Abstract
Laryngeal air sacs have evolved convergently in diverse mammalian lineages including insectivores, bats, rodents, pinnipeds, ungulates and primates, but their precise function has remained elusive. Among cervids, the vocal tract of reindeer has evolved an unpaired inflatable ventrorostral laryngeal air sac. This air sac is not present at birth but emerges during ontogenetic development. It protrudes from the laryngeal vestibulum via a short duct between the epiglottis and the thyroid cartilage. In the female the growth of the air sac stops at the age of 2–3 years, whereas in males it continues to grow up to the age of about 6 years, leading to a pronounced sexual dimorphism of the air sac. In adult females it is of moderate size (about 100 cm3), whereas in adult males it is large (3000–4000 cm3) and becomes asymmetric extending either to the left or to the right side of the neck. In both adult females and males the ventral air sac walls touch the integument. In the adult male the air sac is laterally covered by the mandibular portion of the sternocephalic muscle and the skin. Both sexes of reindeer have a double stylohyoid muscle and a thyroepiglottic muscle. Possibly these muscles assist in inflation of the air sac. Head-and-neck specimens were subjected to macroscopic anatomical dissection, computer tomographic analysis and skeletonization. In addition, isolated larynges were studied for comparison. Acoustic recordings were made during an autumn round-up of semi-domestic reindeer in Finland and in a small zoo herd. Male reindeer adopt a specific posture when emitting their serial hoarse rutting calls. Head and neck are kept low and the throat region is extended. In the ventral neck region, roughly corresponding to the position of the large air sac, there is a mane of longer hairs. Neck swelling and mane spreading during vocalization may act as an optical signal to other males and females. The air sac, as a side branch of the vocal tract, can be considered as an additional acoustic filter. Individual acoustic recognition may have been the primary function in the evolution of a size-variable air sac, and this function is retained in mother–young communication. In males sexual selection seems to have favoured a considerable size increase of the air sac and a switch to call series instead of single calls. Vocalization became restricted to the rutting period serving the attraction of females. We propose two possibilities for the acoustic function of the air sac in vocalization that do not exclude each other. The first assumes a coupling between air sac and the environment, resulting in an acoustic output that is a combination of the vocal tract resonance frequencies emitted via mouth and nostrils and the resonance frequencies of the air sac transmitted via the neck skin. The second assumes a weak coupling so that resonance frequencies of the air sac are lost to surrounding tissues by dissipation. In this case the resonance frequencies of the air sac solely influence the signal that is further filtered by the remaining vocal tract. According to our results one acoustic effect of the air sac in adult reindeer might be to mask formants of the vocal tract proper. In other cervid species, however, formants of rutting calls convey essential information on the quality of the sender, related to its potential reproductive success, to conspecifics. Further studies are required to solve this inconsistency.
Keywords: acoustic communication, air sac, comparative anatomy, courtship behaviour, larynx, reindeer, sound production
Introduction
Eurasian reindeer and New World caribou are cold-adapted gregarious deer (Cervidae) of the northern hemisphere. They are considered to be the same species (Rangifer tarandus Hamilton Smith 1827) consisting of several subspecies (Wilson & Reeder, 2005; cf. Herre, 1956; Banfield, 1974). Domestication of reindeer is thought to have begun about 3000 years ago and to have subsequently spread across all of northern Eurasia (Nowak, 1999; cf. Herre, 1955).
Both non-migratory palaearctic populations of reindeer and migratory nearctic populations of caribou have a polygynous mating system. In Eurasian reindeer the rutting period begins in the last third of September and lasts roughly until the end of October (Pleske, 1884; Jacobi, 1931). During this period dominant males gather a group of several to about 20 (or even more than 100) females and defend it vigorously against intruding males. In sight of a rival male, the dominant male is sometimes seen to tramp rapidly with his hind legs, the hoofs being kept close together and the back hunched, and to urinate in small quantities on its lower legs. The strong odour may both repel rival males and attract female reindeer. In the course of the summer adult male reindeer develop a considerable subcutaneous fat layer up to 80 mm in thickness. It serves as an energy source and is used up during the exhausting rut. Dominant males seldom feed or do not feed at all within the rutting period (Jacobi, 1931; Herre, 1955, 1956; Espmark, 1964). Females do not develop a comparable subcutaneous fat layer.
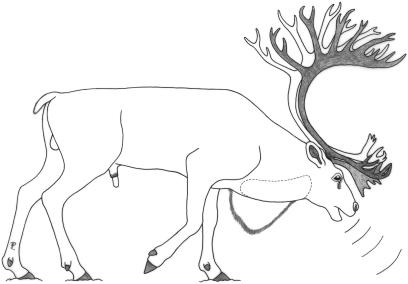
At the beginning of the rut the neck diameter increases considerably owing to a gain in mass of the neck muscles (Pleske, 1884; Jacobi, 1931; Espmark, 1964). In addition, adult males develop a ventral neck mane (‘neck ruff‘) hanging down beard-like from the lower contour of the neck (Lent, 1965; Fig. 1).
Fig 1.
Calling posture of an adult male reindeer. Head and neck are kept low, with the throat region extended. Ventral neck mane and position of large air sac (dotted line) are indicated.
Apart from this ‘seasonal sexual dimorphism’, there are permanent mean body mass differences. Females gain about one-quarter less of body mass than males. Unique among cervid species, both sexes of reindeer have antlers but they are considerably smaller in females.
Courtship behaviour of the male comprises a characteristic vocalization. It has been described as ‘a peculiar, hoarse, guttural roar’ (Austen, 1875), as ‘grunting’ and ‘bugling’ (Dugmore, 1913) or as ‘a series of husky, rapid, rather low-voiced rattles that are brought about by repeated expirations of air’ (Espmark, 1964). Pleske (1884) describes the vocalization as ‘a loud roar still audible at a distance of about 500 meters’. This kind of rutting call is produced in sight of other males, in herding behaviour or when driving harem females. Driving means that a male pursues or closely follows a female and, in addition to the acoustic performance, displays his antlers and neck mane by applying specific head (and body) postures (Pruitt, 1960; Espmark, 1964; Henshaw, 1970; cf. Geist, 1966; Andersson, 1994). The utterance of the rutting call is associated with a conspicuous posture in which head and neck are kept low and the throat region is extended (Jacobi, 1931, fig. 31, p. 233). This is also the characteristic resting posture of the male, which is frequently adopted during the rutting period (Dugmore, 1913; Espmark, 1964).
Vocalizations are also important for adult female reindeer in mother–infant communication (Pruitt, 1960; Ericson, 1972). Mother and infant can identify each other by individual voice characteristics. Accordingly, inter-individual voice variation is great among adult females and young (Espmark, 1971b, 1975).
The vocal tract of reindeer has evolved a peculiar specialization: a laryngeal air sac. It was originally described by Camper as early as 1791 in a 4-year-old male and illustrated by a small but instructive figure (Camper, 1791, pl. VIII, fig. VII). A description and a rough schematic figure have also been provided by Negus (1929, 1962) and by Schneider (1964). Morrow (1877), Lönnberg (1902), Ogura & Yamane (1915) and Skjenneberg & Slagsvold (1968) reported that this air sac, although smaller, is also present in the female. According to Ogura & Yamane, the sternohyoid muscle attaches onto the air sac whereas the caudal constrictor muscles of the pharynx are not connected to the air sac. The air sac protrudes rostroventrally from the larynx between the base of the epiglottis and the rostral rim of the thyroid cartilage. The diameter of the opening in the laryngeal wall is about 5–10 mm (Ogura & Yamane, 1915; Skjenneberg & Slagsvold, 1968). No mention is made concerning the ontogenetic development of the air sac. According to Millais (1907) the laryngeal air sac also occurs in the Newfoundland caribou. To our knowledege, reindeer are the only cervids possessing an air sac.
Normally, younger Scandinavian reindeer herders are ignorant of the reindeer's air sac. However, this feature is still known to old Samisk people, e.g. of north-east Norway, as ‘hubmaseakkha’. This term may have been developed by reindeer herders themselves as it is not registered in a Samisk dictionary that does, however, list ‘hubma’ for ‘talk, chat or indistinct talk’ and ‘hubmai’ for ‘talkative or chatty person’. (Information on etymology and Samisk knowledge transmitted by N. Tyler, pers. comm., 2006).
It would appear that this laryngeal air sac is in some way or other related to the vocalization of reindeer in general, and to the peculiar hoarse rutting calls of the adult dominant males in particular. Gegenbaur (1901) had already referred to the laryngeal ventricles and air sacs in general as resonance devices which increase the amplitude of vocalization. Lönnberg (1902) proposed a ‘sound balloon’ function for the air sac of reindeer and Ogura & Yamane (1915) also promoted a resonance function.
Materials and methods
All specimens of our investigations came either from zoo animals or from semi-domestic reindeer in northern Finland, near Ivalo. All dissections were made with the specimens submersed in water according to the procedure described in Frey & Hofmann (2000). Consecutive dissection steps were documented by a series of analogue photographic slides (Nikon F3) and also by digital images (Minolta Dimage 7 and the appropriate software). Certain stages were recorded in proportional drawings. The vocal tract length was obtained by measurement of a string that was arranged in the airway from the glottis to the upper lip. The error of linear or string measurements of the intrinsic laryngeal structures was ±3 mm (n = 10). Measurements of the isolated epiglottis and laryngeal cartilages were taken by means of a string measure and calipers. Air sac volume was ascertained by filling water into the opening of the excised, formalin-preserved air sac up to maximal filling and by subsequent measuring of the contained water volume in a beaker.
Computer tomographic (CT) scanning was performed by means of a General Electric Lightspeed 4-Slice Spiral CT (GE, Medical Systems, Milwaukee, WI, USA). The slice thickness was 0.6 mm. A volume rendering software (GE VolumeViewer) was applied for processing of the data and for producing the virtual endoscopy.
The isolated vocal tract including the air sac of one adult male (∼6 years) was taken in the Leibniz Institute for Zoo and Wildlife Research (IZW) during routine post-mortem on 5 September 2001 and preserved in 3–4% formalin for subsequent dissection. The animal had died in Tierpark Berlin. Body mass after death was 180 kg. The preserved vocal tract and air sac were dissected in November and December 2001.
One subadult younger male (3–4 years) had been shot near Ivalo, northern Finland, on 4 January 2004. Head and neck were sent deep frozen to the IZW in March 2004. After CT investigation, this specimen was dissected in August 2004. Vocal tract length (VTL) was measured both in the CT images and in the course of dissection.
One adult female (∼8 years) had died on 31 August 2004 in Tierpark Berlin. Body mass was 85 kg. Head and neck were deep frozen and subjected to CT investigation. Prior to dissection in March 2005 the major blood vessels of the specimen were injected via the common carotid arteries and the external jugular veins with coloured silicone solutions according to Schmidt (1981). In addition, the air sac was injected with a coloured silicone solution. Access for the injection cannula was made by an incision into the ventral wall of the air sac just opposite to where the air sac arises from the laryngeal vestibulum. VTL was measured both in the CT images and in the course of dissection.
Another adult male (∼6 years) was shot in September 2004 near Ivalo, northern Finland. Head and neck were sent deep frozen to the IZW in October 2004. CT investigations were made. Measurements of VTL were performed two-dimensionally in sagittal CT sections.
A formalin-fixed neonate male head-and-neck specimen, originating from Tierpark Schönbrunn Vienna, was first investigated by CT scanning and afterwards dissected macroscopically. VTL was measured both in CT images and in the course of the dissection.
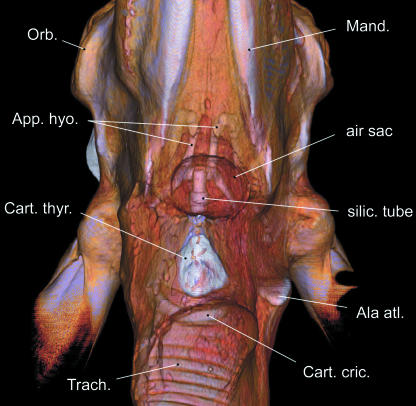
In a second adult female (∼13 years) that had died in October 2005 in Tierpark Berlin of multiple lung tumours, the air sac had been injected with coloured silicone solution via a silicone tube. Prior to injection, the tube was inserted into the trachea of this head-and-neck specimen and pushed rostrally through the glottis and laryngeal vestibulum up into the air sac. After cutting through the skin in the ventral neck region, the ‘neck’ of the air sac was then carefully tied up around the silicone tube to prevent backflow of the injected solution. The injection allowed for documenting the filled air sac in CT images (see Fig. 12).
Fig 12.
Small silicone-injected air sac in an old adult female reindeer, ventral view based upon CT scans. Air sac position is between hyoid apparatus and thyroid cartilage. End piece of silicone tube visible within the air sac.
Samples of the air sac of one adult male and one adult female were processed histologically according to routine procedures. Samples were taken from the air sac wall and from the pneumatic duct of the air sac near its opening. Sections were stained with haematoxylin/eosin and with resorcin/fuchsin.
In the Results section only those muscles exhibiting specific features are described. Additional muscles were investigated and are listed in Table 1. Sternal origins of the mandibular portion of the sternocephalic muscle and of sternohyoid and sternothyroid muscles could not be dissected as we had only head-and-neck specimens at our disposal.
Table 1.
Pharyngolaryngeal region of Rangifer tarandus– origin and insertion of muscles
| Muscle | Origin | Specific features | Insertion | |
|---|---|---|---|---|
| M. cut. fac. | dorsal portion | superficial fascia of the neck obliquely along lateroventral edge of M. sternocephalicus | fibres coursing obliquely in rostrodorsal direction | connective tissue ventral to M. orbic. oculi, touching its most ventral fibres |
| ventral portion | fibres coursing rostrally | connective tissue near the angle of the mouth and the insertion of M. zyg. | ||
| M. parotaur. | ventral edge of cartilaginous part of Meatus acusticus externus | fibres interweave medioventrally with those of the contralateral M. parotaur, thus forming a flexible ribbon around structures within retromandibular fossae and pharyngolaryngeal region | ||
| M. sternoceph., pars mand. | dorsal tendon | cranial part of sternum, Manubrium sterni (not dissected) | stronger | deep to V. fac. and Duct. parot. along rostral edge of M. mass. to its aponeurosis, covered by caudal portion of M. mal. |
| ventral tendon | weaker | ventrocaudal part of Mandibula, near ventral edge of M. mass., tapering rostrally | ||
| M. sternoh. | cranial part of sternum, Manubrium sterni (not dissected) | thin ribbon, 25–35 mm in width, covering the larynx dorsolaterally | caudal aspect of Basihyoid and laterocaudally to ventral half of Thyrohyoid | |
| M. styloh. | caudal band | angle of Stylohyoid | double muscle! – two parallel bands, near their origins enclosing the caudal belly of M. digastr. | Basihyoid, caudally and slightly displaced dorsally relative to rostral band |
| rostral band | dorsorostrally from origin of caudal band | Basihyoid, rostroventral to caudal band | ||
| M. digastr. | Proc. parac. | double-bellied muscle | medial aspect of molar part of Corpus mandibulae | |
| M. myloh. | caudal portion | Linea mylohyoidea, P4 – Tub. max. | transverse and rostrally directed fibres | Basihyoid and median raphe, laterally covered by rostral and transverse portion, aponeurotic caudal edge attached to insertions of both portions of M. styloh. |
| rostral portion | Linea mylohyoidea, Frenul. ling. – P3 | transverse and caudally directed fibres | median raphe | |
| transverse portion | lateral aspect of caudal portion, medial to origin of M. digastr. | transversely directed fibres, caudally attached to rostral portion | lateral aspect of caudal portion, medial to origin of M. digastr. | |
| M. occiph. | Proc. parac. | caudodorsal edge of Stylohyoid, from Tympanohyoid to stylohyoid angle | ||
| M. omoh. | lacking | |||
| M. sternthyr. | together with M. sternoh. from Manubrium sterni (not dissected) | tendinous inscription at the level of 10th and 11th tracheal ring | along laterocaudal edge of Cart. thyr., adjacent to origin of M. thyroh., covering the insertion of M. cricthyr. | |
| M. thyroh. | along laterocaudal edge of Cart. thyr., adjacent to insertion of M. sternthyr., covering the origin of the Mm. constr. phar. caud. | fibres converging towards insertion, reducing dorsoventral muscle width by half | medioventral surface of Thyrohyoid | |
| M. cerath. | along the entire rostral edge of Thyrohyoid | fills triangle between Thyro- and Ceratohyoid | along the entire caudal edge of Ceratohyoid and to ventrocaudal end of Epihyoid | |
| Mm. constr. phar. caud. | M. cricphar. | laterodorsal surface of Cart. cric., dorsal to Art. cricthyr. | Raphe pharyngis | |
| M. thyrphar. | lateral surface of Cart. thyr. along ventrally convex line between its more calcified and less calcified portion | in adult males few rostral bundles may extend onto the surface of the air sac | Raphe pharyngis | |
| M. constr. phar. med. | M. hyophar. | dorsorostral edge of ventral half of Thyrohyoid and dorsal edge of middle portion of Ceratohyoid | Raphe pharyngis | |
| Mm. constr. phar. rostr. | M. stylphar. caud. | medially from dorsal half of Stylohyoid | fibres fan out caudally, covered by those of M. thyrphar. | lateral wall of pharynx and caudal two thirds of mediodorsal edge of Cart. thyr. up to the base of Corn. caud. |
| M. stylphar. rostr. | medially from ventral end of Stylohyoid | Raphe pharyngis | ||
| M. pterphar. | Os pterygoideum | Raphe pharyngis, rostral to M. stylphar. caud. | ||
| M. palphar. | Aponeurosis palatina, caudal edges of Ossa palatina and pterygoidea | rostral edge of Cart. thyr. and Raphe pharyngis | ||
| M. lev. veli pal. | Pars tymp. of Os temporale, caudally adjacent to M. tens. veli pal. | Palat. mol. and contralateral muscle, medial to M. pterphar. | ||
| M. tens. veli pal. | Pars tymp. of Os temporale and cartilage of auditory tube | Aponeurosis palatina | ||
| M. hyoepigl. | Basihyoid and Thyrohyoid | ventrorostral portion of Epigl. | ||
| M. cricthyr. | rostrolateral surface of Cart. cric. | caudal rim of Cart. thyr. and ventral circumference of Corn. caud. | ||
| M. cricaryt. lat. | dorsorostrally from lateral surface of Arc. cart. cric. | short, covering the dorsal half of the oblique rostral edge of Cart. cric. | ventrocaudal aspect of Proc. musc. | |
| M. cricaryt. dors. | Lam. cart. cric. | caudodorsal aspect of Proc. musc., laterally touching the insertion of M. cricaryt. lat. | ||
| M. aryt. transv. | dorsorostral aspect of Proc. musc., lateral surface of Cart. aryt., dorsal to the Crista arcuata, and to the Lig. arycorn. of Cart. aryt. | contralateral muscle, rostral to the Lig. aryt. transv. (between Procc. med. of Cartt. aryt.) | ||
| M. thyraryt. | M. ventric. | ventrolateral surface of epiglottic base, dorsal to opening of air sac (‘epiglottico-arytenoid’ muscle) | wedge-shaped cleft between M. ventric., M. voc. and M. thyrepigl. devoid of muscle fibres | rostrolateral aspect of Proc. musc., ventral to Lig. arycorn. |
| M. voc. | caudodorsal surface of Cart. thyr. and dorsal surface of Lig. cricthyr. | medioventral aspect of Proc. musc. | ||
| M. thyrepigl. | dorsal surface of Cart. thyr., medial to M. voc. | covers the neck of the air sac laterally | lateroventrally to connective tissue at the base of the Epigl. |
Some of the acoustic recordings were made about 20 km south-east of Ivalo near Kuukkelilampi, Moitakurunselkä, in northern Finland (27°34.012′E, 68°28.445′N) during the autumn roundup of c. 1200 reindeer on 10 and 11 October 2004, i.e. within the rutting period. The acoustic recordings were made from about 10:00 to 16:00 h within enclosures spreading radially from a central arena to which the reindeer were driven first. Size of the radial enclosures was about 2 ha. The range of distances between the vocalizing animal and the microphone lay between 10 and 50 m. The roundup destroys the original harem structure, which the dominant males try to restore thereafter. Video recordings were made at the same roundup to document restoration of harem structure and courtship behaviour after calming down of the animals.
In addition, rutting calls of one Eurasian reindeer stag were recorded from 27 September to 12 October 2005 in Tierpark Berlin at distances from 3 to 8 m between microphone and vocalizing stag. The small captive herd consisted of this old adult male, eight adult females and five juveniles. The recording time was from 06:30 to 07:45 h.
All recordings were made by a digital audio tape (DAT) recorder (Sony TCD-D 100) in combination with a directional microphone and wind cover (Sennheiser ME 80 including a K3U pre-amplifier). The sample frequency was 48 kHz with a 16-bit resolution. After transfer to a computer, the recordings were cut by means of a wave editor (Audacity 1.2.3 for Linux; http://audacity.sourceforge.net). The resulting wave files were preprocessed and analysed by means of a voice analyser (Praat 4.3.24 for Linux, P. Boersma & D. Weenink, University of Amsterdam; http://www.praat.org). Spectrograms of representative calls (settings: 0.05-s analysis width, 0.002-s time step, 20-Hz frequency step, Gaussian window function, maximum frequency 20 kHz) were generated after filtering (Hann band pass, settings: 30–25 000 Hz, smoothing 10 Hz). The fundamental frequency of a call was calculated by the reciprocal of the mean distance between signal maxima in the time domain. For more detailed analyses of the frequency distribution, the spectrum from a (subjectively) stationary part of 0.05 s from the middle of a call was calculated. Spectra shown in the figures here were smoothed by transforming them into long-term average spectra (LTAS) with 50-Hz bandwidth. LTAS with 1-kHz bandwidth are used to determine the spectral decay of the signal.
Anatomical terms follow NAV (2005). In the following the paired structures of the larynx and the adjacent musculature are generally referred to in the singular. Anatomical abbreviations are given in the Appendix.
Results
Skull and hyoid apparatus
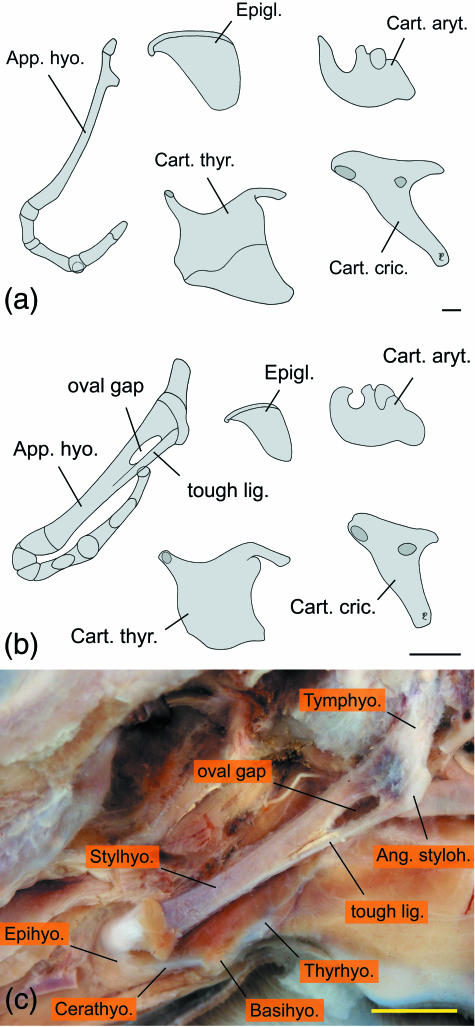
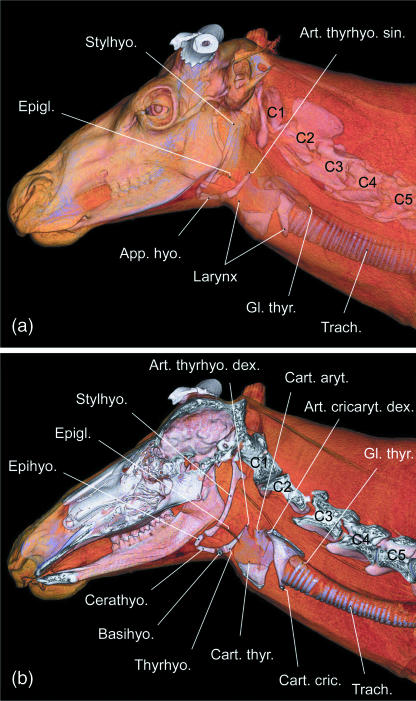
The basic structure of the adult hyoid apparatus of reindeer conforms with that of other ruminants, e.g. in domestic sheep, goats and cattle (Nickel et al. 1984). However, compared with the flat stylohyoid of domestic ruminants, the dorsal four-fifths of the reindeer's stylohyoid is remarkably slender, having only about half the rostrocaudal diameter of its most ventral portion and of epi-, cerato- and thyrohyoid. The thyrohyoid is short and of about equal length as the ceratohyoid. Its caudal third, establishing the hyoid part of the thyrohyoid articulation, consists of cartilage (Fig. 2a). Except for the short cartilaginous parts connecting the individual ossified elements of the adult hyoid apparatus, no interposed flexible elements could be detected. In the neonate male hyoid a tough ligament connected the ventral end of the stylohyoid angle to the ventral edge of the stylohyoid, thereby creating an oval gap between the stylohyoid and this ligament on both sides. This oval gap provides fixation for the passage of the external carotid artery. In the neonate, epi-, cerato- and thyrohyoid consisted mainly of cartilage, but ossification centres were visible in the middle portions of cerato- and thyrohyoid (Fig. 2b,c).
Fig 2.
Hyoid apparatus and laryngeal cartilages of reindeer, left lateral view. Shape of adult female elements (not shown) are largely identical with those of the adult male. (a) Adult male; (b) neonate male; (c) neonate male, hyoid apparatus in situ, the oval gap is for passage of the external carotid artery. Scale bars: 10 mm.
Mandibular portion of sternocephalic muscle
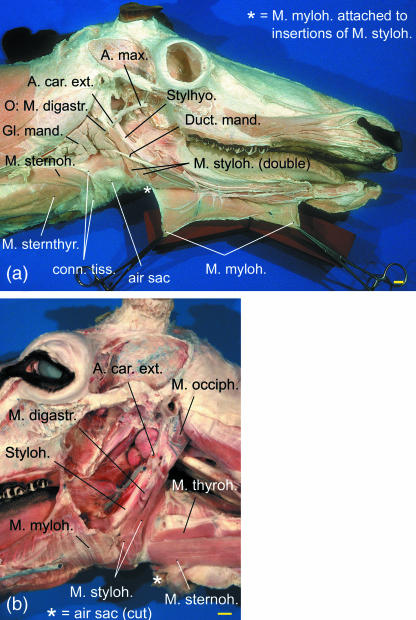
In both sexes the broad and powerful strap-like mandibular portion of the sternocephalic muscle has a double insertion: (1) by a strong tendon onto the rostral aspect of the aponeurosis of the masseter, and (2) by a weaker tendon to the ventrocaudal part of the mandibula (Table 1). In the throat region this muscle, lateral to the sternohyoid muscle, covers the air sac laterally (see Fig. 5c).
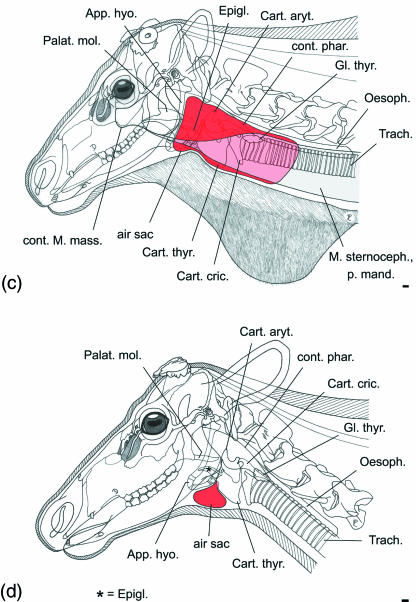
Fig 5.
Ontogeny and sexual dimorphism of the air sac in reindeer. (a) Male neonate, does not yet have an air sac but a small rostral depression, probably a homologue of the median laryngeal recess (see inset upper left); (b) subadult male, small air sac; (c) adult male, large air sac; (d) old adult female, small air sac. Scale bars: 10 mm.
Hyoid muscles
In contrast to the description given by Ogura & Yamane (1915), the sternohyoid muscle does not attach onto the air sac. Instead, this slender strap muscle inserts to the caudal aspect of the basihyoid and, in part, to the thyrohyoid (Table 1). In the adult female the sternohyoid muscle, near to its insertion and medial to the mandibular portion of the sternocephalic muscle, covers the funnel-shaped ‘neck’ of the air sac dorsolaterally. In the adult male the sternohyoid muscle on one side is covered laterally by the dorsally displaced portions of the enlarged air sac and, further laterally, by the mandibular portion of the sternocephalic muscle.
The stylohyoid muscle is remarkable because it consists of two parallel muscular bands separated by a cleft from origin to insertion (Table 1). The dorsal portion of this cleft encloses the caudal belly of the digastric muscle. The excretory duct of the mandibular gland courses laterally to the stylohyoid muscle. The external carotid artery passes through the triangle formed by the origin of the stylohyoid muscle, the stylohyoid and the caudal belly of the digastric muscle (Fig. 3).
Fig 3.
Double stylohyoid muscle in reindeer. (a) Subadult male, right lateral view; (b) old female, left lateral view.
Constrictor muscles of the pharynx
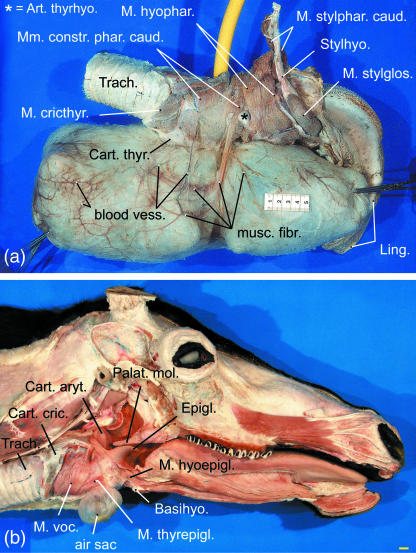
Camper (1791) described two muscles originating from the basihyoid and extending fan-like onto either side of the air sac. According to our dissections the two muscles represent the paired rostral portions of the caudal constrictor muscles of the pharynx, which take their origin from the pharyngeal raphe Fig. 4a). These muscles, inserting onto the connective tissue of the air sac walls, were observed in the adult male but not in the subadult male.
Fig 4.
Air sac in reindeer. (a) Large water-filled air sac of adult male, right lateral view. Yellow: rubber hose used for filling of the air sac, scale bar: 50 mm. (b) Small water-filled air sac in adult female, ventricular muscle removed, right lateral view, scale bar: 10 mm.
For the other constrictor muscles see Table 1.
Laryngeal air sac
In the adult male, the air sac is laterally covered by the strong mandibular portion of the sternocephalic muscle, by the weak ventral cutaneous muscles of the head and neck, and by the integument (Fig. 5c). The air sac takes its origin from the laryngeal vestibulum medioventral to the base of the stemless epiglottis by an opening, 5–10 mm wide in adults of both sexes. From this opening a short rostroventrally directed pneumatic duct traverses the connective tissue between epiglottis and thyroid cartilage, thereby connecting the laryngeal vestibulum with the air sac proper (Fig. 7, see below).
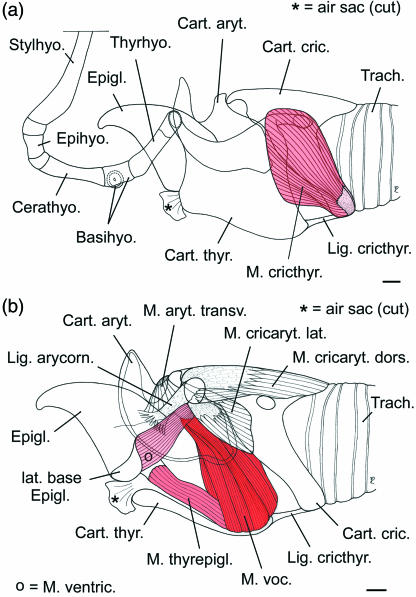
Fig 7.
Topographic relationships of the intrinsic laryngeal muscles in reindeer, left lateral view. (a) Thyroid cartilage in situ, cricothyroid muscle; (b) left half of thyroid cartilage removed. Scale bars: 10 mm.
Histological components of the air sac wall were assessed qualitatively. In adult male and female the inner surface of the air sac is covered with laryngeal mucosa. It is followed by a narrow subepithelial layer of dense connective tissue with a large amount of tangentially orientated elastic fibres. Towards the submucosa and the periphery of the air sac wall, it turns into a thick layer of loose connective tissue with a certain amount of collagen fibres intermingled with a few additional elastic fibres. In the region of the pneumatic duct numerous mucous glands could be identified. Blood vessels are coursing within the submucosa.
In the neonate male an air sac was lacking. However, a slight corrugated depression of the mucous membrane, 3 mm in rostrocaudal length and 1.5 mm in transverse width, occurred in a position corresponding to the opening of the air sac in adults (Fig. 5a). In the adult females the air sac had a volume of c. 100 cm3 in the artificial water-filled or silicone-injected state. In the dissected adult male its volume amounted to about 4000 cm3. The younger male (3–4 years) had an air sac volume of c. 250 cm3. The dorsally directed blind end of the moderately enlarged air sac of the subadult male was sandwiched between the sternohyoid muscle (medially) and the mandibular portion of the sternocephalic muscle (laterally) on the right side of the neck. The large air sac of the adult males extended asymmetrically along one side of the neck, to the left in one individual and to the right in the other. In the dissected adult male an even greater portion of the air sac than in the subadult male was lodged between the sternohyoid muscle and the mandibular portion of the sternocephalic muscle. Linear measurements and respective volumes of three dissected air sacs are given in Table 2.
Table 2.
Air sac of Rangifer tarandus– linear measurements (mm) and volume (ml) (am = adult male, sam = subadult male, af = adult female)
| Length (rostrocaudal) | Height (dorsoventral) | Volume (water-filled) | |
|---|---|---|---|
| am | 440 | 200 | 4000 |
| sam | 80 | 75 | 250 |
| af | 50 | 50 | 100 |
In juveniles and adult females the ventromedian air sac is restricted to the small space rostral to the thyroid cartilage, ventral to the base of the epiglottis and ventral to the basihyoid. Together with the laryngeal prominence, the air sac occupies the most prominent position of the ventral neck region. Contact area of the uninflated air sac with the ventral neck skin is up to approximately 50 × 50 mm. In adult males, however, the extensions of the uninflated aysymmetric air sac cover a large subcutaneous area (c. 400 × 200 mm) along the right or left lateroventral neck region. The rostral portion of the air sac extends up to the stylohyoid and beyond; its caudal portion extends caudally far beyond the thyroid gland (Fig. 5c). Medioventrally attached to the neck region covering the air sac is the white neck mane of adult males consisting exclusively of long hairs. The ventral skin contour of the neck is straight (Figs 1, 5b,c and 6a,b).
Fig 6.
In situ positions of hyoid apparatus and laryngeal cartilages in an adult male reindeer. Three-dimensional reconstructions based upon CT scans. (a) Left lateral view; (b) virtual parasagittal section, medial view of right half.
In an adult male specimen, the swelling of the neck caused by artificial inflation of the air sac could be seen in the course of the dissections.
Laryngeal cartilages
Respective shapes of the epiglottis, of the arytenoid cartilages and of the cricoid cartilage resemble roughly those of other cervids (Köhler, 1982). By contrast, the shape of the thyroid cartilage differs from that of other cervids in so far as its rostral horn is relatively shorter (Figs 2 and 6). The epiglottis of reindeer lacks a stem (Petiolus epiglottidis). Measurements of the laryngeal cartilages are given in Table 3.
Table 3.
Laryngeal cartilages of Rangifer tarandus– measurements (mm) (am = adult male, sam = subadult male, af = adult female, nem = neonate male)
| length (base to apex) | width (maximal transverse diameter) | circumference (along free rostral edge from left to right aryepiglottic fold) | |
| Epiglottis | |||
| (am) | 70 | 60 | 130 |
| (sam) | 55 | 40 | 80 |
| (af) | 48 | 40 | 64 |
| (nem) | 17 | 14 | 26 |
| horn tip distance (rostrocaudal) | maximal height (dorsoventral) | maximal length (diagonal: tip of rostral horn to caudal tip of lamina) | |
| Cart. thyroidea | |||
| (am) | 82 | 78 | 108 |
| (sam) | 67 | 70 | 83 |
| (af) | 62 | 60 | 76 |
| (nem) | 25 | 21 | 26 |
| maximal length (rostrocaudal) | maximal height (dorsoventral) | maximal width (transverse) | |
| Cart. arytenoidea | |||
| (am) | 72 | 40 | 21 |
| (sam) | 58 | 34 | 19 |
| (af) | 60 | 35 | 18 |
| (nem) | 19 | 13 | 7 |
| inside diameter of arch (obliquely dorsoventral along median line of arch) | length of lamina (rostrocaudal) | maximal length (diagonal: rostral tip of lamina to caudal tip of arch) | |
| Cart. cricoidea | |||
| (am) | 75 | 75 | 101 |
| (sam) | 61 | 53 | 85 |
| (af) | 53 | 53 | 75 |
| (nem) | 17 | 17 | 25 |
Extrinsic laryngeal muscles
There are three extrinsic laryngeal muscles, sternothyroid, thyrohyoid and hyoepiglottic, which do not differ markedly from those of other ruminants. Origins and insertions are given in Table 1. In the adult male, sternothyroid and thyrohyoid muscles are located medial to the large air sac on the respective side. In young males and in females the air sac is too small to reach the sternothyroid and thyrohyoid muscles, which course further dorsally.
Intrinsic laryngeal muscles
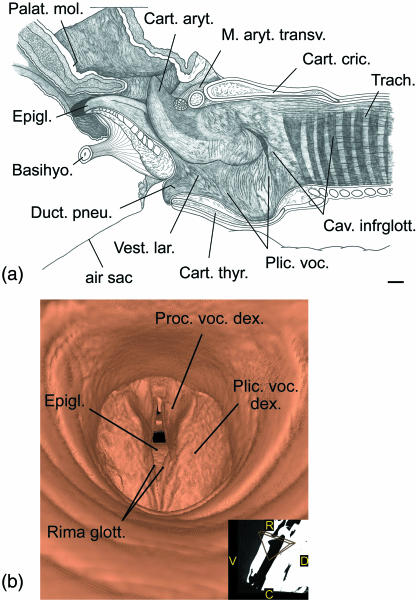
There are six intrinsic laryngeal muscles in reindeer, M. aryt. transv., M. cricthyr., M. cricaryt. dors., M. cricaryt. lat., M. thyraryt and M. thyrepigl. The first four muscles conform with the corresponding muscles in other ruminants (Fig. 7). The thyroarytenoid muscle is divided into a rostral ventricular and a caudal vocalis muscle. The origin of the ventricular muscle is remarkable because it is not from the rostrodorsal surface of the thyroid cartilage but laterally from the base of the epiglottis. (Thus, in the literal sense, it is not a ‘thyro-arytenoid’ but an ‘epiglotto-arytenoid’ muscle.) As in the Mongolian gazelle (Bovidae – Frey & Gebler, 2003; Frey & Riede, 2003) there is a thyroepiglottic muscle in reindeer. In reindeer it covers the opening of the air sac into the laryngeal vestibulum laterally. The wedge-shaped area of the laryngeal vestibulum surrounded by ventricular, vocal and thyroepiglottic muscle is devoid of muscle fibres but there are no lateral laryngeal ventricles (Fig. 7b). Origins and insertions of the intrinsic laryngeal muscles are given in Table 1.
Laryngeal cavity and vocal fold
The laryngeal vestibulum in male and female adult reindeer is different from that in other ruminants: between the base of the epiglottis and the rostroventral rim of the thyroid cartilage there is a short duct (pneumatic duct), 5–10 mm in diameter and 10–15 mm in length, connecting the laryngeal cavity to the lumen of the air sac (Fig. 8a). The longitudinal axis of this pneumatic duct is rostroventrally inclined at c. 45° against the longitudinal axis of the trachea.
Fig 8.
Vocal fold of reindeer. (a) Right vocal fold of adult male, medial view, scale bar: 10 mm; (b) virtual endoscopic view of subadult male's vocal folds based upon CT scans, caudal view, view angle see inset. R = rostral, C = caudal, D = dorsal, V = ventral.
The vocal fold in the neonate extended from about half the rostrocaudal length of the thyroid cartilage to the vocal process, whereas in the adults of both sexes it extended from the caudal portion of the thyroid cartilage and from the cricothyroid ligament to the vocal process. In consequence, the dorsal end of the vocal fold of the neonate is caudally inclined whereas in the adults of both sexes it is rostrally inclined (Fig. 8a). In the subadult male the vocal folds could be imaged by a virtual endoscopy by using three-dimensional reconstructions of CT scans (Fig. 8b). Dorsoventral lengths of the vocal fold were approximately 35 mm in the adult female and in the subadult male, 40 mm in the adult male and 9 mm in the neonate male.
The vocal fold of all dissected males (neonate, subadult, adult) produced no distinct relief of the glottic region. This vocal fold morphology resembles those of domestic ruminants but differs from other cervid species investigated by Köhler (1982). Even the neonate lacked a sharp and flexible rostral edge of the vocal fold, the rostral and caudal edges of which blended smoothly with the mucosa of the laryngeal vestibulum and the infraglottic space, respectively. The dissection of the adult female revealed a somewhat more pronounced rostral edge of the vocal fold.
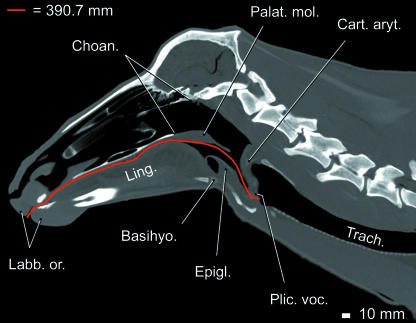
Vocal tract length (VTL)
Measurements of VTL in CT images yielded c. 340 mm for the 8-year-old female and c. 365 mm for the 13-year-old female, c. 350 mm for the subadult male, c. 390 mm for the adult male (Fig. 9) and c. 138 mm for the neonate male. Direct measurements yielded c. 350 mm for the adult female, 370 mm for the subadult male and 145 mm for the neonate male.
Fig 9.
Measurement of VTL in an adult male reindeer. Virtual sagittal section of head and neck based upon CT scans. Two-dimensional measurement of VTL (vocal fold to lips) indicated by red line. VTL is about 390 mm.
Vocalizations
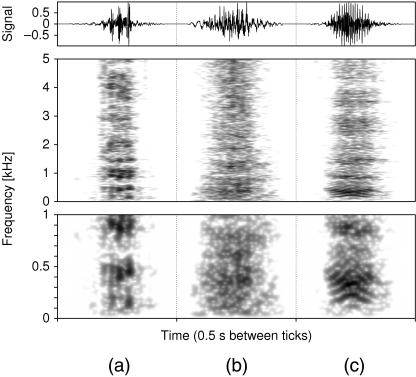
The rutting vocalization of adult male reindeer consists of a series of short calls which sound remarkably hoarse and are produced by a series of exhalations in rapid succession. Spectrograms reveal almost no distinct structures (Fig. 10). If occurring at all, bands of higher sound pressure levels are weak and blurred. The lowest of those bands most often lies between 200 and 500 Hz. Appearances of other bands vary between different calls even of the same individual.
Fig 10.
Narrow band spectrograms of adult male (a), adult female (b), and juvenile reindeer calls (c) normalized to their respective maxima. The lower part of the figure is a magnification of the 0–1-kHz section of the upper part.
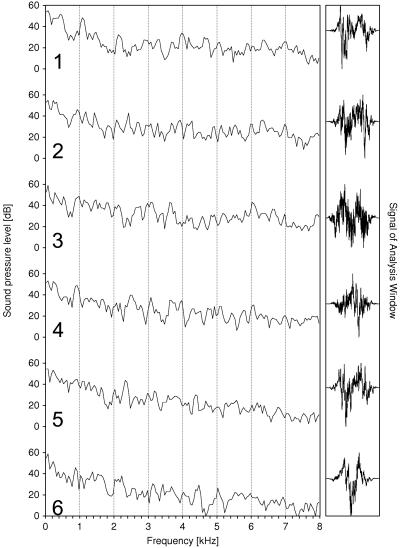
The fundamental frequency, measured by counting the number of glottal beats per second, averages 55 Hz (SD = 11 Hz, n = 37). Single calls have a mean duration of 0.18 s (SD = 0.06 s, n = 37). A call series contains from three to 15 single calls. The time gap between consecutive calls within one series is about 0.2 s. If the amplitudes of successive calls are high, the sound pressure levels near the maxima increase and, consequently, the maxima themselves become less pronounced in the spectra (Fig. 11).
Fig 11.
Spectra of rutting calls from a call series of one adult male reindeer; temporal order is from top to bottom, sound intensity decreases in the following order: 2–3−4–1−5–6.
Adult males produce their rutting calls by taking up a specific calling posture in which the head and neck are kept low and extended. The mouth is kept open during a call and, owing to the large size of the air sac, a swelling of the ventral neck region during the first part of a call series can be observed (Fig. 1, cf. fig. 3 in Bergerud, 1974). H. Persen, an old Samisk reindeer herder at Varanger, north-east Norway, confirmed that air sac inflation is visible when adult males vocalize (transmitted by N. Tyler, pers. comm., 2006; cf. Lönnberg, 1902). Before the onset of a call series the air sac remains deflated. We assume deflation of the air sac towards the end of a call series but this could not be clearly observed. The flanks of adult males contract synchronous to their vocalizations. Clouds of condensed exhalatory air could be observed during the calls. Apparently, most of the air escaped from the mouth but a certain amount might also have escaped from the nostrils.
In the course of three different recording sessions at Tierpark Berlin the observed male uttered a total of about 60 call series per hour (sum of calls within sessions divided by respective recording time).
Females and juveniles normally do not vocalize during the rut. Nonetheless, calls of females and juveniles in the rutting period could be recorded in Finland owing to the stress conditions of the autumn roundup. In contrast to the male calling posture and to male call series, juveniles and females produce their respective non-serial calls mostly with head and neck in an erect position and by means of one single exhalation.
The calls of female reindeer sound similar to those of males but exhibit a lesser extent of hoarseness (Fig. 10). As in adult males, the spectrograms of adult female calls contain no or only weak bands of higher sound pressure levels. The mean fundamental frequency is 75 Hz (SD = 21 Hz, n = 14). Compared with adult males and juveniles, the fundamental frequencies in the calls of females varied considerably (from 55 to 95 Hz). Mean call duration in adult females is 0.28 s (SD = 0.10 s, n = 14).
A listener can clearly distinguish the call of a juvenile from calls of an adult male or female reindeer. The spectral composition of juvenile calls includes distinctly more harmonic parts (Fig. 10). The mean fundamental frequency is 100 Hz (SD = 16 Hz, n = 25).
The frequency at which the sound pressure level of a call reaches −40 dB relative to the level maximum is about 8 kHz in adult males, about 9 kHz in adult females and about 12 kHz in juveniles (LTAS; bandwidth 1 kHz). In general, the frequency-dependent decay characteristic is conspicuously flat at higher frequencies up to an individual cut-off frequency where the sound pressure level suddenly fades away.
To the human ear the loudness of the hoarse rutting calls of an adult male reindeer seemed to be of lower volume than the single calls of an adult female or a juvenile. However, in Tierpark Berlin an increase in loudness of some of the male's calls could be noticed when the male was separated from the females.
Discussion
Comparative anatomical remarks on laryngeal saccules in mammals
Soft-walled side chambers protruding from the contour of the laryngeal cavity in therian mammals have been termed saccules, appendices, recesses, sinus, ventricles or air sacs depending on their respective sizes and on different authors (e.g. Bartels, 1905; Negus, 1929; Göppert, 1937; Starck & Schneider, 1960; Schneider, 1964; Starck, 1995).
According to the topographic relationships and by choosing ‘laryngeal saccules’ as a general term for laryngeal side chambers, irrespective of their sizes, five positions can be distinguished (proceeding from rostral to caudal): (1) ventrorostral laryngeal saccule, protruding ventrally from the laryngeal cavity between the base of the epiglottis and the rostral edge of the thyroid cartilage; (2) lateral laryngeal saccule, protruding laterally from the laryngeal cavity and rostral to the vocal fold; (3) ventral laryngeal saccule, protruding ventrally from the laryngeal cavity into an extension of the thyroid cartilage (a thyroid bulla); (4) ventrocaudal laryngeal saccule, protruding ventrally from the laryngeal cavity between the caudal edge of the thyroid cartilage and the cricoid arc; (5) dorsocaudal laryngeotracheal saccule, protruding dorsally from the laryngeal cavity between the caudal edge of the cricoid lamina and the first tracheal ring (Table 4 – for details and figures see the following references: Dobson, 1881; Milne Edwards & Grandidier, 1875; Bartels, 1905; Sonntag, 1923; Hasskó, 1929; Negus, 1929; Göppert, 1937; Hill & Booth, 1957; Kelemen & Sade, 1960; Starck & Schneider, 1960; Schneider, 1962, 1964; Avril, 1963; Schneider et al. 1967; Schön, 1970; Gautier, 1971; Starck, 1995; Reeb & Best, 1999).
Table 4.
Laryngeal saccules in mammals, different sites of emergence from laryngeal cavity and pre- or post-glottal position. Some species may have two types of saccules, e.g. Alouatta. Unequivocal positive evidence for sexual dimorphism is indicated by an asterisk. Light grey: saccules, dark grey: vocal fold
| Position/type of mucosa | Genera/species | Family | Diagram | |
|---|---|---|---|---|
| 1a | ventrorostral, without hyoid bulla | Arvicola | Arvicolidae |  |
| (preglottal, laryngeal mucosa) | Muridae | |||
| Equus | Equidae | |||
| Rangifer* | Cervidae | |||
| Ovibos | Bovidae | |||
| 1b | ventrorostral, covered by hyoid bulla | Colobus | Colobidae |  |
| (preglottal, laryngeal mucosa) | Presbytis | Colobidae | ||
| Cercopithecus | Cercopithecidae | |||
| Cercocebus | Cercopithecidae | |||
| Macaca | Cercopithecidae | |||
| Papio | Cercopithecidae | |||
| Alouatta | Cebidae | |||
| Lagothrix | Cebidae | |||
| 2a | paired lateral, without hyoid bulla or no connection to it | Erinaceus | Erinaceidae | 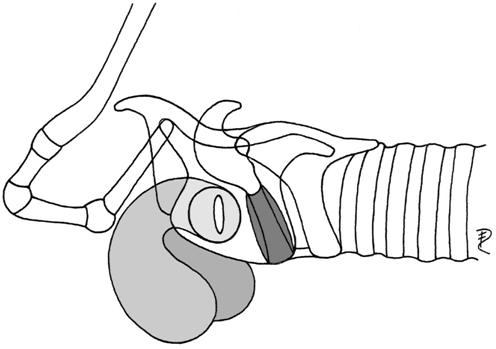 |
| Hypsignathus* | Pteropodidae | |||
| (preglottal, laryngeal mucosa) | Sciurus | Sciuridae | ||
| Marmota | Sciuridae | |||
| Agouti paca | Dasyproctidae | |||
| Mustelidae | ||||
| Viverridae | ||||
| Canis lupus | Canidae | |||
| Ursus | Ursidae | |||
| Otaria byronia | Otariidae | |||
| Rhinocerotidae | ||||
| Tapirus | Tapiridae | |||
| Equus | Equidae | |||
| Sus scrofa | Suidae | |||
| Procapra gutturosa* | Bovidae | |||
| Lemuridae | ||||
| Colobus | Colobidae | |||
| Presbytis | Colobidae | |||
| Cebus albifrons | Cebidae | |||
| Alouatta | Cebidae | |||
| Homo | Hominidae | |||
| 2b | paired lateral, small portion covered by hyoid bulla | Cercopithecus | Cercopithecidae | 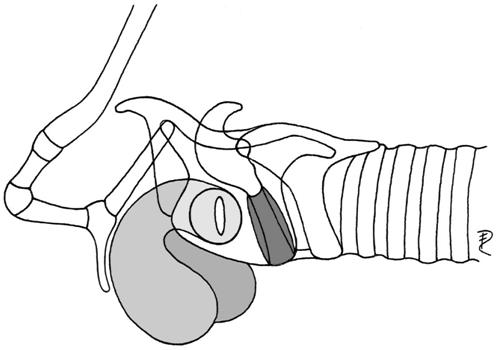 |
| Macaca nemestrina | Cercopithecidae | |||
| (preglottal, laryngeal mucosa) | Papio | Cercopithecidae | ||
| Symphalangus | Hylobatidae | |||
| Pongo pygmaeus* | Pongidae | |||
| Pan troglodytes | Pongidae | |||
| Gorilla* | Pongidae | |||
| 3 | ventral, inside thyroid bulla | Didelphis | Didelphidae | 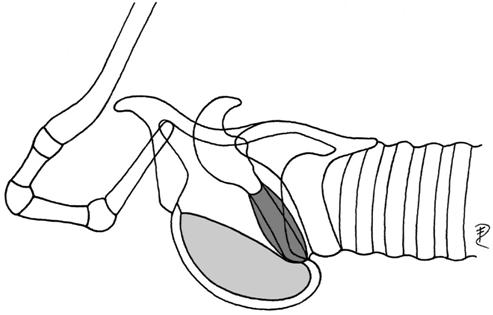 |
| (preglottal, laryngeal mucosa) | Dasyurus | Dasyuridae | ||
| Trichosurus | Phalangeridae | |||
| Phalanger | Phalangeridae | |||
| Wallabia | Macropodidae | |||
| Tragelaphus oryx | Bovidae | |||
| Budorcas taxicolor | Bovidae | |||
| 4 | ventrocaudal | Callithrix | Callitrichidae | 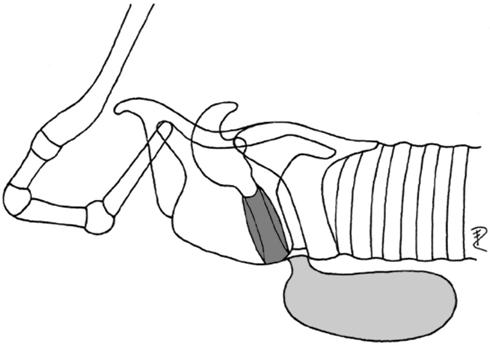 |
| (postglottal, laryngeal mucosa) | Leontocebus | Callitrichidae | ||
| Balaenoptera | Balaenopteridae | |||
| Caperea | Balaenidae | |||
| 5 | dorsocaudal | Indri | Lemuridae | 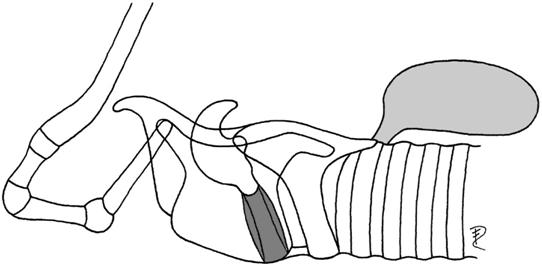 |
| (postglottal, tracheal mucosa) | Varecia | Lemuridae | ||
| Microcebus | Lemuridae | |||
| Ateles | Cebidae |
Ventrorostral saccule, ventral, ventrocaudal and dorsocaudal saccule are unpaired, whereas the lateral saccule is paired. In many primate species, particularly in male pongids, these lateral laryngeal saccules develop into large air sacs, often asymmetric, which may touch ventromedially and, eventually, may even fuse in the midline (Symphalangus, Pongo, Gorilla, Pan). They extend partly beneath the pectoral muscles and may reach up to the axilla (Sonntag, 1923). Air sacs of the vocal tract in mammals must not be confused with the air sacs of birds, which are specialized parts of the avian respiratory system (Duncker, 1978), or with the air (vocal) sacs of frogs, which are diverticula of the buccal cavity communicating with it via specific apertures (Tyler, 1971, 1972; Duellman & Trueb, 1994; Tyler & Duellman, 1995). In both taxa the air sacs are not directly connected to the larynx.
In mammals, there is no sharp definition for the differentiation of small laryngeal ventricles from large air sacs. Considering ventrorostral laryngeal side chambers in colobid primates, Hill & Booth (1957) distinguished between saccules that do not pierce the thyrohyoid membrane and air sacs that do. Unfortunately, this criterion is not valid for the remaining laryngeal side chambers.
Except the dorsocaudal laryngeotracheal saccule, which is lined with tracheal mucosa, all other laryngeal saccules are lined with laryngeal mucosa.
Surrounding structures of the soft-walled laryngeal saccules may differ: (1) hard structures, e.g. parts of the dilated hyoid apparatus, hyoid bulla (Alouatta, Colobus, Gorilla, Macaca, Pan, Papio) or a dilated portion of the thyroid cartilage, thyroid bulla (Dasyurus, Phalanger, Budorcas); and (2) a combination of soft and hard structures, (2a) connective tissue, thyrohyoid membrane, horseshoe-like frame of basi- and thyrohyoid (Colobinae, Ovibos), (2b) connective tissue, thyroarytenoid muscle, lamina of thyroid cartilage (Equus, Procapra), (2c) oesophagus, trachea (Indri, Ateles), (2d) ventral hyoid muscles, trachea (Callithrix, Balaenoptera), (2e) lower jaw, sternum and sternal ribs, pectoral muscles, cutaneous muscles of the neck, integument (Gorilla, Pongo), (2f) lower jaw, thyroid cartilage, trachea, cutaneous muscles of the neck, integument (Symphalangus).
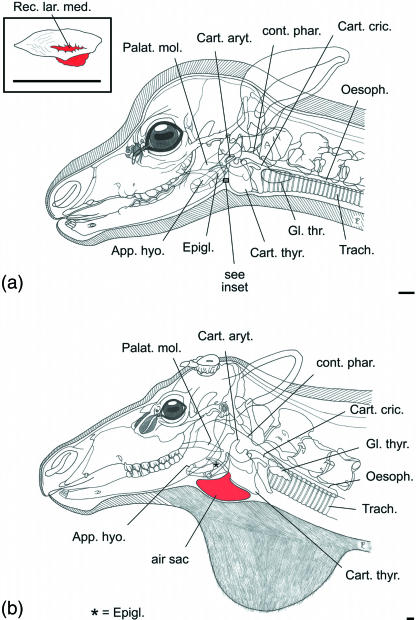
Given the above division, reindeer have a ventrorostral laryngeal saccule (Table 4) that develops into an air sac of moderate size in young animals and females (Fig. 12) and into a large air sac in adult males. The air sac in reindeer is surrounded by soft and hard structures, medially by thyroid cartilage, trachea, ventral hyoid muscles and laterally by the mandibular portion of the sternocephalic muscle, by cutaneous muscles of the neck and the integument.
The ventrorostral laryngeal saccule of reindeer is a homologue of the median laryngeal recess (Recessus laryngis medianus) of other mammals.
Sexual dimorphism and ontogeny of the air sac in reindeer
According to Bergerud (1974) the sexual dimorphism of body mass (male/female) is around 1.6 : 1 (males: n = 35, females: n = 60) in Newfoundland caribou. Body masses of one adult male and one adult female of our Eurasian reindeer zoo specimens differed by 2.1 : 1. According to our results the volumes of the air sac (male/female) differed by c. 40 : 1. Linear dorsoventral height of the air sac differed by 4 : 1 and rostrocaudal length by 8.8 : 1 (male/female). Thus, the sexual dimorphism of the air sac is obviously more pronounced than that of body mass.
In the neonate an air sac has not yet developed. The slight depression in the neonate is probably a homologue of the ventromedian laryngeal recess. We may reasonably assume that the first stages of the air sac's ontogenetic development are comparable between the juveniles of both sexes. In the females ontogenetic growth of the air sac stops at an age of about 2–3 years. In the male, however, growth of the air sac seems to continue beyond physiological sexual maturity up to an age of about 6 years, i.e. when a stag is capable of becoming the dominant male of a harem during the rut. Body size and antler size of males also increase until about 6 years (Bergerud, 1974, p. 425). This extended growth period ultimately leads to the enlarged and asymmetric air sac in older males. Concomitant to ontogenetic growth, the air sac in subadult males begins to invade one neck side laterodorsally and, ultimately, becomes sandwiched between the sternohyoid muscle and the mandibular portion of the sternocephalic muscle. In consequence, only the ventral portions of the air sac are in direct contact with the integument.
In some cases the air sac is also sexually dimorphic with respect to musculature. In one adult male (6 years) some bundles of the right caudal constrictor muscles of the pharynx extended fan-like onto the surface of the air sac. Camper (1791) described a similar, but paired, muscle in a 4-year-old subadult male. The small air sac of our adult female did not show any trace of musculature. However, this feature may be inconsistent because in our subadult male (aged 3–4 years) we also could not find any muscles extending onto the air sac.
Vocalizations
The first impression of a vocalizing adult reindeer to a human listener is the hoarse or atonal character of calls in contrast to the calls of juvenile reindeer or to those of other ruminants, e.g. Procapra gutturosa (Frey & Gebler, 2003), Ovibos moschatus (Frey et al. 2006), Muntiacus reevesi (Gebler 2003), Cervus elaphus (Reby & McComb, 2003a) and domestic cattle. This perception is caused by a broad distribution of sound energy over the frequency spectrum and the lesser extent of audible harmonic parts in the calls (Fig. 10). The decay characteristic of sound pressure level at higher frequencies supports this impression.
Espmark (1975) mentioned calls of adult female reindeer to be ‘too noisy and unstructured to allow reliable measuring’. By contrast, unpublished recordings by other researchers revealed clearer vocal tract resonances in adult reindeer than found by us (D. Reby, pers. comm., 2006). At the moment there is no explanation available for this inconsistency. Therefore, the subsequent discussion is solely based on our own results.
In reindeer acoustic perception has been shown to conform with hearing abilities of other ruminants. The frequency range of sensitivity was relatively flat from 1 to 16 kHz, with greatest sensitivity of 3 dB at 8 kHz (Flydal et al. 2001). In the calls of reindeer the part of sound energy at this frequency is relatively high compared with the more harmonic calls of other ruminants. Field observations and experiments revealed a well-developed individual acoustic recognition of reindeer females and their calves. Individual recognition was considered to be favoured by the high individuality of the calls (Espmark, 1971b; Lent, 1975). This individuality also appeared in our recordings.
During the mating season male rutting calls are most frequently emitted in adult male–male interactions, e.g. challenger stag approaching a herd (Bergerud, 1974), and in adult male–female interactions, e.g. in driving a female (Bergerud, 1974). Travelling single stags searching for females advertize their presence by emitting rutting calls. Both males and females are attracted by male rutting calls. Calling of a dominant stag contributes to preserve the established structure of a travelling group (Bergerud, 1974, pp. 410, 434; Lent, 1975, p. 400).
According to Bergerud (1974, p. 408) various descriptions have been used to characterize adult male rutting calls in reindeer, e.g. ‘bugling’ (Dugmore, 1913), ‘rattling’ (Espmark, 1964), ‘grunting’ (Lent, 1965; Henshaw, 1970) and ‘roaring’ (Fraser, 1968). We prefer not to use any of these terms because they do not refer to the repetitive breathing events during the male rutting call of reindeer. Otherwise, those terms have been used to describe the rutting calls of other cervid species (e.g. ‘bugling’ for wapiti, ‘roaring’ for red deer) which are quite different from the rutting calls of reindeer. Bergerud himself (1974) uses the term ‘panting’ to describe the ‘hoarse guttural sounds’ uttered by the adult males in rut. Although ‘panting’ involves the repetitive breathing aspect, we suggest avoiding this term in the context of male rutting calls as well because ‘panting’ is a technical term in physiology used for rapid breathing of both sexes under heat stress (cf. Aas-Hansen et al. 2000). In contrast to panting under heat stress, in which inhalations and exhalations are of similar duration (Aas-Hansen et al. 2000), exhalations in the course of a male rutting call seem to be enhanced. Indirect evidence comes from pronounced contracting flank movements synchronous to vocalizations.
It needs to be stated that, under natural conditions and contrary to the artificial stress situation during our recordings in Finland, female and juvenile reindeer do not vocalize during the rut (Lent, 1975, p. 402). Under this aspect our investigated calls of females and juveniles may be judged as ‘atypical’. However, the situation of re-finding of mother and young after the roundup seemed to be comparable with re-finding situations within normal mother–young interactions outside the rut. Therefore, we would not expect significant call differences in both situations.
As the anatomical results did not reveal any other potential oscillators, the vocal folds are assumed to be the sole phonative source. Following Lent (1975), the mean ‘fundamentals’ of female calls (‘grunts’) and of juvenile calls (‘bleats’) are 630 and 1100 Hz, respectively. These values are much higher than the fundamental frequencies ascertained by us. Unfortunately, the term ‘fundamental’ used by Lent is not further explained and we feel that his usage of this term characterizes something else than the rate of glottal beats. In the spectrograms of juvenile calls in Lent's fig. 3, clear harmonic structures are visible between formants. In this figure we estimate the mean distance between adjacent harmonics to be at around 120 Hz. This would be in better accordance with the fundamental frequency of juvenile calls determined by us (about 100 Hz), and even more so as Lent had used recordings of July and our recordings were made in October so that the recorded young were of different ages.
Function of the air sac in sound production
Given that in adult male reindeer the inflation of the air sac could be observed solely in connection with rutting calls, the air sac is clearly involved in vocalization.
The increasing size of the air sac from juveniles to subadults to adults correlates with the decrease of harmonic structures in the spectrograms of their respective calls.
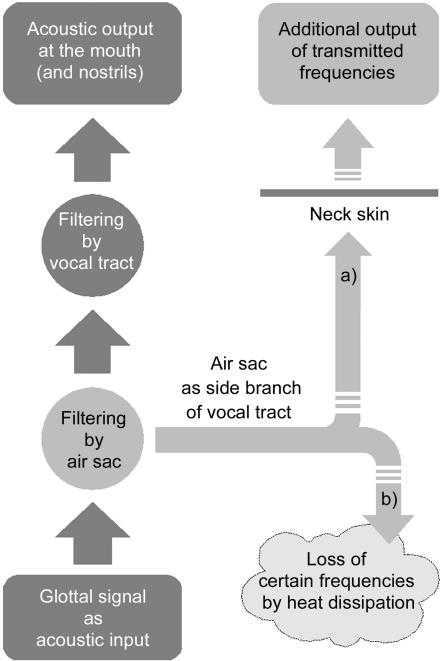
In the source-filter theory of human speech production (Hermann, 1890; Fant, 1960), the vocal tract, i.e. the upper respiratory tract rostral to the glottis, filters the primary signal produced by the source of the acoustic energy, i.e. by the vocal folds. The reindeer's air sac is probably not a source of sound energy but has to be considered as a side branch of the vocal tract and, thus, as an additional filter (Fig. 13). Physically, a filter is a resonator that absorbs energy at certain resonance frequencies. The absorbed energy may be regained from the resonator or may be transformed into heat dissipation. Considering the acoustic signal, specific frequencies may either be transmitted or destroyed by the resonator. The transmission produces so-called formants that are relatively amplified frequencies of the primary sound signal in the acoustic output. The resonances of a side branch-resonator are called anti-resonances or anti-formants and should be seen as zeros in the spectrogram. Applied to an air sac, resonance frequencies decrease with a larger air sac volume.
Fig 13.
Possible signal pathways through the vocal tract of reindeer. The air sac is considered as a side branch. (a) Transmission of resonance frequencies of the air sac to the environment by acoustic coupling; (b) loss of resonance frequencies of the air sac by dissipation to surrounding tissues.
The glottal signal minus the resonance frequencies of the air sac is subsequently filtered by the remaining vocal tract. The outcome makes up the acoustic output at the mouth opening and, perhaps, the nostrils of a reindeer. Considering a possible acoustic coupling between air sac and environment, two signal pathways may be put forward. They do not exclude each other and might occur in combination.
In the first case (Fig. 13a), it is assumed that the air sac in adult males is acoustically coupled to the environment via the soft and flexible skin of the laryngeal region. The larger the contact surface between the air sac wall and the overlying skin the more acoustic energy can be transmitted. Therefore, the air sac may act as a band-pass filter. In consequence, the spectrum of the emitted sound would be a combination of the acoustic output via mouth and, perhaps, the nostrils and of the resonance frequencies transmitted by the air sac wall and the adjacent skin. Mechanisms in which part of the sound is not radiated via mouth (and nostrils) but via tissue vibrations might also be involved, as e.g. in the purring of cats (cf. Frazer Sissom et al. 1991).
In many species, e.g. in the howler monkeys, the evolution of large resonator devices (air sacs) apparently led to a relative increase of call amplitude (Starck & Schneider, 1960; Schön, 1970). This effect can be explained by a transmission of acoustic energy to the environment in addition to that emitted via the mouth and nostril openings. Accordingly, the experimental puncturing of the air sacs in Cercopithecus neglectus resulted in a reduced intensity of calls (Gautier, 1971).
Under this aspect, an interesting behaviour pattern in reindeer occurs. Typically, the courting male does not advance directly towards the female but approaches her from the side in a low-stretch posture, i.e. head low and neck extended (Lent, 1965; Bergerud, 1974; Fig. 1). Assuming a marked transmission of sound energy through the air sac, this position provides the opportunity for an efficient ‘broadside acoustic display’ as the male's enlarged air sac extends subcutaneously along the ventral neck contour. Considering the asymmetry of the air sac, one may ask whether the male turns that side with the larger air sac portion towards the female? To our knowledge, this aspect has not yet been considered.
In contrast to the assumption of a sound transmission via the air sac, the calls of adult reindeer males with a large air sac seemed to be less loud than the calls of juveniles with a smaller air sac. Adult reindeer males may not require high call amplitudes as male–male contests and courtship behaviour are occurring within short-range distances.
In the second case (Fig. 13b), the acoustic coupling between air sac and the environment is weak. Owing to the absorption of the dense winter hair coat and of the mandibular portion of the sternocephalic muscle overlying the air sac, the sound energy of its resonance frequencies is lost by dissipation. As stated above, the sound energy in the calls of adult reindeer is relatively shifted towards higher frequencies. These frequencies are the higher harmonics of the glottal signal and present always much less sound energy than the fundamental frequency itself and its first harmonics. In order to achieve a relative increase of sound energy at higher frequencies, the sound energy at lower frequencies, especially at the lower formants, has to be damped. The potential damping of broad frequency ranges provides a further explanation for the apparent low amplitude of adult male calls.
A large air sac can act as a stop-band filter, which may be tuned to the lower formants by the degree of its inflation. Inflation of the air sac is effected by enhanced phonative exhalations. Apart from expiratory muscles, the peculiar double stylohyoid muscle of reindeer, enclosing the caudal portion of the digastric muscle, may assist in air sac inflation by successive contractions of its two portions (rostral first, then caudal) thereby pulling the basihyoid dorsally and tilting it dorsorostrally. This would tend to widen the subcutaneous space between the opening of the air sac and the skin and to decrease resistance to inflation. The mylohyoid muscle may support such dorsal pulling as its aponeurotic caudal edge is laterally attached to the insertions of both stylohyoid muscle portions (Fig. 3, Table 1). Interestingly, a piercing of the inserting tendon of the, otherwise undivided, stylohyoid muscle by the digastric muscle occurs in the horse (Nickel et al. 1987). Unfortunately, we do not know of any functional explanation for this conspicuous feature.
A narrowing of the pneumatic duct may be effected by contraction of the paired thyroepiglottic muscle. This would aid in regulating the inflation of the large air sac by preventing a fast escape of the inflated air.
As the evolutionarily enlarged air sac in adult males is sandwiched between the larynx and the mandibular portion of the sternocephalic muscle, this powerful muscle should be relaxed during the onset and initial stages of vocalization in order to decrease resistance to inflation. This might explain the characteristic low-head and extended-throat posture of adult calling males, which is probably effected by dorsal extensor muscles of the neck.
Deflation probably occurs owing to the resilience of the ventral neck skin, perhaps supported by contracting lateroventral muscles (cutaneous muscles of the neck, mandibular portion of the sternocephalic muscle). The weak bundles of the caudal pharynx spincters may assist in deflation and may also contribute to keeping the air sac in place.
In the rutting calls of different species of the Cervinae and of different subspecies of red deer formant frequencies are well defined. Position of formant frequencies and formant spacing in the rutting calls of the Cervinae have been identified as key features conveying information on the quality of the sender to conspecifics that is most relevant to the sender's reproductive success (Reby & McComb, 2003a). In our spectrograms of adult reindeer calls, however, only very few and ill-defined formant frequencies were detectable. Apart from larynx position and larynx mobility, the air sac is a major anatomical difference between the vocal tracts of reindeer and of the species of the Cervinae. Provided that our spectrograms are representative of reindeer vocalizations, we may thus conclude that the air sac possibly counteracts the formation of clearly detectable formant frequencies, perhaps by masking formant frequencies of the vocal tract proper. Thus, if not formants, what other acoustic parameters contain that information in reindeer? Further studies are required to elucidate this issue.
Variation of air sac size and shape in different males of similar ages can be expected to modify the respective acoustic signal, thereby facilitating individual recognition of rutting calls by males and females. If necessary, the assumed tuning of air sac resonances by the degree of inflation might compensate for the variety of air sacs.
A preliminary histological investigation of the air sac wall confirmed the results of Ogura & Yamane (1915). The structure of the air sac wall is in accordance with its resilient nature and, thus, can be expected to support inflation and elastic deflation of the air sac.
Evolution of the air sac
All reindeer individuals, juveniles, females and males, have an air sac bulging from the ventrorostral part of their larynx. For what reason did this remarkable structure evolve primarily? In contrast to Negus (1929, p. 99) who promoted a non-acoustic rebreathing function of air sacs in general, we follow an acoustic approach (cf. Starck & Schneider, 1960; Fitch & Hauser, 1995, 2002). Even the small air sacs of juveniles and females are extensions of the vocal tract and, thus, will modify its filter characteristics. Accordingly, the acoustic output will become as specific as size and shape of individual air sacs differ. Considering the high individuality of juvenile and female calls (cf. Espmark, 1971b, 1975; Lent, 1975), we may conclude therefore that the primary function of the air sac in reindeer was individual acoustic recognition.
At some point in the evolution of reindeer, sexual selection is likely to have favoured a shift of function of the air sac in the male sex. Related to this development a considerable size increase of the air sac has occurred and adult male calls became restricted to the rutting period. In addition, a transformation of the primitive single call, retained by juveniles and females, into those frequently emitted husky call series occurred. This resulted in a secondary function of the air sac as an essential part of an acoustic display of adult males during the rut. However, the primitive function may persist in so far as reindeer females may be capable of discriminating between the call series of different reindeer stags.
As in other polygynous species with a harem-like mating system and an acoustic display during the mating season (e.g. red deer, fallow deer, Mongolian gazelle, elephant seals, hammer-headed fruit bat), we may reasonably assume that male rutting calls in reindeer serve to indicate body size, which is usually linked to fighting strength of the sender. Thus, such rutting calls are useful to deter other males and, at the same time, to attract females (cf. Clutton-Brock & Albon, 1979; Fitch, 1997; Fitch & Reby, 2001; Reby & McComb, 2003a). Accordingly, the acoustic display of reindeer is part of male–male contests, e.g. dominance fights (Bergerud, 1973, 1974), and also of male–female courtship interactions, e.g. in the driving of the female (Espmark, 1964).
In harem-like mating systems few males get most of the matings (Andersson, 1994). Thus, a minor initial evolutionary increase of air sac size of a male reindeer that helped to decrease the number of fights by acoustically repelling competing males (intrasexual selection) and, simultaneously, to increase the attractiveness to females (intersexual selection) was clearly advantageous. Accordingly, male–male competition may have resulted in an evolutionary arms race concerning the size increase of the air sac in reindeer stags. Once the process had started, males ‘could not afford’ a smaller air sac because it would have impaired their mating success. This led to the pronounced sexual dimorphism of the air sac. However, it is reasonable to expect a natural physiological limit to the size of the male sound-producing organs beyond which other functions would be impaired. Greatest possible air sac size is dependent upon neck size, which, in one and the same species, can be assumed to correlate with skull size, which is in turn closely correlated with body size in mammals (Fitch, 1997, 2000a). In addition, the inflation of a greatly enlarged air sac cannot be achieved by one single call. Instead, a series of several successive forced phonative exhalations is required. In consequence, the frequent emission of rutting call series with a large air sac is energetically more expensive than it would be with a small or without any air sac. At the physiologically induced end of the arms race any acoustic change of the rutting calls correlated with the size of the air sac will still be an honest signal of body size (cf. Zahavi, 1975, 1977, 1981; Dawkins & Krebs, 1979; Reby & McComb, 2003a,b).
The evolution of a special acoustic display during the rut imposed a handicap on adult males in terms of an increased expenditure of time allocated to vocalizations and of energy used for call production. High-quality males can afford to invest in display (cf. McElligott et al. 1999). Generally, the energy expenditure of dominant males in polygynous mating systems is enormous (cf. McElligott et al. 2003). Male rutting efforts can even be potentially dangerous to survival (Barboza et al. 2004). In red and fallow deer the repetition rate of rutting calls has been shown to be of importance for mating success (Clutton-Brock et al. 1988; McComb, 1991; Reby & McComb, 2003a). As rutting calls are part of most adult male–male and male–female interactions during the rut, this might also hold for reindeer. Considering the almost continuous emission of call series requiring large lung volumes to be accelerated in rapid succession by pronounced coordinated actions of abdominal, pelvic, diaphragmatic, cervical and thoracic muscles, the acoustic display may be an exceptionally strenuous part of the rut for reindeer stags. Stronger, heavier males should be able to achieve large series of intermittent air sac inflations more efficiently than males of lower quality and, thereby, obtain a higher reproductive success (cf. Reby & McComb, 2003a).
Attractiveness to females might not only result from the acoustic nature of the rutting calls but also from call-related visible changes caused by inflation of the air sac (Lönnberg, 1902; Skjenneberg & Slagsvold, 1968). The swelling of the ventral neck region itself and, in addition, the concomitant spreading of the white ventral neck mane may function as optical signals and mediate a ‘strong neck performance’ to the females. The rutting reindeer stag presents its ventral neck region including the neck mane to the female in a modified threat posture (Pruitt, 1960). By this visual display the ventral neck region plus neck mane becomes more conspicuous (Lent, 1965). A basic long-term increase of the male neck circumference is achieved by accumulating subcutaneous neck fat deposits during the pre-rutting period, which subsequently are depleted in the course of the rut. In contrast to red and fallow deer (Fitch & Reby, 2001), most of the matings in reindeer occur in the afternoon, i.e. during daylight hours (Espmark, 1964; Bergerud, 1974, p. 421). A similar optical function has been inferred for the neck bulging caused by the evolutionarily enlarged larynx of the adult male Mongolian gazelle (Frey & Riede, 2003).
It would appear that acoustic and optic signals convey the same message (cf. Lent, 1975, p. 398), namely the presence of a high-quality mating partner. However, irrespective of the possible function of the air sac as an optical signal, the primary function seems to be an acoustic one. Inflations of the air sac exclusively occur during the emission of calls and never by non-phonative exhalations.
Different vocal specializations within the Cervidae
Similar selection factors associated with polygynous mating systems have led to the evolution of acoustically different male mating calls across species (cf. Reby & McComb, 2003a) and of basically different adult male vocal tract specializations in Cervinae, e.g. in red and fallow deer, and in Rangiferinae. Roaring red and fallow deer stags retract the larynx almost down to the thoracic aperture when emitting their loud rutting calls. Accordingly, the vocal tract length increases considerably for the duration of a roar (Fitch & Reby, 2001; Reby & McComb, 2003b; McElligot et al. 2006). While calling the head is kept high and the ventral neck region extended. This effects an additional extension of the vocal tract. Resulting lowered resonance frequencies of the vocal tract and a decreased formant dispersion, together with call repetition rates, were considered to convey information on the quality of the sender to conspecifics (Fitch & Reby, 2001; Reby & McComb, 2003b). Recent studies have confirmed this for intrasexual agonistic interactions in male red and fallow deer (Reby et al. 2005; McElligot et al. 2006). In addition, intersexual preference of high call repetition rates by female choice has been demonstrated to occur in fallow deer (McComb, 1991).
By contrast, reindeer stags evolved a large subcutaneous cervical air sac attached to the larynx via a narrow opening. The reindeer stag's low-amplitude rutting calls consist of a short series of hoarse sounds produced by short exhalations in rapid succession. In the typical calling posture the head is kept low and the throat region extended. The hyoid apparatus of reindeer, including its junction with the larynx, does not comprise any ligamentous or pronouncedly elastic structures. Therefore, we may reasonably conclude that, in contrast to the above Cervinae, the larynx of reindeer cannot be actively retracted as far caudally as in red and fallow deer (cf. Fitch, 2000b; Fitch & Reby, 2001; McElligot et al. 2006). Accordingly, a shifting of formants as a result of vocal tract elongation does not seem to occur in Rangiferinae. Instead, resonance frequencies of the air sac, possibly together with call repetition rates, can be assumed to convey information about the sender to conspecifics.
Apart from vocal tract specializations, Reby & McComb (2003b) pointed to the apparent lack of correlation between investigated polygynous species (or subspecies) body size and fundamental frequency in the Cervidae. In the following row of increasing body mass, respective fundamental frequencies are: fallow deer 35 Hz, Corsican red deer 34 Hz, European reindeer 55 Hz (this study), European red deer 110 Hz, American red deer or wapiti 1 kHz (Reby & McComb, 2003b).
As in red and fallow deer (Fitch & Reby, 2001; McElligot et al. 2006), the rutting calls of reindeer stags seem to be designed for short-range communication. Rutting calls are emitted most frequently when competing stags or attracted females are nearby. Nonetheless, the rutting calls were audible at distances up to 180–270 m on calm days (Bergerud, 1974, p. 410).
Acknowledgments
We are grateful to T. Kempe and his family, reindeer keepers in Finnish Lapland, for providing one subadult and one adult male deep-frozen head-and-neck specimen and also for their hospitality and experience when two of us (R.F., K.N.) made the acoustic recordings during the Kuukkelilampi roundup of 2004 near Ivalo. We thank H. Persen, Samisk reindeer herder at Varanger, Norway, and N. Tyler, Department of Biology, University of Tromsø, Norway, for etymological information, references, and for providing the cover photo. In addition, we would like to thank Dr B. Blaszkiewitz, director of Tierpark Berlin Friedrichsfelde, Germany, for making available one adult male and two adult females. We also thank Dr G. Strauss and Mrs M. Fanke of Tierpark Berlin for allowing access to the reindeer enclosure. A further three male specimens, one neonate, one adult and one isolated larynx, came from Tierpark Schönbrunn, Vienna, for which we are thankful to its director, Dr Helmut Pechlaner. We thank Professor L. Brunnberg, director of the Clinic for small animals in Düppel, Free University of Berlin, for cooperation in use of the computer tomograph. Dr K.-H. Frommolt, archive for animal acoustics, Museum of Natural History, Berlin, lent technical support for making the recordings. Mrs A. Kißmann and Mrs D. Krumnow, technical assistants at the IZW, made the photo library and the histological sections, respectively. B. Paschmionka, former dissector at the IZW, assisted in processing of one of the specimens. Dr G. Wibbelt performed the histological inspection. Mrs C. Greulich and Mrs B. Peters, librarians of the IZW, provided invaluable help in procuring literature sources. In addition, we thank two anonymous referees for their constructive and stimulating comments.
Appendix
| List of abbreviations | List of abbreviations | ||
|---|---|---|---|
| A. car. ext. | external carotid artery | M. myloh. | mylohyoid muscle |
| A. max. | maxillary artery | M. occiph. | occipitohyoid muscle |
| Ala atl. | transverse process of first cervical | M. omoh. | omohyoid muscle |
| Ang. styloh. | angle of stylohyoideum | M. orbic. oculi. | sphincter muscle of eyelids |
| App. hyo. | hyoid apparatus | M. palphar. | palatopharyngeal muscle |
| Arc. cart. cric. | arc of cricoid cartilage | M. parotaur. | parotidoauricularis muscle |
| Art(t). cricaryt. (dex.) | (right) cricoarytenoid articulation(s) | M. pterphar. | pterygopharyngeal muscle |
| Art. cricthyr. | cricothyroid articulation | M. sternoceph., p. mand. | mandibular portion of sternocephalic muscle |
| Art. thyrhyo. (dex., sin.) | (right, left) thyrohyoid articulation | ||
| Basihyo. | basihyoideum | M. sternoh. | sternohyoid muscle |
| blood vess. | blood vessels | M. sternthyr. | sternothyroid muscle |
| C1–C5 | cervical vertebrae 1–5 | M. stylglos. | styloglossal muscle |
| Cart(t). aryt. | arytenoid cartilage(s) | M. styloh. | stylohyoid muscle (two parallel portions) |
| Cart. cric. | cricoid cartilage | M. stylphar. (caud., rostr.) | (caudal, rostral) stylopharyngeal muscle |
| Cart. thyr. | thyroid cartilage | M. tens. veli pal. | tensor muscle of soft palate |
| Cav. infrglott. | infraglottic cavity | M. thyraryt. | thyroarytenoid muscle (M. ventric. + M. voc.) |
| Cerathyo. | ceratohyoideum | ||
| Choan. | choanae | M. thyrepigl. | thyroepiglottic muscle |
| conn. tissue | connective tissue | M. thyroh. | thyrohyoid muscle |
| cont. M. mass. | contour of M. mass. | M. thyrphar. | thyropharyngeal muscle |
| cont. phar. | contour of pharynx | M. ventric. | ventricularis muscle |
| Corn. caudale | caudal horn of Cart. thyr. | M. voc. | vocalis muscle |
| Duct. mand. | duct of mandibular gland | M. zyg. | zygomatic muscle |
| Duct. parot. | duct of parotid gland | Mm. constr. phar. caud. | caudal constrictor muscles of pharynx |
| Duct. pneu. | pneumatic duct connecting Vest. lar. and air sac | Mm. constr. phar. med. | middle constrictor muscles of pharynx |
| Mm. constr. phar. rostr. | rostral constrictor muscles of pharynx | ||
| Epigl. | epiglottis | musc. fibr. | muscle fibres |
| Epihyo. | epihyoideum | O: M. digastr. | origin of digastric muscle |
| Frenul. ling. | frenulum of the tongue | Oesoph. | oesophagus |
| Gl. mand. | mandibular gland | Orb. | orbita |
| Gl. thyr. | thyroid gland | P3, P4 | third, fourth premolar |
| Labb. or. | lips of the mouth | Palat. mol. | soft palate |
| Lam. cart. cric. | lamina of Cart. cric. | Pars tymp. | tympanic part of temporal bone |
| lat. base Epigl. | lateral base of epiglottis | Plic. voc. (dex.) | (right) vocal fold |
| Lig. arycorn. | arycorniculate ligament | Proc(c). med. | medial process(es) of Cart(t). aryt. |
| Lig. aryt. transv. | transverse arytenoid ligament | Proc. musc. | muscular process of Cart. aryt. |
| Lig. cricthyr. | cricothyroid ligament | Proc. parac. | paracondylar process |
| Ling. | tongue | Proc. voc. (dex.) | (right) vocal process of arytenoid cartilage |
| Mand. | mandibula (dentary) | Rec. lar. med. | median laryngeal recess |
| M. aryt. transv. | transverse arytenoid muscle | Rima glott. | glottic cleft |
| M. cerath. | ceratohyoid muscle | silic. tube | silicone tube (for air sac injection) |
| M. cricaryt. dors. | dorsal cricoarytenoid muscle | Stylhyo. | stylohyoideum |
| M. cricaryt. lat. | lateral cricoarytenoid muscle | Thyrhyo. | thyrohyoideum |
| M. cricphar. | cricopharyngeal muscle | tough lig. | tough ligament from Ang. to shaft of Stylhyo. |
| M. cricthyr. | cricothyroid muscle | ||
| M. cut. fac. | facial cutaneous muscle | Trach. | trachea |
| M. digastr. | digastric muscle | Tub. max. | maxillary tuber |
| M. hyoepigl. | hyoepiglottic muscle | Tymphyo. | tympanohyoideum |
| M. hyophar. | hyopharyngeal muscle | V. fac. | facial vein |
| M. lev. veli pal. | levator muscle of palatine velum | Vest. lar. | laryngeal vestibule |
| M. mal. | malaris muscle | VTL | vocal tract length |
| M. mass. | masseter muscle |
References
- Aas-Hansen Ø, Folkow LP, Blix AS. Panting in reindeer (Rangifer tarandus) Am J Physiol Regul Integr Comp Physiol. 2000;279:R1190–R1195. doi: 10.1152/ajpregu.2000.279.4.R1190. [DOI] [PubMed] [Google Scholar]
- Andersson M. Sexual Selection. Princeton, NJ: Princeton University Press; 1994. Monographs in behavior and ecology. [Google Scholar]
- Austen NL. Notes on the Scandinavian reindeer. In: Lartet E, Christy H, Jones TR, editors. Reliquiae Aquitanicae, Being Contributions to the Archaeology and Palaeontology of Périgord and the Adjoining Provinces of Southern France. London: Williams and Norgate; 1875. pp. 213–218. [Google Scholar]
- Avril C. Kehlkopf und Kehlsack des Schimpansen, Pan troglodytes (Blumenbach 1799) (Mammalia, Primates, Pongidae) Morph Jahrb. 1963;105:74–129. [Google Scholar]
- Banfield AWF. The Mammals of Canada. Toronto: National Museum of Natural Sciences. University of Toronto Press; 1974. [Google Scholar]
- Barboza PS, Hartbauer DW, Hauer WE, Blake JE. Polygynous mating impairs body condition and homeostasis in male reindeer (Rangifer tarandus tarandus) J Comp Physiol B. 174:309–317. doi: 10.1007/s00360-004-0416-6. [DOI] [PubMed] [Google Scholar]
- Bartels P. Über die Nebenräume der Kehlkopfhöhle. Z Morph Anthropol. 1905;53:11–61. [Google Scholar]
- Bergerud AT. Movement and rutting behavior of Caribou (Rangifer tarandus) at Mount Albert, Quebec. Can Field Naturalist. 1973;87:357–369. [Google Scholar]
- Bergerud AT. Rutting behaviour of Newfoundland Caribou. In: Geist V, Walther F, editors. The Behavior of Ungulates and its Relation to Management. Morges, Switzerland: International Union for Conservation of Nature and Natural resources IUCN; 1974. pp. 395–435. [Google Scholar]
- Camper P. Naturgeschichte … des Rennthiers. Düsseldorf: Johann Christian Daenzer; 1791. pp. 71–108. [Google Scholar]
- Clutton-Brock TH, Albon SD. The roaring of red deer and the evolution of honest advertising. Behaviour. 1979;69:145–170. [Google Scholar]
- Clutton-Brock TH, Green D, Hiraiwa-Hasegawa M, Albon SD. Passing the buck: resource defense, lek breeding and mate choice in fallow deer. Behav Ecol Sociobiol. 1988;23:281–296. [Google Scholar]
- Dawkins R, Krebs JR. Arms races between and within species. Proc R Soc Lond B. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- Dobson GE. On the structure of the pharynx, larynx, and hyoid bones in the Epomophori – with remarks on its relation to the habits of these animals. Proc Zool Soc London. 1881:685–693. [Google Scholar]
- Duellman WE, Trueb L. Biology of Amphibians. Chapter 4: Vocalization. Baltimore: The Johns Hopkins University Press; 1994. [Google Scholar]
- Dugmore AAR. The Romance of the Newfoundland Caribou. London: W. Heinemann; 1913. [Google Scholar]
- Duncker HR. General morphological principles of amniotic lungs. In: Pijper J, editor. Respiratory Functions in Birds, Adult and Embryonic. Berlin: Springer; 1978. pp. 2–15. [Google Scholar]
- Ericson CA. Some preliminary observations on the acoustic behaviour of semi-domestic reindeer (Rangifer tarandus tarandus) with emphasis on intraspecific communication and the mother–calf relationship. Fairbanks: University of Alaska; 1972. MS thesis. [Google Scholar]
- Espmark Y. Rutting behaviour in reindeer (Rangifer tarandus L.) Anim Behav. 1964;12:159–163. [Google Scholar]
- Espmark Y. Mother–young relationship and ontogeny of behaviour in reindeer (Rangifer tarandus L.) Z Tierpsychol. 1971a;29:42–81. doi: 10.1111/j.1439-0310.1971.tb01723.x. [DOI] [PubMed] [Google Scholar]
- Espmark Y. Individual recognition by voice in reindeer mother-young relationship, field observations and playback experiments. Behaviour. 1971b;40:295–301. doi: 10.1163/156853971x00438. [DOI] [PubMed] [Google Scholar]
- Espmark Y. Individual characteristics in the calls of reindeer calves. Behaviour. 1975;54:50–59. [Google Scholar]
- Fant G. Acoustic Theory of Speech Production. The Hague: Mouton; 1960. [Google Scholar]
- Fitch WT, Hauser MD. Vocal production in nonhuman primates: acoustics, physiology, and functional constraints on ‘honest’ advertisement. Am J Primatol. 1995;37:191–219. doi: 10.1002/ajp.1350370303. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Skull dimensions in relation to body size in nonhuman mammals: the causal bases for acoustic allometry. Zoology. 2000a;103:40–58. [Google Scholar]
- Fitch WT. The phonetic potential of non-human vocal tracts: comparative cineradiographic observations on vocalizing animals. Phonetica. 2000b;57:205–218. doi: 10.1159/000028474. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Reby D. The descended larynx is not uniquely human. Proc R Soc Lond, B. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WT, Hauser MD. Unpacking ‘honesty’: vertebrate vocal production and the evolution of acoustic signals. In: Simmons A, Fay RR, Popper AN, editors. Acoustic Communication. Berlin: Springer; 2002. pp. 65–137. [Google Scholar]
- Flydal K, Hermansen A, Enger PS, Reimers E. Hearing in reindeer (Rangifer tarandus) J Comp Physiol A. 2001;187:265–269. doi: 10.1007/s003590100198. [DOI] [PubMed] [Google Scholar]
- Fraser AF. Reproductive Behavior in Ungulates. London: Academic Press; 1968. [Google Scholar]
- Frazer Sissom DE, Rice DA, Peters G. How cats purr. J Zool Lond. 1991;223:67–78. [Google Scholar]
- Frey R, Hofmann RR. Larynx and vocalization of the takin (Budorcas taxicolor Hodgson, 1850 – Mammalia, Bovidae) Zool Anz. 2000;239:197–214. [Google Scholar]
- Frey R, Gebler A. The highly specialized vocal tract of the male Mongolian gazelle (Procapra gutturosa Pallas, 1777 – Mammalia, Bovidae) J Anat. 2003;203:451–471. doi: 10.1046/j.1469-7580.2003.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Riede T. Sexual dimorphism of the larynx of the Mongolian gazelle (Procapra gutturosa Pallas, 1777) (Mammalia, Artiodactyla, Bovidae) Zool Anz. 2003;242:33–62. [Google Scholar]
- Frey R, Gebler A, Fritsch G. Arctic roars – laryngeal anatomy and vocalization of the muskox (Ovibos moschatus Zimmermann, 1780, Bovidae) J Zool. 2006;268:433–448. [Google Scholar]
- Gautier J-P. Étude morphologique et fonctionelle des annexes extra-laryngées des Cercopithecinae; liaison avec les cris d'espacement. Biol Gabon. 1971;7:230–267. [Google Scholar]
- Gebler A. The vocal tract anatomy and vocalization of the Chinese Muntjac (Muntiacus reevesi OGILBY, 1839). First International Conference on Acoustic Communication by Animals; July 27–30, 2003; University of Maryland, College Park. [Google Scholar]
- Gegenbaur C. Vergleichende Anatomie der Wirbelthiere2Band (Darmsystem und Atmungsorgane, Gefäßsystem, Urogenitalsystem) Leipzig: Wilhelm Engelmann; 1901. [Google Scholar]
- Geist V. The evolution of horn-like organs. Behaviour. 1966;27:175–214. [Google Scholar]
- Göppert E. Atmungssystem (Organe der Luftatmung), I. Kehlkopf und Trachea. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der vergleichenden Anatomie der WirbeltiereBd3. Berlin: Urban & Schwarzenberg; 1937. pp. 797–866. [Google Scholar]
- Hasskó A. Über die Kehlsäcke des Orang-Utan und eine todbringende Erkrankung im Kehlsacke eines jungen Orangs. Z Hals- Nas - Ohrenheilk. 1929;23:258–262. [Google Scholar]
- Henshaw J. Consequences of travel in the rutting of reindeer and caribou (Rangifer tarandus) Anim Behav. 1970;18:256–258. [Google Scholar]
- Hermann L. Phonophotographische Untersuchungen III. Pflügers Arch ges Physiol. 1890;47:347–391. [Google Scholar]
- Herre W. Das Ren als Haustier – eine zoologische Monographie. Leipzig: Akademische Verlagsgesellschaft Geest & Portig; 1955. [Google Scholar]
- Herre W. Rentiere Die Neue Brehm-Bücherei. Lutherstadt: A. Ziemsen; 1956. Heft 180. [Google Scholar]
- Hill WCO, Booth AH. Voice and larynx in African and Asiatic Colobidae. J Bombay Nat Hist Soc. 1957;54:309–321. [Google Scholar]
- Jacobi A. Das Rentier – eine zoologische Monographie der Gattung Rangifer. Leipzig: Akademische Verlagsgesellschaft; 1931. [Google Scholar]
- Kelemen G, Sade J. The vocal organ of the howling monkey (Alouatta palliata) J Morph. 1960;107:123–140. doi: 10.1002/jmor.1051070202. [DOI] [PubMed] [Google Scholar]
- Köhler H. Vergleichend-anatomische Untersuchungen am Kehlkopf von Cerviden: Rotwild (Cervus elaphus Linné, 1758), Damwild (Cervus dama Linné 1758), Rehwild (Capreolus capreolus Linné 1758) und Elchwild (Alces Alces Linné 1758) Schriften des Arbeitskreises für Wildbiologie und Jagdwissenschaft an der Justus-Liebig-Universität Gießen Heft 8. Stuttgart: Ferdinand Enke; 1982. [Google Scholar]
- Lent PC. Rutting behaviour in a barren-ground caribou population. Anim Behav. 1965;13:259–264. doi: 10.1016/0003-3472(65)90044-8. [DOI] [PubMed] [Google Scholar]
- Lent PC. A review of acoustic communication in Rangifer tarandus. In: Luick JR, Lent PC, Klein DR, White RG, editors. Proceedings of the First International Reindeer and Caribou Symposium. Fairbanks: University of Alaska; 1975. pp. 398–408. [Google Scholar]
- Lönnberg E. Zur Kenntnis des Kehlsackes beim Renntier. Anat Anz. 1902;21:467–474. [Google Scholar]
- McComb KE. Female choice for high roaring rates in red deer, Cervus elaphus. Anim Behav. 1991;41:79–88. [Google Scholar]
- McElligott AG, O'Neill KP, Hayden TJ. Cumulative long-term investment in vocalization and mating success of fallow bucks, Dama dama. Anim Behav. 1999;57:1159–1167. doi: 10.1006/anbe.1999.1076. [DOI] [PubMed] [Google Scholar]
- McElligott AG, Naulty F, Clarke WV, Hayden TJ. The somatic cost of reproduction: what determines reproductive effort in prime-aged fallow bucks? Evol Ecol Res. 2003;5:1239–1250. [Google Scholar]
- McElligot AG, Birrer M, Vannoni E. Retraction of the mobile descended larynx during groaning enables fallow bucks (Dama dama) to lower their formant frequencies. J Zool Lond. 2006;270:340–345. [Google Scholar]
- Millais JG. Newfoundland and its Untrodden Ways. 1. London: Longmans, Green; 1907. [Google Scholar]
- Milne Edwards A, Grandidier A. Histoire naturelle des mammiféres. Tome IV, Atlas. I., pl. 99. Tome I, Texte I. In: Grandidier A, editor. Histoire physique, naturelle et politique de Madagascar. Paris: Imprimerie Nationale; 1875. [Google Scholar]
- Morrow R. Notes on the Cariboo. Proc Trans Nova Scotia Inst Nat Sc (Halifax) 1877;4:281–300. [Google Scholar]
- NAV. Nomina anatomica veterinaria [online] World Ass. Vet. Anat.; 2005. Available from http://www.wava-amav.org/Downloads/nav_2005.pdf[Accessed 1 February 2006] [Google Scholar]
- Negus VE. The Mechanism of the Larynx. London: WM Heinemann (Medical Books); 1929. [Google Scholar]
- Negus VE. The Comparative Anatomy and Physiology of the Larynx. New York: Hafner; 1962. [Google Scholar]
- Nickel R, Schummer A, Seiferle E. Lehrbuch der Anatomie der Haustiere. Berlin: Paul Parey; 1984. 5. Aufl. Bd. 1. [Google Scholar]
- Nickel R, Schummer A, Seiferle E. Lehrbuch der Anatomie der Haustiere. Berlin: Paul Parey; 1987. 6. Aufl. Bd. 2. [Google Scholar]
- Nowak RM. 6. Vol. 2. Baltimore: The Johns Hopkins University Press; 1999. Walker's Mammals of the World. [Google Scholar]
- Ogura K, Yamane J. Beiträge zur Kenntnis des Kehlsackes beim Renntiere. J Coll Agr Tohoku Imp University, Sapporo. 1915;6(7):151–155. [Google Scholar]
- Pleske T. Übersicht der Säugethiere und Vögel der Kola-Halbinsel, Teil I. Säugethiere. 37. Rangifer tarandus L. Reprinted 1969. In: Helmersen GV, Schrenck LV, editors. Beiträge zur Kenntnis des Russischen Reiches und der Angrenzenden Länder Asiens. Osnabrück: Biblio Verlag; 1884. pp. 179–195. Zweite Folge, Bd. VII. [Google Scholar]
- Pruitt WO. Behavior of the barren-ground caribou. Biol Pap University Alaska. 1960;3:1–44. [Google Scholar]
- Reby D, McComb K. Vocal communication and reproduction in deer. Adv Stud Behav. 2003a;33:231–264. [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav. 2003b;65:519–530. [Google Scholar]
- Reby D, McComb K, Cargnelutti B, Darwin C, Fitch WT, Clutton-Brock T. Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Proc R Soc London. 2005;272:941–947. doi: 10.1098/rspb.2004.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb D, Best PB. Anatomy of the laryngeal apparatus of the pygmy right whale, Caperea marginata (Gray 1846) J Morph. 1999;242:67–81. doi: 10.1002/(SICI)1097-4687(199910)242:1<67::AID-JMOR5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schmidt K. Kunststoff-, Silikonkautschuk-Perfusorinjektion. Die Darstellung kleinster Blutgefäße durch verbesserte Injektionsmethoden. Der Präparator. 1981;27:117–120. [Google Scholar]
- Schneider R. Vergleichende Untersuchungen am Kehlkopf der Robben (Mammalia, Carnivora, Pinnipedia) Morph Jb. 1962;103:177–262. [Google Scholar]
- Schneider R. Der Larynx der Säugetiere. In: Helmcke J-G, Lengerken HV, Starck D, Wermuth H, editors. Handbuch der Zoologie. Berlin: de Gruyter; 1964. pp. 1–128. 8. Band, 35. Lieferung. [Google Scholar]
- Schneider R, Kuhn H-J, Kelemen G. Der Larynx des männlichen Hypsignathus monstrosus Allen, 1861 (Pteropodidae, Megachiroptera, Mammalia) Z Wiss Zool. 1967;175:1–53. [Google Scholar]
- Schön. MA. On the mechanism of modulating the volume of the voice in howling monkeys. Acta Otolaryng. 1970;70:443–447. doi: 10.3109/00016487009181909. [DOI] [PubMed] [Google Scholar]
- Skjenneberg S, Slagsvold L. Reindriften og dens naturgrunnlag (Reindeer husbandry and its ecological principles) Oslo: Universitets forlaget, 332 pp. English edition: Deehr TT (translator); Anderson CM, Luick JR (eds) (1979) US Department of the Interior, Bureau of Indian Affairs. Juneau, Alaska, 395 pp.
- Sonntag CF. Anatomy, physiology and pathology of the chimpanzee. Proc Zool Soc London. 1923;1923:323–429. [Google Scholar]
- Starck D, Schneider R. Respirationsorgane, A. Larynx. Primatologia. 1960;3/2:423–587. [Google Scholar]
- Starck D, editor. Lehrbuch der speziellen Zoologie – Wirbeltiere. Jena: Gustav Fischer; 1995. pp. 695–1241. 5. Teil: Säugetiere, 5/1, 1–694, 5/2. [Google Scholar]
- Tyler MJ. The phylogenetic significance of vocal sac structure in hylid frogs. Univ Kansas Publ Mus Nat Hist. 1971;19:319–360. [Google Scholar]
- Tyler MJ. Superficial mandibular musculature, vocal sacs and the phylogeny of Australo-Papuan leptodactylid frogs. Rec S Aust Mus. 1972;16:1–20. [Google Scholar]
- Tyler MJ, Duellman WE. Superficial mandibular musculature and vocal sac structure in hemiphractine hylid frogs. J Morph. 1995;224:65–71. doi: 10.1002/jmor.1052240108. [DOI] [PubMed] [Google Scholar]
- Wilson DE, Reeder DM, editors. Mammal Species of the WorldA Taxonomic and Geographic Reference. 3. Baltimore: The Johns Hopkins University Press; 2005. [Google Scholar]
- Zahavi A. Mate selection: a selection for a handicap. J Theoret Biol. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zahavi A. The cost of honesty (further remarks on the handicap principle) J Theoret Biol. 1977;67:603–605. doi: 10.1016/0022-5193(77)90061-3. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Natural selection, sexual selection and the selection of signals. In: Scudder GGE, Reveals JH, editors. Evolution Today. Pittsburgh: Carnegie-Mellon University Press; 1981. pp. 133–138. [Google Scholar]