Abstract
Nucleus pulposus cells of the intervertebral disc have no endogenous vasculature and have thus been hypothesized to be hypoxic. This hypothesis was tested using 2-nitroimidazole, EF5, a drug that at low oxygen concentrations forms covalent adducts with cellular proteins. After administrating EF5 to rats, sections of the intervertebral disc were analysed for EF5 adducts. Drug adducts were quantified in tissue sections using a fluorescent monoclonal antibody. Although the level of EF5 fluorescence in all intervertebral disc tissues was low, the transition zone at the periphery of the nucleus pulposus exhibited the highest level of EF5 binding. To substantiate this result, tissue nitroreductase levels and drug pharmacology were evaluated. Nitroreductase levels were measured in whole discs under severe hypoxia. We noted that there was robust EF5 binding to cells in the annulus fibrosus and transition zone with modest binding to cells of the nucleus pulposus and endplate. High-performance liquid chromatography analysis indicated limitations in EF5 access to the nucleus pulposus, most probably related to the lack of vasculature and slow drug distribution through the gel-like interior of the disc. However, despite diffusion problems, the drug dose was determined to be sufficient to report the oxygen status of the nucleus pulposus cells. Based on these findings, we conclude that despite poor vascularization, the disc cells accommodate to the local environment by displaying a limited need for oxygen. Accordingly, the cells of the intervertebral disc are not severely hypoxic.
Keywords: EF5, hypoxia, intervertebral disc, non-invasive, nucleus pulposus, oxygen utilization, oxygen
Introduction
The human spine comprises 24 vertebrae, each separated by a complex non-mineralized intervertebral disc. The central core of the disc space is occupied by a gel-like structure, the nucleus pulposus (NP). Cells in this tissue secrete aggrecan, a proteoglycan that provides the disc with elasticity while at the same time cushioning applied loads. Surrounding the NP is a ligamentous structure, the annulus fibrosus (AF). The outermost fibres of the AF are attached to the non-vascularized cartilaginous endplates (EP) of the individual vertebra. Because the inner AF and the NP have limited and no vascularity, respectively, cells in both regions have been assumed to be hypoxic (Urban et al. 1977; Guiot & Fessler, 2000; Fogelholm & Alho, 2001; Freemont et al. 2002; Martin et al. 2002). It has been suggested that the limited availability of oxygen to cells in the AF and NP could cause degenerative disc disease (Guiot & Fessler, 2000; Fogelholm & Alho, 2001) and that this degenerative state is exacerbated by smoking, possibly due to the effects of nicotine on the disc vasculature (Guiot & Fessler, 2000; Fogelholm & Alho, 2001; Iwahashi et al. 2002). Other factors associated with disc degeneration include supra-physiological loading of the spine (Hutton et al. 2000; Rannou et al. 2004), vascular disease (Guiot & Fessler, 2000; Fogelholm & Alho, 2001) and calcification of the endplate cartilage (Guiot & Fessler, 2000). Each of these conditions could influence the availability of oxygen to the cells of the NP.
Determination of the oxygen status of the cells populating the intervertebral disc is difficult. Although several invasive probe studies have indicated that disc cells experience an extremely low oxygen tension, the unique structure of the disc makes the use of such sensors problematic (Baumgartl et al. 1997; Jenkins et al. 2000). In this study, we use the biochemical probe 2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3,-pentafluoropropyl) acetamide (EF5), to assess the oxaemic status of the disc tissues. We show that the transition cells exhibit prominent EF5 binding in comparison with the other disc regions and that the NP cells have negligible EF5 binding, suggesting that they are not oxygen-constrained, despite a limited vascular supply.
Materials and methods
Animals and tissue preparation
Eight-to 12-week-old male Fischer or Wistar rats were used for all investigations using a protocol approved by the Institutional Animal Care and Use Committees of Thomas Jefferson University. Rats were anaesthetized with 2% isoflurane. EF5 was administered as a 10 mm solution (in 5% dextrose with 2.4% alcohol) in a volume that corresponded to 1% of the rat weight (Shapiro et al. 1997) at time ‘zero’ by tail vein injection and again at 3 h intraperitoneally. After 6 h, the animals were killed via a cardiac injection of potassium chloride. Lumbar intervertebral discs, blood, liver, spleen, prostate and muscle were harvested at 4 °C from four animals.
Immunohistochemical analysis of EF5 binding to cells of the intervertebral disc
Disc, liver, spleen and muscle tissues were cooled to 4 °C, embedded in OCT and frozen at −80 °C. Frozen tissues were sectioned using a cryostat (Meyers Instruments, Houston, TX, USA) set at a thickness of 10 µm. Transverse and longitudinal disc sections were adhered to poly-l-lysine-coated microscope slides and allowed to air dry for 30 min. The disc sections were fixed in acetone for 5 min and air dried for a minimum of 30 min; sections of the liver, spleen and muscle were fixed with 4% paraformaldehyde for 60 min, followed by two 10-min rinses in PBS (Shapiro et al. 1997; Koch, 2002). The procedures for staining, imaging and analysing tissues for EF5 have been previously reported (Jenkins et al. 2000; Koch, 2002). Briefly, the sections were blocked in PBS containing 0.3% Tween 20, 1.1% albumin, 20% non-fat milk and 5% mouse serum overnight at 4 °C, rinsed once in 0.3% Tween 20 in PBS and incubated for 4–6 h at 4 °C in a solution containing Cy3-conjugated ELK3-51 (anti-EF5 antibody; 75 µg mL−1) (Shapiro et al. 1997). As a control for non-specific binding, representative sections (competed stain or ‘CS’) were stained with ELK3-51 antibody containing 0.5 mm authentic EF5 (Koch, 2002). A no-stain control was used to monitor autofluorescence (none found). Sections were rinsed twice in 0.3% Tween 20 in PBS for 45 min each and once in PBS alone for 45 min. For the CS sections, the first two rinsing solutions also contained 0.25 mm EF5 in PBS. All slides were stored at 4 °C in PBS with 1% paraformaldehyde, which stabilizes the antibody and its fluorochrome and photographed within 2 weeks (Shapiro et al. 1997; Koch, 2002). Additional controls included sections of tissues from animals that had not been injected with EF5. Individual cells were localized by counterstaining their DNA using Hoechst 33342 (20 µm for 5 min). Stained slides were stored in PBS before and during photography. EF5 and DNA fluorescence was recorded using a Nikon fluorescence microscope with filters appropriate for Hoechst and Cy3 and a cooled Photometrics digital camera. To quantify the optics, a solution of Cy3 dye in 1% paraformaldehyde was diluted to an Abs549 = 1.25, added to a haemocytometer and photographed (Koch, 2002). Tissue area was quantified using Imagepro Plus 5.0 software (Media Cybernetics, Newburyport, MA, USA). Brightness values for the Cy3 fluorescence images were converted from 12-bit to 8-bit greyscale images. Pixel brightness above a typical background tolerance of 20 was selected for analysis. Fluorescence values of the image were converted to an absolute scale by accounting for the dye standard intensity and relative camera exposure times: absolute fluorescence = 1000 × (image fluorescence/standard fluorescence) × (exposure time of the standard/exposure time of the image). Image analysis was performed on tissue sections (spleen/liver, n = 3; AF, n = 8; EP, n = 5; NP, n = 8) and normalized to their corresponding EF5 binding brightness when tissues were cultured with 200 µm EF5 in 1% oxygen (n = 3 for each tissue type).
Drug metabolism and diffusion of EF5 into the disc
EF5 diffusion into the disc
For constant oxygen level, EF5 binding is proportional to the product of tissue nitroreductase activity, drug concentration and time (Koch, 2002). Because the intervertebral disc is a poorly vascularized and the AF is a highly ligamentous structure, it was necessary to verify that there was adequate diffusion of the drug into the tissue. As described above, animals were injected with EF5 and the drug was allowed to circulate. At times of interest, the level of isoflurane was increased to 5% and the rat was immediately killed via an intracardiac injection of 0.5 mL 1 m potassium chloride. The animal was cooled on ice and various tissues (disc, muscle, liver, spleen, prostate and blood) were quickly removed and mixed with an equal volume of ice-cold 10% trichloroacetic acid. The tissues were minced, and the suspension centrifuged. The supernatant was analysed for EF5 by high-performance liquid chromatography (HPLC) (Shapiro et al. 1997). This was performed using a reversed-phase C18 column: the mobile phase was 45% methanol, 100 mm ammonium phosphate, pH 4.5. The absorbance of the eluted EF5 was measured at 325 nm and compared with appropriate standards.
Confirmation that disc cells bind EF5
To ascertain that the nitroreductase activity of the disc cells can reduce EF5, two types of controls were performed. First, animals were injected with EF5 and then killed. This experiment was based on the assumption that, following death, as the tissue becomes hypoxic, the cells continue to metabolize EF5. To permit the tissues to take up and metabolize the EF5, and to allow the distributed EF5 to bind to macromolecules in the resident cells, the body was maintained at 37 °C for 40 min; this control was termed the ‘euthanized animal control’. Samples were then collected from the disc and other tissues (spleen, liver and prostate). Tissues were analysed for EF5 adducts using immunohistochemistry (controls not shown) and HPLC was performed as described above. The second control was ‘cube reference binding’. Whole intervertebral discs were obtained from animals that had not been injected with EF5. In this control, it was assumed that the metabolism of the excised tissue was not affected by its transition from in vivo to in vitro conditions and that drug access was not an issue. Although this assumption is reasonable for many homogeneous soft tissues, it was important to ensure that there was minimum metabolic disruption of the intervertebral disc. Whole discs were incubated in stirred vials containing McCoy's 5A medium with 200 µm EF5 at 37 °C in 95% nitrogen/5% carbon dioxide for 3 h, as described in detail elsewhere (Shapiro et al. 1997). These samples were stored frozen until sectioned and stained with ELK3-51 as described above.
Determination of oxygen-dependent fluorescence
Whole lumbar intervertebral discs, as well as small roughly cube-shaped control tissue samples (approximately 5 mm on a side), were immediately harvested from the dead animals (no EF5 injection) and cultured in vitro with 200 µm EF5 in a high-osmolarity medium [high-glucose Dulbecco's minimal essential medium, 15% FBS, 1× ITS (insulin–transferrin–selenium), 150 mm sucrose and 2× PSF antibiotics] for 6 h at 1, 3 and 10%pO2. Discs were frozen, sectioned and stained with antibodies to EF5 as described above. Due to intrinsic cellular oxygen consumption, it was not possible to control the overall oxaemic state of the tissue, only that at its surface.
Analysis of EF5 binding to the disc tissues
Data from the immunohistochemical staining of intervertebral disc sections were quantified as described above, by using Photoshop. Because accurate cellular counts were difficult to obtain in tissues with extremely high cell densities (i.e spleen and liver), the values for each section were normalized by the area of the section as determined by Hoechst staining using the ImagePro system. After normalization to area, to account for total nitroreductase activity in each tissue area, the ‘in vivo’ values were again normalized to their corresponding ‘1%’ values. Where indicated, a one-way anova test was used to determine statistical significance. Significance was assessed at P < 0.05.
Results
Low EF5 binding in the intervertebral tissues
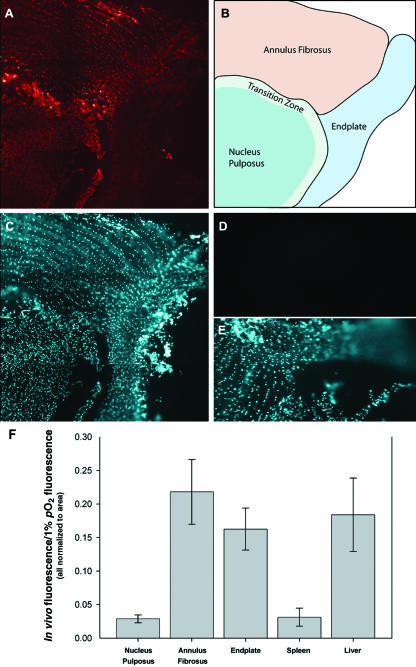
Animals injected with EF5 were killed at 6 h and fluorescence in the NP, AF and EP was analysed (Fig. 1). Figure 1(A) shows NP and EP fluorescence at a lower level than the AF. Furthermore, the transition zone, consisting of the inner annulus and outer NP, displayed the highest level of EF5 fluorescence. Identification of cells using Hoechst 33342 confirmed that the staining was cellular (Fig. 1C). To verify that this binding was specific for EF5, representative sections were stained with a solution containing both ELK3-51 antibody and 0.5 mm authentic EF5 (Koch, 2002) (Fig. 1D,E). Non-specific staining was absent in all three regions of the disc. We also measured EF5 binding in liver and spleen, respectively (Fig. 2), to confirm that the observed fluorescent yield in these animals was comparable with earlier reported values. In those studies, the liver exhibited moderate EF5 binding while spleen showed minimal binding (Laughlin et al. 1996). Because total nitroimidazole levels vary with tissue type, we normalized the level of in vivo binding to maximal tissue binding at a low oxygen tension (Fig. 1F). When normalized, liver exhibited EF5 binding similar to the AF and EP (approximately 20% of maximal tissue binding for each). EF5 binding to the spleen, a tissue that is well oxygenated, was similar to that of the NP (Fig. 1F).
Fig. 1.
EF5 staining of the AF, EP and NP. Longitudinal section through the tissues of the intervertebral disc of EF5-injected rats. The sections were immunostained with ELK3-51 (A) or nuclear stained with Hoechst dye (C). A diagram of the stained intervertebral disc section is shown in B. The images were quantified using fluorescence microscopy (F). Note that the relative fluorescence of the NP and EP is less than that of the AF. When the fluorescence brightness was normalized to area and expressed as a function of maximum fluorescence, the NP fragmented the same fraction of EF5 as spleen, an oxygenated tissue (F). Control for tissue autofluorescence and non-specific binding is indicated in D and E, respectively. Magnification, ×10; 10-µm sections; exposure time: 1570 ms in A.
Fig. 2.
Stained sections of liver and spleen of EF5-injected rats. The liver contains high nitroreductase activity and deoxygenated blood. It fluoresced brightly when immunostained with ELK3-51 (A); the same section was nuclear stained with Hoechst dye (B). By contrast, the brightness of a highly oxygenated spleen tissue was noticeably reduced when immunostained with ELK3-51 for metabolized EF5 (C). The same section nuclear stained with Hoechst (D). Note the brightness of liver in comparison with the highly oxygenated spleen. Magnification, ×4; 10 µm.
Intervertebral discs received adequate amounts of EF5 in vivo
To ensure that the NP received sufficient drug for the oxygen measurement studies, we evaluated tissue levels of EF5. HPLC analysis indicated that 5 min after injection, the blood EF5 concentration was 120 µm. At the 20-min time point, the EF5 level had dropped to 60 µm, a level sustained through 180 min (Fig. 3A). Muscle, spleen, AF and EP reached equilibrium by 60 min (Fig. 3B). The AF and EP equilibrated at 25% of the serum concentration, whereas muscle and spleen were maximal at approximately 74 and 47% of the blood level, respectively. By contrast, the EF5 level in the NP was maximal at 360 min; however, the EF5 concentration was 25% of the serum value. At 20 (Fig. 3C) and 180 min (Fig. 3D), the central region of the NP showed evidence of increased EF5 staining in vivo with time. This staining alleviates the concern that lack of EF5 diffusion into the NP caused regional differences in staining in vivo (Fig. 1A).
Fig. 3.
Time-dependent changes in tissue EF5 levels. Rats were injected intravenously with 10 mm EF5. The volume was adjusted so that at the time of injection the serum concentration was approximately 100 µm and tissues were collected after 5, 20, 60, 180 and 360 min. EF5 concentration in the blood (A), muscle, spleen and disc (B) were measured by HPLC. The vascular accessible tissues such as muscle, spleen, EP and AF reach equilibrium within 5 min of injection. By contrast, the NP was about 50% of maximum by 180 min. Note the EF5-stained NP cells are markedly more fluorescent at 180 min (D) than at 20 min (C). By 6 h, the EF5 concentration in the NP is similar to the other disc tissues. Pmt (photomultiplier tube voltage) = 786 V; Magnification, ×10; 20-µm section; scale bars = 200 µm.
EF5 diffused into the disc and was metabolized in vivo
As we observed a low level of EF5 staining in the NP, it was important to demonstrate that drug metabolism was specific and could be increased in severe hypoxia. To induce artificially whole-body hypoxia while maintaining cell viability, EF5-injected rats were killed 20 min after injection and the body temperature was maintained at 37 °C for 40 min. Whereas in vivo the NP produced a low level of EF5 binding (Fig. 4A), the killed animal (Fig. 4B) demonstrated a marginal elevation in EF5-dependent fluorescence. When quantified, this data reflected a trend toward an increase in EF5 staining in the killed animal (Fig. 4C). However, this difference was not statistically significant. HPLC analysis indicted that, even in the hypoxic state, the intervertebral discs contained excess EF5 (36 µm) at 60 min (Fig. 4D). By contrast, there was no excess drug in any of the control tissues of the killed animal. Because of the presence of residual EF5 in the disc, it is probable that the respiration rate of the resident cells was insufficient to consume all of the drug, and more importantly, the drug concentration was not limiting.
Fig. 4.
Drug metabolism by the rat disc. Rats were injected with EF5 and killed after 20 min. The animal was maintained at 37 °C for 40 min, after which the intervertebral discs were harvested and the fluorescence measured. Immunofluorescent images of disc cells in longitudinal 20-µm sections of disc tissues harvested immediately after death (A) and after 40 min of in vivo hypoxia (B) were captured using a fluorescent microscope. Image brightness and contrast were elevated equally in both images to aid presentation. Fluorescence from these images was quantified (C). Note that while there is a trend toward an increase in fluorescence in the killed animals, that change was not statistically significant. HPLC analysis detected residual EF5 in the discs of hypoxic animals, but not in the control tissues (D). This finding suggests that the disc cells do not metabolize oxygen at a rate sufficient to create a hypoxic environment that would cause a marked increase in EF5 binding. Magnification, ×10; exposure time: 4000 ms, n.s. = not significant, P < 0.05.
Under hypoxic conditions intervertebral disc cells metabolized EF5
As we observed excess EF5 in the intervertebral disc (see above), it was important to demonstrate that, within the disc, EF5 could be metabolized at a low oxygen tension. Whole intervertebral discs were isolated from rats and maintained in organ culture. EF5 binding was evident in the NP, AF and EP (Fig. 5A) when these discs were cultured under anoxia. The AF and EP had similar rates of maximum binding, while that for the NP was two-fold lower. Using the Hoechst stain, we verified that, in all three zones, EF5 staining was cellular (Fig. 5B). Notably, total EF5 binding in each of the different regions of the intervertebral disc, including the transition zone, was varied. As this was most probably due to differences in nitroreductase activity, quantification of fluorescence (relative brightness values in Fig. 1F) was expressed as a function of the maximum EF5 binding seen in cubes maintained at 1% oxygen tension per unit area of tissue. As the in vivo binding was substantially reduced, in comparison with maximum binding (Fig. 1F), drug availability was not a limiting factor. This result suggested that the low level of in vivo EF5 binding was, in fact, due to sufficient oxygen present in the NP to inhibit nitroreductase activity.
Fig. 5.
Intervertebral discs cultured in anoxia demonstrate the presence of bound EF5. Whole intervertebral discs were incubated with 200 µm EF5 under anoxia for 3 h. The sections were treated with (A) Cy3-conjugated ELK3-51 antibody or (B) Hoechst dye. Cartoon of a stained section (C) define the nucleus pulposus (NP), annulus fibrosus (AF) and endplate (EP). The transition zone, an area of tissue that borders the NP, is also shown. As each of the tissue zones binds EF5, it is inferred that they all possess nitroreductase activity. Magnification, ×10; 20-µm section; exposure time: 840 ms in A.
Intervertebral disc cells can bind EF5 in an oxygen-dependent manner
Although cells of the intervertebral disc can bind EF5, it was necessary to confirm that binding can be inhibited by oxygen. At a media oxygen concentration of 10%, cells of the NP exhibited little EF5 binding (Fig. 6A). At 3% oxygen, there was a significant increase in drug binding (Fig. 6B); a further increase in EF5 binding fluorescence was apparent at 1% oxygen (Fig. 6C).
Fig. 6.
The NP bound EF5 in an oxygen-dependent manner. Whole rat intervertebral discs were cultured in media containing 200 µm EF5 at 10% (A), 3% (B) and 1% oxygen (C) for 6 h. EF5 binding was identified by the fluorescence of ELK3-51 antibody-stained sections of the NP. Note there is an increase in fluorescence between 1 and 3% oxygen. In A–C, the left panel shows EF5 staining, and the right panel the corresponding Normarski image. In this study, the Pmt was set at 656 V; Magnification, ×20.
Discussion
We used the oxygen-sensitive probe EF5 to evaluate the oxygenation status of cells in the intervertebral disc. When reduced by oxygen-inhibited nitroreductases, EF5 forms adducts with cellular macromolecules that can be detected using the monoclonal antibody ELK3-51. We expected to find an increase in EF5 adduct levels at the centre of the intervertebral disc. However, using this non-invasive technique, we observed little EF5 binding to cells of the NP. This result was surprising as the NP is the disc tissue most removed from the vascular supply. Although greater EF5 binding was seen in the AF compared with the NP and EP, the transition zone, between the inner AF and the NP, exhibited the highest level of EF5 binding. Despite these observations, for all the tissues of the intervertebral disc, the degree of binding was lower than might be expected for a tissue with a limited oxygen supply. Because the intervertebral disc is (almost) avascular, it must be presumed that the resident cells are metabolically adapted to the low oxygen supply and therefore exhibit reduced EF5 binding.
Although details of EF5 uptake are unknown, it is probable that the drug was delivered to the disc tissues by diffusion from blood vessels serving the outer annulus and, possibly, the avascular EP cartilage (Urban et al. 1977; Holm et al. 1982; Fogelholm & Alho, 2001; Martin et al. 2002). The highly organized ligamentous architecture of the outer annulus would serve to restrict probe diffusion, as would the dense calcified cartilage of the EP. By contrast, the hydrophilic nucleus pulposus should enhance probe diffusion. However, the high concentration of sulphated proteoglycans (aggrecan) within the NP (Guiot & Fessler, 2000; Hayes et al. 2001) could confound EF5 diffusion. For this reason, we enhanced the EF5 level in the disc by doubling the dose previously utilized for studies of cartilage (Shapiro et al. 1997), and by increasing the circulation time from 3 to 6 h. Using this approach, we noted that the disc tissues were not in complete equilibrium with blood. There was at most a three-fold deficit in drug ‘area under curve’ for the NP tissue. Accordingly, this treatment protocol provided sufficient drug to assess the oxygen status of the disc, but insufficient for accurate quantification of the actual tension (see Table 1).
Table 1.
Approximate EF5 binding correlated with pO2
| Oxygen status | Actual oxygen tension | Maximal binding |
|---|---|---|
| Physiological | 10% | 1% |
| Modest hypoxia | 2.5% | 3% |
| Moderate hypoxia | 1% | 10% |
| Severe hypoxia | 0.1% | 30% |
| Anoxia | 0% | 100% |
Table adapted from Evans et al. (2004).
Irrespective of the origin of the drug, its ability to sense oxygen is dependent on the activity of a ubiquitous nitroreductase system that binds the 2-nitroimidazole in an oxygen-dependent manner. Because little was known of the activity of this enzyme in disc cells, especially those of the NP, considerable attention was directed at establishing assessments for both EF5 delivery and binding using ex vivo studies. We verified that the nitroreductase activity of the disc cells would reduce EF5 in an oxygen dose-and time-dependent manner. Although nitroreductase activity, and consequent maximal EF5 binding, differed between the three disc tissues, including the transition zone, it substantiated the need to account for these differences and provided brightness values for normalization. We further ascertained that nitroreductase activity was dose related: thus, there was a proportional increase in ELK3-51 binding between 10, 3 and 1.0% oxygen. In addition to the structural concerns mentioned above, the intradiscal pressure is higher than that of the blood (Holm et al. 1982); this pressure difference could limit nutrient and, presumably, drug diffusion into the disc. Therefore, we verified that adequate amounts of EF5 reached each tissue compartment.
Despite the fact that the intervertebral disc received significant quantities of EF5, it was not known if the drug would accurately signal the oxaemic state of the tissue. For this reason, we analysed EF5 binding in killed animals. Rats were injected with the drug, which was allowed to circulate for 20 min. The animals were then killed, but left intact and warm for 40 min, for a total of 60 min exposure to the drug. In the absence of a functioning circulation, all tissues should continue to consume oxygen and become hypoxic. We noted no increase in EF5 brightness in discs of killed animals compared with animals killed and immediately analysed. During this same time period, the increase in EF5 brightness of the spleen and liver was 2.6-and 3.3-fold, respectively (data not shown). HPLC analysis indicated the presence of excess EF5 in the discs of the killed animals at 60 min, whereas liver, spleen and prostate contained no excess drug. The residual EF5 most probably reflected the low cellularity of the disc and/or its low oxygen consumption. Thus, the tissue hypoxia caused by death resulted in a change in fluorescence for the tissues with completely metabolized EF5. Based on these findings, we conclude that the cells of the intervertebral disc can respond to the local oxygen tension, and that neither EF5 concentration nor nitroreductase activity limit reactivity and binding. Together, these in situ studies indicate that the drug provided a useful signal for measurement of the oxaemic status of the intervertebral tissues.
Based on previous studies of the disc using invasive sensors (Holm et al. 1981, 1982), we expected to find that the actual oxygen tension varied from 0.3 to 1.1% at the core and from 6.8 to 8.1% at the periphery. We were unable to substantiate this gradient using the non-invasive nitroimidazole system. However, our in vivo results are consistent with previous suggestions that disc cells generate almost all of their energy through glycolysis (Rajpurohit et al. 2002) and therefore oxygen consumption by these cells would be expected to be very low (Holm et al. 1981, 1982). Furthermore, due to diffusion limitations based on the mucus-like consistency of the NP, we cannot rule out the possibility that oxygen gradients exist within the NP. Because we used pooled samples for pharmacological measurements, our technique precluded studies of these gradients.
Although gradients were not evaluated, it is clear that disc cells are well adapted to a very low oxygen environment. Using our (C.J.K. & S.M.E.) previously published standards (Table 1) (Evans et al. 2004), the level of EF5 fluorescence exhibited by the AF and EP corresponds to an oxygen tension which is similar to that of the liver (below 1%). Conversely, the NP exhibits a level of EF5 fluorescence similar to that of the spleen (between 2.5 and 10%). Because the surrounding tissues have a lower oxygen status than the NP (below 1%), it would not be unreasonable to predict that the actual oxygen tension in the NP is far lower than that reported here using EF5. In contrast to the NP, AF and EP, the transition zone of the intervertebral disc contains cells that exhibit considerable EF5 staining. The transition zone has been suggested to provide progenitor cells for the NP and therefore may be metabolically more active than the quiescent NP, AF and EP cells (Rajpurohit et al. 2002). Regardless, any event that changes the oxaemic state of this critical group of cells would eventually impact on the cellularity of the mature NP. From this perspective, conditions that promote vascular insufficiency (atherosclerosis or smoking) (Guiot & Fessler, 2000; Fogelholm & Alho, 2001; Iwahashi et al. 2002) can influence the oxygen tension within the disc and impair the ability of the progenitor cells to replace dead or dying NP cells. As the regenerative capacity of the disc is degraded, it would lead to loss of differentiated NP cells, as well as a decrease in cellularity of the AF. If this is the case, then the EF5 technology could be used for the non-invasive detection of disc degeneration. For example, nuclear magnetic resonance imaging and positron emisson tomography techniques, used for tumour detection, could be applied to measurement of the oxaemic status of the degenerate disc (Koch et al. 2001). Its use in a large animal model would permit determination if the results presented here represent a fundamental characteristic of the intervertebral disc, and if degeneration of the disc is linked to changes in the oxaemic state.
Conclusions
Central to our understanding of disc cell function is the role of the local oxygen tension in regulating energy metabolism and extracellular matrix synthesis. However, due to its unique anatomy and isolated location, few studies have attempted to document the effective oxygen concentration of the disc in situ. Here, we utilized the drug EF5 to assess non-invasively the oxaemic status of the intervertebral disc. We document that the NP and AF exhibit low EF5 binding, probably due to low oxygen utilization. By contrast, transition zone cells appear to exhibit a higher level of EF5 binding as well as a higher potential maximal EF5 binding. The oxaemic status of the transitional cells indicates that these cells are hypoxic. These data provide new insight into disc cell metabolism and the results suggest that a modification of the EF5 technology could be used to probe the development of degenerative disc disease.
Acknowledgments
This work was supported by NIH grants DE-13319, DE-10875, DE-05748, AR-051303 + Dupuy Spine + CA-74071 and the Department of Defense grant DAMD17-03-1-0713. We would like to thank Anne Lee Shuman for her technical expertise in the animal injections.
References
- Baumgartl H, Zimelka W, Lubbers DW. Effects of puncturing on the measurement of local oxygen pressure using polarographic microelectrodes. In: LeManna NA, editor. Oxygen Transport to Tissues XVIII. New York: Plenum Press; 1997. pp. 527–539. [DOI] [PubMed] [Google Scholar]
- Evans SM, Judy KD, Dunphy I, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res. 2004;64:1886–1892. doi: 10.1158/0008-5472.can-03-2424. [DOI] [PubMed] [Google Scholar]
- Fogelholm RR, Alho AV. Smoking and intervertebral disc degeneration. Med Hypothesis. 2001;56:537–539. doi: 10.1054/mehy.2000.1253. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196:374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- Guiot BH, Fessler RG. Molecular biology of degenerative disc disease. Neurosurgery. 2000;47:1034–1040. doi: 10.1097/00006123-200011000-00003. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Holm S, Maroudas A, Urban JPG, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- Holm S, Selstam G, Nachemson A. Carbohydrate metabolism and concentration profiles of solutes in the canine lumbar intervertebral disc. Acta Physiol Scand. 1982;115:147–156. doi: 10.1111/j.1748-1716.1982.tb07057.x. [DOI] [PubMed] [Google Scholar]
- Hutton WC, Ganey TM, Elmer WA, et al. Does long-term compressive loading on the intervertebral disc cause degeneration? Spine. 2000;25:2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- Iwahashi M, Matsuzaki H, Tokuhashi Y, Wakabayashi K, Uematsu Y. Mechanism of intervertebral disc degeneration caused by nicotine in rabbits to explicate intervertebral disc disorders caused by smoking. Spine. 2002;27:1396–1401. doi: 10.1097/00007632-200207010-00005. [DOI] [PubMed] [Google Scholar]
- Jenkins WT, Evans SM, Koch CJ. Hypoxia and necrosis in rat 9L glioma and Morris 7777 hepatoma tumors: comparative measurements using EF5 binding and the Eppendorf needle electrode. Int J Radiat Oncol Biol Phys. 2000;46:1005–1017. doi: 10.1016/s0360-3016(99)00342-9. [DOI] [PubMed] [Google Scholar]
- Koch CJ, Hahn SM, Rockwell K, Covey Jr, McKenna WG, Evans SM. Pharmacokinetics of EF5 [2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl acetamide)] in human patients: implications for hypoxia measurements in vivo by 2-nitroimidazoles. Cancer Chemother Pharmacol. 2001;48:177–187. doi: 10.1007/s002800100324. [DOI] [PubMed] [Google Scholar]
- Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and the 2-nitroimidizole EF5. Meth Enzymol. 2002;352:3–31. doi: 10.1016/s0076-6879(02)52003-6. [DOI] [PubMed] [Google Scholar]
- Laughlin KM, Evans SM, Jenkins WT, et al. Biodistribution of the nitroimidazole EF5 (2-[2-nitro-1H-imidazol-1-yl]-N-(2,2,3,3,3-pentafluoropropyl) acetamide) in mice bearing subcutaneous EMT6 tumors. J Pharmacol Exp Ther. 1996;277:1049–1057. [PubMed] [Google Scholar]
- Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus. 2002;13:1–6. doi: 10.3171/foc.2002.13.2.2. [DOI] [PubMed] [Google Scholar]
- Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1, and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- Rannou F, Lee TS, Zhou RH, et al. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro IM, Mansfield KD, Evans SM, Lord EM, Koch CJ. Chondrocytes in the endochondral growth cartilage are not hypoxic. Am J Physiol. 1997;272:C1134–C1143. doi: 10.1152/ajpcell.1997.272.4.C1134. (Cell Physiol41) [DOI] [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Related Res. 1977;129:101–114. [PubMed] [Google Scholar]