Abstract
Histo-blood group antigens Le-x and Le-y are oligosaccharidic terminals that characterize many glycoproteins in the human tissues. In seminal plasma, they are expressed as part of the so-called glycodelin S, which is suggested to regulate sperm capacitation/decapacitation. It has recently been demonstrated that the core protein of glycodelin S is secreted by seminal vesicles. Here we show that epithelial cells of human seminal vesicles also release the Le-x and Le-y antigens. The presence of these substances in secretory material was revealed by means of an immunogold staining method in normal surgical samples. The results suggest that glycodelin S is secreted by seminal vesicles in its finished glycosylated form. Moreover, antigen reactivity was also revealed associated with plasma membranes.
Keywords: human, Le-x, Le-y, seminal vesicle
Introduction
Lewis antigens belong to the class of carbohydrate histo-blood antigens whose determinants are oligosaccharide sequences carried by a variety of proteins or lipids. They are strictly related to ABH antigens, in that they share the origin from type 1 and type 2 precursor chains. Le-a and Le-x derive from direct fucosylation of type 1 and type 2 chains, respectively, while Le-b and Le-y derive from the additional fucosylation of type 1H and type 2H, respectively (Grollman et al. 1969).
Lewis antigens are widely distributed in the human body, both associated with the cell surface glycoconjugates and as secretory products in fluids such as saliva, milk and semen (Oriol, 1987; Lloyd, 2000; Ravn & Dabelsteen, 2000). As part of the glycoproteins and glycolipids of cell membranes, they are believed to act as adhesion molecules or as ligands for microorganisms (Garratty, 1995; Hakomori, 1990). As secretory products, they are generally related to mucosal protection due to their ability to bind microorganisms.
Human semen contains considerable amounts of free and conjugated oligosaccharides, including histo-blood group antigens (Mann, 1964). As a whole, seminal glycoconjugates are functionally involved in the protection of the genital tract mucosa against infections or in preventing immune reactions when adsorbed on sperm (Chalabi et al. 2002). Seminal saccharides are chiefly secreted by seminal vesicles, which also produce mucins, enzymes, prostaglandins, etc., which help the sperm functions by different mechanisms (Aumüller & Riva, 1992; Robertson, 2005). However, our previous results indicated that seminal ABH, Le-a and Le-b substances are secreted by urethral and bulbourethral glands instead of seminal vesicles (Cossu et al. 1994). More recently, indications have emerged that Le-x and Le-y of seminal plasma can also be related to mechanisms that regulate fertility. Morris et al. (1996) found in human seminal plasma Le-x and Le-y oligosaccharides in the so-called glycodelin S, a glycoprotein which probably maintains sperm in an uncapacitated state before they enter the uterine cavity.
Certainly seminal vesicles produce the proteic part of glycodelin S, as immunohistochemically reactive glycodelin as well as glycodelin mRNA have been demonstrated in seminal vesicles and ampulla of the vas deferens (Koistinen et al. 1997). However, we do not know whether glycodelin S is secreted in its complete form or acquires the saccharidic groups successively, given that both glycosyltransferases and free oligosaccharides are present in seminal plasma (Ronquist & Stegmayr, 1983; Chalabi et al. 2002).
Here we demonstrate the presence of Le-x and Le-y sequences in the epithelial cells of human seminal vesicles by immunogold staining (IGS) histochemistry. Observation via electron microscopy was chosen in order to discern the staining of secretory granules from that of the membrane surfaces that often express Lewis antigens.
Materials and methods
Samples of normal seminal vesicles, excised from four consenting patients during operations for cystectomy and prostatectomy, were cut into small pieces and fixed in a mixture of 1% formaldehyde and 1.25% glutaraldehyde in 0.1 m cacodylate buffer, pH 7.2, for 3 h. The secretor status was unknown. Pieces then were embedded in Epon resin following the standard procedures for electron microscopy preparations. Post-fixation with osmium tetroxide was omitted.
Ultrathin sections were treated for immunocytochemistry. Primary antisera were mouse monoclonal antibodies specific for Le-x and Le-y purchased from Signet Laboratories (Dedham, MA, USA), diluted 1: 50 in a Tris-buffered saline (TBS) solution enriched with 1% bovine serum albumin (BSA) and 5% normal goat serum. Secondary antisera were goat anti-mouse (GAM) IgM Auroprobe EM G10 (Amersham International plc, Little Chalfont, UK), diluted 1: 30 in TBS plus BSA. Non-immune mouse serum or mouse anti-HbsAg antibody (Chemicon International, Inc., Temecula, CA, USA) were used as negative controls for staining. Finally, sections were stained with uranyl acetate and bismuth subnitrate and observed with a JEOL 100S electron microscope.
Results
The seminal vesicle epithelium showed normal morphological features. Its aspect, as well as the general cell ultrastructure, exactly corresponded to those described by Riva & Aumuller (1994). Principal and basal cells were clearly distinguishable, and apical cytoplasmic blebs containing variable amounts of secretory granules were seen in principal cells.
All samples showed identical staining intensity and localization of immunoreactivities. Basal cells never reacted.
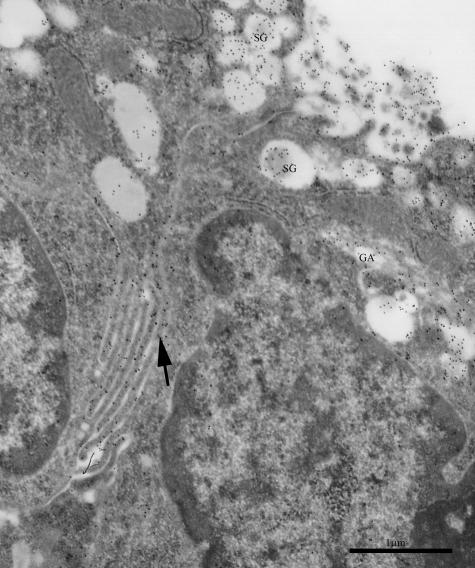
In principal cells, the secretory granules were indeed the most evident site of gold particle deposition (Figs 1 and 2). Both Le-x and Le-y antigens appeared strongly labelled in the inner dense mass surrounded by a peripheral pale halo, which by contrast was unreactive (Fig. 3A). A few gold particles also decorated small vacuoles close to the Golgi apparatus (Fig. 1).
Fig. 1.
Principal cells of human seminal vesicle epithelium. Gold particles reveal the expression of Le-y antigen in secretory granules (SG), Golgi apparatus (GA) and lateral folds of plasma membranes (arrow). Scale bar = 1 µm.
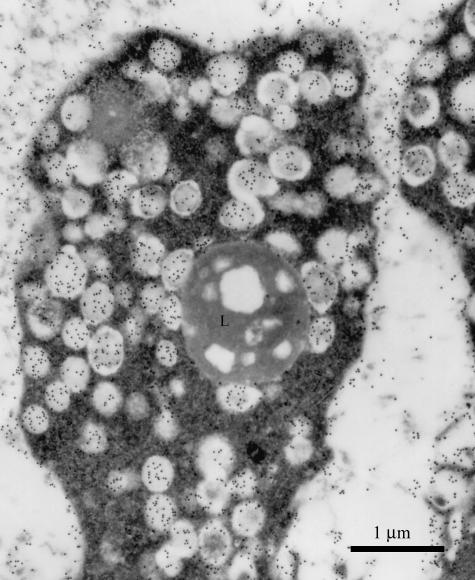
Fig. 2.
Large apical bleb of a principal cell, filled with secretory granules reactive for Le-y antigen. A lipofuscin body (L) is also present. Scale bar = 1 µm.
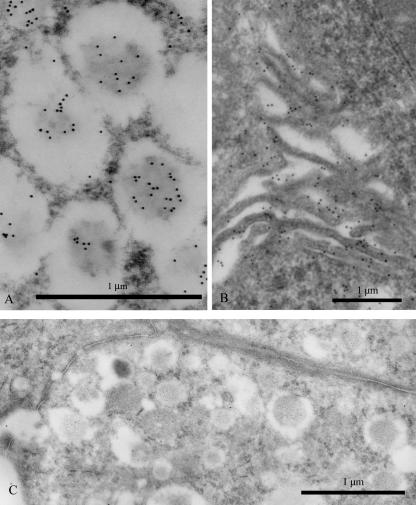
Fig. 3.
(A) Immunoreactivity of Le-x in the dense core of secretory granules. Scale bar = 1 µm. (B) Gold particles showing Le-y antigen decorating basolateral cell membranes. Scale bar = 1 µm. (C) Unlabelled control section incubated with anti-HbsAg-containing medium. Scale bar = 1 µm.
In addition, Le-y antigen was intensely reactive on the cell surfaces, both at the apical and the basolateral levels (Figs 1,2 and 3B), while Le-x reactivity was much weaker at this location.
Control sections were unstained (Fig. 3C).
Discussion
Our results first show that principal cells of human seminal vesicle epithelium express Le-x and Le-y antigens as secretory products.
Several saccharide residues have been identified by means of lectin histochemistry in seminal vesicle epithelium (Arenas et al. 2001), but Lewis antigens were not of concern in these experiments. The study of Le-x and Le-y reactive seminal components has become attractive only recently, when it was found that they were part of the glycodelin S molecule, and thus related to sperm functions. Glycodelin S is closely related to glycodelins A and F, produced in female reproductive tissues, and which have different functional roles in the regulation of fertility (Morris et al. 1996; Seppala et al. 1998, 2002). Glycodelin S has the same core protein as glycodelin A, but differs in the saccharide groups on which the protein function depends (Morris et al. 1996). Peculiar features of glycodelin S are the absence of sialylated groups and the abundance in Le-x and Le-y. Although other seminal fluid proteins carry Lewis antigens (Hanisch et al. 1986), to our knowledge glycodelin S is the only seminal vesicle product for which Le-x and Le-y have thus far been documented.
The present results do not prove that Le-x and Le-y antigens are associated with glycodelin, but at least suggest that principal cells of the seminal vesicle epithelium secrete not only the protein core (Koistinen et al. 1997) but also the fucose-rich saccharidic components of glycodelin S. A key role in the regulation of sperm capacitation has been recently proposed for glycodelin S (Chiu et al. 2005). After binding to sperm, glycodelin S should maintain sperm in a decapacitated condition, in order to avoid the occurrence of an acrosomal reaction before reaching the site of fertilization. The mechanism of this effect has not yet been demonstrated, but a likely hypothesis has been proposed. Chiu et al. (2005) suggested that glycodelin S binds to the sperm glycocalyx and renders the membrane cholesterol unavailable to its acceptor. In this way, sperm capacitation cannot begin.
Although we had no information on the secretor status of the patients, we presume that Lewis antigen secretion in seminal vesicles does not depend on the secretor status given that the secretor-dependence appears to be restricted to tissues of endodermal origin (Oriol, 1987). Secretor status can also be deduced from significant differences between the reactivities of mono-and difucosylated antigens, but difficulties in obtaining human biopsy tissues did not allow us to make this evaluation on a congruous number of samples.
Two distinct forms of antigen reactivity were discerned, one associated with the secretory material and the other with cell surfaces. This double localization is generally ascribed to Lewis as well as all histo-blood group antigens, although in fact the distinction between surface-bound and secretory antigens has been shown clearly only in a few studies at the EM level (Cossu & Lantini, 1996). By contrast, surface-associated Lewis antigens have received considerable attention because of their involvement in the mechanisms that mediate relationships of the cells with other cells and their environment. Attempts have been made to define some of them as markers for embryonal stem cells (Fenderson et al. 1986; Muramatsu & Muramatsu, 2004) and to relate their presence to apoptosis (Hiraishi et al. 1993; Azuma et al. 2004). As aberrant expression of Lewis antigens and their sialylated derivatives is often indicative of tumour progression, their distribution has been analysed chiefly by oncologists in order to identify tumour markers (Dabelsteen, 1996; Lloyd, 2000; Le Pendu et al. 2001). In male accessory organs, several studies have been carried out from an oncological perspective, and reported greatly altered Le-y expression in prostate carcinoma (Martensson et al. 1995; Myers et al. 1995). Our results clearly show that cell surfaces of seminal vesicle epithelium normally express Le-x and Le-y. Thus, as surface histo-blood group antigens, they should participate in the regulation of intercellular adhesion by interacting with surface lectins. An intriguing hypothesis is that membrane-bound Le-x or Le-y or both mediate the recognition of sperm stored in the seminal vesicle cavities. This might represent the initial step of spermiophagy, which can occur in seminal vesicle epithelium, as suggested many years ago by the observation of phagocytosed sperm residuals within principal cells (Riva et al. 1981).
Acknowledgments
We are grateful to Mrs S. Bernardini and to Mr A. Cadau for their excellent technical assistance. This investigation was supportd by the Ministero dell’Università e della Ricerca.
References
- Arenas MI, Royuela M, Fraile B, Paniagua R, Wilhelm B, Aumuller G. Identification of N-and O-linked oligosaccharides in human seminal vesicles. J Androl. 2001;22:79–87. [PubMed] [Google Scholar]
- Aumüller G, Riva A. Morphology and functions of the human seminal vesicle. Andrologia. 1992;24:183–196. doi: 10.1111/j.1439-0272.1992.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Ito M, Taniguchi A, Matsumoto K. Expression of cell surface Lewis X and Y antigens and FUT4 mRNA is increased in Jurkat cells undergoing apoptosis. Biochim Biophys Acta. 2004;1672:157–163. doi: 10.1016/j.bbagen.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Chalabi S, Easton RL, Patankar MS, et al. The expression of free oligosaccharides in human seminal plasma. J Biol Chem. 2002;277:32562–32570. doi: 10.1074/jbc.M205152200. [DOI] [PubMed] [Google Scholar]
- Chiu PC, Chung MK, Tsang HY, et al. Glycodelin-S in human seminal plasma reduces cholesterol efflux and inhibits capacitation of spermatozoa. J Biol Chem. 2005;280:25580–25589. doi: 10.1074/jbc.M504103200. [DOI] [PubMed] [Google Scholar]
- Cossu M, Lantini MS, Migliari R. ABH and Lewis antigens in human male accessory sex glands. In: Riva A, Testa Riva F, Motta PM, editors. Ultrastructure of Male Urogenital Glands. Boston: Kluwer Academic Publishers; 1994. pp. 177–185. [Google Scholar]
- Cossu M, Lantini MS. Ultrastructural localization of blood group antigens in human salivary glands. Eur J Morph. 1996;34:191–195. doi: 10.1076/ejom.34.3.191.13030. [DOI] [PubMed] [Google Scholar]
- Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179:358–369. doi: 10.1002/(SICI)1096-9896(199608)179:4<358::AID-PATH564>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Fenderson BA, Holmes EH, Fukushi Y, Hakomori S. Coordinate expression of X and Y haptens during murine embryogenesis. Dev Biol. 1986;114:12–21. doi: 10.1016/0012-1606(86)90379-9. [DOI] [PubMed] [Google Scholar]
- Garratty G. Blood group antigens as tumor markers, parasitic/bacterial/viral receptors, and their association with immunologically important proteins. Immunol Invest. 1995;24:213–232. doi: 10.3109/08820139509062774. [DOI] [PubMed] [Google Scholar]
- Grollman EF, Kobata A, Ginsburg V. An enzymatic basis for Lewis blood types in man. J Clin Invest. 1969;48:1489–1494. doi: 10.1172/JCI106115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids: modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990;256:18713–18716. [PubMed] [Google Scholar]
- Hanisch FG, Egge H, Peter-Katalinic J, Uhlenbruck G. Structure of neutral oligosaccharides derived from mucus glycoproteins of human seminal plasma. Eur J Biochem. 1986;155:239–247. doi: 10.1111/j.1432-1033.1986.tb09482.x. [DOI] [PubMed] [Google Scholar]
- Hiraishi K, Suzuki K, Hakomori S, Adachi M. Ley antigen expression is correlated with apoptosis (programmed cell death) Glycobiology. 1993;3:381–390. doi: 10.1093/glycob/3.4.381. [DOI] [PubMed] [Google Scholar]
- Koistinen H, Koistinen R, Kämäräinen M, Salo J, Seppälä M. Multiple forms of messenger ribonucleic acid encoding glycodelin in male genital tract. Lab Invest. 1997;76:683–690. [PubMed] [Google Scholar]
- Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Moullac-Vaidye B, Clement M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9–31. doi: 10.1111/j.1600-0463.2001.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Lloyd KO. The chemistry and immunochemistry of blood group A, B, H, and Lewis antigens: past, present and future. Glycoconj J. 2000;17:531–541. doi: 10.1023/a:1011066308591. [DOI] [PubMed] [Google Scholar]
- Mann T. The biochemistry of semen and the male reproductive tract. J Reprod Immunol. 1964;59:253–265. [Google Scholar]
- Martensson S, Bigler SA, Brown M, Lange PH, Brawer MK, Hakomori S. Sialyl-Lewis(x) and related carbohydrate antigens in the prostate. Hum Pathol. 1995;26:735–739. doi: 10.1016/0046-8177(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Morris HR, Dell A, Easton RL, et al. Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J Biol Chem. 1996;271:32159–32167. doi: 10.1074/jbc.271.50.32159. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Muramatsu H. Carbohydrate antigens expressed on stem cells and early embryonic cells. Glycoconj J. 2004;21:41–45. doi: 10.1023/B:GLYC.0000043746.77504.28. [DOI] [PubMed] [Google Scholar]
- Myers RB, Srivastava S, Grizzle WE. Lewis Y antigen as detected by the monoclonal antibody BR96 is expressed strongly in prostatic adenocarcinoma. J Urol. 1995;153:1572–1574. [PubMed] [Google Scholar]
- Oriol R. Tissular expression of ABH and Lewis antigens in humans and animals: expected value of different animal models in the study of AB0-incompatible organ transplants. Transplant Proc. 1987;19:4416–4420. [PubMed] [Google Scholar]
- Ravn V, Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000;108:1–28. doi: 10.1034/j.1600-0463.2000.d01-1.x. [DOI] [PubMed] [Google Scholar]
- Riva A, Cossu M, Usai E, Testa-Riva F. Spermatophagy by epithelial cells of the seminal vesicle and of the ampulla ductus deferentis in man: a scanning and transmission EM study. In: Frajese G, et al., editors. Oligozoospermia: Recent Progress in Andrology. New York: Raven Press; 1981. pp. 45–53. [Google Scholar]
- Riva A, Aumuller G. Epithelium of the distal portion of the human spermatic pathway: seminal vesicle, ampulla ductus deferentis, and ejaculatory duct. In: Riva A, Testa Riva F, Motta PM, editors. Ultrastructure of Male Urogenital Glands. Boston: Kluwer Academic Publishers; 1994. pp. 35–49. [Google Scholar]
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- Ronquist G, Stegmayr B. High fucosyl transferase activity in human seminal plasma. Int J Fertil. 1983;28:239–242. [PubMed] [Google Scholar]
- Seppala M, Bohn H, Tatarinov Y. Glycodelins. Tumour Biol. 1998;19:213–220. doi: 10.1159/000030009. [DOI] [PubMed] [Google Scholar]
- Seppala M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23:401–430. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]