Abstract
The purpose of this study was to examine and describe the sequence of events involved in long-term biological reconstruction of a tendon–bone interface following surgical reattachment. Patellar tendon re-attachment in the adult sheep was used to investigate and describe the biological components involved in healing and repair of a tendon enthesis. Light microscopy was used to describe the healing morphology at time intervals of 8, 12, 26, 52 and 104 weeks. By 8 weeks a collagen continuum was observed between the tendon and bone. Over time this fibrous bridge became anchored into the original tissues (tendon and bone), with the resultant enthesis resembling more a fibrous rather than the original fibrocartilagenous enthesis. The associated collagen fibrils between the two tissues gradually changed in morphology over time to reflect the fibres seen in the original tendon tissue. The fibrous tissue of the forming enthesis remained hypercellular when compared with the controls. The resultant long-term morphology may be a reflection of functional adaptation rather than anatomical replication.
Keywords: bone–tendon junction, enthesis, fibrocartilagenous insertion, healing, long-term morphology, tendon-to-bone healing
Introduction
The bony insertion of soft tissue – be it tendon, ligament or capsule – is described as an enthesis. The enthesis tissue includes collagen fibres that extend from the tendon through two specialized zones of fibrocartilage to anchor into the underlying bone. The literature describes two basic types of insertions depending on the presence or absence of fibrocartilage (Knese & Biermann, 1958; Woo et al. 1987; Benjamin & Ralphs, 1997) and the biomechanical loads placed on the insertion during articulation. Not only is the anatomical relocation of a tendon or ligament important for the success of any repair or reconstruction of the enthesis, but so too is the morphological replication of this unique zone of connective tissue.
Due to the advances in polymer sciences, the use of bio-absorbable implants in orthopaedic surgery has become more frequent. These implants offer the advantages of initial biomechanical protection and gradual loading of the biological interface between the two tissues, as well as a reduced need for hardware removal. With the use of these biological ‘anchors’, the formation of collagen fibres that anchor a tendon into the bone following surgical reconstruction has generally been regarded as a critical event in the reconstruction of a bone–tendon interface (Whiston & Walmsley, 1960; Forward & Cowan, 1963; Oguma et al. 2001).
At the time of surgery, initial soft tissue fixation is achieved mechanically using a surgical implant such as interference screws or suture anchors. These devices anchor the soft tissue to the bone and provide early strength and optimum healing by opposing the two tissues, thereby enabling formation of the subsequent biological anchor. This process is time-dependent and will be responsible for the long-term anchoring of the tendon or graft to the bone. The perceived effectiveness and success of this subsequent biological fixation is reflected in the continued use of bio-absorbable implants.
Several animal studies have described the process of biological fixation of soft tissue to bone as an invasion of a non-specific cuff of hypercellular, hypervascular granulation tissue of short disorganized collagen fibrils that fill the space between the bone and a tendon that has either been sutured to bone or held within a tunnel of cancellous bone (Whiston & Walmsley, 1960; Forward & Cowan, 1963; Jackson et al. 1993; Rodeo et al. 1993; St. Pierre et al. 1995; Nagano et al. 1997; Aoki et al. 1998; Uhthoff et al. 2000). With time, healing between the two tissues occurs through a process of progressive re-establishment of an integrated collagen bridge between the tendon and bone (Jones et al. 1987; Aoki et al. 1998; Park et al. 1998; Uhthoff et al. 2000). Bony integration of this interface tissue appears to occur via progressive ossification of the extracellular matrix (ECM) (Forward & Cowan 1963; Rodeo et al. 1993; Grana et al. 1994; Blickenstaff et al. 1997; Pinczewski et al. 1997; Oguma et al. 2001) that gradually matures to resemble a ‘normal’ insertion by 12–26 weeks (St. Pierre et al. 1995; Kang et al. 1996; Aoki et al. 1998). However, very little information is available regarding the long-term morphology (greater than 26 weeks) following anatomical reconstruction of a bone–tendon interface. The literature that does exist presents differing findings, with some authors describing the re-establishment of what could be referred to as a normal physiological enthesis (Jones et al. 1987; Park et al. 1998), whereas others describe the re-establishment of a collagen continuum between the tendon and bone but not the reconstruction of the original fibrocartilagenous enthesis (St. Pierre et al. 1995; Blickenstaff et al. 1997).
The aim of this study was to evaluate the long-term morphology of a reconstructed enthesis and compare these results with the morphology of a normal enthesis. It is hypothesized that the reconstructed enthesis would reflect the native enthesis and will offer biological support to the continued use of bio-absorbable devices in tendon transplant surgery.
Materials and methods
A prospective observational study utilizing an animal model was used to investigate the transition and long-term outcome of the re-attachment of tendon to bone. Ten skeletally mature 2–4-year-old female sheep (weight 70–80 kg) were used in this study and all procedures were undertaken with the prior approval from the University of Otago Animal Ethics Committee. The experimental surgical technique was undertaken in a strict aseptic environment and animal care was in accordance with the Animal Ethics requirements.
Animal surgical procedure
The animals were anaesthetized using an intravenous injection of thiopentone sodium (Bomathal, BOMAC Laboratories, Auckland, New Zealand), 1 g per 20 mL isotonic saline, and then intubated through an endotracheal tube (ET) and connected to the anaesthetic machine. Oxygen was administered at a flow rate of 1.0 L min−1, and anaesthesia maintained with 2% halothane gas, and during the operative procedure the animal was connected to an oximeter to monitor oxygen levels.
With the sheep anaesthetized, the right hip and hind leg was shorn to expose the hind limb (medially and laterally) and the skin aseptically prepared for surgery. Four 5.0-mm Schanz pins were then inserted along the midline of the femur and along the midline of the tibia. A 3.0-mm Kirschner wire with a trocar point was then inserted into the middle of the thickest portion of the patella. The two femoral pins and two tibial pins were joined by an 8.0-mm-diameter clean, but non-sterile, straight mild steel rod and held in place with non-sterile polymethylmethacrylate cement (Ostron 100 T. Blue, GC Dental Industrial Corp., Tokyo, Japan). The stifle joint was then maximally extended and the patella placed under longitudinal traction to produce a palpable slackness in the patellar tendon. Once in this position, the Kirschner wire was attached to the external fixator (Fig. 1) to maintain this position.
Fig. 1.
The external fixation used to maintain the stifle in extension in initial stages of healing. (A) The triangular arrangement of the steel rods that formed the external fixator on the sheep; and (B) the location of the four 5.0-mm Schanz pins along the midline of the femur, and the tibia (pins 1, 2, 3 and 4) and the 3.0-mm Kirschner wire in the patella.
With an aseptic technique, the patellar tendon and the tibial attachment were then exposed through a skin incision made immediately below the tibial tuberosity. A scalpel blade was used to pare the patellar tendon off the tibial attachment as close as possible to the underlying bone, and the calcified fibrocartilage was removed with a saline-cooled dental burr to expose the subchondral bone. The patellar tendon was gently apposed to the bone (as close to the anatomical position as possible) using two bio-absorbable TAG 3.7-mm Rod II anchors (Acufex, USA). The incision was then closed. Post-operatively the sheep recovered in a partial weight-bearing position assisted by a sling system, and once the external fixator was removed the sheep was moved to deep litter until it demonstrated full mobility. It was then returned to the farm until the designated time interval. The morphological characteristics of the healing tendon-to-bone interface were evaluated by conventional histological methods.
Morphological analysis
Two sheep were killed at time intervals 8, 12, 26, 52 and 104 weeks from the time of surgery, along with a further two ‘control’ sheep, in accordance with the University Animal Ethics Code (Andrews et al. 1993; Reilly, 1993). A 30-mm length of the intact patellar tendon along with the tibial insertion was harvested bilaterally and these blocks of tissue further divided lengthwise along the midline between the suture anchors. Control samples were obtained from the opposite (non-operated) stifle, and the right stifle of two sheep with intact tendons. A series of 500-µm-thick samples for histological examination were placed in 10% neutral buffered formalin for 1 week. After fixation the samples were decalcified using formic formalin decalcification solution. When decalcification was complete the tissues were subjected to a routine dehydration process of a graded series of ethanol, embedded in paraffin, and 7-µm serial sections (sagittal plane) taken. These sections were then stained with haematoxylin and alcoholic eosin (H&E), Mallory trichome (MALT), alcian blue counterstained with H&E and toluidine blue phloxinate (TBP) and viewed and photographed with an Olympus AX70 ‘Provis’ Microscope and Panasonic digital CCD video camera. The digital images were then processed on a Power Macintosh 8500/120 computer.
Results
All animals made a full recovery post surgery, regaining full mobility and a ‘normal’ gait pattern. At post-mortem the operated stifle was examined and compared with the control stifle in all animals. Experimental stifles in all sheep demonstrated a full and equal passive range of movement and no ligamentous laxity. Only a small amount of soft tissue thickening was observed over the surgical incisions in all sheep.
Normal/control enthesis
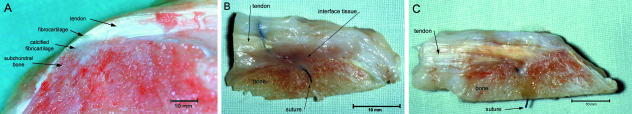
When viewed macroscopically, the transition between the two tissues within the control sheep demonstrated a gradual change in morphology along the enthesis. A layer of white tissue indicated the possible band of enthesis fibrocartilage that separated the bone and tendon. As the direction of tendon fibres at this morphological junction changed to become perpendicular to the bone surface, the tendon appeared to ‘flare’ and become thicker and cover a large area of bone surface at the insertion. At the insertion site the individual fibres of the tendon were unrecognizable as the tissue became more dense and uniform, and the ‘subchondral’ bone seen at the insertion of the patellar tendon was cancellous in appearance. Directly under the tendon insertion was a triangular layer of calcified fibrocartilage whose apex penetrated some distance from the fibrocartilage layer into the subchondral bone (Fig. 2A).
Fig. 2.
Macroscopic healing of the tendon–bone junction. (A) The macroscopic view of the insertion of the normal patellar tendon enthesis in the sheep. (B) At 8 weeks, macroscopically a large band of scar tissue was seen between the tendon and the bone in samples taken from around the suture anchors. (C) At 52 weeks, macroscopically the interface seen between the tendon and the bone showed an observable collagen continuity between the tendon and bone.
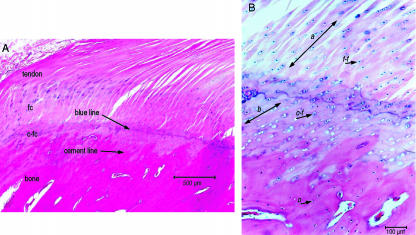
The insertion of the patellar tendon into the tibial tubercle could be divided histologically into four regions: (a) tendon, (b) fibrocartilage, (c) calcified fibrocartilage and (d) bone (Fig. 3A) and reflected the findings made by other authors. The longitudinal sections of the tendon showed compact, parallel collagen fibres with a characteristic elongated ‘crimp’ pattern. These fibres were grouped into recognizable bundles separated from the neighbouring fascicles by loose interfascicular connective tissue containing small blood vessels and elongated spindle-shaped fibrocytes. The division between the tendon tissue and the fibrocartilage was indicated by a change in cell morphology from fibroblasts into chondrocytes, and was accompanied by a change in orientation of the collagen fibres, becoming perpendicular to the bone surface.
Fig. 3.
Histological appearance of the normal enthesis of the patella tendon. (A) The histological appearance of the zones of tissue within a normal patellar tendon enthesis of the sheep (H&E stain); (B) the change in cellular appearance from across the ‘blue line’ from the rounder cells within lacunae (f-f) of the zone of fibrocartilage, small cells within the zone of calcified fibrocartilage (c-f), to the small oseocytes of the bone (o), and the change in fibre orientation through fibrocartilage layer (a) and calcified fibrocartilage (b) in the normal enthesis (alcian blue/H&E stain).
The next two histological boundaries observed between the fibrocartilage and the calcified fibrocartilage, and between the calcified fibrocartilage and the subchondral bone, were indicated by the ‘blue line’ and the by the ‘cement line’, respectively (Fig. 3B). The collagen of the fibrocartilage zone of tissue had a more compact, ‘crimped’ appearance compared with the more elongated, ‘crimped’ appearance of fibres in the tendon. The cells of this zone were characteristic chondrocytes arranged in columns containing dark-staining nuclei. The ECM stained light blue with the MALT and H&E/TBP stains. The collagen fibres within the calcified fibrocartilage zone were arranged perpendicular to the bone surface but were less distinguishable than in the previous fibrocartilage zone, while the cellular components were small, round and surrounded by a ‘blue halo’ with H&E stains (Fig. 3B). The cells became increasingly separated from other cells by ECM but were still arranged in columns between ‘brick-red’ collagen fibres.
The delineation between the calcified fibrocartilage and bone had the appearance of interdigitating ‘fingers’ within the underlying cancellous bone. Directly under the insertion site the bone appeared denser when compared with the surrounding cancellous bone and resembled a layer of compact cortical bone covering the bone. This layer of compact bone was made up of small osteocytes housed within individual lacunae and incorporated within a mixture of parallel and circular lamellae of no conclusive or common orientation to the lamellae. The collagen of the previous zone did not appear to integrate with the individual lamella but appeared to ‘butt-up’ against the lamellae. It appeared that the ECM was ossified around the collagen fibres without interdigitation of fibres.
Eight weeks
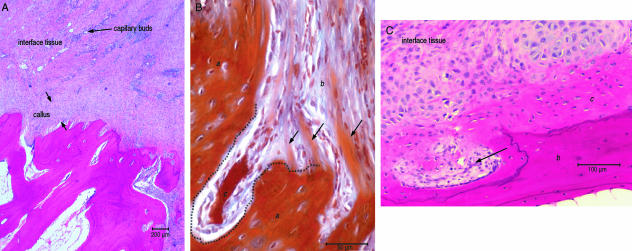
Microscopically the junction between the tendon and the bone could be divided into three tissue zones: the tendon, a band of interface tissue and the underlying bone (Fig. 2B). The demarcation between tendon and interface tissue was determined by a change in orientation of the collagen fibres. However, the peripheral edge of the tendon was difficult to determine as there was a gradual change in morphology from the mature tendon to a layer of tissue that consisted of thin elongated fibroblasts and light staining fibres seen next to the tendon tissue. Covering the bone surface was a callus of tissue consisting of small osteoblasts and distinguishable fibres representing a layer of new woven bone. A faint cement line was observed in places across the bone surface between the callus of bone tissue and the subchondral bone. Covering this layer was a layer of hypercellular fibrous tissue that contained fibroblasts and small blood vessels. Furthermore, there was a gradual change in the morphology of the fibroblasts, with those at the bone surface exhibiting a rounder appearance compared with a more elongated appearance at the tendon surface (Fig. 4A).
Fig. 4.
Histological appearance of the healing junction between bone and tendon at 8 and 12 weeks. (A) The interfacing scar tissue between the tendon stump and the bone at 8 weeks and the formation of a callus tissue covering the bone surface (alcian blue/H&E stain). (B) The vertically orientated fibre within the callus (b) butted-up to, but integrate within, the original bone (a). What appeared to be new cancellous cavities (c) are seen at 12 weeks (MALT stain). (C) The cells within the callus of woven bone (c) seen against the original bone (b) and the cells of the interface tissue that resembled chondrocytes. What appeared to be small cancellous cavities formed (arrow) across the bone surface at 12 weeks (H&E stain).
It is worth noting that the position where the samples were taken in relation to the suture anchor resulted in different macroscopic observations of interface morphology. In samples taken lateral to the suture anchors no interfacing scar tissue was observed between the tendon and bone and the tendon appeared to unite directly to the underlying bone. The samples taken between the suture anchors showed an interfacing scar tissue between the tendon stump and the bone.
Eight weeks
The interfacing tissue was still distinguishable macroscopically from the original tendon and bone. The scar tissue was separated from the bone surface by what appeared to be a thin layer of uneven cortical bone or calcified fibrocartilage that contoured the bone surface. Under low-power magnification (×10 objective), the interface between the tendon and the bone was filled with organized tissue that appeared to have fibrous integration with the bone and tendon. At higher magnification, fibrous integration was not consistent across the entire tendon or bone interfaces. The tendon–interface tissue junction was indicated by randomly placed small capillaries and a change in collagen fibre orientation from being horizontally orientated and reflecting the orientation of the tendon, to a more perpendicular orientation in relation to the bone surface. The cellular component of the periphery of the tendon consisted of thin elongated fibroblasts sparsely distributed between collagen fibres. In comparison, the interface tissue contained a large number of fibroblasts that were rounder and less elongated than those within the tendon. They were also surrounded by short collagen fibres. Across the bone interface tissue junction, collagen fibres integrated with a newly formed calcified callus of woven bone and the collagen fibres associated with this callus were entrapped within the ECM. The cells within the callus tissue closest to the interface tissue were large, active cells housed within individual lacunae that were suggestive of chondrocytes (Fig. 4B), whereas closer to the underlying bone tissue, the cells were smaller and had the appearance of osteoblasts.
No ‘blue line’ was seen to indicate a demarcation between the fibrocartilage zone and calcified layers of the callus, although there was an irregular cement line between the underlying bone and the calcified callus. At this junction vertically orientated fibres within the callus butted-up to, but did not integrate with, the original bone (Fig. 4C).
Twenty-six weeks
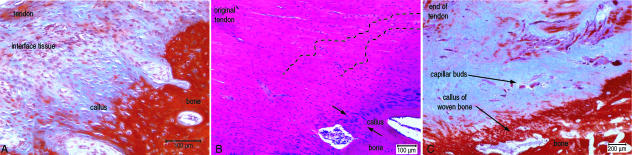
At the macroscopic level, a layer of pale interface tissue still separated the tendon from the bone, but this interface tissue appeared thinner than observed in the previous time intervals. Microscopically, none of the disorganized hypercellular interface tissue described in the earlier time intervals was seen. A band of interface tissue was observed that differed morphologically from the bone and tendon, exhibiting disorganized collagen fibrils within more ECM compared with the tendon and containing large, oval-shaped cells. This band of tissue exhibited poor staining properties (Fig. 5A).
Fig. 5.
Histological appearance of the healing junction between bone and tendon at 26, 52 and 104 weeks. (A) Microscopically the formation of a callus of what appeared woven bone integrated the fibrous component of the interface tissue over the bone surface at 26 weeks (MALT stain). (B) The interface between the tendon and bone at 52 weeks was filled by a layer of tissue that resembled woven bone (between arrows) (H&E stain). (C) The interface between the tendon and the bone was still disorganized and hypercellular especially around the vascular buds compared with controls at 102 weeks (MALT stain).
The tissue seen distal to the suture material was still disorganized in appearance. At the recognizable end of the original tendon, tendon fibres merged indistinguishably with the peripheral fibres of the interface tissue. The feature that differentiated the tendon from the interface tissue was the change in cell shape, with the spindle-shaped fibroblasts of the tendon being replaced by elongated oval-shaped fibroblasts of the interface tissue.
At the bone interface tissue junction, the interface tissue was incorporated in a callus of woven bone. At the tendon side of the callus there were exposed fibres separated from each other by fibroblasts continuous with that seen in the callus tissue. Fibres were arranged parallel to each other and orientated perpendicular to the bone surface, and these fibres extended from the original bone layer through the callus of woven bone into the interfacing tissue. No ‘blue line’ or ‘cement line’ was observed in the histological sections. Below the callus tissue, cancellous bone of normal appearance was observed.
Fifty-two weeks
A continuity of collagen was observed macroscopically between the tendon and bone in all samples. The surface of the surgical bone bed was covered with a white cap of thin, transparent tissue which contoured the bone surface and resembled a callus of new bone. The tendon merged unrecognizably with the bone tissue, forming what appeared to be a completed enthesis (Fig. 2C).
At low magnification the bone–tendon interface still differed from the control interface, with the junction between the calcified tissue and fibrous tissue being indicated by a blue line that reflected the shape and topography of the bone surface. The band of the fibrocartilaginous tissue was narrower and, in places, non-existent. The bone surface was covered by a callus of fibrous tissue that appeared calcified and resembled woven bone (Fig. 5B). A definite cement line was not consistently seen with all samples, and when examined, the ECM of this band of tissue was more dense than the interface tissue but less dense than the underlying bone. Fibres were less distinct within this ECM, but were still recognizable. The cellular component of this layer consisted of oval-shaped cells within blue staining lacunae at the soft tissue side of the callus, with the cell morphology gradually changing to small osteoblast-like cells at the junction of the original bone and callus. As in samples taken at earlier time intervals, the collagen fibres contained within this callus did not appear to integrate with, or become continuous with, the existing lamellae of the underlying cancellous bone.
One hundred and four weeks
Similar observations were made with samples taken at this time interval compared with those recorded at 52 weeks. The interface between the tendon and the bone was similar macroscopically to that of the control group. However, microscopic observations revealed differences between the control and the surgical groups. Compared with the controls, the interface between the tendon and bone was still hypercellular and contained areas of disorganization that were associated with vascular buds and contained short, irregularly orientated light staining fibres (Fig. 5C). The cells in this area were round fibroblasts with an orientation that reflected that of the fibres. The cellular morphology and collagen alignment of the rest of the interface tissue demonstrated small osteoblast-like cells separated by vertically orientated fibres anchored in the callus of woven bone at the bone surface, and elongated fibroblast between collagen fibres orientated in line with the fibres of the original tendon. No layer of fibrocartilage tissue was seen between the tendon and the bone at 104 weeks (Table 1).
Table 1.
Summary of the key findings at a macro and micro level of the healing tendon bone interface at time intervals of 8, 12, 26, 52 and 104 weeks post-surgery
| Time interval | Key histological findings | |
|---|---|---|
| 8 weeks | Macroscopic | Lateral to the suture anchors, the tendon united directly to the underlying bone. |
| Around the suture anchors, interfacing scar tissue was present between the tendon stump and the bone | ||
| Microscopic | Gradual change in morphology from the mature tendon to a layer of new woven bone. | |
| An intermediate zone of hypercellular fibrous tissue. | ||
| 12 weeks | Macroscopic | Interfacing scar tissue still separated the original tendon from the bone. |
| Scar tissue was separated from the bone surface by a thin layer of uneven cortical bone or calcified fibrocartilage. | ||
| Microscopic | Fibrous integration with the tendon had occurred. | |
| Across the bone surface, collagen fibres integrated with a newly formed calcified callus of | ||
| woven bone. The fibres entrapped within the callus did not integrate with lamellar of the underlying original bone, but instead finished adjacent to the lamella. | ||
| 26 weeks | Macroscopic | Fibrous integration occurred between the tendon and bone. |
| A thin layer of pale interface tissue separated the tendon from the bone. | ||
| Microscopic | Fibrous integration occurred with the bone and tendon. | |
| A band of disorganized interface tissue exhibiting disorganized collagen fibrils, extensive extracellular and oval-shaped cells was observed between the tendon and calcified callus of resembling woven bone. | ||
| 52 weeks | Macroscopic | The tendon merged unrecognizably with the bone tissue forming a completed enthesis. |
| Microscopic | Fibrous integration with the tendon was continuous across the interface but integration across the bone surface was interrupted. Where fibrous integration occurred an histological ‘blue line’ was observed that reflected the topography of the bone surface. | |
| The bone surface was covered by a callus of fibrous tissue that resembled woven bone. | ||
| 104 weeks | Macroscopic | The tendon merged unrecognizably with the bone tissue forming a completed enthesis. |
| Microscopic | The interface between the tendon and bone was still hypercellular and contained areas of fibre disorganization associated with vascular buds. | |
| The cells in this area were round fibroblasts. | ||
| No layer of fibrocartilage tissue was observed. |
Discussion
This study provides an insight into the long-term healing of a tendon to bone in the animal model and describes the sequence of events involved in the development of the long-term morphology. The long-term healing of a re-attached patellar tendon did not result in the re-establishment of the original fibrocartilage enthesis with interfacing layers of fibrocartilage-calcified fibrocartilage, but ultimately resulted in a fibrous interface. Despite the absence of a layer of fibrocartilage, there existed a population of cells that resembled chondrocytes seen at the interface between the relative flexible fibrous tissue and the rigid callus of calcified tissue and represented the boundary between the calcified callus and tendon. The lack of a clear band of fibrocartilage and the re-establishment of a fibrocartilagenous enthesis seen in the control stifles, or as described in the literature (Woo et al. 1987; Benjamin & Ralphs, 1997; Benjamin et al. 2004), differs from studies of healing of the bone–tendon interface that have described the re-establishment of an enthesis (St. Pierre et al. 1995; Kang et al. 1996; Aoki et al. 1998). This difference may reflect a combination of the animal model used in this study, the functional mechanics of the stifle joint, the properties of the tendon or the process of healing in a skeletally mature animal. The long-term healing between the patellar tendon and tibial attachment in this study may represent functional repairing to resist biomechanical loading as opposed to anatomical regeneration. If the latter were the case, it could be said that the resultant morphology reflects a poor healing result.
The functionality of this resultant morphology was not tested biomechanically, but no sheep were reported to be lame or have an affected gait pattern, suggesting that the long-term healing was not dysfunctional. Although this observation might suggest functional morphology, it does raise the question of whether a fibrocartilaginous patellar tendon–tibial insertion as seen in the normal sheep is required. Current views regarding the functional purpose and biomechanical role of the fibrocartilage have been influenced to some extent by the two-dimensional nature of investigations to date. These studies have suggested the layer of fibrocartilage offers an intrinsic two-tiered defence mechanism against wear and tear (Schneider, 1956), and as Benjamin & Ralphs (1998) concluded, the amount of fibrocartilage contained in an insertion site reflected the greater angular change experienced by the inserting fibres during articulation. The lack of fibrocartilage developed after 2 years in the present model may reflect this lack of ‘bend’ experienced by the insertion post-surgery, which may have occurred as a result of limited range of tibio-femoral or patello-femoral movement following surgery. Although no detectable stiffness or limitation in the functional range of movement was detected at post-mortem to confirm this, the possibility of a limitation in accessory movement such as anterior translation/glide of the tibio-femoral joint, or accessory movements of the patello-femoral joint required for normal functional movements, was not assessed. Restriction of these movements may have reduced the change in insertion angle of the patellar tendon with functional movement, eliminating the need for a layer of interfacing fibrocartilage. Also, subtle changes may have occurred to the tendon viscoelastic properties or in compensatory movements of proximal segments following surgery, resulting in different loading forces on the patellar tendon and the tibial insertion. Although there were no observable gait abnormalities or limited range of movements of the stifle detected at post-mortem, these were not quantified and therefore cannot be confirmed.
A three-dimensional investigation (Milz et al. 2002) shows a complex pattern of interlocking between the fibrocartilage, calcified fibrocartilage and subchondral bone, with regional differences in the amounts of calcified fibrocartilage across the enthesis organ. This suggests the importance of the complexity of the interface between calcified fibrocartilage and subchondral bone in the anchoring of a tendon or ligament to bone. The complexity of the interface between these two tissues is well recognized (Gao & Messner, 1996; Benjamin & Ralphs, 1998; Woo et al. 1987; Milz et al. 2002) and is possibly the integral component necessary for enthesis integrity. It appears that this complex interlocking of the two tissues at the ‘cement line’ augments the collagenous link between the tendon or ligament and bone. The results from the current long-term study showed the development of a collagen continuum between the tendon fibres and the invading interface tissue and the fibres within a callus of woven bone. The fibres at the woven bone interface did not integrate with the individual lamellae of the existing bone, but instead were found to abut them. The development of a callus of new bone appears to be integral to the integrity of the remodelling soft tissue–bone interface and is a commonly reported event involved in the fixation of soft tissue to bone (Rodeo et al. 1993; Schiavone et al. 1993; St. Pierre et al. 1995; Blickenstaff et al. 1997). This new bone development has been described as the continued development and maturity of the layer of woven bone (St. Pierre et al. 1995), as trabecular bone development (Rodeo et al. 1993; Blickenstaff et al. 1997), or progressive calcification of the layer of fibrocartilage seen against the bone (Schiavone et al. 1993). Whatever the description used in the literature, a callus of calcified tissue or new bone interlocks the fibres of the interface tissue and this interlocking of this callus with the bone is important for remodelling tendon–bone interface integrity.
The observations made in this study represent a process of functional repair that reflects subtle post-operative changes in the biomechanics of the stifle. However, the results cannot explain the long-term observation of a fibrous rather than a fibrocartilagenous enthesis developing, and this anomaly requires further investigation. As the stifle joint in the sheep operates in flexion it would suggest that the developing enthesis of the patellar tendon in the sheep model would be subjected to more tensile than bending forces. If, as Benjamin et al. (1986) postulated, the reason fibrocartilage was characteristic of epiphysial tendons was because of the change in angle at the insertion that occurred during limb movement, and represented an adaptation to protect against bending (Benjamin et al. 1986, 1991; Woo et al. 1987; Evans et al. 1990), then the development of fibrocartilage in a flexed joint would be redundant. The development of a fibrous insertion reflected the biomechanical environment of the flexed stifle that was possibly exposed to more tensile than bending forces, and therefore the interpolation of a biological outcome of the present study to healing in the functionally extended knee of the human requires cautious inference.
Conclusion
The results from this study suggest that a functional enthesis is not only dependent on the replication of the original insertion morphology, but is dependent on the formation of a fibrous continuum between the tendon and the bone. By 8 weeks, a collagen continuum was observed in this model between the tendon and bone and over the long term this fibrous bridge became anchored into the original tendon and bone tissues, with the resultant enthesis resembling a fibrous rather than a fibrocartilagenous enthesis. The associated collagen fibres between the two tissues gradually changed in morphology to reflect the fibres seen in the original tendon tissue, but the fibrous tissue between the original tendon and forming enthesis remained hypercellular compared with the controls. In this respect the results of the present study reflect those recorded by other authors (Rodeo et al. 1993; Grana et al. 1994; Blickenstaff et al. 1997). However, studies of the long-term healing between a tendon and bone have used an ACL (anterior cruciate ligament) model of a tendon attached within a bone tunnel. Few studies have looked at the extra-articular reattachment of a tendon to bone using procedures other than a tunnel, and have not investigated the healing morphology 12 months post-reattachment. The significance of the hypercellular appearance of the tendon and the fibrous enthesis 2 years after reconstruction suggests that the important feature of a re-attachment of a tendon in terms of functional outcome is not the histological appearance.
References
- Andrews EJ, Bennett BT, Clark JD, et al. Report of the AVMA panel on euthanasia. J Am Vet Med Assoc. 1993;202:229–249. [PubMed] [Google Scholar]
- Aoki M, Isogai S, Okamura K, Fukushima S, Ishii S. Healing of the rotator cuff at tendon insertion to bone: a study using canine infraspinatus. Proceedings of the 44th Annual Meeting, Orthopaedic Res Soc New Orleans; 1998; Louisiana. p. 627. [Google Scholar]
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Dontheneni RR, Findlay JA, Pemberton DJ. Quantitative differences in the histology of the attachment zones of the meniscal horns in the knee joint of man. J Anat. 1991;177:127–134. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Tendons and ligaments – an overview. Histol Histopathol. 1997;12:1135–1144. [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The ‘enthesis organ’ concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheumatism. 2004;50:3306–3313. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinous autograft in a rabbit model. Am J Sports Med. 1997;25:554–559. doi: 10.1177/036354659702500420. [DOI] [PubMed] [Google Scholar]
- Evans EJ, Benjamin M, Pemberton DJ. Fibrocartilage in the attachment zones of the quadriceps tendon and patellar ligament of man. J Anat. 1990;171:155–162. [PMC free article] [PubMed] [Google Scholar]
- Forward AD, Cowan RJ. Tendon suture to bone. An experimental investigation in rabbits. J Bone Joint Surg. 1963;45A:807–823. [Google Scholar]
- Gao J, Messner K. Quantitative comparison of soft tissue–bone interface at chondral ligament insertions in the rabbit knee joint. J Anat. 1996;188:367–373. [PMC free article] [PubMed] [Google Scholar]
- Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344–351. doi: 10.1177/036354659402200309. [DOI] [PubMed] [Google Scholar]
- Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- Jones JR, Smibert JG, McCullough CJ, Price AB, Hutton WC. Tendon implantation into bone: An experimental study. J Hand Surgery. 1987;12-B:306–312. doi: 10.1016/0266-7681_87_90179-3. [DOI] [PubMed] [Google Scholar]
- Kang Y-K, Kim I, Woo Y-K, et al. The role of fibrocartilage of insertion sites in the tendon to bone healing. An experimental study of rabbits. Proceedings of the 42nd AnnMeeting, Orthop ResSocAtlanta,; Georgia. p. 367. [Google Scholar]
- Knese K-H, Biermann H. Die knochenbildung an sehnen und bandansätzen im bereich ursprünglich chondraler apophysen. As cited Benjamin M., Evans E.J., Copp L. (1986) J Anat. 1958;149:89–100. [PubMed] [Google Scholar]
- Milz S, Rufai A, Buettner A, Putz R, Ralphs JR, Benjamin M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 2002;200:145–152. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Yoshiya S, Kuroda R, Kurosaka M, Mizuno K. Remodelling and healing process of bone-patellar tendon-bone graft in a bone tunnel: a histological study in dogs. Proceedings Transactions of the 43rd AnnuMeeting of the OrthopResSoc,; San Francisco, California. p. 78. (13. [Google Scholar]
- Oguma H, Murakami G, Takahashi-Iwanaga H, Aoki M, Ishii S. Early anchoring collagen fibres at the bone–tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res. 2001;19:873–880. doi: 10.1016/S0736-0266(01)00021-3. [DOI] [PubMed] [Google Scholar]
- Park MJ, Seong SC, Lee MC. A comparative study on healing of bone-tendon autograft and bone-tendon autograft unsing patellar tendon in rabbits. Proceedings of the 44th Annual Meeting, Orthop ResSocNew Orleans; Louisiana. p. 610. [Google Scholar]
- Pinczewski LA, Clingeleffer AJ, Otto DD, Bonar SF, Corry IS. Integration of hamstring tendon graft with bone in reconstruction of the anterior cruciate ligament. Arthroscopy. 1997;13:641–643. doi: 10.1016/s0749-8063(97)90194-8. [DOI] [PubMed] [Google Scholar]
- Reilly JS. Euthanasia of Animals Used for Scientific Purposes. Queensland, Australia: Australian and New Zealand Council for the Care of Animals in Research and Teaching (ANZCCART); 1993. [Google Scholar]
- Rodeo SA, Arnosczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon healing in a bone tunnel. J Bone Joint Surg. 1993;75-A:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- Schiavone PA, Fabbriciani C, Delcogliano A, Fanzese S. Bone–ligament interaction in patellar tendon reconstruction of the ACL. Knee Surg Sports Traumatol Arthroscopy. 1993;1:4–8. doi: 10.1007/BF01552150. [DOI] [PubMed] [Google Scholar]
- Schneider H. Zur Struktur der Sehnenansatzzonen. Z Anat Entwicklungsgeschichte. 1956;119:431–456. [PubMed] [Google Scholar]
- St Pierre P, Olson EJ, Elliott JJ, O’Hairs KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Joint Surg. 1995;77-A:1858–1866. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- Uhthoff HK, Sano H, Trudel G, Ishii H. Early reactions after reimplantation of the tendon of supraspinatus into bone. J Bone Joint Surg. 2000;82-B:1072–1076. doi: 10.1302/0301-620x.82b7.9986. [DOI] [PubMed] [Google Scholar]
- Whiston TB, Walmsley R. Some observations on the reaction of bone and tendon after tunnelling of bone and insertion of tendon. J Bone Joint Surg. 1960;42-B:377–386. doi: 10.1302/0301-620X.42B2.377. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Maynard J, Butler D, et al. Ligament, tendon, and joint capsule insertions to bone. In: Woo SL-Y, Buckwalter JA, editors. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopedic Surgeons; 1987. pp. 133–166. [Google Scholar]