Abstract
Current surgical treatment of spinal root injuries aims at reconnecting ventral roots to the spinal cord while severed dorsal roots are generally left untreated. Reactive changes in dorsal root ganglia (DRGs) and in injured dorsal roots after such complex lesions have not been analysed in detail. We studied dorsal root remnants and lesioned DRGs 6 months after C7 dorsal rhizotomy, ventral root avulsion and immediate ventral root replantation in adult rabbits. Replanted ventral roots were fixed to the spinal cord with fibrin glue only or with glue containing ciliary neurotrophic factor and/or brain-derived neurotrophic factor. Varying degrees of degeneration were observed in the deafferented dorsal spinal cord in all experimental groups. In cases with well-preserved morphology, small myelinated axons extended into central tissue protrusions at the dorsal root entry zone, suggesting sprouting of spinal neuron processes into the central dorsal root remnant. In lesioned DRGs, the density of neurons and myelinated axons was not significantly altered, but a slight decrease in the relative frequency of large neurons and an increase of small myelinated axons was noted (significant for axons). Unexpectedly, differences in the degree of these changes were found between control and neurotrophic factor-treated animals. Central axons of DRG neurons formed dorsal root stumps of considerable length which were attached to fibrous tissue surrounding the replanted ventral root. In cases where gaps were apparent in dorsal root sheaths, a subgroup of dorsal root axons entered this fibrous tissue. Continuity of sensory axons with the spinal cord was never observed. Some axons coursed ventrally in the direction of the spinal nerve. Although the animal model does not fully represent the situation in human plexus injuries, the present findings provide a basis for devising further experimental approaches in the treatment of combined motor/sensory root lesions.
Keywords: dorsal rhizotomy, dorsal root and ganglia changes, ventral root avulsion, ventral root replantation
Introduction
Traumatic brachial plexus injuries in human accident cases or patients with obstetric complications often affect ventral and dorsal roots of several spinal cord segments (Terzis et al. 1999; El Gammal & Fathi, 2002; Bae et al. 2003; Dunham, 2003; Kim et al. 2003). Treatment of motor deficits in patients, and in experimental models of root injuries, has been attempted by nerve transfer (Narakas, 1985; Fantis & Slezk, 1967) or surgical replantation of ventral rootlets (Carlstedt et al. 1995). Regeneration of motor axons into replanted ventral roots with some recovery of motor functions was observed in both patients and animal models (Cullheim et al. 1989; Hallin et al. 1999; Carlstedt et al. 2000).
By contrast, replantation of an injured dorsal root does not lead to spontaneous regeneration of central sensory axons into the spinal cord in animal models (e.g. Fraher, 1999; Ramer et al. 2002). This is due to at least two major problems: (1) an unfavourable glial environment at the peripheral–central nervous system (PNS-CNS) transition zone, caused by axon growth-inhibiting effects of a glial scar, predominantly composed of reactive astrocytes and microglia, and of oligodendrocytes and CNS myelin (Pesheva et al. 1989; Bandtlow et al. 1990; Schnell & Schwab, 1990); and (2) a lack of adequate signals that stimulate regrowth of central sensory processes and restoration of synapses. Recently, it was shown that application of neurotrophic factors may be a possible therapeutic approach to overcome these problems and to induce ingrowth of rhizotomized dorsal root axons into the CNS in animal models (Romero et al. 2001; Donnerer, 2003; Tang et al. 2004).
Although promising, these novel strategies are still in an experimental phase and the functional outcome is uncertain. Therefore, the treatment of root avulsions in clinical practice to date remains restricted to replantation of ventral roots to restore motor functions, while injured dorsal roots are usually left untouched (Carlstedt et al. 2000). Nevertheless, some patients who had undergone ventral root replantation regained sensory modalities (e.g. proprioception, temperature, pain) in avulsed dermatoms (Carlstedt et al. 2000). A conclusive explanation for this phenomenon is not at hand. It has been suggested that it may be caused by localized intraspinal sprouting of previously ‘silent’ spared fibres coursing through bordering adjacent rootlets (Darian-Smith, 2004), or by sprouting of dorsal horn neuronal axons along the replanted ventral root (Carlstedt et al. 2000). Ventral roots contain a population of thinly myelinated and unmyelinated axons deriving from sensory ganglia (Hildebrand et al. 1997; Schenker & Birch, 2000). Particularly if replantation is combined with neurotrophic factor application, regeneration of primary sensory axons within or along the replanted ventral root also appears theoretically possible.
The effect of isolated dorsal rhizotomy on dorsal root ganglia (DRGs) and dorsal root remnants has been analysed extensively (Cragg, 1970; Jenkins et al. 1993; Chong et al. 1996, 1999; Broude et al. 1997; Zhang et al. 2000; Aldskogius & Kozlova, 2002; Wallquist et al. 2004). In most animal models for ventral root avulsion and replantation, the dorsal roots were left intact (e.g. Cullheim et al. 1989; Carlstedt et al. 1993b; Risling et al. 1993; Hoffmann et al. 1996; Hallin et al. 1999; Bergerot et al. 2004) or the post-lesional morphology of dorsal roots and DRGs were not analysed (Chai et al. 2000; Gu et al. 2004, 2005).
We recently devised a model of brachial plexus injury and treatment in adult rabbits in which, unilaterally, ventral roots of segment C7 were avulsed, immediately replanted, and fixed to the spinal cord with fibrin glue, or with fibrin glue containing ciliary neurotrophic factor (CNTF) and/or brain-derived neurotrophic factor (BDNF) (Lang et al. 2005b). Segmental dorsal roots were severed close to the spinal cord, but were left untouched in the further operation procedure. Spinal cord morphology, neuron loss and pathways of myelinated axons that had regenerated from the spinal cord into the replanted ventral root 6 months after lesion were documented in a previous investigation: we found inter-individual differences of segment C7 damage, a ventral horn motoneuron survival rate of around 30%, and substantial regeneration of myelinated axons into the replanted ventral root, without significant differences between neurotrophic factor treated or non-treated experimental groups (Lang et al. 2005b). The present study was aimed at documenting in detail the morphology of dorsal root remnants and DRGs in our model.
Materials and methods
Operative approach
All animal experiments were carried out in accordance with German law on the protection of animals conforming to international ethical guidelines. A detailed description of the technical approach has been given in recent publications and is reported briefly here (Lang et al. 2005a,b): 28 female New Zealand white rabbits (2500 g) were operated in a dorsal approach exposing segment C7 after hemilaminectomy of the vertebra C6. C7 dorsal roots were severed close to the spinal cord and ventral roots were avulsed. Severed dorsal roots were left untreated. The ventral rootlets were replanted ventrolaterally to the spinal cord (Carlstedt et al. 1993a; Carlstedt, 2000). The replanted rootlets were fixed in position with 0.2 mL fibrin glue (Tissuecol Duo S, Baxter, Vienna, Austria) containing 10 µL CNTF (recombinant rat CNTF, 0.78 mg mL−1; n = 7), 10 µL BDNF (recombinant human BDNF, 1 mg mL−1; n = 7) or 10 µL each of both factors (n = 7). In the control group (n = 7), fibrin glue without additional factors was applied.
Two animals of the CNTF group suffered from postoperative circulation problems and had to be killed. All of the other animals except one of the BDNF group, which died 8 weeks after operation, recovered completely.
After 6 months, the remaining animals were anaesthetized, ventilated via tracheostomy and perfused transcardially using 4% paraformaldehyde and 0.2% glutaraldehyde in 0.1 m posphate buffer, pH 7.4.
Tissue preparation
Segments C6–C8 were dissected together with the spinal roots, DRGs and the proximal part of the spinal nerve. Both on the lesioned and on the unlesioned side, the DRGs and the adhering ventral roots of segment C7 were cut perpendicular to their long axes at DRG midlevel (Fig. 1a). The fluorescent tracer DiI (1,1′dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine-perchlorate) was applied as solitary crystals to the proximal section plane of the DRG/ventral roots. The crystals were fixed in position with a drop of 2% agar solution. The preparations were incubated in 4% buffered paraformaldehyde at 37 °C. After at least 12 months of incubation, the cervical segments with the DRGs and ventral roots on both sides were washed in PBS, transferred to 10 and 20% sucrose in PBS, placed in a drop of Tissue Freezing Medium (Leica Instruments, Nussloch, Germany), and frozen in liquid nitrogen-cooled isopentane.
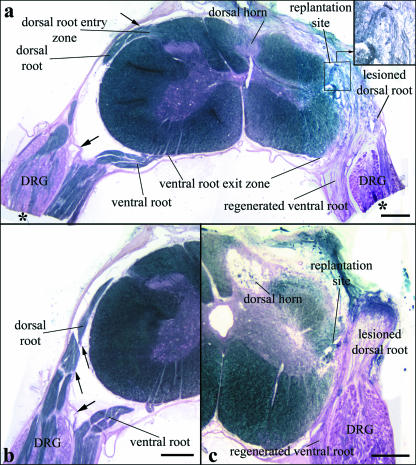
Fig. 1.
(a) Transverse section of segment C7 with attached dorsal and ventral roots and DRGs 6 months after operation displaying minimal alterations of the dorsal horn on the lesioned side (myelin sheath staining). The distal parts of the DRGs and adjacent ventral roots (asterisks) were embedded in Epon and used to prepare semithin sections for DRG morphological and morphometric analyses (see Materials and methods). The replantation site of the avulsed ventral root and the regenerated ventral root axons are clearly seen (inset). Loose fibrous tissue surrounds the replantation site and extends to the original dorsal root entry zone (DREZ). The dorsal root stump is attached to this fibrous tissue. (b) Morphology of C7 roots and their sheaths on the unlesioned sides. Ventral and dorsal roots are enveloped by thin sheaths, which are in continuity with the spinal cord pia, particularly clearly visible at the dorsal root (small arrows). A thicker sheath, resembling the dura, is interposed between the distal ventral root and the DRG and the dorsal root, separating the two roots completely (large arrows in a and b). (c) Section of C7 in an animal with severe degeneration of the dorsal horn. Scale bars: 500 µm.
Serial cryostat sections (30 µm) transverse to the cord were prepared from the C7 segments and thawed onto Superfrost™ (Menzel, Braunschweig, Germany) microscopic slides. These preparations included longitudinal sections of the proximal parts of the DRGs and ventral and dorsal roots on lesioned and unlesioned sides (Fig. 1). After mounting, the sections were observed via an Olympus BHS fluorescence microscope. Micrographs were taken from individual sections to document the fluorescent structures.
Every fourth section was processed using a recently described modified Klüver–Barrera staining technique for combined documentation of neuronal morphology and of myelinated axons (Geisler et al. 2002). In brief, the sections were dehydrated in 70% ethanol for 24 h, incubated overnight in prewarmed Luxol Fast Blue at 56 °C, washed in PBS, ethanol and in distilled water, and subsequently stained for 30 min in cresyl violet, dehydrated in an ascending series of alcohol and coverslipped in Entellan. The remaining sections were stained using Sudan black B and Kernecht red. This procedure produces black myelin staining. Cellular cytoplasm and nuclei are stained red. A detailed description of the staining method is given in Böck (1989).
The distal parts of the DRGs and adhering ventral roots were osmicated, dehydrated and embedded in Spurr's medium according to conventional methods. Transverse ∼1-µm semithin sections were prepared and stained with toluidine blue.
Quantitative analysis
Digital photomicrographs were taken with a Zeiss Axioskop equipped with a Spot digital camera (Visitron Systems, Puchheim, Germany) and analysed using Spot Advanced® software. For the analysis of numbers and areas of myelinated axons and of cell body sizes of sensory neurons in DRGs, toluidine-blue-stained transverse semithin sections of the distal parts of lesioned and unlesioned DRGs (see Fig. 1) were used. DRG sections were first observed at 1.25× magnification. At this magnification, neuron groups and axon bundles can be recognized (see also below), but morphological details cannot be discerned. At two randomly chosen positions within the outline of the DRG, magnification was increased to 20×, and micrographs were taken. These micrographs were non-overlapping, represented a total area of 145 000 µm2 of each DRG section, and always encompassed peripheral and central neuron DRG groups and intervening axon bundles (see below). Cell body areas of DRG neurons with recognizable nucleus and nucleolus were determined in the two micrographs. For the construction of histograms, the total number of neurons analysed in each DRG was taken as 100%, then size ranges of 500 µm2 were defined, and the percentage of neurons falling into these size ranges was calculated for each individual DRG. From the percentage values of each animal, mean values ± SEM were calculated for each size range (e.g. 0–500 µm2, 500–1000 µm2, etc.) in the different experimental groups.
For axon density determination, two regions of interest (ROIs; 2500 µm2) were defined within the axonal areas of each micrograph according to previous studies (Novikov et al. 1997; Geuna et al. 2001; Vogelin et al. 2006). All myelinated axons situated within these ROIs were counted according to established methods (Geuna et al. 2001), and cross-sectional areas of the axons (excluding their myelin sheaths) were measured. The number of myelinated axons in the total analysed area was determined for each DRG, and mean values of axon densities in axon bundles (numbers of myelinated axons per 5000 µm2) were calculated for each experimental group. Mean values of proportions of axons in different size ranges (bin size 5 µm2) were calculated from individual DRGs for each experimental group as described for neuron areas above, and histograms were constructed.
Neuron density in DRG neuron groups was analysed in one Klüver–Barrera-stained section from the midrostrocaudal level of the proximal part of each DRG. Again, the sections were observed at low magnification, and an area of 120 000 µm2 was delineated by positioning four non-overlapping ROIs (30 000 µm2 each) within the peripheral neuron groups. The number of neurons with visible nuclei in the ROIs was determined for each DRG, and mean values of neuron densities in neuron groups (numbers of neurons per 120 000 µm2) were calculated for each experimental group.
Statistical analysis was performed applying all pairwise multiple comparison procedures (Tukey test) using Sigma Stat® Software.
Micrographs of myelin-stained cryostat sections were used to document pathways of dorsal root axons. Axon diameters of myelinated axons were determined in the dorsal roots on unlesioned and in the regenerated dorsal root stumps on lesioned sides in at least two cases of each experimental group. Analysis of all experimental animals was not possible because in most cases the unlesioned dorsal roots formed extremely compact bundles, rendering it impossible to recognize individual axons in the sections. Data assessment for normality and significance of differences between lesioned and unlesioned sides was carried out by a Mann–Whitney rank sum test using Sigma Stat® Software.
Results
Postoperative alterations of segment C7 dorsal spinal cord and nerve roots
Lesion-induced alterations are illustrated in representative sections shown in Fig. 1. Whereas the unlesioned sides appeared completely unaffected (Fig. 1a,b), dorsal horns and dorsal root entry zones (DREZs) on the lesioned side showed inter-individually varying alterations, from almost normal layering of the dorsal horn and moderate changes of posterior, posterolateral and lateral tracts (Figs 1a and 2) to complete destruction and cyst formation of the dorsal horn and DREZ areas (Fig. 1c). These varying degrees of alterations were seen in all experimental groups. A comparatively well-preserved dorsal horn structure as represented in Figs 1(a) and 2 was found in 3/7 controls, in 3/5 CNTF-treated, in 4/6 BDNF-treated, and in 5/7 CNTF + BDNF-treated animals.
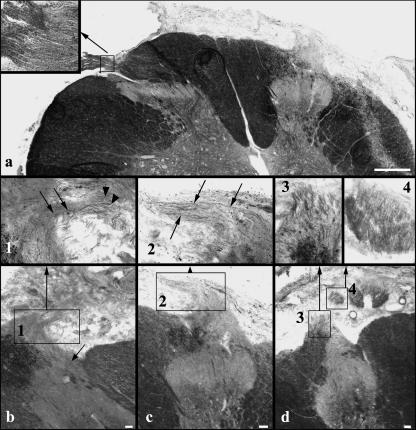
Fig. 2.
(a) C7 section showing the DREZ and entering dorsal root fibres on the unlesioned side (left; myelin sheath staining). On the lesioned side (right), a small conical tissue protrusion from the DREZ is visible. The inset shows the unlesioned central–peripheral transition zone at higher magnification. Note the more intensely stained peripheral myelin. (b–d) Deafferented dorsal horns and DREZs in different animals. Insets 1–4 are higher magnifications of the boxed areas in (b)–(d). (b) Thin fibres course from the dorsal horn into the tissue projection (arrows in the inset). The arrow in (b) points to fibres running from the medial part of the lateral tract into the tissue protrusion. Numerous myelinated axons without obvious continuity with the spinal cord are seen in the vicinity of the tissue protrusion (arrowheads inset 1). (c, inset 2) In some animals, the thin fibres extend for some distance within the tissue projection. (d, insets 3 and 4) At rostral and caudal segmental levels in some animals, myelinated axons passing through the DREZ are found. Although the bundle formed by these axons differs in morphology from dorsal root bundles on the unlesioned sides (cf. a), the axons display typical peripheral myelination characteristics. In close vicinity, in the subarachnoid space, cross-sections of axon bundles are found, which in serial sections can be seen to be sections through a bundle continuous with the DREZ. Scale bars: 500 µm in (a), 50 µm in (b)–(d).
Extraspinally, loose fibrous tissue surrounded the ventral root replantation site, extending to the original DREZ (Fig. 1a,c). Close to the spinal cord, myelinated axons were observed running from the region of the replantation site and from the original ventral exit zone into the replanted ventral root (Fig. 1a; cf. Lang et al. 2005b). In all cases, numerous Sudan-black-stained nerve fibres issued from the DRGs in a dorsal direction (Fig. 1a,c; see also below).
The dorsal root stumps were always closely attached to or even enveloped by the fibrous tissue surrounding the replantation site, but were always completely disconnected from the original DREZ, indicating complete intraoperative transection of the dorsal rootlets in segment C7 (Fig. 1a,c) (Carlstedt, 1997).
Alterations of the dorsal spinal cord were most extensive at midlevels of the lesioned segment, extending with decreasing severity to rostral and caudal segmental levels. In 14 animals (controls: three, CNTF-treated: three, BDNF-treated: two, CNTF + BDNF-treated: six), all but two with well-preserved dorsal horn structure, small conical tissue protrusions were observed issuing from the deafferented DREZ (Fig. 2a–c). Varying numbers of thin Sudan-black-stained fibres were found in these protrusions. In serial sections it appeared that many of these thin fibres were in continuity with deep layers of the dorsal horn, or with the medial part of the lateral tract (Fig. 2b). In some cases, the fibres appeared to extend quite far peripherally without turning back towards the DREZ (Fig. 2b,c). Also, numerous myelinated fibres were observed in the vicinity of the tissue protrusion. However, the origin of these fibres could not be determined (Fig. 2b, inset 1). A connection of fibres within the tissue protrusion with the peripheral dorsal root stump was never found (see above). At rostral and caudal segmental levels, numerous comparatively large myelinated fibres were found issuing from the lesioned DREZ in several animals, forming broad bundles in the subarachnoid space (Fig. 2d). These bundles displayed a distinctly different morphology from dorsal rootlet bundles on the unlesioned side (Fig. 1a,b and 2a), but their strongly Sudan-black-stained peripheral myelin sheath was similar (Fig. 2a,d).
C7 dorsal root ganglia (DRG)
Morphology
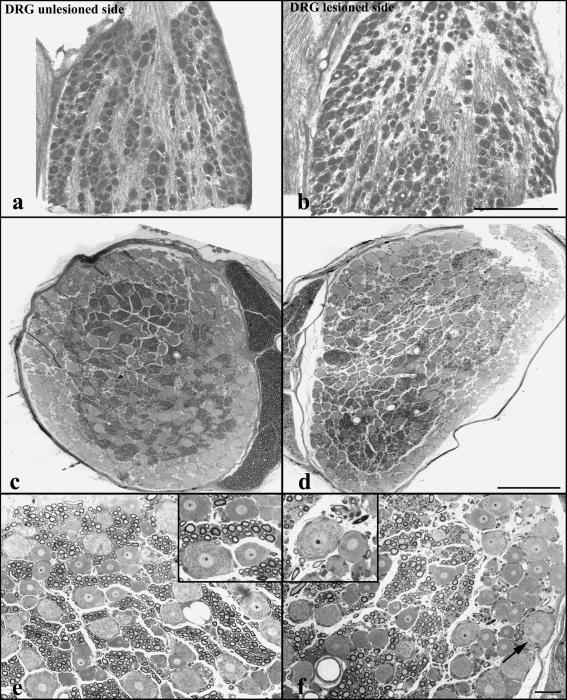
In unlesioned DRGs, neuronal perikarya of different sizes were localized peripherally, arranged in a compact layer three to four neurons deep without intervening cross-sectioned axons and little endoneurial connective tissue (Fig. 3a,c). Additionally, compact groups of neuronal perikarya separated by fibre tracts were localized in the central DRG (Fig. 3a,c). The arrangement of the neuron groups and fibre tracts was well recognizable but slightly less compact in most lesioned DRGs (Fig. 3b,d). Distorted or obviously degenerating neuronal or axonal profiles were rare. In all animals, pseudounipolar neurons on the lesioned side showed perfectly normal morphology with rounded cell bodies, centrally localized nuclei and distinct nucleoli, clearly recognizable Nissl bodies and axon origins (Fig. 3f). Non-myelinated small axons and myelinated axons of different sizes were observed in cross-sectioned axon tracts in semithin sections of DRGs of both sides (Fig. 3c–f). It appeared that in subregions of some lesioned DRGs their number was reduced and replaced by connective tissue, and that the size distribution of myelinated axons varied more than on the unlesioned sides (Fig. 3d,f).
Fig. 3.
Klüver–Barrera-stained cryostat (a,b) and semithin sections (c,f) of DRGs on unlesioned (a,c,e) and lesioned sides in control animals (b,d,f). The arrangement of neurons and fibre tracts appears little disturbed (b). (c–f) Semithin sections show comparatively unaltered arrangement and morphology of neurons in lesioned DRGs. The density and size of cross-sectioned myelinated axonal profiles appears somewhat reduced in subregions of some lesioned DRGs (d,f). The arrow in (f) points to an axon hillock identified by its lack of Nissl substance. Scale bars: 500 µm in (a)–(d); 50 µm in (e) and (f).
Quantitative investigations
The qualitative impression of changes in neuron and fibre arrangements in lesioned DRGs led us to study changes in lesioned DRGs in more detail using morphometric characteristics available in the present material. As the intimate contact of the peripheral dorsal root remnant with the scar tissue surrounding the replantation site indicated that injured sensory neurons may have had access to neurotrophic factors in the fibrin glue, comparisons were carried out both between results from all lesioned and unlesioned DRGs and between results from lesioned DRGs of animals of the different experimental groups.
Comparison of neuron densities determined in defined areas within DRG neuron groups showed no significant differences between all unlesioned DRGs and all lesioned DRGs (P = 0.16). No significant differences were found when data from all unlesioned DRGs were compared with data from lesioned DRGs of the different experimental groups, and when data from lesioned DRGs of the different experimental groups were compared (Table 1).
Table 1.
Quantitative analyses of neuron and axon densities and of the relative proportions of very small axons (≤ 5 µm2) in all unlesioned and lesioned DRGs and in lesioned DRGs of the different experimental groups (means ± SEM)
| Unlesioned sides | Lesioned sides | Control lesioned sides | CNTF-lesioned sides | BDNF-lesioned sides | CNTF + BDNF-lesioned sides | |
|---|---|---|---|---|---|---|
| Neuron density | 25.0 ± 1.0 | 22.2 ± 0.9 | 20.6 ± 1.2 | 24.0 ± 1.8 | 23.6 ± 2.8 | 21.6 ± 1.6 |
| Axon density/5000 µm2 | 31.4 ± 1.6 | 30.2 ± 1.8 | 33.6 ± 4.2 | 35.2 ± 4.2 | 26.8 ± 2.0 | 25.4 ± 1.8 |
| % axons ≤5 µm2 | 15.7 ± 2.1 | 25.7 ± 3.0* | 36.3 ± 2.4** | 33.5 ± 3.8+ | 16.2 ± 5.0++ | 16.1 ± 6.1# |
Neuron numbers are totals of counts in four ROIs (30 000 µm2each) in neuron groups for each DRG, and axon numbers were counted in two ROIs (2500 µm2each) in axon groups per DRG. Mean values of axon densities in axon bundles (numbers of myelinated axons/5000 µm2)were calculated for each experimental group. Significance levels for percentages of axons ≤ 5 µm2:
P = 0.003,
P =0.001
P =0.017 compared with all unlesioned DRGs;
P =0.015
P = 0.014 compared with control lesioned DRGs.
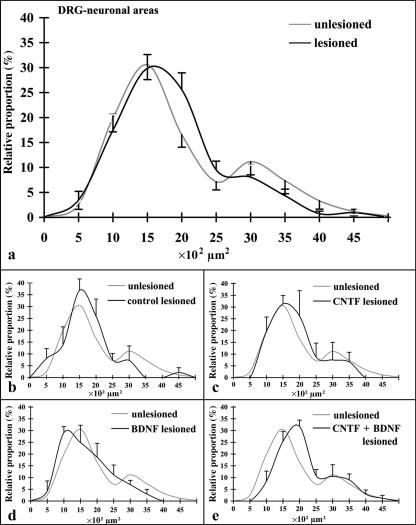
Cell body area histograms documented a large peak at approximately 1500 µm2, and a smaller, broad peak at approximately 3000 µm2, with a flat shoulder slope slowly decreasing towards larger areas in unlesioned DRGs (Fig. 4a). Area histograms derived from lesioned DRGs showed similar size distributions (Fig. 4a–e). Large neurons with perikaryal areas higher than 2500 µm2 constituted about 20% of all neurons in unlesioned (61 of 301 neurons analysed) and about 15.5% of neurons analysed in all lesioned DRGs (34 of 219 neurons). Analysis of area histograms from lesioned DRGs of the different treatment groups separately showed decreased relative proportions of large neurons in all but the CNTF + BDNF groups (Fig. 4). However, when data from individual animals were statistically analysed, the differences did not reach significance.
Fig. 4.
Histograms of relative proportions of sensory neuron cell body areas in all unlesioned and lesioned DRGs (a; means ± SEM) and of neurons in lesioned DRGs (black lines; means ± SEM) of controls (b), CNTF-treated (c), BDNF-treated (d), and CNTF + BDNF-treated animals (e). Light grey lines in (b)–(e): histogram derived from all unlesioned DRGs (means only; cf. a).
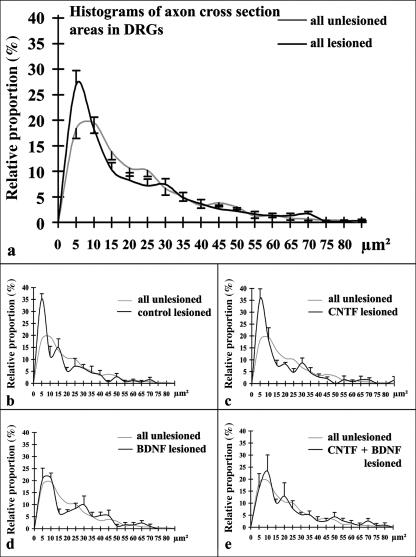
Despite the visual impression (see above), no significant side or group differences were noted with respect to DRG myelinated axon density (Table 1). Histograms of relative proportions of myelinated axon areas in unlesioned and lesioned DRGs indicated that the size distribution was similar (Fig. 5), with a first peak between 5 and 10 µm2, and a second smaller but broader peak between 20 and 30 µm2. The percentage of very small axons (areas up to 5 µm2) was significantly higher in lesioned than in unlesioned DRGs (Fig. 5, Table 1). When data from lesioned DRGs of the different experimental groups were compared with data from all unlesioned DRGs, the relative frequency of very small axons was significantly increased in the control and CNTF groups whereas in the BDNF and CNTF + BDNF groups it was only somewhat higher (Fig. 5, Table 1).
Fig. 5.
Histograms of relative proportions of axon cross-section areas in all unlesioned and lesioned DRGs (a; means ± SEM) and in lesioned DRGs (black lines; means ± SEM) of controls (b), CNTF-treated (c), BDNF-treated (d), and CNTF + BDNF-treated animals (e). Light grey lines in (b)–(e): histogram derived from all unlesioned DRGs (means only; cf. a). Only myelinated axons were analysed.
Dorsal root axons
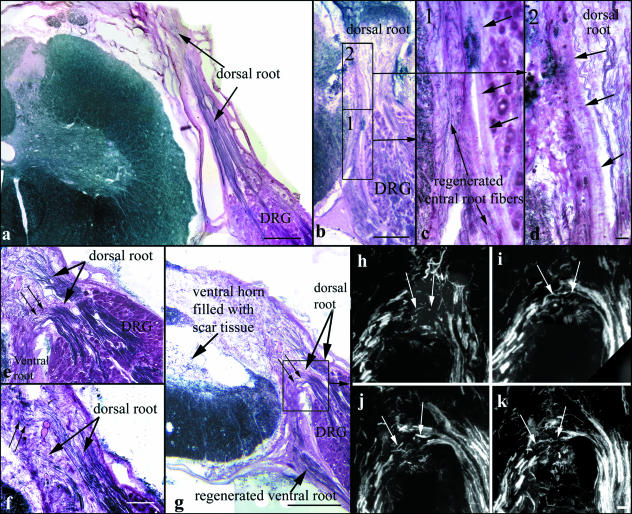
On the unlesioned sides, myelinated dorsal root axons were seen to course from the DRGs directly into the dorsal root, entering the spinal cord via the DREZ (Fig. 1a,b). Ventral and dorsal roots were completely separated throughout their course and were enveloped by thin but recognizable root sheaths, which were in continuity with the spinal cord pia as has been described previously (Kaar & Fraher, 1986). The proximal DRG and dorsal root origin were additionally separated from the ventral root by a sheath resembling the dura mater (Fig. 1a,b) as has been described for human thoracal spinal nerve roots (Scharf & Bargmann, 1958). Thus, a common radicular nerve containing both ventral and dorsal root fibres was not formed, and nerve fibres connecting ventral root and DRG or dorsal root were never observed (Fig. 1a,b).
On the lesioned sides, numerous myelinated axons were observed extending from the DRGs in a dorsal direction (Figs 1 and 6). In all cases analysed, diameters of myelinated axons in the lesioned dorsal root stumps were significantly reduced compared with those in the unlesioned dorsal roots (P = 0.001; Table 2). The myelinated axons exited the DRGs in several bundles throughout the rostrocaudal extent of the ganglion. In most animals, myelinated axons in the dorsal root stump reached considerable length, occasionally extending to the dorsal circumference of the spinal cord (Fig. 6a). The axons always appeared to end blindly, sometimes forming axonal swirls within the dorsal root sheaths, without regaining connectivity with the spinal cord (Figs 1a,c and 6a–d). The dorsal root stump formed by the axon bundles adhered to the replanted ventral root and the fibrous tissue surrounding it, but appeared for the most part to be separated from this tissue by a sheath resembling the dorsal root sheath on the unlesioned side (Fig. 6b–d). The replanted ventral root usually lacked a clear sheath up to its contact point with the DRG. There, a sheath resembling the dura separated lesioned DRGs and dorsal root origins from regenerated ventral roots (Fig. 6b,g). In fluorescence tracings, dorsal root axons could be followed for some distance (not shown). Again, continuity of fluorescent axons between the dorsal root stump and the spinal cord was not observed, and fluorescent axons were not found in the region of the DREZ or the dorsal horn.
Fig. 6.
(a) Myelin sheath staining shows substantial lengths of lesioned dorsal root axons in many animals. (b) The dorsal root stump is in close contact to the spinal cord at the replantation site. Higher magnification of the boxed areas 1 and 2 (c,d) shows that the dorsal root fibres are separated from the regenerated fibres within the replanted ventral root by clear sheaths (arrows in c,d). (e–g) Analysis of serial sections shows that in some animals dorsal root axons appear to course into the fibrous tissue surrounding the replantation site and replanted ventral root. The dorsal root sheath is not recognizable in this area. Some of these axons turn ventrally (small arrows in e,g) and appear to run distally with the replanted ventral root. Some axons do not turn ventrally (small arrows in f). None of the axons connects with the spinal cord. (h–k) Serial sections demonstrating fluorescence tracing results in the boxed area in (g). Small arrows point to fluorescent axons or axon parts apparently turning ventrally from the DRG into the ventral root. Scale bars: 500 µm in (a), (b) and (g), 50 µm in (c) and (d), 250 µm in (e), 150 µm in (f), 100 µm in (h)–(k).
Table 2.
Mean (± SEM) diameters of dorsal root axons (µm) of unlesioned and lesioned sides ± SEM in individual animals of the different experimental groups
| Animal | Diameter of dorsal root axons | |
|---|---|---|
| Unlesioned side | Lesioned side | |
| Control 1 | 3.9 ± 0.2 | 2.9 ± 0.1* |
| Control 3 | 5.2 ± 0.4 | 2.2 ± 0.1* |
| CNTF 1 | 5.6 ± 0.4 | 2.8 ± 0.2* |
| CNTF 3 | 5.4 ± 0.5 | 2.8 ± 0.1* |
| BDNF 1 | 6.3 ± 0.6 | 2.8 ± 0.7* |
| BDNF 3 | 4.6 ± 0.4 | 2.8 ± 0.1* |
| BDNF 4 | 6.0 ± 0.5 | 3.0 ± 0.2* |
| Combination 5 | 4.6 ± 0.4 | 2.4 ± 0.1* |
| Combination 7 | 3.7 ± 0.1 | 2.7 ± 0.1* |
P≤ 0.001 compared with the unlesioned side.
In 13 animals (three control, four CNTF-, two BDNF-and three CNTF + BDNF-treated animals), a subgroup of myelinated axons which had exited the DRG in a dorsal direction performed a sharp, medially directed turn, and some axons performed a complete u-turn to run in a ventral direction. In two cases with extensive intra-and extraspinal scarring, analyses of myelin-sheath stainings (Fig. 6e–g) and of fluorescent tracings in serial sections (Fig. 6h–k) suggested that dorsal root axons coursed into the fibrous tissue surrounding the replantation site at a point where a sheath was not clearly recognizable. Some of these axons appeared to run ventrally in the direction of the spinal nerve with the myelinated axons in the replanted ventral root (Fig. 6e,g–k). Others were observed to turn dorsally again in the fibrous tissue and could be followed for some distance running outside the spinal cord pia (Fig. 6f). Continuity of these axons into the spinal cord in the region of the replantation site or the DREZ was not observed.
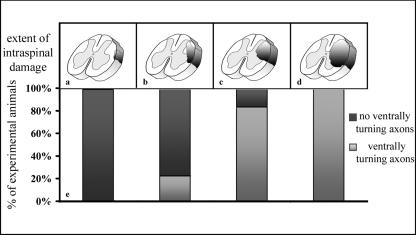
Ventrally turning axons were found in animals of all groups. The occurrence of ventrally turning dorsal root axons appeared to be correlated with the extent of spinal cord damage (Fig. 7): ventral turns were observed in 100% of the animals that showed very severe spinal cord alterations (degree 4 according to Lang et al. 2005b), in 83.3% of animals showing severe alterations (degree 3) and in 22.2% of the animals with moderate spinal cord alterations (degree 2). Ventrally turning dorsal root axons in animals showing light alterations (degree 1) were never observed.
Fig. 7.
Diagram illustrating a correlation between mild (a), moderate (b), severe (c) and very severe (d) spinal cord alterations and the proportion of cases with ventrally turning dorsal root axons in the different categories. For details see text.
Discussion
Analysis of lesioned DRGs and dorsal root remnants yielded insights into alterations and regeneration responses in sensory pathways after dorsal rhizotomy in an animal model for replantation treatment of ventral root avulsion injuries. The results document the presence of numerous myelinated nerve fibres in central and peripheral dorsal root remnants 6 months after operation. Slight alterations of DRG morphology on the lesioned side were observed which, unexpectedly, appeared to be affected by neurotrophic factors applied in the glue fixing the replanted ventral roots.
Spinal cord and central dorsal root remnants
Alterations of the lesioned dorsal horns varied between experimental animals. Different degrees of spinal cord alterations may be due to differences in the vascular supply of the segments and roots (Lang et al. 2005b), an anatomical variation well known in humans (Lasjaunias & Berenstein, 1990; Lang et al. 2005b). Differences in the extent of spinal cord injuries in human root lesion victims and in animal models caused by variations in vascularization have previously been suggested by Carlstedt et al. (2000) and should be taken into account when the results of treatments and animal experiments are interpreted.
Rhizotomy leads to degeneration of central and peripheral axon segments (Murray et al. 1990; Fraher, 1999). We found considerable regenerative responses in the central dorsal root remnant. Thin Sudan-black-stained fibres extended from the spinal cord far into conical tissue protrusions at the deafferented DREZs. Their straight course suggested that these fibres represented newly sprouted processes from spinal cord neurons rather than propriospinal axons taking a looping course through central tissue projections at the DREZ (Fraher, 1999; Carlstedt, 1997; Fraher, 2002). Carlstedt and colleagues induced outgrowth of dorsal horn neuron processes into a cut dorsal root re-implanted into the spinal cord in adult rats (Carlstedt, 1985; Carlstedt et al. 1991) and reported that these sprouts connected with pacinian receptors (Carlstedt, 1997). After dorsal root injury without replantation, i.e. without lesion to the spinal cord (crush or ganglionectomy), axons were absent from the central part of dorsal roots (Carlstedt, 1985, 1997; Carlstedt et al. 1989). In accordance with this, Smith et al. (1992) showed that mild spinal cord trauma was both necessary and sufficient to induce sprouting of fibres into a chronically denervated dorsal root. Thus, the spinal cord lesion induced by replantation of the ventral root in our model may well have induced sprouting reactions of intrinsic spinal neurons. Although some of these sprouts may have been directed towards the replanted ventral root (Carlstedt et al. 2000), our findings provide evidence that sprouting occurred into the central dorsal root remnant even without the availability of growth-promoting activity derived from peripheral nervous tissue at the former DREZ. Carlstedt (1997) proposed that sprouting reactions of secondary sensory neurons may be useful for attempts to re-establish afferent connectivity between the periphery and the spinal cord without the necessity for regeneration of primary sensory neurons. However, undirected sprouting may also lead to untoward excitation of spinal cord neurons.
One difficulty in interpreting apparent axonal regeneration through the dorsal root CNS–PNS transition zone arises from the existence of anastomoses between cut dorsal roots and rootlets of serially adjacent spinal nerves (Fraher, 1999). Our careful analyses of sections into the adjacent segments indeed showed that some axon bundles which appeared to connect the central dorsal root remnant with the periphery were most probably pre-existing undamaged axons of neighbouring intact dorsal roots.
DRGs and peripheral dorsal root stumps
When their peripheral processes are injured, DRG neurons show chromatolysis, alterations of their electrophysiological properties, and increased expression of the immediate early gene cJun and the growth-associated protein GAP43 (for a review, see (Lieberman, 1971; see also Himes & Tessler, 1989; Chong et al. 1994, 1996; Broude et al. 1997; Vestergaard et al. 1997; McKay et al. 2002; Ma et al. 2003)). This response is followed by robust regeneration of the peripheral process (Belyantseva & Lewin, 1999). If target re-innervation is not achieved, many neurons die (e.g. Karchewski et al. 2002). By contrast, disruption of their central processes does not affect the DRG neurons profoundly (Broude et al. 1997; Zhang et al. 2000; Wallquist et al. 2004), and significant neuronal degeneration was not noted even after prolonged survival periods (Cragg, 1970; Aldskogius & Kozlova, 2002). Axon regeneration into the Schwann cell environment of the distal dorsal root remnant appears to be limited (Jenkins et al. 1993; Broude et al. 1997; Aldskogius & Kozlova, 2002; Wallquist et al. 2004), but can be enhanced by providing growth-supporting matrices such as peripheral nerve or fetal spinal tissue grafts (Chong et al. 1996; Broude et al. 1997), or through a peripheral nerve injury (Chong et al. 1999). Electrophysiological changes after rhizotomy were restricted to large neurons (Ma et al. 2003), and expression of cJun and of the adhesion molecule CHL1 (close homologue of L1) was up-regulated only in small and medium-sized DRG neurons after fetal tissue grafting or dorsal root injury, respectively (Broude et al. 1997; Zhang et al. 2000). Moreover, medium/large sensory neurons, which express trkB, the specific high-affinity BDNF receptor, do not produce significant levels of BDNF under physiological conditions, but up-regulate their BDNF production after peripheral lesions (Michael et al. 1999; Zhou et al. 1999). This feature has been suggested to subserve a sustained autocrine survival function for these obviously particularly vulnerable sensory neuron subgroups (Karchewski et al. 2002).
Morphological alterations in DRGs 6 months after lesion were mainly restricted to a slight disarrangement of the fibre tracts and neuron groups. Neuronal morphology and neuronal density appeared unaltered within the DRG neuron groups. Thus, in this respect, our results confirm previous investigations demonstrating a comparative lack of effect of dorsal rhizotomy on DRG neurons. Our size distribution measurements yielded populations of small and medium-sized (cell body area up to 2500 µm2) and large DRG neurons (cell body areas >2500 µm2) of about 80 and 20%, respectively, on the unlesioned sides. The tendency towards a decreased relative proportion of large neurons on the lesioned sides indicates that these neurons may not only be more prone to respond acutely to central process injury as indicated previously (see above), but may also be subject to some degeneration or shrinkage after prolonged survival periods. This observation is supported by recent findings of selective vulnerability of large DRG neurons 30 and 60 days after rhizotomy (Guseva & Chelyshev, 2006).
Myelinated axons in the peripheral dorsal root stumps had a significantly smaller diameter than axons on the unlesioned sides in all animals analysed. As regenerated axons are reduced in size relative to the original axons (Morris et al. 1972a,b; Schroder, 1972), this indicates that the analysed axons represented regenerated or shrunken primary axons, and possibly reactive axon sprouts (e.g. Lekan et al. 1997). The size distribution measurements of myelinated axon areas within the DRGs documented significantly more very small axons in the lesioned than in the unlesioned ganglia. Because neuron and axon densities did not differ significantly between the unlesioned and lesioned DRGs, it is unlikely that intraganglionic sprouting of central or peripheral sensory processes caused the increase in relative proportions of very small axons in our material. Therefore, we propose that many small myelinated axons represented regenerated or shrunken central processes. A necessary prerequisite for this line of reasoning would be that the rhizotomy led to retrograde degeneration of the central process up to the level of the sensory neuron cell bodies, a supposition which needs to be tested in future studies.
Our morphological studies showed that the fibrin glue fixing replanted ventral roots to the spinal cord had apparently been replaced by loose fibrotic tissue after 6 months, as has been described for glue fixing dorsal roots to a lesion in the spinal cord (Iwaya et al. 1999). The peripheral dorsal root stumps were attached to the fibrous tissue, in some cases completely engulfed by it. Because this indicated that DRG neurons may have had access to the neurotrophic factors in the fibrin glue, we decided to investigate whether any differences were to be found between the lesioned DRGs in the different experimental groups. These analyses yielded some interesting observations, which are difficult to interpret at the moment. The decrease in the relative proportion of large neurons was absent in the CNTF + BDNF-treated group. Also, the proportion of very small axons was almost normal in the BDNF-and CNTF + BDNF-treated groups. Although it cannot be excluded that technical aspects influenced the data (e.g. interindividually varying degrees of contact of severed dorsal roots with the fibrin glue, slight differences in the proximo-distal position of the DRG transverse sectional level), the high level of significance at least of the differences in the axonal proportions indicates that these findings represent real effects of neurotrophic factors. Thus, availability of neurotrophic factors may have contributed to preventing death or shrinkage of large sensory neurons, and may have attenuated the extent of degeneration of central processes. These hypotheses need to be tested in experiments specifically designed to assess the effects of the neurotrophic factors on sensory neuron lesion and regeneration.
Pathways of axons in peripheral dorsal root stumps
Significant extension of myelinated axons from the lesioned DRGs was found in all cases. The axons were localized in what appeared to be the remnant of the original dorsal root. They usually formed several distinct bundles, and sometimes reached substantial length. Enhanced regeneration of central sensory axons is observed after a preconditioning injury of peripheral processes (see above). In our model, the motoric denervation may have led to altered afferent activity, for instance of proprioreceptor neurons in the rhizotomized DRG, supporting regeneration.
A surprising finding was that some dorsal root axons coursed medially and ventrally after exiting the lesioned DRG. This was found in animals of all groups, indicating that neurotrophic factor treatment had no influence. However, it was more commonly observed in animals in which spinal cord damage was particularly pronounced. In two cases with extensive scarring, it appeared that dorsal root axons crossed through gaps in the dorsal root sheath into the fibrous tissue surrounding the replanted ventral root. None of these axons was observed to connect with the spinal cord, but some joined regenerated fibres in the replanted ventral root, extending towards the spinal nerve. One possible explanation for the described findings is that differences in vascularization and in the subsequent injury response in general may affect both the spinal cord integrity, the involvement of the severed dorsal root remnant in the extraspinal scarring, the closure of the severed root sheaths and the subsequent axon course.
Thus, we have no evidence that regenerating dorsal root axons are able to grow into the spinal cord along the replanted ventral rootlet bundles. However, we also suggest that, under particular circumstances, regenerating dorsal root axons may be able to enter the replanted ventral roots and may occupy ventral root conduits to grow out in a distal direction. Thus, if reconnecting a severed dorsal root is not intended, it may be advisable to resect the DRG when replantation of an avulsed ventral root is carried out to prevent misdirected growth of dorsal root axons.
In conclusion, 6 months after dorsal rhizotomy, ventral root avulsion and replantation, thin myelinated fibres were found extending from the spinal cord into the central dorsal root remnants, indicating that the spinal cord injury in our model induces a sprouting reaction of spinal neurons toward the periphery. Numerous myelinated fibres were also observed extending from the lesioned DRGs into the peripheral dorsal root stump. In some cases, subgroups of these axons appeared to have entered fibrous tissue surrounding replanted ventral roots, but re-establishment of connectivity with the spinal cord was not observed. In accordance with findings reported after isolated dorsal rhizotomy, DRG neuron morphology was comparatively unaffected in our model. Quantitative analyses of lesioned DRGs indicated a slight decrease in the proportion of large neurons and a significant increase in the proportion of intraganglionic small-calibre myelinated axons, presumably lesioned central sensory neuron processes. These changes appeared to have been attenuated by neurotrophic factors, particularly BDNF, applied to the replantation site in our experimental model. The results of the present study provide novel information about sensory pathway reactions to rhizotomy in the context of replantation treatment of avulsed ventral roots. Although the animal model does not fully represent the situation in human plexus avulsion injuries, the findings may contribute to the improvement of future strategies for experimental therapeutic approaches in this clinically important field.
Acknowledgments
We are indebted to Rita Herrmann, Karin Reinfurt-Gehm and Sieglinde Schenk for excellent technical support and Dr Jürgen Peters for his advice concerning statistics. We are grateful to Professor M. Sendtner, Institute of Clinical Neurobiology, Julius-Maximilians-University, Würzburg, and to other members of Sonderforschungsbereich (SFB) 581 for useful advice and critical comments. This project was granted from the BMBF (Bundesministerium für Bildung und Forschung) and supported by the Deutsche Forschungsgemeinschaft (SFB 581, Z3).
References
- Aldskogius H, Kozlova EN. Strategies for repair of the deafferented spinal cord. Brain Res Brain Res Rev. 2002;40:301–308. doi: 10.1016/s0165-0173(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Bae DS, Waters PM, Zurakowski D. Reliability of three classification systems measuring active motion in brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85-A:1733–1738. doi: 10.2106/00004623-200309000-00012. [DOI] [PubMed] [Google Scholar]
- Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–3848. doi: 10.1523/JNEUROSCI.10-12-03837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur J Neurosci. 1999;11:457–468. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Bergerot A, Shortland PJ, Anand P, Hunt SP, Carlstedt T. Co-treatment with riluzole and GDNF is necessary for functional recovery after ventral root avulsion injury. Exp Neurol. 2004;187:359–366. doi: 10.1016/j.expneurol.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Böck P. Romeis Mikroskopische Technik. München: Urban und Schwarzenberg; 1989. [Google Scholar]
- Broude E, McAtee M, Kelley MS, Bregman BS. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp Neurol. 1997;148:367–377. doi: 10.1006/exnr.1997.6665. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Dorsal root innervation of spinal cord neurons after dorsal root implantation into the spinal cord of adult rats. Neurosci Lett. 1985;55:343–348. doi: 10.1016/0304-3940(85)90459-8. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Cullheim S, Risling M, Ulfhake B. Nerve fibre regeneration across the PNS–CNS interface at the root–spinal cord junction. Brain Res Bull. 1989;22:93–102. doi: 10.1016/0361-9230(89)90133-0. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Aldskogius H, Hallin RG, Nilsson-Remahl I. Novel surgical strategies to correct neural deficits following experimental spinal nerve root lesions. Brain Res Bull. 1993a;30:447–451. doi: 10.1016/0361-9230(93)90277-i. [DOI] [PubMed] [Google Scholar]
- Carlstedt TP, Hallin RG, Hedstrom KG, Nilsson-Remahl IA. Functional recovery in primates with brachial plexus injury after spinal cord implantation of avulsed ventral roots. J Neurol Neurosurg Psychiatry. 1993b;56:649–654. doi: 10.1136/jnnp.56.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt T. Nerve fibre regeneration across the peripheral–central transitional zone. J Anat. 1997;190:51–56. doi: 10.1046/j.1469-7580.1997.19010051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt T, Aldskogius H, Rosario CM. Extension of dorsal horn neurons into the severed and implanted dorsal root. Restorative Neurol Neurosci. 1991;3:205–209. doi: 10.3233/RNN-1991-3405. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Grane P, Hallin RG, Noren G. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346:1323–1325. doi: 10.1016/s0140-6736(95)92342-x. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Approaches permitting and enhancing motoneuron regeneration after spinal cord, ventral root, plexus and peripheral nerve injuries. Curr Opin Neurol. 2000;13:683–686. doi: 10.1097/00019052-200012000-00012. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Anand P, Hallin R, Misra PV, Noren G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237–247. doi: 10.3171/spi.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- Chai H, Wu W, So KF, Yip HK. Survival and regeneration of motoneurons in adult rats by reimplantation of ventral root following spinal root avulsion. Neuroreport. 2000;11:1249–1252. doi: 10.1097/00001756-200004270-00021. [DOI] [PubMed] [Google Scholar]
- Chong MS, Reynolds ML, Irwin N, Coggeshall RE, Emson PC, Benowitz LI, Woolf CJ. GAP-43 expression in primary sensory neurons following central axotomy. J Neurosci. 1994;14:4375–4384. doi: 10.1523/JNEUROSCI.14-07-04375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MS, Woolf CJ, Turmaine M, Emson PC, Anderson PN. Intrinsic versus extrinsic factors in determining the regeneration of the central processes of rat dorsal root ganglion neurons: the influence of a peripheral nerve graft. J Comp Neurol. 1996;370:97–104. doi: 10.1002/(SICI)1096-9861(19960617)370:1<97::AID-CNE9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chong MS, Woolf CJ, Haque NS, Anderson PN. Axonal regeneration from injured dorsal roots into the spinal cord of adult rats. J Comp Neurol. 1999;410:42–54. [PubMed] [Google Scholar]
- Cragg BG. What is the signal for chromatolysis? Brain Res. 1970;23:1–21. doi: 10.1016/0006-8993(70)90345-8. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Carlstedt T, Linda H, Risling M, Ulfhake B. Motoneurons reinnervate skeletal muscle after ventral root implantation into the spinal cord of the cat. Neuroscience. 1989;29:725–733. doi: 10.1016/0306-4522(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Primary afferent terminal sprouting after a cervical dorsal rootlet section in the macaque monkey. J Comp Neurol. 2004;470:134–150. doi: 10.1002/cne.11030. [DOI] [PubMed] [Google Scholar]
- Donnerer J. Regeneration of primary sensory neurons. Pharmacology. 2003;67:169–181. doi: 10.1159/000068405. [DOI] [PubMed] [Google Scholar]
- Dunham EA. Obstetrical brachial plexus palsy. Orthop Nurs. 2003;22:106–116. doi: 10.1097/00006416-200303000-00007. [DOI] [PubMed] [Google Scholar]
- El Gammal TA, Fathi NA. Outcomes of surgical treatment of brachial plexus injuries using nerve grafting and nerve transfers. J Reconstr Microsurg. 2002;18:7–15. doi: 10.1055/s-2002-19703. [DOI] [PubMed] [Google Scholar]
- Fantis A, Slezk Z. [On the surgical management of brachial plexus injuries] Acta Chir Orthop Traumatol Cech. 1967;34:301–309. [PubMed] [Google Scholar]
- Fraher JP. The transitional zone and CNS regeneration. J Anat. 1999;194:161–182. doi: 10.1046/j.1469-7580.1999.19420161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher J. Axons and glial interfaces: ultrastructural studies. J Anat. 2002;200:415–430. doi: 10.1046/j.1469-7580.2002.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Heilmann H, Veh RW. An optimized method for simultaneous demonstration of neurons and myelinated fiber tracts for delineation of individual trunco-and palliothalamic nuclei in the mammalian brain. Histochem Cell Biol. 2002;117:69–79. doi: 10.1007/s00418-001-0357-z. [DOI] [PubMed] [Google Scholar]
- Geuna S, Tos P, Guglielmone R, Battiston B, Giacobini-Robecchi MG. Methodological issues in size estimation of myelinated nerve fibers in peripheral nerves. Anat Embryol (Berl) 2001;204:1–10. doi: 10.1007/s004290100188. [DOI] [PubMed] [Google Scholar]
- Gu HY, Chai H, Zhang JY, et al. Survival, regeneration and functional recovery of motoneurons in adult rats by reimplantation of ventral root following spinal root avulsion. Eur J Neurosci. 2004;19:2123–2131. doi: 10.1111/j.0953-816X.2004.03295.x. [DOI] [PubMed] [Google Scholar]
- Gu HY, Chai H, Zhang JY, et al. Survival, regeneration and functional recovery of motoneurons after delayed reimplantation of avulsed spinal root in adult rat. Exp Neurol. 2005;192:89–99. doi: 10.1016/j.expneurol.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Guseva D, Chelyshev Y. The plasticity of the DRG neurons belonging to different subpopulations after dorsal rhizotomy. Cell Mol Neurobiol. doi: 10.1007/s10571-006-9005-4. 10.1107/s10571-006-9005-4. [DOI] [PubMed] [Google Scholar]
- Hallin RG, Carlstedt T, Nilsson-Remahl I, Risling M. Spinal cord implantation of avulsed ventral roots in primates; correlation between restored motor function and morphology. Exp Brain Res. 1999;124:304–310. doi: 10.1007/s002210050627. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Karlsson M, Risling M. Ganglionic axons in motor roots and pia mater. Prog Neurobiol. 1997;51:89–128. doi: 10.1016/s0301-0082(96)00052-4. [DOI] [PubMed] [Google Scholar]
- Himes BT, Tessler A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J Comp Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- Hoffmann CF, Marani E, van Dijk JG, vd Kamp W, Thomeer RT. Reinnervation of avulsed and reimplanted ventral rootlets in the cervical spinal cord of the cat. J Neurosurg. 1996;84:234–243. doi: 10.3171/jns.1996.84.2.0234. [DOI] [PubMed] [Google Scholar]
- Iwaya K, Mizoi K, Tessler A, Itoh Y. Neurotrophic agents in fibrin glue mediate adult dorsal root regeneration into spinal cord. Neurosurgery. 1999;44:589–595. doi: 10.1097/00006123-199903000-00085. [DOI] [PubMed] [Google Scholar]
- Jenkins R, McMahon SB, Bond AB, Hunt SP. Expression of c-Jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact primary sensory neurons in the rat. Eur J Neurosci. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Kaar GF, Fraher JP. The sheaths surrounding the attachments of rat lumbar ventral roots to the spinal cord: a light and electron microscopical study. J Anat. 1986;148:137–146. [PMC free article] [PubMed] [Google Scholar]
- Karchewski LA, Gratto KA, Wetmore C, Verge VM. Dynamic patterns of BDNF expression in injured sensory neurons: differential modulation by NGF and NT-3. Eur J Neurosci. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Cho YJ, Tiel RL, Kline DG. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003;98:1005–1016. doi: 10.3171/jns.2003.98.5.1005. [DOI] [PubMed] [Google Scholar]
- Lang EM, Asan E, Plesnila N, Hofmann GO, Sendtner M. Motoneuron survival after C7 nerve root avulsion and replantation in the adult rabbit: effects of local ciliary neurotrophic factor and brain-derived neurotrophic factor application. Plast Reconstr Surg. 2005a;115:2042–2050. doi: 10.1097/01.prs.0000163328.51271.dd. [DOI] [PubMed] [Google Scholar]
- Lang EM, Schlegel N, Sendtner M, Asan E. Effects of root replantation and neurotrophic factor treatment on long-term motoneuron survival and axonal regeneration after C7 spinal root avulsion. Exp Neurol. 2005b;194:341–354. doi: 10.1016/j.expneurol.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Lasjaunias P, Berenstein A. Surgical Neuroangiography, Functional Vascular Anatomy of Brain, Spinal Cord and Spine. Vol. 3. Berlin: Springer; 1990. [Google Scholar]
- Lekan HA, Chung K, Yoon YW, Chung JM, Coggeshall RE. Loss of dorsal root ganglion cells concomitant with dorsal root axon sprouting following segmental nerve lesions. Neuroscience. 1997;81:527–534. doi: 10.1016/s0306-4522(97)00173-5. [DOI] [PubMed] [Google Scholar]
- Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- McKay HA, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat. timecourse of cell death and elimination. Exp Brain Res. 2002;142:308–318. doi: 10.1007/s00221-001-0929-0. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Averill S, Shortland PJ, Yan Q, Priestley JV. Axotomy results in major changes in BDNF expression by dorsal root ganglion cells. BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur J Neurosci. 1999;11:3539–3551. doi: 10.1046/j.1460-9568.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- Morris JH, Hudson AR, Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. I. The traumatic degeneration of myelin in the proximal stump of the divided nerve. Z Zellforsch Mikrosk Anat. 1972a;124:76–102. [PubMed] [Google Scholar]
- Morris JH, Hudson AR, Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the ‘regenerating unit’. Z Zellforsch Mikrosk Anat. 1972b;124:103–130. [PubMed] [Google Scholar]
- Murray M, Wang SD, Goldberger ME, Levitt P. Modification of astrocytes in the spinal cord following dorsal root or peripheral nerve lesions. Exp Neurol. 1990;110:248–257. doi: 10.1016/0014-4886(90)90036-r. [DOI] [PubMed] [Google Scholar]
- Narakas AO. The treatment of brachial plexus injuries. Int Orthop. 1985;9:29–36. doi: 10.1007/BF00267034. [DOI] [PubMed] [Google Scholar]
- Novikov L, Novikova L, Kellerth JO. Brain-derived neurotrophic factor promotes axonal regeneration and long-term survival of adult rat spinal motoneurons in vivo. Neuroscience. 1997;79:765–774. doi: 10.1016/s0306-4522(96)00665-3. [DOI] [PubMed] [Google Scholar]
- Pesheva P, Spiess E, Schachner M. J1–160 and J1–180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol. 1989;109:1765–1778. doi: 10.1083/jcb.109.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Bishop T, Dockery P, et al. Neurotrophin-3-mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol Cell Neurosci. 2002;19:239–249. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- Risling M, Fried K, Linda H, Carlstedt T, Cullheim S. Regrowth of motor axons following spinal cord lesions: distribution of laminin and collagen in the CNS scar tissue. Brain Res Bull. 1993;30:405–414. doi: 10.1016/0361-9230(93)90272-d. [DOI] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf JH, Bargmann W. Handbuch der Mikroskopischen Anatomie des Menschen. Berlin: Springer; 1958. Sensible Ganglien; pp. 117–120. [Google Scholar]
- Schenker M, Birch R. Intact myelinated fibres in biopsies of ventral spinal roots after preganglionic traction injury to the brachial plexus. A proof that Sherrington's ‘wrong way afferents’ exist in man? J Anat. 2000;197:383–391. doi: 10.1046/j.1469-7580.2000.19730383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schroder JM. Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res. 1972;45:49–65. doi: 10.1016/0006-8993(72)90215-6. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kodama RT, Liuzzi FJ. Is CNS trauma a prerequisite for the elongation of CNS axons into denervated peripheral nerve? Brain Res. 1992;575:79–85. doi: 10.1016/0006-8993(92)90426-a. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Cai J, Nelson KD, Peng XJ, Smith GM. Functional repair after dorsal root rhizotomy using nerve conduits and neurotrophic molecules. Eur J Neurosci. 2004;20:1211–1218. doi: 10.1111/j.1460-9568.2004.03595.x. [DOI] [PubMed] [Google Scholar]
- Terzis JK, Vekris MD, Soucacos PN. Outcomes of brachial plexus reconstruction in 204 patients with devastating paralysis. Plast Reconstr Surg. 1999;104:1221–1240. doi: 10.1097/00006534-199910000-00001. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Tandrup T, Jakobsen J. Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol. 1997;388:307–312. [PubMed] [Google Scholar]
- Vogelin E, Baker JM, Gates J, Dixit V, Constantinescu MA, Jones NF. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp Neurol. 2006;199:348–353. doi: 10.1016/j.expneurol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Zelano J, Plantman S, Kaufman SJ, Cullheim S, Hammarberg H. Dorsal root ganglion neurons up-regulate the expression of laminin-associated integrins after peripheral but not central axotomy. J Comp Neurol. 2004;480:162–169. doi: 10.1002/cne.20345. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Roslan R, Lang D, Schachner M, Lieberman AR, Anderson PN. Expression of CHL1 and L1 by neurons and glia following sciatic nerve and dorsal root injury. Mol Cell Neurosci. 2000;16:71–86. doi: 10.1006/mcne.2000.0852. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Chie ET, Deng YS, et al. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92:841–853. doi: 10.1016/s0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]