Abstract
Disuse (i.e. inactivity) causes bone loss, and a recovery period that is 2–3 times longer than the inactive period is usually required to recover lost bone. However, black bears experience annual disuse (hibernation) and remobilization periods that are approximately equal in length, yet bears maintain or increase cortical bone material properties and whole bone mechanical properties with age. In this study, we investigated the architectural properties of bear femurs to determine whether cortical structure is preserved with age in bears. We showed that cross-sectional geometric properties increase with age, but porosity and resorption cavity density do not change with age in skeletally immature male and female bears. These findings suggest that structural properties substantially contribute to increasing whole bone strength with age in bears, particularly during skeletal maturation. Porosity was not different between skeletally immature and mature bears, and showed minimal regional variations between anatomical quadrants and radial positions that were similar in pattern and magnitude between skeletally immature and mature bears. We also found gender dimorphisms in bear cortical bone properties: females have smaller, less porous bones than males. Our results provide further support for the idea that black bears possess a biological mechanism to prevent disuse osteoporosis.
Keywords: ageing, black bear, cortical bone, disuse osteoporosis, porosity
Introduction
Decreased skeletal loading, which occurs during bedrest, spaceflight and limb immobilization (e.g. in patients with stroke or spinal cord injury), alters normal bone remodelling processes (Bikle et al. 2003). Bone lost during disuse in small animals such as rats and mice can be the result of decreased bone formation alone (Wronski et al. 1987; Turner et al. 1995), or both increased bone resorption and decreased bone formation (Weinreb et al. 1989; Rantakokko et al. 1999). Immobilization of canine forelimbs increases both resorption and formation, but bone loss occurs because of increased remodelling space, unbalanced relative increases in resorption and formation, and possibly because of an abnormally long lag time between resorption and formation (Li et al. 2005a,b). Thus, in all of these scenarios, disuse causes unbalanced bone remodelling, which leads to net bone loss and increased fracture risk (Vico et al. 1987; Collet et al. 1997; de Bruin et al. 2000; Kanis et al. 2001; Modlesky et al. 2005). Disuse-induced bone loss affects bones on both the microstructural (e.g. porosity) and the macrostructural (e.g. cross-sectional area) level. Common changes in long bones during unloading include multifold increases in porous area and porous cavity size, loss of cortical area, and reductions in moments of inertia (Gross & Rubin, 1995; Rubin et al. 1996; de Bruin et al. 2000; Garber et al. 2000; Li et al. 2005a).
Previous studies have shown that a remobilization period of approximately 2–3 times the length of the unloading period is usually required to recover completely bone lost during disuse (Kaneps et al. 1997; Weinreb et al. 1997). For example, canine humeri that had been immobilized for 16 weeks required 32 weeks of remobilization, half of which included treadmill exercise, to match control values for ultimate stress (Kaneps et al. 1997). This requisite time span for remobilization would seemingly create problems for black bears (Ursus americanus); bears in northern climates experience annual periods of disuse (hibernation) and activity that are each approximately 6 months in length. Because bears do not have a remobilization period of the relative length (2–3 times longer than the disuse period) that other animals require for complete bone recovery, it would be expected that their bones would lose mechanical properties with age due to the annual deficit in recovered bone. However, bears retain their material and whole-bone mechanical properties with age (Harvey & Donahue, 2004; Harvey et al. 2005; Donahue et al. 2006b; McGee et al. 2006c). Additionally, bears maintain balanced bone formation and resorption during hibernation (Donahue et al. 2006a). Bears increase serum type I collagen crosslinked carboxy-terminal telopeptide (ICTP, a marker of bone resorption) approximately 160% during hibernation relative to prehibernation levels, but also increase serum osteocalcin (a marker of bone formation) approximately 320% during hibernation compared with prehibernation (Donahue et al. 2006a). This can be contrasted with human bedrest, in which serum ICTP levels increase by approximately 20–30% and serum osteocalcin levels do not change (Zerwekh et al. 1998), and human spaceflight, in which serum CTX (another marker of bone resorption) levels increase by 40% and serum osteocalcin levels decrease by approximately 7% (Caillot-Augusseau et al. 2000). Taken together, these findings suggest that bears possess a unique mechanism for avoiding the detrimental consequences of disuse by favouring a positive bone balance despite increased turnover.
It has been clearly demonstrated that the mechanical properties of bear cortical bone are not compromised by annual periods of disuse (i.e. ageing) (Harvey & Donahue, 2004; Harvey et al. 2005; Donahue et al. 2006b; McGee et al. 2006c), but it is unknown how bear cortical bone structural properties are affected by ageing. Therefore, we quantified the porosity, resorption cavity density, and cross-sectional properties of black bear femurs from a wide age range of bears (1–20 years old) to determine whether they are affected by annual periods of disuse. We hypothesized that cortical bone properties would be preserved with age because bone formation remains balanced with resorption in bears during disuse (hibernation) (Donahue et al. 2006a).
Methods
Twenty-seven femurs were obtained from black bears that were hunter-killed or that had died a natural death in Utah and Alaska. Twelve were from female bears (age range 2–20 years, mean 8.8 years) and 15 were from male bears (age range 1–17 years, mean 4.1 years). Epiphyseal fusion occurs in bears between 6 and 8 years of age (Westwood, 1996); for the purposes of this study, skeletal maturity was defined as 8 years of age. Thus, five of the female bears and two of the male bears were skeletally mature (i.e. 8 years of age or older). The length of each bone was measured (males: 302 ± 30 mm, females: 291 ± 10 mm) and midshaft was defined as half total bone length.
Cross-sectional properties
A 15-mm section immediately proximal to midshaft was removed from the diaphysis, and the midshaft cross-section was imaged with a digital camera. Cross-sectional properties including the periosteal area (Ps.Ar), cortical area (Ct.Ar), endosteal area (Es.Ar), endosteal area fraction (Es.Ar/PsAr), section modulus (SM), maximum moment of inertia (Imax), mediolateral moment of inertia (IML) and anteroposterior moment of inertia (IAP) were determined using measurement tools and a custom macro in Scion Image (Frederick, MD, USA) (Fig. 1). As the bending axes of long bones during gait can vary from the anatomical and principal axes (Demes et al. 2001), moments of inertia at 45° to the anatomical axes (IAM-LP and IAL-MP) (Fig. 1) were also calculated to characterize better the response of the cross-section to annual unloading periods. Cortical thickness (Ct.Th), the average perpendicular distance between the endosteal and periosteal borders, was measured in 1-mm intervals around the entire cortex and averaged for each sample using image analysis software (BIOQUANT OSTEO, Nashville, TN, USA).
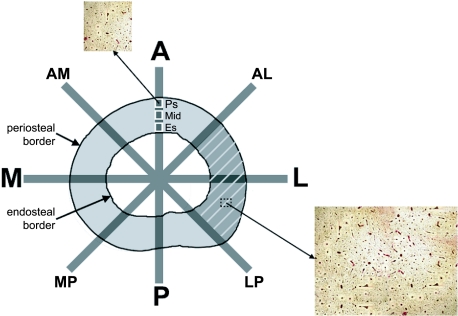
Fig 1.
Femoral cross-section designating the anatomical axes and axes at 45° to the anatomical axes, thus dividing each cross-section into octants. Moments of inertia were calculated about each axis and section modulus was calculated about the mediolateral axis. Periosteal area is inside the periosteal border, endosteal area is inside the endosteal border, and cortical area is between the two borders. Cortical thickness is the average perpendicular distance between the periosteal and endosteal borders. Porosity was quantified by anatomical quadrant (e.g. shaded area for lateral quadrant), as well as for the entire cross-section. Porosity was also quantified at three radial locations (designated by white squares: Ps, periosteal; Mid, midcortical; Es, endosteal) along each octant line. The inset images show basic fuchsin-stained sections of bone at 40× magnification that were used to calculate porosity digitally.
Porosity and resorption cavity density
The bone segments were cleaned of marrow, dehydrated for 48 h in 70% ethanol, and embedded in methylmethacrylate. Thin sections were removed using a diamond saw (Isomet 1000, Buehler, Lake Bluff, IL, USA) and were ground to 70–90 µm. The sections were stained in four increasing ethanol concentrations of a 1% basic fuchsin stain (70–100% ethanol) for 30 s each, and were then rinsed in 100% ethanol for 30 s. This staining method provided good contrast between the bone and porous spaces (Fig. 1). The sections were mounted on glass microscope slides, and the anatomical quadrants and directions were marked on each sample such that each cross-section was divided into eight octants (Fig. 1). Slides were imaged at 40× magnification with a digital camera (SPOT Insight QE, Diagnostic Instruments, Sterling Heights, MI, USA) and microscope (Leica LABORLUX S, Leica Microsystems, Bannockburn, IL, USA). The bone microstructure was analysed using a semi-automated image analysis software package (BIOQUANT OSTEO). Porosity, which included all porous spaces except osteocyte lacunae and canaliculi, was determined separately for each anatomical quadrant (PorA, PorM, PorP, PorL), as porosity shows regional variation in the femur (Thomas et al. 2005), and increases non-uniformly in long bone cross-sections during disuse (Gross & Rubin, 1995). The porous areas (in mm2) from each quadrant in a given cross-section were added together and divided by cortical area to obtain a measure of total cross-sectional porosity (Portotal) for each bone. Porosity was also measured at three sampling sites [periosteal (PorPs), midcortical (PorMid) and endosteal (PorEs)] along each octant line to determine the radial variation in porosity; the average area analysed at each sampling site was 1.6 ± 0.5 mm2 (Fig. 1). Resorption cavities were identified as porous spaces with a scalloped border without a surrounding cement line and were quantified at 100× magnification. Resorption cavity density (Rp.Ca.Dn) was calculated for the entire cross-section as the number of resorption cavities divided by cortical area.
Statistics
Bone properties for the skeletally immature bears were regressed against age (which indicates the number of annual disuse periods experienced). Males and females were separated for the regression analyses due to the potential for synergistic age- and sex-related structural changes, because many animals (including bears) display gender-related dimorphism during growth (Derocher et al. 2005). As the age distribution was skewed for the immature male and female bears, Cook's distance was calculated for each data point to identify influential samples which would impact the regression model parameter estimates. Points for which Cook's distance was greater than 1 were dropped from the model and the regression was refit to the remaining samples. Linear regressions were not performed for the skeletally mature bears due to their small sample sizes. Instead, the mean values for each bone property were compared between skeletally immature and mature bears with one-factor anova. Comparisons between skeletally immature male and female bears were made using ancova, treating age as the covariate. In order to validate the assumptions of the ancova analyses, the regression slopes between males and females were compared by evaluating the significance of the age × sex interaction term in a two-factor anova. One-factor anova was used to compare the bone properties of skeletally mature male and female bears. One-factor anova with Fisher's protected least significant difference (PLSD) post-hoc test was used to assess the radial and quadrant-related distribution of porosity in the bear femurs. Male and female bears, as well as skeletally immature and mature bears, were separated for these regional and radial porosity analyses.
Results
Cross-sectional properties – age-related changes
Only one data point in one regression had to be excluded based on Cook's distance (a 7-year-old male in the endosteal area fraction regression, Cook's distance = 1.05). All cross-sectional properties significantly increased with age in skeletally immature male bears (P < 0.027, r > 0.611) except endosteal area (P = 0.134, r = 0.438) and endosteal area fraction (P = 0.781, r = 0.090) (Table 1). Similarly, all cross-sectional properties increased with age in immature female bears (P < 0.019, r > 0.837) except endosteal area (P = 0.298, r = 0.461), endosteal area fraction (P = 0.890, r = −0.065) and cortical thickness (P = 0.089, r = 0.686) (Table 1). A representative plot can be seen in Fig. 2. Skeletally mature male bears had significantly greater values (P < 0.033) for each property compared with immature male bears except for endosteal area fraction (P = 0.498) and endosteal area, which approached significance (P = 0.053) (Table 2). Conversely, none of the cross-sectional properties was different between skeletally immature and mature female bears (P > 0.254) (Table 2).
Table 1.
Summary of femoral cross-sectional properties linearly regressed against age for skeletally immature male and female bears
| Property | P-value | r-value |
|---|---|---|
| Male | ||
| Ps.Ar | 0.018 | 0.643 |
| Ct.Ar | 0.015 | 0.654 |
| Es.Ar | 0.134 | 0.438 |
| Es.Ar/PsAr | 0.781 | 0.090 |
| Ct.Th | 0.022 | 0.625 |
| SM | 0.012 | 0.669 |
| Imax | 0.026 | 0.614 |
| IML | 0.013 | 0.663 |
| IAP | 0.027 | 0.611 |
| IAM-LP | 0.016 | 0.650 |
| IAL-MP | 0.018 | 0.643 |
| Female | ||
| Ps.Ar | 0.019 | 0.837 |
| Ct.Ar | 0.010 | 0.876 |
| Es.Ar | 0.298 | 0.461 |
| Es.Ar/PsAr | 0.890 | −0.065 |
| Ct.Th | 0.090 | 0.686 |
| SM | 0.008 | 0.884 |
| Imax | 0.009 | 0.877 |
| IML | 0.013 | 0.859 |
| IAP | 0.009 | 0.883 |
| IAM-LP | 0.005 | 0.903 |
| IAL-MP | 0.010 | 0.873 |
Most properties significantly increased with age in both male and female bears. See text for explanations of abbreviations.
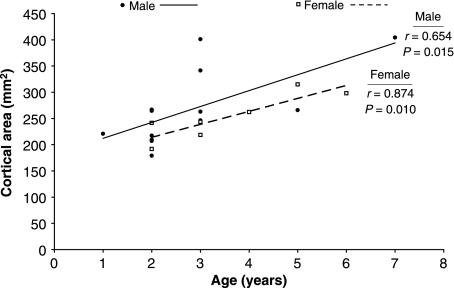
Fig 2.
Cortical area increased with age for skeletally immature male and female bears. The male and female regression slopes were not significantly different (P = 0.735). Similar trends with age were observed for all other cross-sectional properties in skeletally immature bears.
Table 2.
Mean femoral cross-sectional properties for skeletally immature and mature male and female bears
| Property | Immature male | Immature female | P-value (M : F) | Mature male | Mature female | P-value (M : F) |
|---|---|---|---|---|---|---|
| Ps.Ar (mm2) | *388 | 365 | 0.118 | 579 | 389 | 0.001 |
| Ct.Ar (mm2) | *268 | 253 | 0.131 | 412 | 258 | 0.004 |
| Es.Ar (mm2) | 120 | 112 | 0.280 | 167 | 131 | 0.144 |
| Es.Ar/PsAr (%) | 0.31 | 0.30 | 0.898 | 0.29 | 0.34 | 0.209 |
| Ct.Th (mm) | *4.64 | 4.58 | 0.343 | 6.02 | 4.46 | 0.0003 |
| SM (mm2) | *927 | 844 | 0.105 | 1661 | 904 | 0.0005 |
| Imax (mm2) | *13 711 | 11 581 | 0.103 | 29 262 | 13 208 | 0.0003 |
| IML (mm2) | *9635 | 8353 | 0.104 | 20 472 | 9071 | 0.0002 |
| IAP (mm2) | *13 608 | 11 527 | 0.107 | 29 060 | 13 101 | 0.0003 |
| IAM-LP (mm2) | *11 365 | 9772 | 0.091 | 23 923 | 11 532 | 0.002 |
| IAL-MP (mm2) | *12 094 | 10 448 | 0.109 | 26 206 | 11 058 | 0.0002 |
See text for explanations of abbreviations. P-values are for comparisons of male versus female bears within a given age grouping (i.e. immature or mature).
P < 0.05 comparing the immature male value with its corresponding value in mature male bears.
Cross-sectional properties – comparison of male and female bears
The differences between the cross-sectional properties of skeletally immature male and female bears were not statistically significant for any properties (0.091 < P < 0.898) although male bears consistently had a larger mean value for each property (Table 2). The regression slopes were not significantly different between skeletally immature male and female bears (P > 0.675), thus validating the ancova analyses. For the skeletally mature bears, males had significantly greater mean values for all properties (0.0002 < P < 0.004) except endosteal area (P = 0.144) and endosteal area fraction (P = 0.209) (Table 2).
Porosity and resorption cavity density – age-related changes
Total cross-sectional porosity did not change significantly with age for skeletally immature male (P = 0.179) or female (P = 0.798) bears (Fig. 3), and was not different between skeletally mature and immature bears for either males or females (male: P = 0.203, female: P = 0.440). Resorption cavity density also did not change significantly with age for skeletally immature male (P = 0.257) or female (P = 0.337) bears, and was not different between skeletally immature and mature male bears (P = 0.543). However, the difference in resorption cavity density between skeletally immature and mature female bears approached significance (P = 0.054), with mature female bears having a greater mean value. Porosity did not change with age (0.078 < P < 0.981) for any quadrant in skeletally immature male or female bears. The difference between skeletally immature and mature males approached significance in the posterior quadrant (P = 0.058) with the mature males having a lower mean value. No other changes in quadrant porosity with respect to skeletal maturity were significant (P > 0.169). When examined by radial position, porosity did not change significantly (P > 0.557) with age for any position in skeletally immature female bears. Periosteal porosity decreased significantly (P = 0.027, r = −0.610) with age in skeletally immature male bears, whereas midcortical and endosteal porosity did not change significantly with age (midcortical: P = 0.115, endosteal: P = 0.165). The difference between skeletally immature and mature males approached significance for the periosteal position (P = 0.060) with the mature males having a lower mean value. No other changes in quadrant porosity with respect to skeletal maturity were significant (P > 0.171).
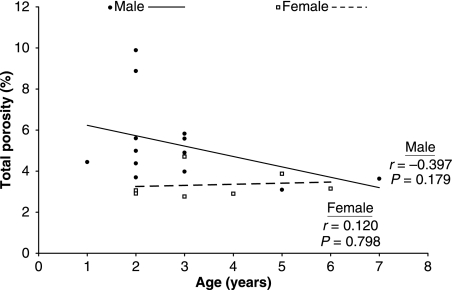
Fig 3.
Total cross-sectional porosity did not significantly change with age for skeletally immature male or female bears. The male and female regression slopes were not significantly different (P = 0.312).
Porosity and resorption cavity density – comparison of male and female bears
Total cross-sectional porosity and resorption cavity density were both greater in skeletally immature male bears than in immature female bears (P = 0.045 and P = 0.034, respectively) (Table 3). Quadrant and radial porosities also tended to be greater in immature males, although not all locations reached statistical significance (Table 3). The skeletally immature male and female regression slopes were not significantly different (P > 0.221) from each other in any quadrant or for the entire cross-section. The regression slopes were also not significantly different for the radial porosities (P > 0.085). Resorption cavity density and all quadrant, radial and total porosities were not significantly different (P > 0.421) between skeletally mature male and female bears.
Table 3.
Male femurs were consistently more porous and had a higher resorption cavity density than female femurs for skeletally immature bears
| Property | Male mean | Female mean | P-value (ancova) |
|---|---|---|---|
| Portotal (%) | 5.30 | 3.34 | 0.045 |
| PorA (%) | 4.86 | 3.17 | 0.070 |
| PorM (%) | 4.05 | 2.56 | 0.141 |
| PorP (%) | 6.93 | 3.39 | 0.005 |
| PorL (%) | 6.41 | 3.78 | 0.018 |
| PorPs (%) | 6.00 | 2.69 | 0.002 |
| PorMid (%) | 4.44 | 3.13 | 0.016 |
| PorEs (%) | 5.75 | 4.35 | 0.136 |
| Rp.Ca.Dn (no. mm−2) | 0.31 | 0.13 | 0.034 |
See text for explanations of abbreviations.
Porosity – comparison of anatomical quadrants and radial positions
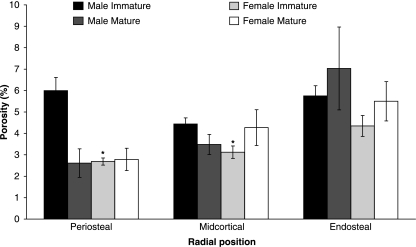
Variation in quadrant porosity was significant for skeletally immature male bears (P = 0.005), but not for immature female (P = 0.085), mature male (P = 0.068) or mature female bears (P = 0.178). The lowest porosities were consistently found in the medial quadrant, whereas the highest porosities were in the lateral quadrant (except for skeletally immature males) (Fig. 4). Variation in radial porosity was significant for skeletally immature female bears (P = 0.009), but not for immature male (P = 0.060), mature female (P = 0.084) or mature male bears (P = 0.153). All female bears and skeletally mature male bears demonstrated a progressive increase in porosity from the periosteal to endosteal radial positions, whereas high porosity values were prevalent in both the periosteal and the endosteal radial positions for the skeletally immature male bears (Fig. 5). Total cross-sectional porosity and resorption cavity density were correlated (P < 0.0001, r = 0.858).
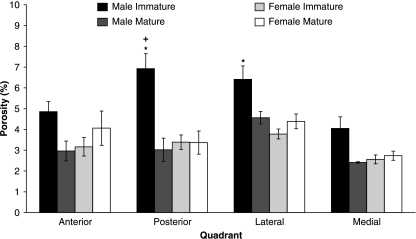
Fig 4.
Mean porosities (with SE error bars) for each anatomical quadrant for skeletally immature and mature male and female bears. The anova model was only significant for skeletally immature male bears, so post-hoc analyses were only conducted for this group. *Significantly different (P < 0.05) from medial quadrant, +: significantly different (P < 0.05) from anterior quadrant.
Fig 5.
Mean porosities (with SE error bars) for each radial position for skeletally immature and mature male and female bears. The anova model was only significant for skeletally immature female bears, so post-hoc analyses were only conducted for this group. *Significantly different (P < 0.05) from endosteal position.
Discussion
In most animals, disuse-induced cortical bone loss is manifest as increased porosity and decreased cross-sectional properties. For example, previous studies have reported three- to five-fold increases in intracortical porosity, 42% loss of cortical area and 13–37% decreases in moments of inertia from reduced skeletal loading (Gross & Rubin, 1995; Rubin et al. 1996; de Bruin et al. 2000; Li et al. 2005a; Modlesky et al. 2005). Without sufficient remobilization (i.e. an active period that is 2–3 times longer than the disuse period), this lost bone cannot be completely recovered (Kaneps et al. 1997; Jaworski & Uhthoff, 1986). Thus, annual net bone loss would be expected in black bears, as they experience annual disuse (hibernation) and active periods that are approximately equal in length. Instead, our study showed that bears do not suffer bone loss with ageing; porosity did not change with age and cross-sectional properties increased with age in skeletally immature male and female bears, and porosity was not higher in skeletally mature bears than in immature bears.
By examining bone properties with age in the skeletally immature bears, there is a possibility of normal age-related changes in bone structure masking disuse-related changes. This is particularly a problem with the cross-sectional properties, as geometrical properties increase with age in maturing animals (Keller et al. 1986). Thus, a limitation of this experiment is that small reductions in cross-sectional properties from insufficient remobilization could be overshadowed by age-related bone growth. However, disuse typically prevents normal age-related increases in bone geometry during growth (Abram et al. 1988; Biewener & Bertram, 1994), which does not appear to occur in bears. In addition, bears did not demonstrate an age-related increase in porosity (Fig. 3) or resorption cavity density, and porosity was not higher in skeletally mature compared with immature bears, although previous work has shown that porosity increases with age in many animals including humans (Srinivasan et al. 2000; Frank et al. 2002; Wang & Ni, 2003; Thomas et al. 2005). Thus, the bear porosity data provide support for the idea that bears counteract the deleterious effects of disuse and ageing on bone structure.
Gender-related dimorphism was apparent in the cross-sectional properties (Table 2). The differences in cross-sectional properties are probably related to body size differences between male and female bears, as bone cross-sectional properties in the femoral diaphysis are proportional to body weight (Stein et al. 1998), and male bears generally have a greater body mass than female bears (Blanchard, 1987; Parkhurst, 1998; Derocher et al. 2005). Prior work in polar bears has shown that gender differences in body mass are small at birth, but diverge further during growth (Derocher et al. 2005). A similar trend was seen in the cross-sectional properties investigated in this study (Table 2); gender-related differences were much larger in the mature bears than in the immature bears. Age-related changes in the cross-sectional properties were also different between male and female bears. Whereas cross-sectional properties were significantly greater in mature male bears than in immature male bears, there were no significant differences between immature and mature female bears (Table 2). This is in agreement with different growth patterns in male and female bears (Derocher et al. 2005); female black bears obtain peak body mass near 6 years of age (i.e. near skeletal maturity), whereas male bears can continue to gain weight until at least 12 years of age (Rogers, 1999). Because bone cross-sectional properties are proportional to body weight (Stein et al. 1998), these properties probably continued to increase in response to body weight in the mature male bears, but did not change past skeletal maturity in the female bears.
It is noteworthy that female bears had less porous (Table 3) or equally porous bones compared with male bears (depending on age). Female bears (3.5 years of age and older) give birth to 2–3 cubs every other year and nurse them during winter denning (Nelson, 1973; Rogers, 1981). Previous studies have shown that increasing parity is positively correlated with porosity, which consequently leads to a decrease in bone mechanical properties (McCalden et al. 1993; Hawkins & Stover, 1997). However, the material properties of female bear cortical bone are not different from those of male bears (Harvey & Donahue, 2004; Harvey et al. 2005), and female bears have less or equally porous bones compared with male bears (Table 3). Thus, the increased metabolic demand placed on the skeleton of female bears does not appear to impact negatively on cortical microstructure or material properties. The maintenance of porosity in female bears may help to preserve bone strength during pregnancy and lactation, and may indicate that female bears have a more rigorous compensation mechanism to deal with both the unloading associated with hibernation and the mineral losses from cub birth.
Because the femurs in this study were stored in a dried state, we were unable to obtain mechanical data to correlate directly bone structural properties to mechanical integrity. However, several properties were calculated that provide good estimates of bone strength. Section modulus (the cross-sectional moment of inertia divided by the distance between the neutral axis and periosteal border) is a good indicator of a bone's resistance to bending (i.e. bone strength) (Petit et al. 2005). Section modulus increased with age in both male and female skeletally immature bears (Table 1), suggesting increasing bone strength with age. Moments of inertia were also quantified about several different axes, as disuse can cause localized reductions in femoral cortical wall thickness (Modlesky et al. 2005), and the true femoral bending axis in bears during gait is unknown. All moments of inertia increased with age in skeletally immature bears (Table 1), suggesting that annual hibernation does not preferentially affect bone strength in any direction. We previously investigated the effect of annual hibernation on the bending and tensile material properties of black bear tibia cortical bone (Harvey & Donahue, 2004; Harvey et al. 2005), and on the whole bone mechanical properties of black bear femurs (McGee et al. 2006c). As black bears in Utah and Michigan experience similar hibernation durations, it is reasonable to compare the structural properties investigated in this study with the mechanical properties determined previously. These studies suggest that preserving bone porosity and increasing mineral content, material strength and cross-sectional properties are responsible for the increase in whole bone strength with age in bears.
Regional variation in porosity was quantified because localized increases in porosity can weaken whole bone strength. Porosity differences between the anatomical quadrants and radial positions were small (most did not achieve statistical significance), and were similar in pattern and magnitude for skeletally immature and mature bears (Figs 4 and 5), indicating that bears prevent localized porosity increases with age. The differences in porosity between anatomical quadrants may be related to structural adaptation to routine loading or the need to repair microdamage by targeted bone remodelling, as the local mechanical strains during gait and microdamage densities both vary between anatomical quadrants of the femur (Manley et al. 1982; Schaffler et al. 1995; Norman & Wang, 1997). Normal transcortical variations in porosity were noted in all female bears and mature male bears (Fig. 5), where porosity increased from the periosteal to endosteal borders (Bousson et al. 2001). It is unclear why the trends for skeletally immature male transcortical porosities were different, with high porosities found in both the endosteal and the periosteal radial positions (Fig. 5). One possible explanation is that because the majority of male bears in this study were 2–3 years old, the high periosteal and endosteal porosities may reflect the rapid apposition of bone on these surfaces as occurs in other large mammals such as horses (Stover et al. 1992). In both sexes, we qualitatively observed that large intracortical pores (i.e. larger than a typical Haverisan remodelling cavity), which can appear throughout the cortex during disuse in other animals (Rubin et al. 1996), were rare. The few that did occur were mainly located near the endosteal border, which corresponds with the higher endosteal porosities we observed in a majority of the bears (Fig. 6). These cavities are possibly related to marrow cavity expansion, although expansion appears to occur slowly because it did not statistically increase with age in male or female bears. Resorption cavity density was not significantly different between skeletally immature and mature male bears. Although resorption cavity density approached a significant difference between skeletally immature and mature female bears (P = 0.054), endosteal area, endosteal area fraction, cortical thickness and porosity were not different between mature and immature female bears, suggesting that the increased resorption cavity density was not detrimental to the cortical structure as a whole.
The age-related trends noted in the cross-sectional properties and porosity in bear femurs suggest that either bears lose less bone during disuse or are able to recover lost bone at a faster rate during remobilization than other animals. Prior and ongoing work in bears supports the first possibility. Black bear and grizzly bear trabecular bone volume is not different before, during or after hibernation (Pardy et al. 2004; McGee et al. 2006b), and cortical porosity decreases during hibernation in grizzly bears (McGee et al. 2006a). Thus, bears appear to preserve trabecular and cortical bone mass during hibernation. This may be the result of a unique bone metabolism process. Unlike most animals, bears show balanced bone remodelling during disuse (Floyd et al. 1990; Donahue et al. 2006a). The mechanism by which bears can maintain balanced bone remodelling during hibernation is not yet known, but previous work suggests the involvement of the anabolic effects of parathyroid hormone (Donahue et al. 2006a). Parathyroid hormone levels are positively correlated with the bone formation marker osteocalcin in bears, suggesting that the maintenance of bone formation during hibernation may be regulated in part by this hormone (Donahue et al. 2006a). It is also possible that bears have the ability to prevent increases in cortical osteoclastic resorption during disuse, as evidenced by the fact that cortical porosity is decreased in hibernating compared with active grizzly bears (McGee et al. 2006a) and neither porosity nor endosteal area fraction increases with age in black bears. Further research into the biological mechanisms by which bears are able to avoid disuse-related loss of bone strength may form the basis for development of therapeutic treatments of metabolic bone diseases.
Acknowledgments
This project was supported by Grant Number AR050420 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; its contents are solely the responsibility of the authors. Additional funding was received from NASA, the Michigan Space Grant Consortium, the National Science Foundation Graduate Research Fellowship Program, and the Michigan Technological University Department of Educational Opportunity. We thank Brian Willard for assistance with Scion Image.
References
- Abram AC, Keller TS, Spengler DM. The effects of simulated weightlessness on bone biomechanical and biochemical properties in the maturing rat. J Biomech. 1988;21:755–767. doi: 10.1016/0021-9290(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Bertram JE. Structural response of growing bone to exercise and disuse. J Appl Physiol. 1994;76:946–955. doi: 10.1152/jappl.1994.76.2.946. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravit Space Biol Bull. 2003;16:45–54. [PubMed] [Google Scholar]
- Blanchard BM. Size and growth patterns of the Yellowstone grizzly bear. Proceedings from the International Conference on Bear Research and Management. 1987;7:99–107. [Google Scholar]
- Bousson V, Meunier A, Bergot C, et al. Distribution of intracortical porosity in human midfemoral cortex by age and gender. J Bone Miner Res. 2001;16:1308–1317. doi: 10.1359/jbmr.2001.16.7.1308. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Herzog R, Rozendal RH, Michel D, Stussi E. Estimation of geometric properties of cortical bone in spinal cord injury. Arch Phys Med Rehabil. 2000;81:150–156. [PubMed] [Google Scholar]
- Caillot-Augusseau A, Vico L, Heer M, et al. Space flight is associated with rapid decreases of undercarboxylated osteocalcin and increases of markers of bone resorption without changes in their circadian variation: observations in two cosmonauts. Clin Chem. 2000;46:1136–1143. [PubMed] [Google Scholar]
- Collet P, Uebelhart D, Vico L, et al. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547–551. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- Demes B, Qin YX, Stern JT, Jr, Larson SG, Rubin CT. Patterns of strain in the macaque tibia during functional activity. Am J Phys Anthropol. 2001;116:257–265. doi: 10.1002/ajpa.1122. [DOI] [PubMed] [Google Scholar]
- Derocher AE, Andersen M, Wiig O. Sexual dimorphism of polar bears. J Mamm. 2005;86:895–901. [Google Scholar]
- Donahue SW, Galley SA, Vaughan MR, et al. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J Exp Biol. 2006a;209:1630–1638. doi: 10.1242/jeb.02185. [DOI] [PubMed] [Google Scholar]
- Donahue SW, McGee ME, Harvey KB, Vaughan MR, Robbins CT. Hibernating bears as a model for preventing disuse osteoporosis. J Biomech. 2006b;39:1480–1488. doi: 10.1016/j.jbiomech.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Floyd T, Nelson RA, Wynne GF. Calcium and bone metabolic homeostasis in active and denning black bears (Ursus americanus) Clin Orthop. 1990;8:301–309. [PubMed] [Google Scholar]
- Frank JD, Ryan M, Kalscheur VL, Ruaux-Mason CP, Hozak RR, Muir P. Aging and accumulation of microdamage in canine bone. Bone. 2002;30:201–206. doi: 10.1016/s8756-3282(01)00623-8. [DOI] [PubMed] [Google Scholar]
- Garber MA, McDowell DL, Hutton WC. Bone loss during simulated weightlessness: a biomechanical and mineralization study in the rat model. Aviat Space Environ Med. 2000;71:586–592. [PubMed] [Google Scholar]
- Gross TS, Rubin CT. Uniformity of resorptive bone loss induced by disuse. J Orthop Res. 1995;13:708–714. doi: 10.1002/jor.1100130510. [DOI] [PubMed] [Google Scholar]
- Harvey KB, Donahue SW. Bending properties, porosity, and ash fraction of black bear (Ursus americanus) cortical bone are not compromised with aging despite annual periods of disuse. J Biomech. 2004;37:1513–1520. doi: 10.1016/j.jbiomech.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Harvey KB, Drummer TD, Donahue SW. The tensile strength of black bear (Ursus americanus) cortical bone is not compromised with aging despite annual periods of hibernation. J Biomech. 2005;38:2143–2150. doi: 10.1016/j.jbiomech.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hawkins DL, Stover SM. Pregnancy-associated changes in material properties of the third metacarpal cortical bone in mares. Am J Vet Res. 1997;58:182–187. [PubMed] [Google Scholar]
- Jaworski ZF, Uhthoff HK. Reversibility of nontraumatic disuse osteoporosis during its active phase. Bone. 1986;7:431–439. doi: 10.1016/8756-3282(86)90003-7. [DOI] [PubMed] [Google Scholar]
- Kaneps AJ, Stover SM, Lane NE. Changes in canine cortical and cancellous bone mechanical properties following immobilization and remobilization with exercise. Bone. 1997;21:419–423. doi: 10.1016/s8756-3282(97)00167-1. [DOI] [PubMed] [Google Scholar]
- Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32:702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- Keller TS, Spengler DM, Carter DR. Geometric, elastic, and structural properties of maturing rat femora. J Orthop Res. 1986;4:57–67. doi: 10.1002/jor.1100040107. [DOI] [PubMed] [Google Scholar]
- Li CY, Majeska RJ, Laudier DM, Mann R, Schaffler MB. High-dose risedronate treatment partially preserves cancellous bone mass and microarchitecture during long-term disuse. Bone. 2005a;37:287–295. doi: 10.1016/j.bone.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Li CY, Price C, Delisser K, Nasser P, Laudier D, Clement M, Jepsen KJ, Schaffler MB. Long-term disuse osteoporosis seems less sensitive to bisphosphonate treatment than other osteoporosis. J Bone Miner Res. 2005b;20:117–124. doi: 10.1359/JBMR.041010. [DOI] [PubMed] [Google Scholar]
- Manley PA, Schatzker J, Sumner-Smith G. Evaluation of tension and compression forces in the canine femur in vivo. Arch Orthop Trauma Surg. 1982;99:213–216. doi: 10.1007/BF00379211. [DOI] [PubMed] [Google Scholar]
- McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- McGee ME, Castillo AB, Nelson OL, Robbins CT, Donahue SW. 52nd Annual Meeting of the Orthopaedic Research Society. Chicago, IL: Orthopaedic Research Society; 2006a. The effects of disuse (hibernation) on trabecular architecture and mineral density in grizzly bear (Ursus arctos horribilis) femurs. [Google Scholar]
- McGee ME, Magic KW, Miller DL, Maki AJ, Donahue SW. Black bear femoral porosity decreases and mechanical properties increase with age despite annual periods of disuse (hibernation) Eng Frac Mech. 2006b. in press. 19 June 2006 (Epub ahead of print)
- McGee ME, Miller DL, Maki AJ, et al. 52nd Annual Meeting of the Orthopaedic Research Society. Chicago, IL: Orthopaedic Research Society; 2006c. Cortical bone porosity, mechanical and cross-sectional properties are preserved during disuse (hibernation) and do not show loss with age in grizzly and black bear femurs. [Google Scholar]
- Modlesky CM, Slade JM, Bickel CS, Meyer RA, Dudley GA. Deteriorated geometric structure and strength of the midfemur in men with complete spinal cord injury. Bone. 2005;36:331–339. doi: 10.1016/j.bone.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nelson RA. Winter sleep in the black bear. A physiologic and metabolic marvel. Mayo Clin Proc. 1973;48:733–737. [PubMed] [Google Scholar]
- Norman TL, Wang Z. Microdamage of human cortical bone: incidence and morphology in long bones. Bone. 1997;20:375–379. doi: 10.1016/s8756-3282(97)00004-5. [DOI] [PubMed] [Google Scholar]
- Pardy CK, Wohl GR, Ukrainetz PJ, Sawers A, Boyd SK, Zernicke RF. Maintenance of bone mass and architecture in denning black bears (Ursus americanus) J Zool Lond. 2004;263:359–364. [Google Scholar]
- Parkhurst J. Virginia Polytechnic Institute and State University; 1998. Managing wildlife damage. Black bears Ursus americanus. Virginia Cooperative Extension Publication 420-200. [Google Scholar]
- Petit MA, Beck TJ, Kontulainen SA. Examining the developing bone: what do we measure and how do we do it? J Musculoskelet Neuronal Interact. 2005;5:213–224. [PubMed] [Google Scholar]
- Rantakokko J, Uusitalo H, Jamsa T, Tuukkanen J, Aro HT, Vuorio E. Expression profiles of mRNAs for osteoblast and osteoclast proteins as indicators of bone loss in mouse immobilization osteopenia model. J Bone Miner Res. 1999;14:1934–1942. doi: 10.1359/jbmr.1999.14.11.1934. [DOI] [PubMed] [Google Scholar]
- Rogers L. A bear in its lair. Natural History. 1981;90:64–70. [Google Scholar]
- Rogers LL. American black bear, Ursus americanus. In: Wilson DE, Ruff S, editors. The Smithsonian Book of North American Mammals. Washington, DC: Smithsonian Institution Press; 1999. pp. 157–160. [Google Scholar]
- Rubin C, Gross T, Qin YX, Fritton S, Guilak F, McLeod K. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J Bone Joint Surg Am. 1996;78:1523–1533. doi: 10.2106/00004623-199610000-00010. [DOI] [PubMed] [Google Scholar]
- Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Keilin SA, Judex S, Bray RC, Zernicke RF, Gross TS. Aging-induced osteopenia in avian cortical bone. Bone. 2000;26:361–365. doi: 10.1016/S8756-3282(00)00237-4. [DOI] [PubMed] [Google Scholar]
- Stein MS, Thomas CD, Feik SA, Wark JD, Clement JG. Bone size and mechanics at the femoral diaphysis across age and sex. J Biomech. 1998;31:1101–1110. doi: 10.1016/s0021-9290(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Stover SM, Pool RR, Martin RB, Morgan JP. Histological features of the dorsal cortex of the third metacarpal bone mid-diaphysis during postnatal growth in thoroughbred horses. J Anat. 1992;181:455–469. [PMC free article] [PubMed] [Google Scholar]
- Thomas CD, Feik SA, Clement JG. Regional variation of intracortical porosity in the midshaft of the human femur: age and sex differences. J Anat. 2005;206:115–125. doi: 10.1111/j.1469-7580.2005.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Evans GL, Wakley GK. Spaceflight results in depressed cancellous bone formation in rat humeri. Aviat Space Environ Med. 1995;66:770–774. [PubMed] [Google Scholar]
- Vico L, Chappard D, Alexandre C, et al. Effects of a 120 day period of bed-rest on bone mass and bone cell activities in man: attempts at countermeasure. Bone Miner. 1987;2:383–394. [PubMed] [Google Scholar]
- Wang X, Ni Q. Determination of cortical bone porosity and pore size distribution using a low field pulsed NMR approach. J Orthop Res. 2003;21:312–319. doi: 10.1016/S0736-0266(02)00157-2. [DOI] [PubMed] [Google Scholar]
- Weinreb M, Rodan GA, Thompson DD. Osteopenia in the immobilized rat hind limb is associated with increased bone resorption and decreased bone formation. Bone. 1989;10:187–194. doi: 10.1016/8756-3282(89)90052-5. [DOI] [PubMed] [Google Scholar]
- Weinreb M, Patael H, Preisler O, Ben-Shemen S. Short-term healing kinetics of cortical and cancellous bone osteopenia induced by unloading during the reloading period in young rats. Virchows Arch. 1997;431:449–452. doi: 10.1007/s004280050122. [DOI] [PubMed] [Google Scholar]
- Westwood S. Loss of Bone mass with aging and femoral sexual dimorphism in the American black bear (Ursus americanus) Provo, UT: Department of Zoology, Brigham Young University; 1996. PhD Thesis. [Google Scholar]
- Wronski TJ, Morey-Holton ER, Doty SB, Maese AC, Walsh CC. Histomorphometric analysis of rat skeleton following spaceflight. Am J Physiol. 1987;252:R252–R255. doi: 10.1152/ajpregu.1987.252.2.R252. [DOI] [PubMed] [Google Scholar]
- Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13:1594–1601. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]