Abstract
As part of the hip joint, the proximal femur is an integral locomotor component. Although a link between locomotion and the morphology of some aspects of the proximal femur has been identified, inclusive shapes of this element have not been compared among behaviourally heterogeneous hominoids. Previous analyses have partitioned complex proximal femoral morphology into discrete features (e.g. head, neck, greater trochanter) to facilitate conventional linear measurements. In this study, three-dimensional geometric morphometrics are used to examine the shape of the proximal femur in hominoids to determine whether femoral shape co-varies with locomotor category. Fourteen landmarks are recorded on adult femora of Homo, Pan, Gorilla, Pongo and Hylobates. Generalized Procrustes analysis (GPA) is used to adjust for position, orientation and scale among landmark configurations. Principal components analysis is used to collapse and compare variation in residuals from GPA, and thin-plate spline analysis is used to visualize shape change among taxa. The results indicate that knucklewalking African apes are similar to one another in femoral shape, whereas the more suspensory Asian apes diverge from the African ape pattern. The shape of the human and orangutan proximal femur converge, a result that is best explained in terms of the distinct requirements for locomotion in each group. These findings suggest that the shape of the proximal femur is brought about primarily by locomotor behaviour.
Keywords: Generalized Procrustes analysis, Gorilla, hindlimb, hominoid, Homo, Hylobates, locomotion, morphology, Pan, Pongo, thin plate spline
Introduction
Many studies link primate proximal femoral morphology with the role of the hindlimb in locomotion, indicating that a strong functional signal underlies the configuration of this element. For instance, primate diaphyseal structure (Schaffler et al. 1985; Ruff, 1987, 1988, 2002; Gebo, 1989; Anemone, 1990, 1993; Ruff et al. 1991; Ruff & Runestad, 1992; Rafferty & Ruff, 1994; Lieberman et al. 2001) and relative joint surface area (Ruff, 1988; Godfrey et al. 1995; MacLatchy & Bossert, 1996) are shown to vary according to locomotor patterns. Morphology of the recent human proximal femur relates to bipedal locomotion, such as the relatively large head that resists ground reaction forces, and the long neck and superior position of the head relative to the greater trochanter that provide advantageous leverage for hip-stabilizing musculature (Lovejoy, 1975; McHenry & Corruccini, 1976, 1978; Corruccini & McHenry, 1978; Stern & Susman, 1981; Jungers, 1988, 1990; MacLatchy, 1996; Rafferty, 1998; Lovejoy et al. 2002). Compared with humans, great apes have a greater trochanter that projects superiorly in relation to a smaller femoral head (McHenry & Corruccini, 1976, 1978; Lovejoy et al. 2002). This configuration may provide greater leverage for actions of the gluteus medius as an extensor of the thigh in climbing (Preuschoft, 1970; Stern & Susman, 1981), although the relationship between these aspects of femoral morphology and locomotion in apes is not well documented.
Previous studies have provided a general understanding of the relationship between locomotion and morphology among humans and apes in the proximal femur. However, variation in the shape of this element among the behaviourally heterogeneous and size-variable great apes has not been fully explored. Furthermore, it is not known how the great ape proximal femur compares with that of the more distantly related gibbons.
In terms of forelimb skeletal morphology, gibbons have been characterized as divergent relative to other apes (Larson, 1998; Young, 2003). Divergent morphology is consistent with the highly specialized, and potentially secondarily derived, suspensory adaptation of gibbons (Szalay & Delson, 1979; Cartmill, 1985; Pilbeam, 1996). If forelimb morphology is secondarily derived, it is possible that hindlimb anatomy similarly diverges from that of the great apes. Indeed, a different pattern of gluteal muscle recruitment occurs during climbing in gibbons and great apes (Stern & Susman, 1981). The lower body mass of gibbons may contribute to deviation from great ape femoral shape as well. Thus, the shape of the gibbon's proximal femur may differ from that of other apes because of separate evolutionary trajectories or because of size differences. On the other hand, unlike African apes, Asian apes use their hindlimbs primarily for climbing, although the gibbon repertoire also includes leaping and bipedality, and orangutans incorporate quadrupedalism (Fleagle, 1976; Sugardjito & van Hooff, 1986; Gebo, 1989). Because orangutans and gibbons have a common suspensory and climbing locomotor pattern they could have similar femoral morphology.
In this study, three-dimensional coordinates were used to summarize the shape of the proximal femur in order to ascertain shape variation and its relationship to locomotion among hominoids. The hypothesis was that phenetic affinities in the shape of the proximal femur reflect the degree of similarity in locomotor behaviour. This hypothesis follows from previous studies of the hominoid femur that have documented some locomotor-related differences among apes and humans. Thus, the expectation was that knucklewalking apes would be found to be similar in proximal femoral shape, while the more suspensory orangutans and gibbons may share aspects of proximal femoral shape. The shape of the human proximal femur was expected to diverge from that of other hominoids owing to the derived locomotor pattern of bipedalism.
Geometric morphometric analyses of multidimensional shape data are used widely in studies of bony morphology of primate crania and postcrania (Lynch et al. 1996; Delson et al. 2001; Lague, 2002; Lockwood et al. 2002; Penin et al. 2002; Berge & Penin, 2004). The proximal femur is an appropriate candidate for three-dimensional analysis because its complex convex and concave morphology is not easily partitioned into discrete linear segments. Although the femur has been successfully analysed in discrete linear segments, three-dimensional data allow greater resolution of overall shape of the proximal femur, including aspects that are difficult to quantify, such as the relative positions of the greater and lesser trochanter, head, and trochanteric fossa.
The structure of the proximal epiphysis during development is broadly similar across the hominoids studied here (Schultz, 1970; Reno et al. 2003; Serrat et al. 2004; M. A. Serrat, personal communication), although the tempo of epiphyseal development does vary (Schultz, 1970; Scheuer & Black, 2000). Cross-taxon similarity in epiphyseal structure suggests that variation in adult specimens arises from an apparently homologous developmental origin. Ontogenetic study would provide a sense of when and how variation develops, and should be undertaken in the future. However, an initial study of intergeneric variation was considered appropriate to establish the pattern of variation in adult specimens. The present shape analysis allowed a quantitative study of shape differences within and between hominoid genera and a statistical study of morphological traits that are difficult to measure with more traditional methods.
Materials and methods
Samples
Proximal femoral samples were obtained from the following taxa: Hylobates, Pongo, Gorilla, Pan and Homo (Table 1). All specimens were adult or near-adult, meaning that epiphyses of the head and greater trochanter were fused to the diaphysis, although the epiphyseal line was visible in a few specimens. The ape sample included only wild-shot individuals. The numbers of males and females were approximately equal for each species and subspecies. When available, subspecies were analysed separately.
Table 1.
Femoral samples
| Taxon | n |
|---|---|
| Homo sapiens* | 130 |
| Gorilla gorilla gorilla†‡§ | 19 |
| Gorilla gorilla beringei†‡§ | 13 |
| Pan troglodytes†‡§ | 59 |
| Pan paniscus†‡ | 24 |
| Pongo pygmaeus pygmaeus†§¶ | 26 |
| Pongo pygmaeus abeliiঠ| 18 |
| Hylobates lar lar§ | 22 |
Terry Collection, National Museum of Natural History, Washington, DC; Nubian Collection, Arizona State University, Tempe; Dart Collection, University of Witwatersrand, Johannesburg.
Musée Royal de l'Afrique Centrale, Tervuren.
National Museum of Natural History, Washington, DC.
Museum of Comparative Zoology, Harvard University, Cambridge.
Museum für Naturkunde der Humboldt Universität, Berlin.
The locomotor behaviours practised most frequently by apes have been collected from behavioural reports by others (e.g. Gebo, 1996), and most recently by Ruff (2002). Table 2 presents the characteristic locomotor repertoire of each taxon modified from Ruff (2002: 308). These frequent behaviours form the basis of locomotor categories for comparison with femoral shape.
Table 2.
Locomotor behaviours by taxon in order of frequency (modified from Ruff, 2002)
| Taxon | Locomotor category | Reference |
|---|---|---|
| Homo | Bipedalism | |
| Pan | Quadrupedalism (knucklewalking), climbing, bimanual suspension | Doran (1996), Hunt (1992) |
| Gorilla | Quadrupedalism (knucklewalking), climbing, some bimanual suspension | Remis (1995, 1999) |
| Pongo | Climbing, bimanual suspension, quadrupedalism (fistwalking) | Sugardjito & van Hooff (1986), Cant (1987) |
| Hylobates | Bimanual suspension, climbing | Fleagle (1976) |
Data acquisition
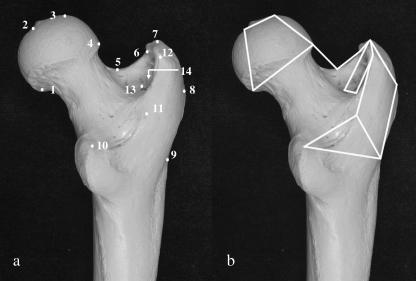
Fourteen landmarks on the head, neck, lateral surface of the greater trochanter, lateral diaphysis, lesser trochanter and intertrochanteric crest were identified for three-dimensional recording (Fig. 1a; Table 3). These landmarks were chosen to provide an adequate summary of aspects of femoral morphology that differ among hominoids, such as the shape of the head, neck, and greater trochanter, individually, and in relation to one another (McHenry & Corruccini, 1978; Lovejoy et al. 2002). The landmarks were Type III (Bookstein, 1991), meaning that they were not demonstrably homologous, but they were geometrically equivalent across study specimens. The landmarks could be located repeatedly based on some spatial property – in this case, points of maximum curvature and anatomical boundaries (Bookstein, 1991; Dryden & Mardia, 1998; O'Higgins, 2000). Landmarks were recorded using a Microscribe 3DX portable digitizer with a manufacturer-reported accuracy of ±0.23 mm (Immersion Corporation, 1998).
Fig 1.
Landmark locations (a) and wireframe diagram of linked landmarks (b). See Table 3 for definitions of landmarks.
Table 3.
Definitions of landmarks
| 1 | Inferiormost point on head–neck border at midline |
| 2 | Fovea capitis centre at midline (in Pongo, centre of femoral head) |
| 3 | Superiormost point of head |
| 4 | Head–neck border at neck midline |
| 5 | Neck midpoint between head and greater trochanter |
| 6 | Neck–greater trochanter border anterior to trochanteric fossa |
| 7 | Superiormost point of greater trochanter, above anterior border of trochanteric fossa |
| 8 | Lateralmost point on greater trochanter, between superior and inferior borders |
| 9 | Greater trochanter base at level of inferior edge of gluteus minimus attachment |
| 10 | Posterosuperior point of lesser trochanter |
| 11 | Superoinferior midpoint of intertrochanteric crest |
| 12 | Posterosuperior point on intertrochanteric crest |
| 13 | Posterior point on trochanteric fossa |
| 14 | Deepest point of trochanteric fossa |
Intra-observer error
In order to verify that the 14 landmarks were replicable, three digitizer trials were conducted on two human and two chimpanzee femora. The initial two trials were separated by 2 h and the final trial took place 1 day later. The mean Euclidean distance between landmark configurations among repeats of the same specimens was 0.04 (range: 0.03–0.06). The mean Euclidean distance between landmark configurations of different specimens was 0.21 (range 0.15–0.26). In no case did the Euclidean distance between landmark configurations of the same specimen overlap with those of different specimens. These results confirmed that the 14 landmarks were replicable with minimal difference on the same specimens, and adequately expressed difference between discrete specimens.
Analytical procedures
Three analyses were conducted. The first included all hominoid taxa and the second involved non-human hominoids, allowing identification of variation among the groups with and without the inclusion of humans. The third focused on African apes to identify variation between apes that shared a locomotor category, but were very different in body size.
In each analysis the recorded landmarks were scaled, rotated and translated using generalized Procrustes analysis (GPA), a procedure that reduced the sum of squared differences between corresponding landmarks (Rohlf & Slice, 1990; Bookstein et al. 1999; O'Higgins, 2000; Delson et al. 2001). To conduct statistical analysis, the shape data were projected onto a plane tangent to their original high-dimensional location (Dryden & Mardia, 1998; O'Higgins & Jones, 2006). Principal components analysis (PCA) was used to collapse and compare variation among landmark configurations (O'Higgins, 2000; Delson et al. 2001). The shape analysis software Morphologika was used for GPA and PCA (O'Higgins & Jones, 2006). The analysis of all taxa included an unweighted pair group (UPGMA) cluster analysis of 39 principal components scores (100% of total variance), executed using the Statistica software package (Release 7.1, Statsoft, Inc.). A cluster diagram depicted phenetic relationships among taxa based on Euclidean distances among species mean PC scores (100% of variation).
Variation was visualized through thin plate spline analysis (TPSA). TPSA is an interpolation function that generates a surface grid of the landmarks around a reference shape and, using minimum bending energy, deforms the grid to match the surface associated with landmarks of a target shape (Bookstein, 1989; Dryden & Mardia, 1998). In this analysis, TPSA was based on landmark configurations calculated in PCA, and reflected shape transformation from reference points to target points represented on PCA axes. Shape transformations between reference points and target points allowed for better precision in visualizing differences among shapes that were of particular interest. Landmark configurations were displayed as wireframe diagrams of linked landmarks (Fig. 1b).
With GPA, size effects related to isometry were removed, but allometric size differences were retained (O'Higgins, 2000). Allometry in proximal femoral shape, if present, points to shape changes that were greater or smaller than expected at a given size (Jungers et al. 1995). In order to determine if size and shape departed from isometry, least squares regressions of centroid size computed from the GPA and PC scores were conducted. Regressions of centroid size (independent variable) on PC scores (dependent variable) were carried out across all hominoid taxa, all apes, African apes and within each taxon for PCs that were informative about shape variation among groups. Because proportional size was removed in GPA, if shape did not depart from geometric similarity, the regression slope and correlation coefficient should not differ significantly from zero.
Results
PCA of all hominoids
Cluster analysis
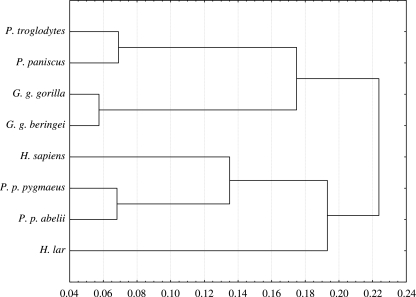
Two major clusters were formed, one consisting of African apes and the other consisting of Asian apes and Homo sapiens (Fig. 2). In the African ape cluster, Pan troglodytes and P. paniscus grouped together, as did the subspecies of Gorilla. In the other major cluster Hylobates was the most distant taxon, and relative to Hylobates, Pongo and Homo formed a group. Subspecies of Pongo grouped most closely together within the Asian ape and Homo cluster. In light of earlier work based on classic biometric methods (e.g. McHenry & Corruccini, 1978; Lovejoy et al. 2002), it was surprising that humans were not separated from other taxa, but instead clustered with Asian apes.
Fig 2.
Cluster analysis of species mean PC scores based on 39 principal components (100% of variance). UPGMA clustering, Euclidean distances.
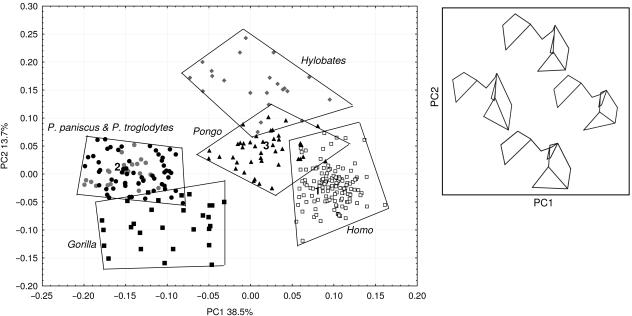
Significant principal components
The first two principal components comprised 52.2% of the total variance. These provided good shape discrimination among hominoid taxa (Fig. 3), while subsequent PCs were not informative about differences between groups. Variation on PC1, which comprised 38.5% of the total variance, separated Homo and Asian apes from the African apes (Fig. 3). Asian apes were not separated from one another on PC1, but Homo cases were distinct from the Asian ape group. The variation summarized by PC1 related to the size of the head, and of the size, shape and position of the greater trochanter. Homo and the Asian apes, compared with African apes, had higher scores on PC1 and had a larger head diameter and a shorter greater trochanter that was low relative to the femoral head (Fig. 3).
Fig 3.
PCA scatterplot of all hominoids for PC1 and PC2. Numbers 1–3 correspond to shapes in TPSA; see Fig. 4. The box depicts wireframe diagrams (posterior view) that correspond to landmark configurations in the PCA scatterplot. Left wireframe, low PC1; right wireframe high PC1. Top wireframe, high PC2; bottom wireframe, low PC2.
The variation that underlay PC2 most distinctly separated gorillas from gibbons, with 13.7% of the total variance. The PC scores of orangutans, chimpanzees, humans and, to a lesser degree, gorillas overlapped extensively on PC2, while scores for gibbons were higher. Variation on PC2 related to the shape of the intertrochanteric crest, changes in the mediolateral breadth of the greater trochanter and the shape of the head (Fig. 3). Pan, Pongo and particularly Hylobates, taxa that were higher on PC2, had mediolaterally inflated femoral heads in comparison with those of Gorilla and Homo. Hylobates was separated from other taxa on PC2 because the intertrochanteric crest in this taxon was barely pronounced and did not project posteriorly as was the case in the other groups, especially gorillas (Fig. 3). No discrimination was evident between P. troglodytes and P. paniscus.
TPSA: H. sapiens vs. Pan and Pongo
The TPS transformations in Fig. 4 illustrate shape differences between humans and apes. The grid depicts the deformation required to transform the reference shape (Homo) to the target shapes (Pan and Pongo). The reference and target shapes are simply representatives for each group chosen from the centre of the cloud of landmark configurations. All 14 landmarks are included. The photograph and TPS wireframe views are posterior. Not included in TPSA are gorillas, which relative to Homo are quite similar in shape to Pan, and Hylobates, which is similar to Pongo.
Fig 4.
TPS transformation from reference shape Homo (1) to target shapes Pan (2) and Pongo (3). Photographs indicate the relationship between the proximal femur and the wireframe of linked landmarks for each taxon. Dotted lines depict linkages between landmarks that are not observable in photo view. The photograph views, wireframes and grid orientations are posterior. All landmarks are depicted. See text for discussion.
In Homo, the head extends above the superior point of the greater trochanter. The greater trochanter is superoinferiorly short and mediolaterally broad. The distance between the head–neck junction and the neck–greater trochanter junction is comparatively long. The lesser trochanter is positioned inferiorly on the shaft. Pan has a head that is at the level of the superior point of the greater trochanter. The distance between the superior and inferior borders of the greater trochanter is long, while the distance between the head–neck and neck–greater trochanter junctions is short. The lesser trochanter is superiorly positioned on the shaft. The Pan TPS transformation grid is elongated superoinferiorly at the lateral edge in response to deformation associated with the longer greater trochanter, and the position of this feature relative to the head. The transformation grid is shortened mediolaterally to reflect the shorter neck and reduced breadth of the greater trochanter. The head, as captured by landmarks 1–4 (head diameter) is smaller in Pan than in Homo, which is part of the variation underlying PC1 but is not obvious in the TPS transformation (see Fig. 3).
Compared with the TPS transformation involving Pan, the transformation from Homo to Pongo requires less grid deformation. The primary change to the target grid is slight elevation of the greater trochanter, which illustrates two related differences between Homo and Pongo: the angle of the neck from the midpoint to the junction of the greater trochanter is reduced, and in conjunction, the greater trochanter is slightly higher relative to the head compared with Homo.
PCA of all apes
The first three principal components summarized 59.2% of the total variance. Variation on PC1 (31.1%) separated Asian from African apes (Fig. 5a). Separation between Asian and African apes was due largely to differences in greater trochanteric shape and position, and the length of the neck from the midpoint to the border with the head (Fig. 1a, landmarks 4 and 5). In comparison with Asian apes, African apes had a longer greater trochanter and the superior point of this feature was high relative to the head (Fig. 5a). The Asian ape neck was longer than that of the African ape from the midpoint to head border (Fig. 5a).
Fig 5.
PCA scatterplot of all apes, PC1 and 2 (a), and PC1 and 3 (b). Numbers 1–4 correspond to shapes in TPSA; see Figs 6 and 7. The boxes depict wireframe diagrams (posterior view) that correspond to landmark configurations in the PCA scatterplots. Left wireframes, low PC1; right wireframes, high PC1. Top wireframes, high PC2; bottom wireframes, low PC2.
Gorillas were separated from other apes on PC2, which comprised 16.3% of the total variance (Fig. 5a). To some extent, gibbons were different from orangutans on this component. Pan paniscus cases were clustered at the low end of the Pan distribution of PC2 scores. Principal component 2 was related to the orientation of the head on the neck and the depth of the trochanteric fossa. Gorillas, which were highest on the component, had a medially orientated head and shallow intertrochanteric fossa (Fig. 5a). Other taxa were lower on this component, reflecting head orientation that was comparatively superior relative to the neck, and a shallow trochanteric fossa (illustrated below).
PC3 explained 11.8% of the variance and separated Hylobates from Pongo (Fig. 5b). As illustrated below, the shape variation summarized by this component related to the degree of posterior projection of the intertrochanteric crest, which was minimal in gibbons. No separation was evident among subspecies of Gorilla or of Pongo on the first three PCs.
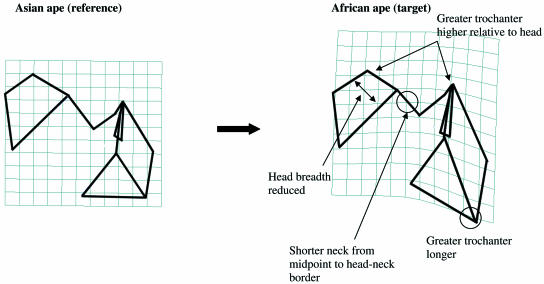
TPSA: Asian apes vs. African apes
Figure 6 illustrates the differences (in posterior view) between Asian and African apes using a wireframe diagram that is derived from the area of overlapping PC1 and PC2 scores of Hylobates and Pongo, and a wireframe diagram derived from the area of overlapping PC1 and PC2 scores of Pan and Gorilla (see Fig. 5a). Asian apes are characterized by a superoinferiorly shorter greater trochanter, in contrast to the long greater trochanter of African apes. Relative to Pongo and Hylobates, the greater trochanter of African apes extends higher in relation to the head. The superiorly positioned head in Asian apes is due, in part, to the greater distance between the head and midpoint of the neck than is the case for African apes.
Fig 6.
TPS transformation from Asian ape reference shape (1) to African ape target shape (2). The wireframe view and grid orientation are posterior. All landmarks are depicted. See text for discussion.
Transformation from an Asian ape to an African ape results in shrinking of the target grid at the neck and lengthening of the grid at the greater trochanter, reflecting the shorter neck and longer greater trochanter of African apes. The grid is also compressed at the head, reflecting the reduced mediolateral dimension of the head in the African ape. This transformation is similar to the TPS transformation from H. sapiens to Pan in Fig. 4.
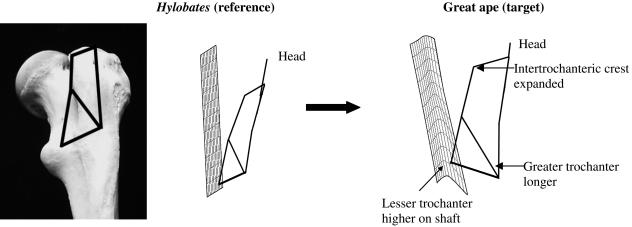
TPSA: Hylobates vs. great apes
Hylobates diverges from other taxa in having a compressed intertrochanteric crest that does not project posteriorly. Figure 7 depicts a TPS transformation between Hylobates (reference) and a ‘generic great ape’ target shape centrally located in the cluster of great ape taxa in Fig. 5(b). The grid representing this transformation is positioned along the posterior axis, and the wireframe view is lateral, as is approximated in the photograph of the gibbon femur. The wireframe links landmarks 4–12 (Fig. 1a), which describe the shape of the greater trochanter, and its relationship to the lesser trochanter and the intertrochanteric crest.
Fig 7.
TPS transformation from Hylobates reference shape (3) to great ape target shape (4) in lateral view. The wireframe view is lateral, as approximated in the photograph at left. The transformation grid orientation is posterior. Landmarks 4–12 are depicted. See text for discussion.
The transformation grid demonstrates that the intertrochanteric crest projects posteriorly in great apes compared with Hylobates, because it is pushed posteriorly in the target shape. The lower corner of the grid is deformed inferiorly, which reflects the lengthening of the greater trochanter from the gibbon reference to the great ape target shape. At the base, the target grid is morphed upward, indicating the higher position of the lesser trochanter on the shaft in the great apes.
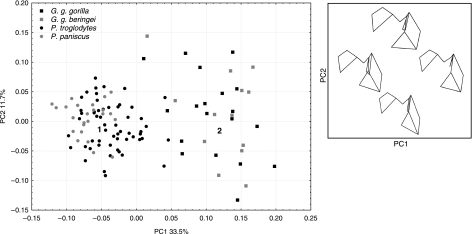
PCA of African apes
The first two components explained 45.2% of the variance. The first component adequately summarized the shape variation between the genera (Fig. 8). The remaining components did not result in separation between P. troglodytes and P. paniscus, or between subspecies of Gorilla. Variation on PC1 (33.5%) distinguished Gorilla from Pan on the basis of head and intertrochanteric crest shape and orientation, and intertrochanteric fossa depth, while PC2 (11.7%) consisted of variation in gorillas related to the breadth and length of the greater trochanter (Fig. 8).
Fig 8.
PCA scatterplot of African apes for PC1 and PC2. Numbers 1 and 2 correspond to shapes in TPS transformation; see Fig. 9. The box depicts wireframe diagrams (posterior view) that correspond to landmark configurations in the PCA scatterplot. Left wireframe, low PC1; right wireframe, high PC1. Top wireframe, high PC2; bottom wireframe, low PC2.
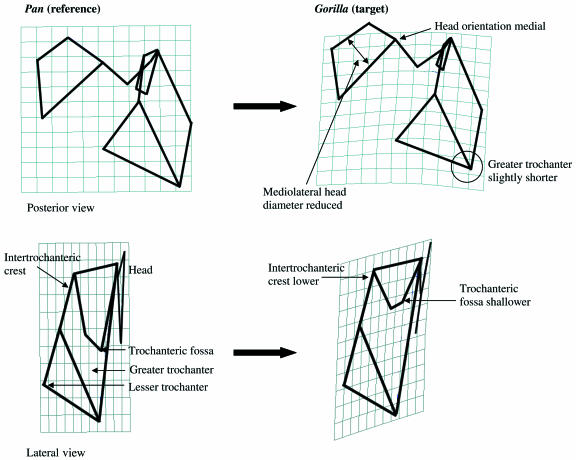
TPSA: Pan vs. Gorilla
Femoral shape is similar in the two groups with some exceptions that are illustrated in the TPS transformations in Fig. 9. In these transformations Pan is the reference shape and Gorilla is the target shape. The posterior view transformation indicates that, in Gorilla, the border of the head at the neck is medially positioned relative to that of Pan. This difference relates to the fact that head in Gorilla is slightly medially directed relative to the neck in comparison with the more superiorly directed head of Pan. Additionally, the mediolateral length of the Gorilla head is narrower than that of Pan. Finally, the target grid illustrates that the greater trochanter of Gorilla is slightly shorter than that of Pan.
Fig 9.
TPS transformation from Pan reference shape (1) to Gorilla target shape (2). In the top transformation, wireframe view and grid orientation are posterior, and all landmarks are depicted. In the bottom transformation, the wireframe view is lateral and grid orientation is posterior. Landmarks 7–12 are depicted. See text for discussion.
The lateral view of the TPS transformation demonstrates that the Gorilla intertrochanteric crest, including the lesser trochanter, is different from that of Pan. In lateral view the Gorilla intertrochanteric crest is caudally and laterally placed relative to the head. The lesser trochanter, which marks the inferior point of the intertrochanteric crest, is low relative to the head in Gorilla, and higher relative to the head in Pan. Gorilla is also characterized by a very shallow trochanteric fossa compared with Pan.
Allometry
The least squares regression of PC1 scores on centroid size of all taxa resulted in a slope that was not significantly different from zero, but this was not the case for PC2 (Table 4). Centroid size and PC2 scores were highly correlated, suggesting the presence of intergeneric allometry. An allometric relationship existed between centroid size and the first three PCs in the analysis of all apes. The same was not evident in the Pan–Gorilla analysis, which produced non-significant slopes for PC1 and PC2. Intrageneric slopes were also not significantly different from zero, with the exception of that of Gorilla (Table 4). The Gorilla slope was positive, and was significantly different from zero on the first two PCs, indicating that GPA scaling of landmark configurations did not completely remove size in this group. These results suggested that size-related shape variation underlies the separation of taxa in all analyses except that of Pan–Gorilla.
Table 4.
Tests for isometry: least squares regression of selected PC scores and centroid size
| n | Slope | SE | R | |
|---|---|---|---|---|
| PC1 | ||||
| All taxa | 265 | −0.01 | 0.01 | 0.12 |
| All apes | 171 | −0.04* | 0.01 | 0.53 |
| Pan–Gorilla | 108 | 0.01 | 0.01 | 0.11 |
| Homo | 93 | 0.00 | 0.01 | 0.01 |
| Pan | 79 | 0.00 | 0.02 | 0.01 |
| Gorilla | 29 | 0.05* | 0.01 | 0.54 |
| Pongo | 41 | 0.02 | 0.02 | 0.15 |
| Hylobates | 22 | −0.08 | 0.14 | 0.12 |
| PC2 | ||||
| All taxa | 265 | −0.05* | 0.00 | 0.80 |
| All apes | 171 | 0.02* | 0.00 | 0.42 |
| Pan–Gorilla | 108 | −2.64 | 1.91 | 0.13 |
| Homo | 93 | −0.01 | 0.01 | 0.09 |
| Pan | 79 | 0.02 | 0.01 | 0.20 |
| Gorilla | 29 | 0.03* | 0.01 | 0.41 |
| Pongo | 41 | 0.00 | 0.02 | 0.01 |
| Hylobates | 22 | −0.04 | 0.17 | 0.06 |
| PC3 | ||||
| All apes | 171 | −0.01* | 0.01 | 0.22 |
Significantly different from isometry (P < 0.05).
Discussion
Implications of allometry
The tests for isometry reveal that positive allometry is a factor in PCA of all taxa and of all apes, suggesting that some of the shape variation discussed below is due to size differences among taxa. Analyses of Pan–Gorilla, and of Pongo, Pan and Hylobates individually, appear to be free of allometric effects, while Gorilla exhibits positive allometry. Positive allometry is not surprising in the analysis of all taxa and all apes because the body mass of hylobatids, and in particular Hylobates lar, is much lower than that of the other included taxa (Smith & Jungers, 1997). Although the size of the femora under comparison is a factor, the phenetic results show that overall, small-bodied gibbons are similar to large-bodied orangutans, and relatively small-bodied chimpanzees are most similar to large-bodied gorillas. This observation indicates that all of the identified shape variation is not due to size (Oxnard, 1978), although the current results do not allow precise discrimination between aspects of shape that are size-related and size-independent. The size-related shape variation described below is functionally important and is desirable to discuss (Jungers & Susman, 1984; Jungers, 1988).
Comparative femoral shape among taxa
Together, the results show that the morphology of quadrupedal/climbing African apes is different from that of suspensory/climbing Asian apes, suggesting that locomotor category is a good predictor of ape femoral shape. Femoral shape in Homo and Pongo is broadly similar, which is not predicted on the basis of their different locomotor categories. The relationship between shape variation, locomotor categories and taxon is summarized in Table 5.
Table 5.
Shape characteristics of the proximal femur by locomotor category and taxon. Shape is partitioned into discrete characters based on PCA and TPSA
| Biped | |
| Homo sapiens | |
| Large superiorly projecting head | |
| Long neck between head and greater trochanter | |
| Greater trochanter superoinferiorly short, mediolaterally expanded | |
| Posteriorly projecting intertrochanteric crest | |
| Suspensory/climbing apes | Hylobates |
| Long neck between head and greater trochanter | Intertrochanteric crest does not project posteriorly |
| Greater trochanter superoinferiorly short | Pongo |
| Head projects superiorly relative to the head | Large head |
| Posteriorly projecting intertrochanteric crest | |
| Quadrupedal/climbing apes | Gorilla |
| Small nonsuperiorly projecting head | Head mediolaterally short |
| Short neck between head and greater trochanter | Trochanteric fossa shallow |
| Greater trochanter superoinferiorly long, mediolaterally narrow | Lesser trochanter and intertrochanteric crest inferiorly placed |
| Posteriorly projecting intertrochanteric crest | Pan |
| Head mediolaterally long | |
| Trochanteric fossa deep | |
| Lesser trochanter and intertrochanteric crest superiorly placed | |
The relative position of the greater trochanter and head, and the configuration of the greater trochanter distinguish suspensory/climbing Asian apes from quadrupedal/climbing African apes. The superoinferior length of the greater trochanter is short in Hylobates and Pongo, and is longer in Pan and Gorilla. In the predominately suspensory/climbing apes, the superior end of the greater trochanter is positioned lower than the femoral head. The functional outcome of the relative position of the head and greater trochanter in Asian apes is greater hip mobility, as described below. High neck-shaft angle has been invoked to explain the superior projection of the head of Pongo (Lovejoy et al. 2002). The results here show how this morphology might be achieved. In Asian apes, the distance between the centre of the neck and the border of the head and neck is longer than in African apes, which appears to contribute to the superior projection of the head relative to the greater trochanter. In the predominately quadrupedal/climbing apes, the superior aspects of the femoral head and greater trochanter are at the same level, or the greater trochanter may project above the head. Although it is unclear what, if any, significance this arrangement has for locomotion, there may be some advantage for extension of the thigh (Preuschoft, 1970). In Pan this muscle is engaged in extension as well as medial rotation in climbing (Stern & Susman, 1981).
As demonstrated in the cluster analysis phenogram (Fig. 2), the shape of the bipedal proximal femur is most similar to that of Asian apes, and Pongo in particular. The small number of landmarks in this study suggests that cautious interpretation of the similarities between Homo and Pongo is warranted. Pongo and Homo have comparatively large femoral head diameters, superiorly projecting heads, longer necks and short greater trochanters. The shared shape of Pongo and Homo is likely to be convergent, resulting from similar adaptive solutions for bipedality, on the one hand, and climbing, on the other. Independent evolution is supported by the absence of a ligamentum teres in Pongo, which promotes joint mobility, and presence of this feature in humans, which promotes joint stability.
A locomotor pattern of bimanual suspension and quadrumanual climbing can explain much of the femoral anatomy of Pongo (Schaffler et al. 1985; Ruff, 1987, 1988; MacLatchy & Bossert, 1996; Ruff, 2002). The relatively large femoral head is consistent with analyses by Ruff (2002) and Godfrey et al. (1995), demonstrating that climbers, in contrast to predominately quadupedal primates, have larger hindlimb joint surface areas relative to diaphyseal strength. Although not empirically demonstrated, the shorter greater trochanter of Pongo could relate to the gluteus minimus configuration. The orangutan gluteus minimus is separated into gluteus scansorius (a thigh flexor, abductor and medial rotator), and the gluteus minimus proper, which medially rotates and abducts the thigh (Sigmon, 1974). According to Sigmon, the effect of the divided gluteus minimus is enhanced freedom of movement at the hip joint, which is adaptive for quadrumanual climbing. The two muscle insertions in orangutans, rather than the single, linear insertion in other apes, could explain the shorter greater trochanter (Sigmon, 1974; Aiello & Dean, 1990). That the head is above, rather than below, the greater trochanter in Pongo relates to joint mobility, as a lower greater trochanter interferes less with abduction of the thigh (Aiello & Dean, 1990).
Most aspects of the proximal femoral shape of Homo relate to bipedal locomotion. The short superoinferior extent of the greater trochanter can be associated with the proportionately smaller percentage of hip musculature that comprises gluteus medius and minimus in Homo (Stern, 1972; Sigmon, 1974; Stern & Susman, 1983). These muscles attach on the lateral and anterior aspects of the greater trochanter, and thus define the anteroposterior and superoinferior dimensions of this feature. In apes the deeper gluteals involve a greater percentage of the overall gluteal musculature (Stern, 1972; Sigmon, 1974; Stern & Susman, 1983). Although not directly demonstrated, the low position of the greater trochanter relative to the head may be the developmental outcome of the bipedalism-induced valgus knee (Tardieu & Preuschoft, 1996; Tardieu & Damsin, 1997). It is also likely that this arrangement enhances the action of the abductors in stabilizing the stance-phase hip during bipedal progression (Lovejoy et al. 2002).
The shape of the proximal femur in Hylobates is not greatly different from that of Pongo and Homo. It would be interesting to determine whether, with the inclusion of other gibbon taxa and siamangs, the common Asian ape pattern of proximal femoral shape holds. Shape differences that exist between Hylobates and other taxa are demonstrably influenced by size. Hylobates is distinguished from other taxa primarily by a very slight intertrochanteric crest and an inferiorly positioned lesser trochanter. The slight intertrochanteric crest of gibbons, compared with the more exaggerated version in other taxa, is likely to be an allometric effect. The intertrochanteric crest is the point of insertion for the quadratus femoris, a lateral thigh rotator and abductor (Sigmon, 1974). Sigmon reported that Hylobates musculature is long and slender in comparison with that of great apes. This characteristic, which is perhaps related to body size, could explain the lack of intertrochanteric crest robustness in gibbons.
Comparative femoral shape among African apes
This analysis reveals morphological similarity between Gorilla and Pan, which is consistent with their common locomotor mode of quadrupedal knucklewalking and climbing. There are some shape differences between Pan and Gorilla, such as in the shape of the head and its relationship to the neck, the depth of the trochanteric fossa, and the position of the intertrochanteric crest relative to the head. The larger articular surface in the Pan femoral head is linked to the capability for a significant degree of abduction at the hip joint (MacLatchy & Bossert, 1996; MacLatchy, 1996). In Gorilla, the articular surface is less mediolaterally deep, suggesting a more limited capacity for abduction. In keeping with this morphology and with larger body size, G. g. gorilla reportedly climbs much less often than does Pan (Gebo, 1996).
Pan has a very deep trochanteric fossa that is not shared by Gorilla. The deep trochanteric fossa has been attributed to a large insertion of the lateral thigh rotator, obturator externus (Aiello & Dean, 1990). By contrast, Lovejoy et al. (2002: 107) argued that the unusually deep trochanteric fossa of Pan is a ‘developmental-modeling feature’ of no biomechanical or phylogenetic consequence. Thus, it remains unclear why Pan would be exaggerated in this regard relative to Gorilla.
That Pan and Gorilla are knucklewalkers, but at very different body sizes, is an explanation for the differences in joint morphology, yet intergeneric allometry is not detected in this analysis. Lovejoy et al. (2002: 115) found differences between Pan and Gorilla in their analysis of a few characters and few specimens, and as explanation, suggested sampling factors, differential adaptation, morphological drift or body size effects. The discrete attributes of Pan and Gorilla joint morphology, which cannot easily be attributed to allometry in this analysis of numerous specimens, eliminates sampling effects and body size effects as plausible explanations. Better explanations for the differences between Pan and Gorilla lie in their different behaviours, which are probably the consequence of substrate-use constraints imposed on large-bodied gorillas (Gebo, 1996).
In most analyses gorillas are widely dispersed across axes of variation, suggesting that they are highly variable in proximal femoral shape. One potential source of the variation is between the subspecies G. g. gorilla and G. g. beringei, which might be more appropriately considered separate species (Groves, 2001; Grubb et al. 2003). Differences between them have been identified in the cranium and mandible, the distal humerus, and in the femoral head surface area (Lague & Jungers, 1999; Ruff, 2002; Guy et al. 2003; Taylor & Groves, 2003). Although G. g. gorilla and G. g. beringei are differentiated from one another in the cluster analysis based on all variation, the difference was not reflected in the significant principal components. The test for intrageneric isometry reveals an allometric relationship among gorillas, which could relate to the presence of the two subspecies. Sexual dimorphism is another possible explanation, although allometry is not detected among similarly sexually dimorphic orangutans.
Implications for the evolution of the proximal femur
The results have implications for the evolution of the proximal femur. Vertical climbing, such as practised by orangutans, has been identified as a pre-adaptation for bipedalism (Stern & Susman, 1981; Fleagle et al. 1981). Therefore, shape similarities in Homo and Pongo could be primitive characters not found in African apes owing to their derived quadrupedal knucklewalking adaptation. The observation that Hylobates shares a relatively long neck, a short greater trochanter and a superiorly projecting head with Pongo and Homo indicates that these features could be primitive traits. Although Hylobates engages in leaping and bipedal walking and Pongo in arboreal quadrupedalism (Fleagle, 1976; Sugardjito & van Hooff, 1986; Cant, 1987), Asian apes are mainly suspensory and infrequently load their hindlimbs (Schaffler et al. 1985; Ruff, 1987), suggesting that locomotor pattern is an equally plausible explanation for their shared morphology. If the last common ancestor of African apes and humans was a quadrupedal knucklewalker (e.g. Richmond et al. 2001), it could be argued that the femoral morphology of chimpanzees and gorillas represents the ancestral state for the African ape and human clade, and that femoral shape in humans converges on that of Asian apes, whose morphology is primitive.
At least three evolutionary scenarios are consistent with the identified pattern of shape: (1) femoral shape in Pongo and Hylobates is primitive, while African ape morphology and that of Homo are secondarily derived, either independently or from a common ancestor; (2) the morphology of African apes is primitive, while that of Hylobates, Pongo and Homo developed independently in response to locomotor requirements; and (3) femoral morphology in each lineage evolved independently and the morphology of the last common ancestor of all apes remains elusive. These alternatives cannot be completely evaluated without consideration of additional hindlimb elements. At least for the proximal femur, function, rather than phylogeny, is likely to make the greater impact on shape.
Conclusions
In this study, the relationship between locomotor behaviour and proximal femoral shape is identified among extant hominoids. The results suggest that there is a strong functional signal in the shape of the proximal femur that is accompanied by intergeneric allometry. The Asian apes, which mostly practise bimanual suspension and climbing, are more similar to one another in shape, whereas the largely quadrupedal and climbing African apes are similar in shape. The differences in shape between the two groups can be linked to functional adaptation. Asian ape morphology includes relatively high head diameter, long neck and a short greater trochanter, consistent with locomotor behaviours that require joint mobility. In African apes the head is smaller, the neck shorter and the greater trochanter longer. This morphology is consistent with greater joint stability and muscle leverage associated with knucklewalking quadrupedalism. Small size does influence some of the differences between gibbons and other taxa. That great apes are not more similar to one another than orangutans are to gibbons further suggests that function, not evolutionary relatedness, is the primary arbiter of the shape of the proximal femur.
The proximal femoral shape of recent Homo is similar in many ways to that of Pongo. The same morphology is associated with different functions in these taxa, which suggests that homoplasy is the best explanation for the shared femoral shape. In the case of Homo, the large femoral head, long neck and short greater trochanter reflect body mass transmission and ground reaction force resistance at the hip, changes in hip abductor musculature, and bicondylar angle of the knee, all of which are advantageous in bipedal locomotion. The shape of the proximal femur reflects locomotor behaviour in Pongo and Homo, but results from different functional requirements.
Acknowledgments
I would like to thank Diane Hawkey, Judy Chupasko, Linda Gordon, David Hunt and Wim Van Neer for access to collections in their care. Charlie Lockwood and Michelle Drapeau provided insightful comments on earlier versions of the manuscript. Suggestions from three anonymous reviewers greatly improved this work. Research was supported by National Science Foundation grant BCS-0333296.
References
- Aiello LC, Dean C. An Introduction to Human Evolutionary Anatomy. London: Academic Press; 1990. [Google Scholar]
- Anemone RL. The VCL hypothesis revisited: patterns of femoral morphology among quadrupedal and saltatorial prosimian primates. Am J Phys Anthropol. 1990;83:373–393. doi: 10.1002/ajpa.1330830310. [DOI] [PubMed] [Google Scholar]
- Anemone RL. The functional anatomy of the hip and thigh in primates. In: Gebo DL, editor. Postcranial Adaptation in Nonhuman Primates. DeKalb: Northern Illinois University; 1993. pp. 150–174. [Google Scholar]
- Berge C, Penin X. Ontogenetic allometry, heterochrony, and interspecific differences in the skull of African apes, using tridimensional Procrustes analysis. Am J Phys Anthropol. 2004;124:124–138. doi: 10.1002/ajpa.10333. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Principal warps: thin-plate splines and the decomposition of deformations. IEEE Trans Pattern Anal Machine Intelligence. 1989;11:567–585. [Google Scholar]
- Bookstein FL. Morphometric Tools for Landmark Data. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Bookstein F, Schafer K, Prossinger H, et al. Comparing frontal cranial profiles in Archaic and Modern Homo by morphometric analysis. Anat Record. 1999;257:217–224. doi: 10.1002/(SICI)1097-0185(19991215)257:6<217::AID-AR7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Cant JG. Effects of sexual dimorphism in body size on feeding postural behavior of Sumatran Orangutans (Pongo pygmaeus) Am J Phys Anthropol. 1987;74:143–148. [Google Scholar]
- Cartmill M. Climbing. In: Hildebrand M, Bramble DM, Liem KF, Wake DG, editors. Functional Vertebrate Morphology. Cambridge: Belknap Press; 1985. pp. 73–88. [Google Scholar]
- Corruccini RS, McHenry HM. Relative femoral head size in early hominids. Am J Phys Anthropol. 1978;49:145–148. doi: 10.1002/ajpa.1330490123. [DOI] [PubMed] [Google Scholar]
- Delson E, Harvati K, Reddy D, et al. The Sambungmacan 3 Homo erectus calvaria: a comparative morphometric and morphological analysis. Anat Record. 2001;262:380–397. doi: 10.1002/ar.1048. [DOI] [PubMed] [Google Scholar]
- Doran D. Comparative positional behavior of African apes. In: McGrew W, Marchant L, Nishida T, editors. Great Ape Societies. Cambridge: Cambridge University Press; 1996. pp. 213–224. [Google Scholar]
- Dryden IL, Mardia KV. Statistical Shape Analysis. New York: Wiley; 1998. [Google Scholar]
- Fleagle J. Locomotion and posture of the Malayan siamang and implications for hominoid evolution. Folia Primatol. 1976;26:245–269. doi: 10.1159/000155756. [DOI] [PubMed] [Google Scholar]
- Fleagle J, Stern J, Jungers WL, Susman R, Vangor AK, Wells JP. Climbing: a biomechanical link with brachiation and bipedalism. Symp Zool Soc London. 1981;48:359–373. [Google Scholar]
- Gebo DL. Postcranial adaptation and evolution in Lorisidae. Primates. 1989;30:347–367. [Google Scholar]
- Gebo DL. Climbing, brachiation, and terrestrial quadrupedalism: historical precursors of hominid bipedalism. Am J Phys Anthropol. 1996;101:55–92. doi: 10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Godfrey LR, Sutherland MR, Paine RR, Williams FL, Boy DS, Vuillaume-Randriamanantena M. Limb joint surface areas and their ratios in Malagasy lemurs and other mammals. Am J Phys Anthropol. 1995;97:11–36. doi: 10.1002/ajpa.1330970103. [DOI] [PubMed] [Google Scholar]
- Groves CP. Primate Taxonomy. Washington, DC: Smithsonian Institution; 2001. [Google Scholar]
- Grubb P, Butynski TM, Oates JF, et al. Assessment of the diversity of African primates. Int J Primatol. 2003;24:1301–1357. [Google Scholar]
- Guy F, Brunet M, Schmittbuhl M, Viriot L. New approaches in hominoid taxonomy: morphometrics. Am J Phys Anthropol. 2003;121:198–218. doi: 10.1002/ajpa.10261. [DOI] [PubMed] [Google Scholar]
- Hunt K. Positional behavior of Pan troglodytes in the Mahale Mountains and Gombe Stream National Parks, Tanzania. Am J Phys Anthropol. 1992;87:83–105. doi: 10.1002/ajpa.1330870108. [DOI] [PubMed] [Google Scholar]
- Immersion Corporation. Microscribe 3D User's Guide. San Jose, CA: Immersion Corporation; 1998. [Google Scholar]
- Jungers WL, Susman RL. Body size and skeletal allometry in African apes. In: Susman RL, editor. Pygmy Chimpanzee. New York: Plenum; 1984. pp. 131–177. [Google Scholar]
- Jungers WL. Relative joint size and hominoid locomotor adaptations with implications for the evolution of hominid bipedalism. J Hum Evol. 1988;17:247–265. [Google Scholar]
- Jungers WL. Scaling of hominoid femoral head size and the evolution of hominid bipedalism. Am J Phys Anthropol. 1990;8:246. [Google Scholar]
- Jungers WL, Falsetti A, Wall C. Shape, relative size and size adjustments in morphometrics. Yearb Phys Anthropol. 1995;38:137–161. [Google Scholar]
- Lague MR, Jungers WL. Patterns of sexual dimorphism in the hominoid distal humerus. J Hum Evol. 1999;36:379–399. doi: 10.1006/jhev.1998.0274. [DOI] [PubMed] [Google Scholar]
- Lague MR. Another look at shape variation in the distal femur of Australopithecus afarensis: implications for taxonomic and functional diversity at Hadar. J Hum Evol. 2002;42:609–626. doi: 10.1006/jhev.2002.0545. [DOI] [PubMed] [Google Scholar]
- Larson SG. Parallel evolution in the hominoid trunk and forelimb. Evol Anthropol. 1998;6:87–99. [Google Scholar]
- Lieberman DE, Devlin MJ, Pearson OM. Articular area responses to mechanical loading: effects of exercise, age, and skeletal location. Am J Phys Anthropol. 2001;116:266–277. doi: 10.1002/ajpa.1123. [DOI] [PubMed] [Google Scholar]
- Lockwood CA, Lynch J, Kimbel WH. Quantifying temporal bone morphology of great apes and humans: an approach using geometric morphometrics. J Anat. 2002;201:447–464. doi: 10.1046/j.1469-7580.2002.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy CO. Biomechanical perspectives on the lower limb of early hominids. In: Tuttle RH, editor. Primate Functional Morphology and Evolution. The Hague: Mouton; 1975. pp. 291–326. [Google Scholar]
- Lovejoy CO, Meindl RS, Ohman JC, Heiple KG, White TD. The Maka femur and its bearing on the antiquity of human walking: applying contemporary concepts of morphogenesis to the human fossil record. Am J Phys Anthropol. 2002;119:97–133. doi: 10.1002/ajpa.10111. [DOI] [PubMed] [Google Scholar]
- Lynch JM, Wood CG, Luboga SA. Geometric morphometrics in primatology: craniofacial variation in Homo sapiens and Pan troglodytes. Folia Primatol. 1996;67:15–39. doi: 10.1159/000157203. [DOI] [PubMed] [Google Scholar]
- MacLatchy LM. Another look at the australopithecine hip. J Hum Evol. 1996;31:455–476. [Google Scholar]
- MacLatchy LM, Bossert WH. An analysis of the articular surface distribution of the femoral head and acetabulum in anthropoids with implications for hip function in Miocene hominoids. J Hum Evol. 1996;31:425–453. [Google Scholar]
- McHenry HM, Corruccini RS. Fossil hominid femora and the evolution of walking. Nature. 1976;259:657–658. doi: 10.1038/259657a0. [DOI] [PubMed] [Google Scholar]
- McHenry HM, Corruccini RS. The femur in early human evolution. Am J Phys Anthropol. 1978;49:473–488. doi: 10.1002/ajpa.1330490407. [DOI] [PubMed] [Google Scholar]
- O'Higgins P. The study of morphological variation in the hominid fossil record: biology, landmarks and geometry. J Anat. 2000;197:103–120. doi: 10.1046/j.1469-7580.2000.19710103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Higgins P, Jones N. Tools for Statistical Shape Analysis. Hull: York Medical School; 2006. [Google Scholar]
- Oxnard CE. One biologist's view of morphometrics. Ann Rev Evol Syst. 1978;9:219–241. [Google Scholar]
- Penin X, Berge C, Baylac M. Ontogenetic study of the skull in modern humans and common chimpanzees: neontenic hypothesis reconsidered with a tridimensional Procrustes analysis. Am J Phys Anthropol. 2002;118:50–62. doi: 10.1002/ajpa.10044. [DOI] [PubMed] [Google Scholar]
- Pilbeam D. Genetic and morphological records of the Hominoidea and hominid origins: a synthesis. Mol Phylogenet Evol. 1996;1:155–168. doi: 10.1006/mpev.1996.0010. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. Functional anatomy of the lower extremity. In: Bourne GH, editor. The Chimpanzee: Immunology, Infections, Hormones, Anatomy, and Behavior. Baltimore: University Park Press; 1970. pp. 221–294. [Google Scholar]
- Rafferty KL, Ruff CB. Articular structure and function in Hylobates, Colobus, and Papio. Am J Phys Anthropol. 1994;94:395–408. doi: 10.1002/ajpa.1330940308. [DOI] [PubMed] [Google Scholar]
- Rafferty KL. Structural design of the femoral neck in primates. J Hum Evol. 1998;34:361–383. doi: 10.1006/jhev.1997.0202. [DOI] [PubMed] [Google Scholar]
- Remis MJ. Effects of body size and social context on the arboreal activities of lowland gorillas in the Central African Republic. Am J Phys Anthropol. 1995;97:413–434. doi: 10.1002/ajpa.1330970408. [DOI] [PubMed] [Google Scholar]
- Remis MJ. Tree structure and sex differences in arboreality among western lowland gorillas (Gorilla gorilla gorilla) at Bai Hokou, Central African Republic. Primates. 1999;40:383–396. [Google Scholar]
- Reno PL, Serrat MA, Meindl RS, Cohn MJ, Lovejoy CO. Hominoids, hindlimbs and Hox: implications for hominid evolution. Am J Phys Anthropol. 2003;120(S36):177–178. [Google Scholar]
- Richmond BG, Begun DR, Strait DS. Origin of human bipedalism: the knuckle-walking hypothesis revisited. Yrbk Phys Anthropol. 2001;44:70–105. doi: 10.1002/ajpa.10019.abs. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice DE. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- Ruff CB. Structural allometry of the femur and tibia in Hominoidea and Macaca. Folia Primatol. 1987;48:9–49. doi: 10.1159/000156283. [DOI] [PubMed] [Google Scholar]
- Ruff CB. Hindlimb articular surface allometry in Hominoidea and Macaca. Folia Primatol. 1988;17:687–714. [Google Scholar]
- Ruff CB, Scott W, Liu A. Articular and diaphyseal remodeling of the proximal femur with changes in body mass in adults. Am J Phys Anthropol. 1991;86:397–413. doi: 10.1002/ajpa.1330860306. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Runestad JA. Primate limb bone structural adaptations. Annu Rev Anthropol. 1992;21:407–433. [Google Scholar]
- Ruff CB. Long bone articular and diaphyseal structure in Old World Monkeys and apes. I: Locomotor effects. Am J Phys Anthropol. 2002;119:305–342. doi: 10.1002/ajpa.10117. [DOI] [PubMed] [Google Scholar]
- Schaffler MB, Burr DB, Jungers WL, Ruff CB. Structural and mechanical indicators of limb specialization in primates. Folia Primatol. 1985;45:61–75. doi: 10.1159/000156218. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Black S. Developmental Juvenile Osteology. London: Academic Press; 2000. [Google Scholar]
- Schultz AH. The skeleton of the chimpanzee. In: Bourne GH, editor. The Chimpanzee: Anatomy, Behavior, and Diseases of the Chimpanzee. Baltimore: University Park Press; 1970. pp. 50–103. [Google Scholar]
- Serrat MA, Reno PL, McCollum MA, Meindl RS, Lovejoy CO. Multivariate comparisons of divergent ossification patterns in the mammalian proximal femur. Am J Phys Anthropol. 2004;123(S38):178–179. [Google Scholar]
- Sigmon BA. A functional analysis of pongid hip and thigh musculature. J Hum Evol. 1974;3:161–185. [Google Scholar]
- Smith RJ, Jungers WL. Body mass in comparative primatology. J Hum Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Stern JT. Anatomical and functional specializations of the human gluteus maximus. Am J Phys Anthropol. 1972;36:315–340. doi: 10.1002/ajpa.1330360303. [DOI] [PubMed] [Google Scholar]
- Stern J, Susman R. Electromyography of the gluteal muscles in Hylobates, Pongo and Pan: implications for the evolution of hominid bipedality. Am J Phys Anthropol. 1981;55:153–166. [Google Scholar]
- Stern JT, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol. 1983;60:279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- Sugardjito J, van Hooff J. Sex–age class differences in positional behavior of Sumatran orangutan (Pongo pygmaeus abelii) in Gunung Leuser National Park, Indonesia. Folia Primatol. 1986;47:14–25. doi: 10.1159/000156260. [DOI] [PubMed] [Google Scholar]
- Szalay FS, Delson E. Evolutionary History of the Primates. New York: Academic Press; 1979. [Google Scholar]
- Tardieu C, Preuschoft H. Ontogeny of the knee joint in humans, great apes and fossil hominids: pelvi-femoral relationships during postnatal growth in humans. Folia Primatol. 1996;66:68–81. doi: 10.1159/000157186. [DOI] [PubMed] [Google Scholar]
- Tardieu C, Damsin JP. Evolution of the angle of obliquity of the femoral diaphysis during growth – correlations. Surg Radiol Anat. 1997;19:91–97. [PubMed] [Google Scholar]
- Taylor AB, Groves CP. Patterns of mandibular variation in Pan and Gorilla and implications for African ape taxonomy. J Hum Evol. 2003;44:529–561. doi: 10.1016/s0047-2484(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Young NM. A reassessment of living hominoid postcranial variability: implications for ape evolution. J Hum Evol. 2003;45:441–464. doi: 10.1016/j.jhevol.2003.09.001. [DOI] [PubMed] [Google Scholar]