Abstract
Selective transfer of the two products of the ColIb primase gene, sog, from donor to recipient cell during conjugation was demonstrated by two independent methods. The transfer of these tra proteins was unidirectional and dependent on DNA transfer. The Sog polypeptides were localized to the cytoplasm of the donor cell, but they appeared to interact with other tra gene products located in the inner membrane. After cell mating, the transferred polypeptides were found to be in the cytoplasm of the recipient cell, and it is estimated that as many as 500 Sog polypeptides were transferred per round of conjugation. It is proposed that these proteins are transferred as a result of an interaction with the single-stranded DNA and that the transferred strand may be coated with Sog polypeptides.
Full text
PDF


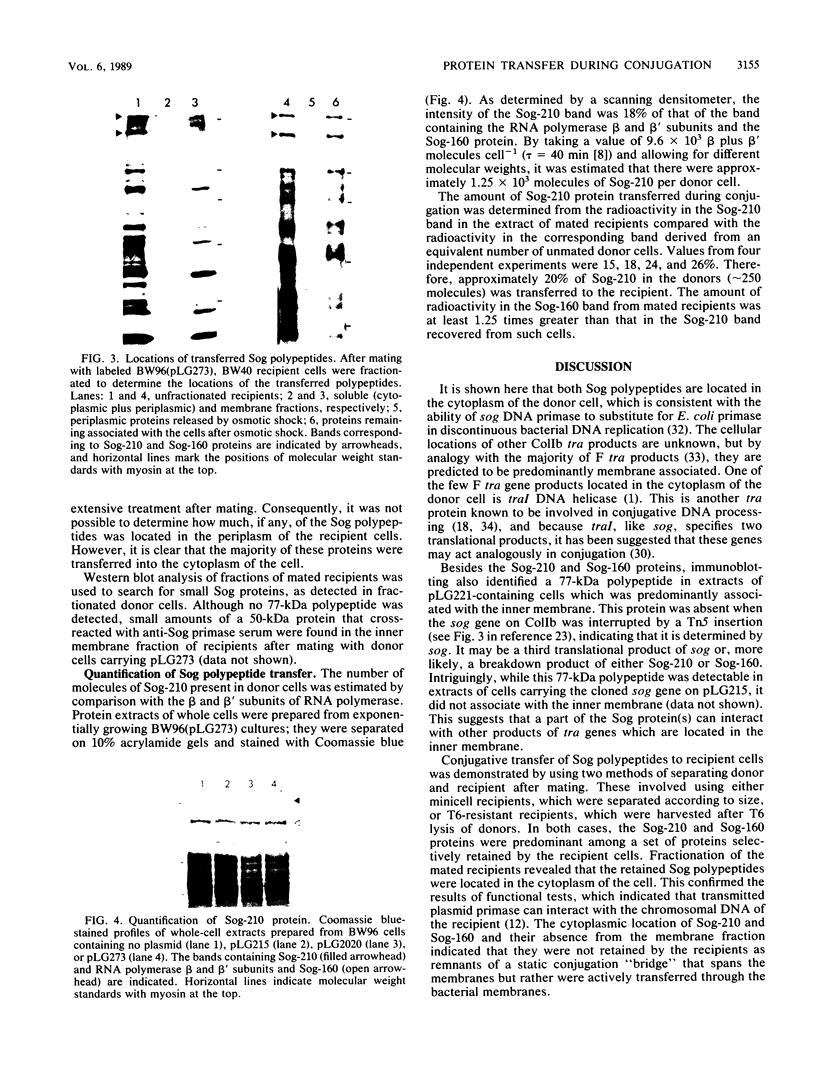


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Morelli G., Schwuchow S. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J Bacteriol. 1978 Sep;135(3):1053–1061. doi: 10.1128/jb.135.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Baker K., Mackman N., Jackson M., Holland I. B. Role of SecA and SecY in protein export as revealed by studies of TonA assembly into the outer membrane of Escherichia coli. J Mol Biol. 1987 Dec 20;198(4):693–703. doi: 10.1016/0022-2836(87)90210-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M. A colI-specified product, synthesized in newly infected recipients, limits the amount of DNA transferred during conjugation of Escherichia coli K-12. J Bacteriol. 1978 Jan;133(1):1–9. doi: 10.1128/jb.133.1.1-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M., Lanka E. Overlapping genes at the DNA primase locus of the large plasmid ColI. Nucleic Acids Res. 1982 Feb 11;10(3):855–869. doi: 10.1093/nar/10.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaregola S., D'Ari R., Huisman O. Quantitative evaluation of recA gene expression in Escherichia coli. Mol Gen Genet. 1982;185(3):430–439. doi: 10.1007/BF00334135. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Chatfield L. K., Orr E., Boulnois G. J., Wilkins B. M. DNA primase of plasmid ColIb is involved in conjugal DnA synthesis in donor and recipient bacteria. J Bacteriol. 1982 Dec;152(3):1188–1195. doi: 10.1128/jb.152.3.1188-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield L. K., Wilkins B. M. Conjugative transfer of IncI1 plasmid DNA primase. Mol Gen Genet. 1984;197(3):461–466. doi: 10.1007/BF00329943. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: stimulation in dnaB(ts) donors by minicells. J Bacteriol. 1973 Dec;116(3):1212–1223. doi: 10.1128/jb.116.3.1212-1223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland C. J., Rees C. E., Barth P. T., Wilkins B. M. The ssb gene of plasmid ColIb-P9. J Bacteriol. 1989 May;171(5):2466–2473. doi: 10.1128/jb.171.5.2466-2473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanka E., Fürste J. P. Function and properties of RP4 DNA primase. Adv Exp Med Biol. 1984;179:265–280. doi: 10.1007/978-1-4684-8730-5_27. [DOI] [PubMed] [Google Scholar]
- Lanka E., Scherzinger E., Günther E., Schuster H. A DNA primase specified by I-like plasmids. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3632–3636. doi: 10.1073/pnas.76.8.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Kilner J. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B. Biochim Biophys Acta. 1978 Dec 4;514(1):117–127. doi: 10.1016/0005-2736(78)90081-0. [DOI] [PubMed] [Google Scholar]
- Merryweather A., Rees C. E., Smith N. M., Wilkins B. M. Role of sog polypeptides specified by plasmid ColIb-P9 and their transfer between conjugating bacteria. EMBO J. 1986 Nov;5(11):3007–3012. doi: 10.1002/j.1460-2075.1986.tb04599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees C. E., Bradley D. E., Wilkins B. M. Organization and regulation of the conjugation genes of IncI1 plasmid colIb-P9. Plasmid. 1987 Nov;18(3):223–236. doi: 10.1016/0147-619x(87)90065-5. [DOI] [PubMed] [Google Scholar]
- SILVER S. D., MOODY E. E., CLOWES R. C. LIMITS ON MATERIAL TRANSFER DURING F+ X F- MATINGS IN ESCHERICHIA COLI K12. J Mol Biol. 1965 May;12:283–286. doi: 10.1016/s0022-2836(65)80300-x. [DOI] [PubMed] [Google Scholar]
- Traxler B. A., Minkley E. G., Jr Revised genetic map of the distal end of the F transfer operon: implications for DNA helicase I, nicking at oriT, and conjugal DNA transport. J Bacteriol. 1987 Jul;169(7):3251–3259. doi: 10.1128/jb.169.7.3251-3259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M., Boulnois G. J., Lanka E. A plasmid DNA primase active in discontinuous bacterial DNA replication. Nature. 1981 Mar 19;290(5803):217–221. doi: 10.1038/290217a0. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]






