOI] [PubMed] [Google Scholar]
- Harris PC, Rossetti S. Molecular genetics of autosomal recessive polycystic kidney disease. Mol Genet Metab. 2004;81:75–85. doi: 10.1016/j.ymgme.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Holthofer H. Lectin binding sites in kidney. A comparative study of 14 animal species. J Histochem Cytochem. 1983;31:531–537. doi: 10.1177/31.4.6827083. [DOI] [PubMed] [Google Scholar]
- Holthofer H, Schulte BA, Spicer SS. Expression of binding sites for Dolichos biflorus agglutinin at the apical aspect of collecting duct cells in rat kidney. Cell Tissue Res. 1987;249:481–485. doi: 10.1007/BF00217319. [DOI] [PubMed] [Google Scholar]
- Holthofer H. Cell type-specific glycoconjugates of collecting duct cells during maturation of the rat kidney. Cell Tissue Res. 1988a;253:305–309. doi: 10.1007/BF00222286. [DOI] [PubMed] [Google Scholar]
- Holthofer H. Cell type-specific glycoconjugates of collecting duct cells during maturation of the rat kidney. Cell Tissue Res. 1988b;253:305–309. doi: 10.1007/BF00222286. [DOI] [PubMed] [Google Scholar]
- Holthofer H, Schulte BA, Spicer SS. Heterogeneity of apical glycoconjugates in kidney collecting ducts: further studies using simultaneous detection of lectin binding sites and immunocytochemical detection of key transport enzymes. Histochem J. 1988;20:471–477. doi: 10.1007/BF01002645. [DOI] [PubMed] [Google Scholar]
- Imberty A, Casset F, Gegg CV, Etzler ME, Perez S. Molecular modelling of the Dolichos biflorus seed lectin and its specific interactions with carbohydrates: alpha-D-N-acetyl-galactosamine, Forssman disaccharide and blood group A trisaccharide. Glycoconj J. 1994;11:400–413. doi: 10.1007/BF00731275. [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Kovacs J, Zilahy M, Gomba S. Morphology of cystic renal lesions. Lectin and immuno-histochemical study. Acta Chir Hung. 1997;36:176–178. [PubMed] [Google Scholar]
- Laitinen L, Virtanen I, Saxen L. Changes in the glycosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochem Cytochem. 1987;35:55–65. doi: 10.1177/35.1.3794309. [DOI] [PubMed] [Google Scholar]
- Lin Y, Zhang S, Rehn M, et al. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: association with sonic hedgehog and ectopic surfactant protein C. Development. 2001;128:1573–1585. doi: 10.1242/dev.128.9.1573. [DOI] [PubMed] [Google Scholar]
- Michael L, Davies JA. Pattern and regulation of cell proliferation during murine ureteric bud development. J Anat. 2004;204:241–255. doi: 10.1111/j.0021-8782.2004.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli CV, Kispert A, Rowitch DH, McMahon AP. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol. 1997;192:193–198. doi: 10.1006/dbio.1997.8745. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Plendl J, Schoenleber B, Schmahl W, Murray AB, Sinowatz F. Sexual dimorphism of the kidney in the NMRI mouse as shown by Dolichos biflorus agglutinin labelling. Anat Histol Embryol. 1992;21:118–126. doi: 10.1111/j.1439-0264.1992.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Sainio K, Suvanto P, Davies J, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev Biol. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. GDNF and its receptors in the regulation of the ureteric branching. Int J Dev Biol. 1999;43:413–418. [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Schumacher K, Strehl R, Minuth WW. Detection of glycosylated sites in embryonic rabbit kidney by lectin chemistry. Histochem Cell Biol. 2002;118:79–87. doi: 10.1007/s00418-002-0422-2. [DOI] [PubMed] [Google Scholar]
- Stuart RO, Bush KT, Nigam SK. Changes in gene expression patterns in the ureteric bud and metanephric mesenchyme in models of kidney development. Kidney Int. 2003;64:1997–2008. doi: 10.1046/j.1523-1755.2003.00383.x. [DOI] [PubMed] [Google Scholar]
- Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA. 1996;93:10657–10661. doi: 10.1073/pnas.93.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vize PD, Woolf AS, Bard JBL. The Kidney: from Normal Development to Congenital Disease. San Diego: Elsevier; 2003. [Google Scholar]
- Watanabe M, Muramatsu T, Shirane H, Ugai K. Discrete distribution of binding sites for Dolichos biflorus agglutinin (DBA) and for peanut agglutinin (PNA) in mouse organ tissues. J Histochem Cytochem. 1981;29:779–780. doi: 10.1177/29.7.7053086. [DOI] [PubMed] [Google Scholar]