Abstract
The spinal nucleus of the accessory nerve (SNA) comprises the group of somata (perikarya) of motor neurons that supply the sternocleidomastoid and trapezius muscles. There are many conflicting views regarding the longitudinal extent and topography of the SNA, even in the same species, and these disagreements prompted the present investigation. Thirty Sprague–Dawley rats (15 males, 15 females) were used. The SNA was localized by retrograde axonal transport of horseradish peroxidase. Longitudinally, the SNA was found to be located in the caudal part (caudal 0.9–1.2 mm) of the medulla oblongata, the whole lengths of cervical spinal cord segments C1, C2, C3, C4, C5 and rostral fourth of C6. In the caudal part of the medulla oblongata, the SNA was represented by a group of perikarya of motor neurons lying immediately ventrolateral to the pyramidal fibres that were passing dorsolaterally after their decussation. In the spinal cord, the motor neuronal somata of the SNA were located in the dorsomedial and central columns at C1, in the dorsomedial, central and ventrolateral columns at C2 and in the ventrolateral column only at C3, C4, C5 and rostral quarter of C6. The perikarya of motor neurons supplying the sternocleidomastoid were located in the caudal part (caudal 0.9–1.2 mm) of the medulla oblongata ventrolateral to the pyramidal fibres that were passing dorsolaterally after their decussation. They were also located in the dorsomedial and central columns at C1, in the dorsomedial, central and ventrolateral columns at C2 and only in the ventrolateral column at the rostral three-quarters of C3. The perikarya of motor neurons supplying the trapezius muscle were located in the ventrolateral column only in the caudal three-quarters of C2, the whole lengths of C3, C4 and C5, and in the rostral quarter of C6.
Keywords: accessory nerve, HRP, medulla oblongata, rat, spinal cord, spinal nucleus
Introduction
The spinal nucleus of the accessory nerve (SNA) comprises the group of somata (perikarya) of motor neurons that supply the sternocleidomastoid (SCM) and trapezius (TRAP) muscles. The SNA has been investigated in experimental animals by many workers using different techniques [e.g. retrograde axonal transport of horseradish peroxidase (HRP) by Kitamura & Sakai, 1982; retrograde cobalt labelling by Matesz & Szekely, 1983; retrograde transport of fluorescent tracer by Clavenzani et al. 1994; retrograde degeneration technique by Ullah & Salman, 1986]. The SNA has also been identified in human embryos by Pearson (1938) and in human cadavers by Routal & Pal (2000) but these studies lack experimental support. There are many conflicting views regarding the longitudinal extent and topography of the SNA, even in the same species. For example, Flieger (1964) in sheep, Augustine & White (1986) in Japanese baboon, Ueyama et al. (1990) in monkey and Ullah & Salman (1986) in rabbit have localized the SNA in the caudal part of the medulla oblongata and upper cervical segments of the spinal cord, whereas Satomi et al. (1985) in cat, Jenny et al. (1988) in monkey and Clavenzani et al. (1994) in sheep, among others, have localized it in the upper cervical segments of the spinal cord only.
There is also disagreement regarding the number of groups of neuronal somata representing the SNA. Dubois & Foley (1936), Holomanova et al. (1972), Augustine & White (1986), for example, have found that the SNA comprises only one group of neuronal somata. Flieger (1964), Clavenzani et al. (1994) and others have found that the SNA comprises two groups of neuronal somata (dorsal and ventral groups). Liinamaa et al. (1997) have found three groups of neuronal somata representing the SNA.
Kitamura & Sakai (1982) and Matesz & Szekely (1983) located the SNA in rat. There is disagreement among them regarding the longitudinal extent of the SNA and location of its neuronal somata. Kitamura & Sakai (1982) found them in the upper six cervical segments of the spinal cord forming three longitudinal columns, columns M, L and 5, whereas Matesz & Szekely (1983) located them in the caudal part of the medulla oblongata and upper six cervical segments of the spinal cord forming three columns, medial, lateral and ventral. These disagreements prompted the present investigation in rat.
Materials and methods
Surgical procedures were approved by the Animal Ethics Committee of the Medical Campus, Universiti Sains Malaysia.
Thirty Sprague–Dawley rats were used. Under general anaesthesia (30 mg per kg of nembutal sodium solution, i.p.) and aseptic conditions, the right SCM or right TRAP or both were exposed in the neck and either of the two muscles or both muscles were injected with 0.05 mL of 30% HRP (Sigma type VI) solution as per details given in Table 1.
Table 1.
Amount of HRP solution injected into sternocleidomastoid (SCM) or trapezius (TRAP) or both
| Group | No. of animals | Muscle(s) injected | Amount of HRP solution |
|---|---|---|---|
| IA | Ten (5 males, 5 females) | Right SCM and right TRAP | 0.05 mL of 30% HRP in each muscle |
| IB | Ten (5 males, 5 females) | Right SCM | 0.05 mL of 30% HRP |
| IC | Ten (5 males, 5 females) | Right TRAP | 0.05 mL of 30% HRP |
After 48 h of survival, the animals were re-anaesthetized, perfused transcardially (through the left ventricle), first with normal saline at room temperature, then with 1.25% glutaraldehyde and 1% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4, at room temperature, and finally with 10% sucrose in the same buffer at 4 °C. After perfusion, the medulla oblongata and the 1st, 2nd, 3rd, 4th, 5th and 6th cervical segments of the spinal cord were removed by dorsal approach, placed in the above sucrose buffer solution at 4 °C for 24 h. Thereafter, their serial transverse sections were cut in a cryostat at 60 µm. The sections were collected in the above phosphate buffer without sucrose and treated according to the tetramethyl benzidine (TMB)-HRP method of Mesulam (1978). The sections were examined microscopically to identify the HRP-labelled neuron somata and to compare the experimental right side with the control left side. From the serial transverse sections the groups of HRP-labelled neurons were reconstructed using the method of Elliott (1942) in which a series of sections are summed.
Classification and nomenclature of groups of neuron somata in the ventral grey horn of the upper six cervical segments of the spinal cord
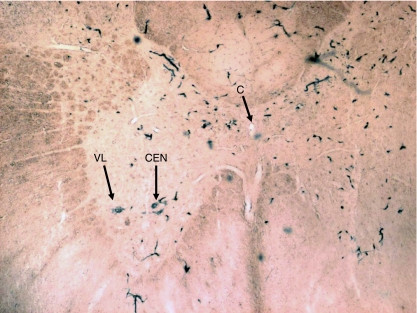
In the present study, the classification and nomenclature of the groups of neuron somata of the ventral grey horn in the upper six cervical segments of the spinal cord are based on our previous study in the same species of rat (Ullah et al. 2006). According to this study, the neuron somata of the ventral grey horn of the upper six cervical segments (C1–C6) were arranged in three groups, lateral, central and medial (Figs 1 and 2). The lateral group included the ventrolateral (VL) and dorsolateral (DL) columns. The VL extended from the cranial end of C2 (second cervical segment) to caudal end of C6. The DL extended from the cranial end of C4 to caudal end of C6. The central group was represented by a single longitudinal column, which had two parts, cranial and caudal. Its cranial part was present in the caudal three-quarters of C1 and whole length of C2, whereas its caudal part (or phrenic column) was located in the whole lengths of C4 and C5. The medial group included two longitudinal columns, the ventromedial (VM) and dorsomedial (DM). Both these columns extended from the cranial end of C1 to caudal end of C6.
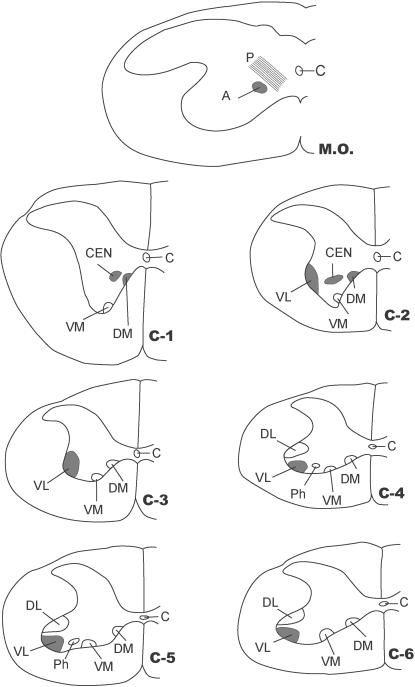
Fig. 1.
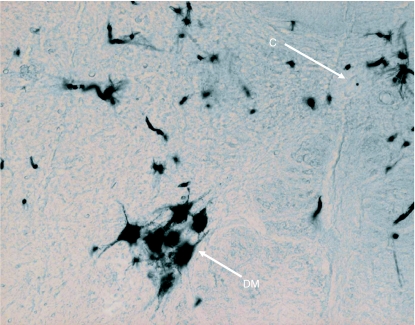
Transverse sections of the caudal end of the medulla oblongata (M.O.), middle of 1st (C1), 2nd (C2), 3rd (C3), 4th (C4) and 5th (C5) cervical segments and rostral part of 6th (C6) cervical segment of spinal cord. The arrangement of cell groups shown here in ventral grey horn is based on our previous study in rat (Ullah et al. 2006). C = central canal; P = pyramidal fibres; A = location of motor neuron somata of SNA in medulla oblongata; DM = dorsomedial column; VM = ventromedial column; VL = ventrolateral column; DL = dorsolateral column; CEN = central column in C1 and C2; Ph = central column (or phrenic column) in C4 and C5. Solid black areas indicate the locations of neuron somata of SNA found in the present study.
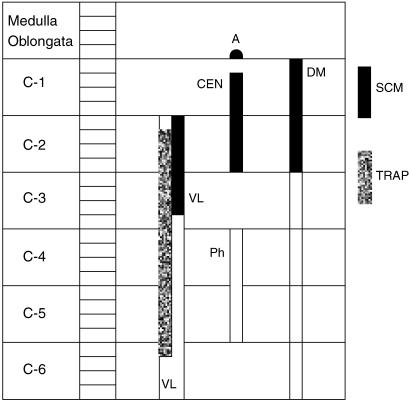
Fig. 2.
Longitudinal extents of the locations of motor neuron somata of the sternocleidomastoid (SCM) shown as solid black areas and trapezius (TRAP) shown as grey area. A = location in lower part of medulla oblongata; C1 to C6 = first to sixth cervical segments of the spinal cord; DM = dorsomedial column; VL = ventrolateral column; CEN = central column in C1 and C2; Ph = central column (or phrenic column) in C4 and C5.
Results
The HRP-labelled neuron somata were observed on the right (experimental) side only and were absent on the left (control) side. These neuron somata were multipolar and in transverse sections their long and short diameters varied from 28 to 42 µm and 15 and 35 µm, respectively.
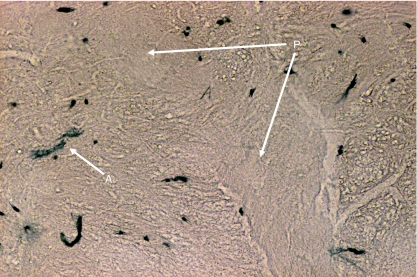
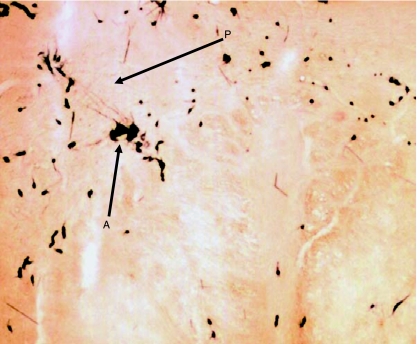
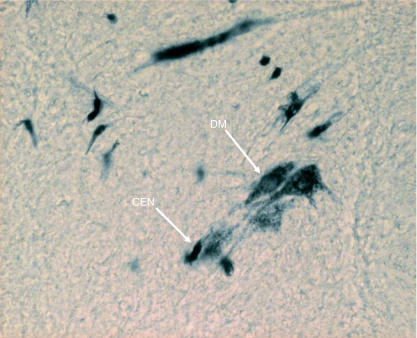
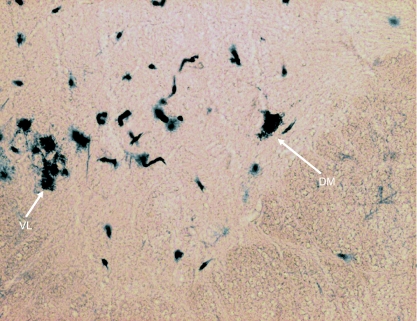
In Group IA animals (in which HRP was injected in both sternocleidomastoid and trapezius muscles), the HRP-labelled neuron somata were observed in the caudal part (caudal 0.9–1.2 mm) of the medulla oblongata, the whole lengths of C1, C2, C3, C4 and C5 and the rostral quarter of C6 (Figs 1 and 2). In the medulla oblongata, they formed a small group that was located at a site immediately ventrolateral to the pyramidal fibres that were passing dorsolaterally after their decussation (Figs 1, 2, 11 and 12). In C1, the HRP-labelled neuron somata were located in the dorsomedial (DM) and central (CEN) columns of the ventral grey horn (Figs 1–4). In C2, they were located in three columns, the dorsomedial (DM), central (CEN) and ventrolateral (VL) columns (Figs 1, 2, 5 and 6). In C3, C4, C5 and rostral quarter of C6, they were located in the ventrolateral (VL) column only (Figs 7–10).
Fig. 11.
Transverse section of lower part of medulla oblongata showing two HRP-labelled neuron somata (A) of SCM lying ventrolateral to pyramidal fibes (P) that are passing dorsolaterally after decussation.
Fig. 12.
Transverse section of lower part of medulla oblongata showing HRP-labelled neuron somata (A) of SCM lying ventrolateral to pyramidal fibres (P) that are passing dorsolaterally after decussation.
Fig. 4.
Location of HRP-labelled neuron somata of SCM in the dorsomedial (DM) and central (CEN) columns at C1.
Fig. 5.
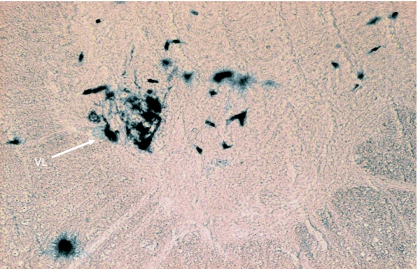
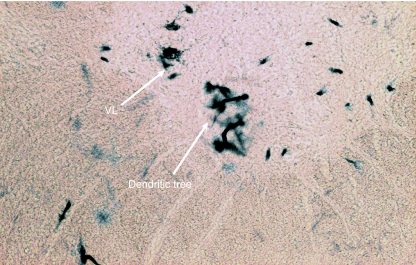
Location of HRP-labelled neuron somata of SCM in the dorsomedial (DM) and ventrolateral (VL) columns at C2.
Fig. 6.
Transverse section of the spinal cord showing the location of HRP-labelled neuron somata of SCM in the central (CEN) and ventrolateral (VL) columns at C2. C = central canal.
Fig. 7.
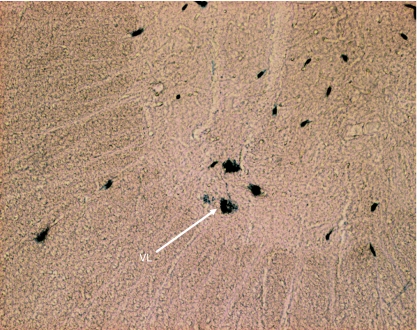
Transverse section of spinal cord showing HRP-labelled neuron somata of trapezius in the ventrolateral column (VL) at C3.
Fig. 10.
Photomicrograph of a transverse section of spinal cord showing one HRP-labelled neuron soma of trapezius in the ventrolateral column (VL) at C6.
Fig. 3.
Location of HRP-labelled neuron somata of SCM in the dorsomedial (DM) column at C1. C = central canal.
Fig. 8.
Transverse section of spinal cord showing HRP-labelled motor neuron somata of trapezius in the ventrolateral column (VL) at C4.
Fig. 9.
Transverse section of spinal cord showing HRP-labelled motor neuron somata of trapezius in the ventrolateral column (VL) at C5.
In Group IB animals (in which HRP was injected into the sternocleidomastoid only), the HRP-labelled neuron somata were located in the caudal part (caudal 0.9–1.2 mm) of the medulla oblongata, whole lengths of C1 and C2 and rostral three-quarters of C3 (Figs 1 and 2). In the medulla oblongata, they were located at a site immediately ventrolateral to the pyramidal fibres that were passing dorsolaterally after their decussation (Figs 1, 2, 11 and 12). In C1, they were located in dorsomedial (DM) and central (CEN) columns, in C2, they were found in dorsomedial (DM), central (CEN) and ventrolateral (VL) columns and in the rostral three-quarters of C3, they were located in the ventrolateral (VL) column only (Figs 1–6).
In animals of Group IC (in which the HRP was injected into trapezius only), the HRP-labelled neuron somata were located only in ventrolateral column (VL) in the caudal three-quarters of C2, the whole lengths of C3, C4 and C5 and the rostral quarter of C6 (Figs 1, 2 and 7–10).
Discussion
Longitudinal extent of SNA
There is disagreement among many investigators regarding the longitudinal extent of the SNA in various species of mammals. It has been located in the caudal part of the medulla oblongata and upper cervical segments of the spinal cord by Flieger (1964) in sheep, Flieger (1966) in horse, Flieger (1967) in cow, Matesz & Szekely (1983) in rat, Ullah & Salman (1986) in rabbit, Augustine & White (1986) in Savanna baboon, Ueyama et al. (1990) in Japanese monkey and Routal & Pal (2000) in human cadaver. In our study the SNA was found to be located in the caudal part of the medulla oblongata and upper cervical segments of the spinal cord.
Pearson (1938) in human embryo, Romanes (1941) in rabbit, Holomanova et al. (1972) in cat, Ruminska-Kowalska et al. (1976) in dog, Kitamura & Sakai (1982) in rat, Satomi et al. (1985) in cat, Jenny et al. (1988) in monkey, Clavenzani et al. (1994) in sheep and Liinamaa et al. (1997) in feline located the SNA in the cervical segments of the spinal cord only and the results of our study are in disagreement with them in this respect.
Topography of SNA
Dubois & Foley (1936) in cat, Pearson (1938) in human embryo, Romanes (1941) in rabbit, Holomanova et al. (1972) in cat, Ruminska-Kowalska et al. (1976) in dog, Ullah & Salman (1986) in rabbit, Augustine & White (1986) in baboon and Routal & Pal (2000) in human cadavers reported only one group of neuronal perikarya representing the SNA but in our study three longitudinal groups of neuronal perikarya were found to represent the SNA. The results of our study are also in disagreement with Flieger (1964, 1966, 1967) and Clavenzani et al. (1994) who found two groups of neuronal perikarya representing the SNA.
Kitamura & Sakai (1982) in rat, Matesz & Szekely (1983) in rat and Liinamaa et al. (1997) in feline found three longitudinal cell columns representing the SNA. Kitamura & Sakai (1982) located the motor neuronal perikarya of SCM and TRAP muscles based on retrograde axonal transport of HRP in rat. Their cell column G1 corresponds to the DM column, G2 corresponds to the CEN column and G4, G5, G6, G7 together correspond to the VL column of the present study. They located HRP-labelled neuronal perikarya of SNA in G1 at C1, in G2 and G6 at C2, in G5 and G6 at C3, in G5 at C4, in G4 and G5 at C5, and in G4 at the rostral part of C6. The results of our study in the spinal cord are nearly in agreement with those of Kitamura and Sakai except that in the present study the central column (CEN) containing SNA motor neuronal somata was also found in the caudal three-quarters of C1. The major disagreement with Kitamura and Sakai is the fact that in our study the SNA was located in the caudal part of the medulla oblongata also but they did not find it there. It is difficult to explain the reason for this disagreement but it may be due to different strains of rat used in the two studies. However, support for the finding of our study comes from Matesz & Szekely (1983; vide infra) who also found the SNA perikarya of rat in the caudal part of the medulla oblongata.
From the above description, it is evident that Kitamura & Sakai (1982) found that the rostral part of the SNA in rat was located in the medial part of the ventral grey horn (in their columns G1 and G2) whereas its caudal part was located in the lateral part of the ventrolateral column. A more or less similar positional shift of SNA was also reported in rat by Matesz & Szekely (1983) and in cat (Holomanova et al. 1972), sheep (Romanes, 1940; Flieger, 1964), rabbit (Romanes, 1941; Ullah & Salman, 1986), horse and cow (Flieger, 1966, 1967), although there are some minor differences among these species as to the degree and level at which this positional shift occurs. In our study, it was observed that in the rostral quarter of C1, the SNA was located in the dorsomedial column, in caudal three-quarters of C1, it was located in dorsomedial and central columns. In C2, it was located in dorsomedial, central and ventrolateral columns and in C3 to C6, it was present in the ventrolateral column only. Thus, in the spinal cord, a positional shift of the SNA perikarya was also observed in our study. The sternocleidomastoid (SCM) is located ventrally as compared with the trapezius (TRAP). The above lateral shift of the SNA suggests that the motor neuronal perikarya supplying the ventrally located muscle (SCM) tend to lie medially and those supplying the dorsally located muscle (TRAP) tend to lie laterally in the ventral grey horn (Fig. 2).
Matesz & Szekely (1983) located the SNA in rat by cobalt labelling (using cobaltic lysine complex solution) and found three longitudinal cell columns containing cobalt-labelled neuronal perikarya: (1) medial, (2) lateral and (3) ventral. Their medial column started at the level of pyramidal decussation in the medulla oblongata and terminated at C2, and their lateral column was found in the ventrolateral part of the ventral grey horn and extended from C2 to C6. According to them, their ventral column was not easily distinguished from the lateral column and comprised a thin thread of neuronal perikarya lying ventral to the lateral column. Their ventral column extended from C2 to C6. Their medial column corresponds to the group of neuronal perikarya of SNA observed in the DM column at C1 and C2 and their lateral and ventral columns combined together correspond to the neuronal perikarya of SNA observed in the VL column of our study. However, no continuity was observed between the dorsomedial column and the group of neuronal perikarya of SNA observed in the medulla oblongata in our study. Matesz & Szekely (1983) also observed a few interposed labelled neuronal perikarya between their medial and lateral columns. These interposed neuronal perikarya correspond to neuronal perikarya observed in the central (CEN) column of our study. In conclusion, the findings of our study are more or less in agreement with Matesz and Szekeley except for some minor details.
Location of sternocleidomastoid and trapezius motor neuron somata within the SNA
There are only a few reports regarding the locations of sternocleidomastoid (SCM) and trapezius (TRAP) motor neuronal perikarya within the SNA. In rat, Kitamura & Sakai (1982) located the SCM perikarya in C1, C2 and C3, and TRAP perikarya in C2 (caudal half), C3, C4, C5 and C6 (rostral half). They did not find SCM perikarya in the medulla oblongata. Regarding the longitudinal extents of motor neuronal perikarya of SCM and TRAP, the findings of our study are in disagreement with them because in our study the SCM perikarya were located in the caudal part of the medulla oblongata, in whole lengths of C1 and C2 and rostral three-quarters of C3, and TRAP perikarya were located in the caudal three-quarters of C2, whole lengths of C3, C4 and C5 and rostral quarter of C6. Kitamura & Sakai (1982) found that the SCM perikarya were located in their column M (corresponding to dorsomedial and central columns of the present study) and in columns L and 5 (corresponding to ventrolateral column of the present study) and that the TRAP perikarya were located in their columns L and 5 (corresponding to ventrolateral column of the present study). Regarding the locations of motor neuronal perikarya of SCM and TRAP in respect of the cell columns of the spinal cord, the results of our study are in agreement with the above observations of Kitamura & Sakai (1982) because in our study also these neuronal somata were located in the corresponding positions.
The sternocleidomastoid (SCM) has two parts, the sternomastoid (SM) and cleidomastoid (CM), while the trapezius has three parts, the cleidotrapezius (CT), acromiotrapezius (AT) and spinotrapezius (ST). All parts of the sternocleidomastoid and trapezius are innervated by motor neuronal somata of the spinal nucleus of the accessory nerve (SNA) that has been localized in rat in the present study. However, there is some controversy as to whether all fibres arising from neuronal somata of SNA pass through the accessory nerve or some of them also pass through cervical spinal nerves. There are many conflicting reports on the presence or absence of motor innervation of SCM and TRAP through the cervical spinal nerves. Kitamura & Sakai (1982) reported that the CM part of SCM and all three parts of TRAP receive double motor innervation, i.e. through the accessory nerve and ventral rami of cervical spinal nerves. According to them, the motor fibres of the cervical part of the accessory nerve arise from their columns M and L and of the cervical spinal nerves from their column 5. Russel (1897) in monkey and Straus & Howell (1936, quoted by Kitamura & Sakai 1982) in chimpanzee and dog reported that the cervical spinal nerves contribute motor innervation to SCM and TRAP but Corbin & Harrison (1938) and Rapoport (1978) reported that his was not the case in the cat. Motor innervation of TRAP through cervical spinal nerves was reported by Sternberg (1898, quoted by Kitamura & Sakai 1982) in monkey and by Brodal (1969, quoted by Kitamura & Sakai 1982) in human. Yashizaki (1961) in human and rabbit and Kumaki (1970) in rat reported that the cervical spinal nerves contribute motor innervation to SCM whereas this was denied by Chauveau (1891) in horse and Sternberg (1898, quoted by Kitamura & Sakai 1982) in monkey. In the present study, all the motor neuron perikarya of SNA supplying the SCM and TRAP muscles were localized but we did not investigate whether the SNA sends fibres to these muscles through the accessory nerve only or through both accessory nerve and cervical spinal nerves.
Matesz & Szekely (1983) investigated the SNA in rat as described above but did not locate the SCM and TRAP motor neuronal somata within the SNA.
In monkey, rabbit and rat, Karim & Nah (1981) found that the SCM perikarya chiefly occupied a medial position in the ventral grey horn of the upper cervical segments of spinal cord, and TRAP perikarya were located at the lateral part of this horn at a more caudal level of the cervical spinal cord. They did not find any SCM perikarya in the medulla oblongata. Regarding the location of SCM perikarya, the findings of our study do not agree with Karim & Nah (1981) because in our study the SCM perikarya were found to be located in the medulla oblongata and the VM, CEN and VL columns of the spinal cord. The findings of our study are in agreement with them regarding the location of TRAP perikarya in that the TRAP perikarya were located in the ventrolateral column (VL) situated in the lateral part of ventral grey horn.
The findings of our study are also in disagreement with Satomi et al. (1985) who found in cat that the SCM perikarya were located only in C1 to C3 spinal segments, whereas, in our study, they were found to be located in the medulla oblongata as well as C1 to C3 spinal segments. Regarding the location of TRAP perikarya, the results of our study are nearly in agreement with Satomi et al. who found them located chiefly more caudally as compared with SCM perikarya.
The results of our study in rat are in agreement with Ueyama et al. (1990) who found that the SCM perikarya in monkey were located in the medulla oblongata and upper three cervical segments and TRAP perikarya were located in C2 to C6.
In feline, Liinamaa et al. (1997) found three motor nuclei laminated mediolaterally representing sternomastoid (SM), cliedomastoid (CM) and trapezius (TRAP), respectively. The results of our study in rat are in disagreement with them because no such laminated arrangement was found in our study. Liinamaa et al. also found SM and CM motor neuronal perikarya in upper cervical segments and TRAP perikarya in C1 to C6 spinal segments. The results of our study in rat are in disagreement with them because in our study the SCM perikarya were found to be located in the caudal part of the medulla oblongata and upper cervical spinal segments and the TRAP perikarya were located in C2 to C6 spinal segments.
Acknowledgments
This study was financed by a short-term grant from the Universiti Sains Malaysia (USM), Penang, Malaysia. We would also like to thank Mr Mohamed Zafrualam Bin Mohd Zain, Graphics Unit, School of Medical Sciences, Health Campus, USM, for his help in the preparation of the vector graphics.
References
- Augustine JR, White JF. The accessory nerve nucleus in the baboon. Anat Record. 1986;214:312–320. doi: 10.1002/ar.1092140311. [DOI] [PubMed] [Google Scholar]
- Chauveau A. On the sensorimotor nerve circuit of muscles. Brain. 1891;14:145–178. [Google Scholar]
- Clavenzani P, Scapolo A, Callegari E, et al. Motoneuron organization of the muscles of the spinal accessory complex of the sheep investigated with the fluorescent retrograde tracer technique. J Anat. 1994;184:381–385. [PMC free article] [PubMed] [Google Scholar]
- Corbin KB, Harrison F. Proprioceptive component of cranial nerves: the spinal accessory nerve. J Comparative Neurol. 1938;69:315–328. [Google Scholar]
- Dubois FS, Foley JO. Experimental studies on the vagus and spinal accessory nerves in the cat. Anat Record. 1936;64:285–307. [Google Scholar]
- Elliott HC. Studies on the motor cells of the spinal cord. I. Distribution in the normal human cord. Am J Anat. 1942;70:95–117. [Google Scholar]
- Flieger S. Experimental determination of the site and extension of the nucleus of accessory nerve in sheep. Acta Anat. 1964;57:220–231. [PubMed] [Google Scholar]
- Flieger S. Experimental demonstration of the position and extent of the n. accessorius (XI) nucleus in Horse. Acta Anat. 1966;63:89–100. [PubMed] [Google Scholar]
- Flieger S. Experimental determination of the topography and range of the nucleus nervi accessorii in the cow. J Hirnforschung. 1967;9:187–196. [PubMed] [Google Scholar]
- Holomanova A, Cierny G, Zlatos J. Localization of the motor cells of the spinal root of the accessory nerve in the cat. Folia Morph. 1972;20:232–234. [PubMed] [Google Scholar]
- Jenny A, Smith J, Decker J. Motor organization of the spinal accessory nerve in the monkey. Brain Res. 1988;441:352–356. doi: 10.1016/0006-8993(88)91413-8. [DOI] [PubMed] [Google Scholar]
- Karim MA, Nah SH. Localization of motoneurons innervating the sternocleidomastoid muscle in the monkey, rat and rabbit: a horseradish peroxidase study. Brain Res. 1981;206:145–148. doi: 10.1016/0006-8993(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Sakai A. A study on the localization of the sternocleidomastoid and trapezius motoneurons in the rat by means of the HRP method. Anat Rec. 1982;202:527–536. doi: 10.1002/ar.1092020412. [DOI] [PubMed] [Google Scholar]
- Kumaki K. The cervical and the spinal accessory nerves: an account by means of fiber analysis. Acta Anat Nippon. 1970;45:311–344. [PubMed] [Google Scholar]
- Liinamaa TL, Keane J, Richmond FJ. Distribution of motoneurons supplying feline neck muscles taking origin from the shoulder girdle. J Comparative Neurol. 1997;377:298–312. doi: 10.1002/(sici)1096-9861(19970113)377:2<298::aid-cne10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Matesz C, Szekely G. The motor nuclei of the glossopharyngeal-vagal and the accessorius nerves in the rat. Acta Biol Hungarica. 1983;34:215–230. [PubMed] [Google Scholar]
- Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Pearson AA. The spinal accessory nerve in human embryos. J Comp Neurol. 1938;68:243–266. [Google Scholar]
- Rapoport S. Location of the sternocleidomastoid and trapezius motoneurons in the cat. Brain Res. 1978;156:339–344. doi: 10.1016/0006-8993(78)90515-2. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. The spinal accessory nerve in the sheep. J Anat. 1940;74:336–347. [PMC free article] [PubMed] [Google Scholar]
- Romanes GJ. The development and significance of the cell columns in the ventral horn of the cervical and upper thoracic spinal cord of the rabbit. J Anat. 1941;76:112–133. [PMC free article] [PubMed] [Google Scholar]
- Routal RV, Pal GP. Location of the spinal nucleus of the accessory nerve in the human spinal cord. J Anat. 2000;196:263–268. doi: 10.1046/j.1469-7580.2000.19620263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruminska-Kowalska G, Wozniac W, Godynicka M. Localization of the motor nucleus of the accessory nerve in the spinal cord of dogs. Folia Morph. 1976;35:211–218. [PubMed] [Google Scholar]
- Russel JSR. An experimental investigation of the cervical and thoracic nerve roots in relation to the subject of wry neck. Brain. 1897;20:35–55. doi: 10.1136/bmj.2.1921.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomi H, Takahashi K, Kasaba T, Kurosawa Y, Otsuka K. Localization of the spinal accessory motoneurons in the cervical cord in connection with the phrenic nucleus: an HRP study in cats. Brain Res. 1985;344:227–230. doi: 10.1016/0006-8993(85)90799-1. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Satoda T, Tashiro T, Matsushima R, Mizuno N. Infrahyoid and accessory motoneurons in the Japanese monkey (Macaca fuscata) J Comp Neurol. 1990;291:373–382. doi: 10.1002/cne.902910305. [DOI] [PubMed] [Google Scholar]
- Ullah M, Salman SS. Localisation of the spinal nucleus of the accessory nerve in the rabbit. J Anat. 1986;145:97–107. [PMC free article] [PubMed] [Google Scholar]
- Ullah M, Mansor M, Ismail ZIM, Kapitonova MY, Sirajudeen KNS, Asari MA. Nerve cell groups in the ventral grey horn of cervical spinal cord of rat. The Malaysian J Med Sci. 2006;13(Suppl. 1):184. [Google Scholar]
- Yashizaki F. Innervation of the sternocleidomastoid muscle of man and the rabbit. Okayama Igakkai Zasshi. 1961;73:159–171. [Google Scholar]