Abstract
We provide quantitative anatomical data on the muscle–tendon architecture of the hare thoracic limb (specifically muscle mass, fascicle length, pennation angle, tendon mass and length). In addition, moment arms of major thoracic limb muscles were measured. Maximum isometric force and power of muscles, the moment of force about a joint, and tendon stress and strain were estimated. Data are compared with those from other cursorial mammals. The thoracic limb of the hare consists predominantly of extrinsic musculature with long parallel fascicles, specialised for generating force over a large range. A large shoulder flexor/elbow extensor muscle mass is present, in particular Triceps brachii. The pennate nature of the long head of this muscle suggests it has an important role in stabilising the elbow joint during stance, whilst moment arm curves suggest that it may also play a role in initiating shoulder flexion. In addition, Supraspinatus and Infraspinatus are capable of generating high forces, potentially to stabilise the shoulder joint during the stance phase of locomotion. Supraspinatus may in addition play an important role in forelimb protraction. The Subscapularis muscle was capable of generating surprisingly high forces, suggesting that the hare must be able to withstand/produce high forces during activities that need medio-lateral stability, such as turning. Distally, tendons were relatively short, showing little potential for elastic energy storage when compared with both their pelvic limb counterparts and their equivalents in the horse thoracic limb. Thus, a ‘stiffer’ thoracic limb may be beneficial in terms of behaving like a strut, simply supporting and deflecting the body during high-speed running. This more distal/less proximal distribution of limb mass is also likely to be important in retaining the manipulative/adaptive/non-locomotor capabilities of the limb.
Keywords: architecture, biomechanics, hare, locomotion, moment arms, muscle, tendon
Introduction
The hare (Lepus europeus) can be considered a cursorial animal, capable of high-speed running (Hildebrand & Hurley, 1985), and with a top speed of 20 m s−1 (Garland, 1983). In addition, hares are very manoeuvrable and excel in changing direction and accelerating quickly. They can also jump, and are able to locomote bipedally at times (Grange, 1932). We might therefore expect the musculoskeletal anatomy of the hare to reflect this requirement for non-steady state locomotion and very diverse range of locomotor activities. Upon visual inspection, the hare thoracic limb appears more generalised, but no information is available regarding either its gross musculoskeletal anatomy, or its muscle–tendon architecture to enable comparison with the pelvic limb.
The pelvic and thoracic limbs of the horse, a highly specialised cursorial animal, are adapted for different functional roles, with the proximal pelvic limb muscles (large volumes) appearing specialised for producing large amounts of force, whilst the proximal thoracic limb muscles (long fascicles), particularly the extrinsic muscles, are more suited to generating (smaller amounts of) force over a large range of motion (Payne et al. 2005a). These architectural differences reflect the different functional roles of the pelvic and thoracic limbs. The pelvic limb is thought to act as the primary propulsor of the animal [consider the animal a ‘rear-wheel drive’ system (Merkens & Schamhardt, 1988a,b; Merkens et al. 1993; Usherwood & Wilson, 2005)], whilst the thoracic limb has more of a role in weight support and deceleration (Merkens & Schamhardt, 1988b; Merkens et al. 1993).
The evolution and phylogenetics of the Lagamorpha are still very much in question. However, it is generally agreed that they shared a common ancestor with ungulates over 85 million years ago and so any evolution that has taken place since is likely to be independent of that of other cursors such as the horse. Hares diverged from rabbits much later, sharing a common ancestor in the late Miocene (Halanych et al. 1999; Asher et al. 2005). Thus, the hare (Lepus europeus) may have had the chance to evolve locomotor specialisations that are not present in rabbits and some other members of the Lagamorpha. The hare possesses a large amount of musculature proximally within the pelvic limb, which suggests specialisation for high power production (and hence accelerative ability), and may also contribute to its ability as a saltator (Williams et al. 2007). In fact, in the ankle extensors in particular, parallels can be drawn with the anatomy of the Macropodids. This family of animals characteristically have large powerful pelvic limbs, whilst possessing comparatively small forelimbs. Upon visual inspection, the hare thoracic limb also appears somewhat smaller and more generalised when compared with the pelvic limb, but no information is available regarding either its gross musculoskeletal anatomy, or its muscle–tendon architecture in order to enable comparison.
This study aims to provide detailed information on muscle–tendon architectural properties and moment arms of the major muscles of the thoracic limb of the hare. We will consider interspecies differences in thoracic limb anatomy/function, and also functional differences between hare thoracic and pelvic limbs. These data will increase our understanding of the roles of muscles in cursorial locomotion whilst also allowing future musculoskeletal modelling, and inverse dynamics calculations to be undertaken.
Materials and methods
Eight hares [mass 3454 ± 500 g (mean ± SD)] were obtained from a game supplier (Freemantle Farm, North Oakley, UK) less than 24 h post-mortem during the early autumn. Due to the time of year hares were obtained it is likely that most were juveniles. Hares were immediately frozen (−20 °C), and stored for 24 h at 4 °C prior to dissection. Seven right thoracic limbs were skinned, and individual muscles were identified, exposed and cleared of fascia. They were then removed systematically and detailed dissections of muscle–tendon architecture were carried out in accordance with methodology described in Williams et al. (2007). In addition, moment arms of major muscles were measured via the tendon-travel method in four left thoracic limbs [also see Williams et al. (2007), for full details of methodology].
Results
Muscle mass
Thirty-eight muscles were identified in total, with origins, insertions and actions (as estimated from our dissections) given in Table 1. Figure 1 shows the superficial musculature of the thoracic limb. All muscle data are given in Table 2. Total (individual) thoracic limb locomotor muscle mass was small at 140.4 ± 19.2 g (all shown are mean ± SD), accounting for only 4.64 ± 0.27% of total body mass. The largest muscle in the thoracic limb was the long head of Triceps brachii (TBLO; 16.5 ± 2.3 g), 6% of total thoracic limb muscle mass. This was closely followed by Pectoralis profundus (PP; 16.0 ± 6.0 g), Latissimus dorsi (LD; 13.9 ± 2.1 g) and Supraspinatus (SSP; 13.3 ± 1.6 g). The smallest muscles in the thoracic limb were distal muscles such as Extensor carpi ulnaris (ECU; 0.16 ± 0.03 g), Abductor pollicis longus (AP; 0.23 ± 0.11 g) and Flexor carpi radialis (FCR; 0.45 ± 0.07 g).
Table 1.
Origin, insertion and suggested actions of the major muscles of the hare thoracic limb
| Muscle | Abbreviation | Origin | Insertion | Action |
|---|---|---|---|---|
| Trapezius cervicis | TPC | Nuchal ligament | Metachromion and fascia overlying scapula | Scapular elevation and limb protraction |
| Trapezius thoracis | TPT | Lumbodorsal fascia | Dorsal portion of scapula spine | Scapular elevation and limb retraction |
| Rhomboideus cervicis | RHC | Nuchal ligament | Anterior two-thirds of vertebral border of scapula | Scapular elevation and limb protraction |
| Rhomboideus thoracis | RHT | Spinous processes of thoracic vertebrae T1 to T7 | Posterior one third of vertebral border of scapula | Scapula elevation and limb retraction |
| Serratus ventralis cervicis | SVC | Transverse processes of posterior five cervical vertebrae and anterior two ribs. | Anterior four fifths of medial surface of ventral border of scapula | Suspends limb from trunk; scapular rotation |
| Serratus ventralis thoracis | SVT | Middle portion of 3rd–6th ribs, alternating with external abdominal oblique muscle | Deep surface of posterior 2/5 of ventral border of scapula | Suspends limb from trunk; raises thorax; scapular rotation |
| Latissimus dorsi | LD | Posterior four ribs and lumbo-dorsal fascia | Deltoid tuberosity and fascia over brachium | Limb retraction; when limb fixed, advances trunk |
| Pectoralis profundus | PP | Entire lateral portion of sternum; distal extremeties of ribs 4–9 | Anterior/anterior-medial surface of middle third of humerus | Adduction and retraction of limb |
| Pectoralis descedens | PD | Manubrium of sternum | Deltoid tuberosity | Adduction and protraction of limb |
| Pectoralis transversus | PTV | Sternum from the attatchment of 4th−7th costal cartilages. | Anterior surface of head of humerus and antebrachial fascia | Adduction and retraction of limb |
| Subclavius | SC | Manubrium of sternum and costal cartilages 1–4 | Remnant of clavicle and ventral 1/4 of scapula spine; supraspinous fascia and medial angle of scapula | Adduction and protraction of limb |
| Brachiocephalicus | BCP | Mastoid process | Anterior surface of humerus; remnant of clavicle within muscle | Limb protraction; lateral neck flexion |
| Deltoideus – Acromial portion | DA | Acromion | Deltoid tuberosity | Shoulder flexion; limb abduction(?) |
| – Scapular portion | DS | Mid portion of scapula spine and fascia overlying scapula | Suprahamate process and deltoid tuberosity | As above |
| Infraspinatus | ISP | Entire lateral surface if posterior portion of of scapula and scapula spine | Greater tubercle of humerus | Shoulder flexion; limb abduction |
| Supraspinatus | SSP | Lateral surface of anterior portion of scapula and scapula spine | Greater tubercle of humerus | Shoulder extension |
| Subscapularis | SS | Entire medial surface of scapula | Greater tubercle of humerus | Limb adduction |
| Teres major | TMJ | Anterior portion of dorsal boarder of scapula | Anterio-medial surface of humerus | Shoulder flexion |
| Teres minor | TMN | Ventral part of anterior border of scapula | Greater tubercle of humerus | Shoulder flexion |
| Triceps brachii – Long head | TBLO | Ventral part of anterior boarder of scapula | Olecranon | Shoulder flexion; Elbow extension |
| – Lateral head | TBLA | Greater tubercle and lateral portion of humerus | Olecranon | Elbow extension |
| – Medial head | TBM | Posterior surface of humerus | Olecranon | As above |
| Biceps brachii | BB | Supraglenoid tubercle | Ventromedial surface of ulna and medial surface of radius | Shoulder extension; elbow flexion |
| Brachialis | BCH | Lateral surface of humerus | As above | Elbow flexion |
| Extensor carpi radialis | ||||
| – brevis | ECRB | Lateral epicondyle | Base of second metacarpal | Carpal extension |
| – longus | ECRL | As above | Base of third metacarpal | As above |
| Abductor pollicis | AP | Antero-lateral surface of radius and ulna | Base of first metacarpal | Abducts and extends first digit |
| Extensor digitorum communis | EDC | Lateral epicondyle and proximal end of ulna | Distal phalanges of digits 2–5 | Digital extension |
| Extensor digitorum lateralis | ||||
| – quarti proprius | EDL4 | Lateral epicondyle | Distal phalanx of digit 4 | Extends digit 4 |
| – quinti proprius | EDL5 | Lateral epicondyle and lateral surface of ulna | Base of first phalanx of digit 5 and head of fifth metacarpal bone. | Extends digit 5 |
| Extensor carpi ulnaris | ECU | Lateral epicondyle and proximal portion of lateral surface of ulna | Base of fifth metacarpal | Extends carpus |
| Pronator teres | PT | Medial epicondyle | Ventral surface of radius | Pronation |
| Flexor carpi radialis | FCR | As above | Base of second metacarpal | Carpal flexion |
| Flexor digitorum superficialis | FDS | Medial epicondyle and proximal portion of ulna | Bases of 2nd phalanges of the four lateral digits | Digital flexion |
| Flexor digitorum profundus | ||||
| – humeral head | FDPH | Medial epicondyle | Bases of distal phalanges | Digital flexion |
| – middle head | FDPM | Ventral surface of ulna | ||
| – radial head | FDPR | Ventral surface of radius | ||
| – ulnar head | FDPU | Medial epicondyle | ||
| Flexor carpi ulnaris | FCU | Medial epicondyle and medial surface of olecranon | Accessory carpal bone | Carpal flexion |
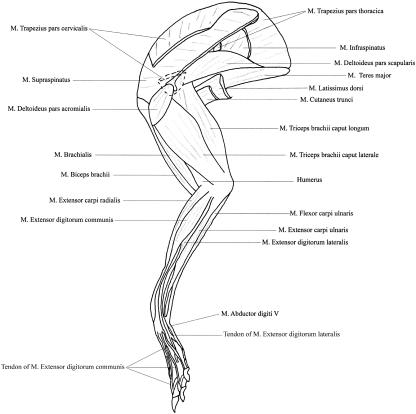
Fig. 1.
Line drawing showing the superficial musculature of the lateral aspect of the hare thoracic limb.
Table 2.
Muscle data: muscle mass, volume, belly length, fascicle length, physiological cross-sectional area (PCSA), pennation angle, and estimated maximum isometric force and power. Values are calculated as per methods described in the text. Values indicate means and range (n = 7)
| Muscle | Muscle mass (g) | Volume (cm3) | Belly length (mm) | Fascicle length (mm) | PCSA (mm2) | Pennation angle (°) | Force (N) | Power (W) |
|---|---|---|---|---|---|---|---|---|
| Anconeus | 0.72 (0.59–0.91) | 0.68 | 26 (22–31) | 7 (5–8) | 10.1 | 33 (23–40) | 30 | 0.24 |
| Abductor policis longus | 0.23 (0.13–0.35) | 0.22 | 47 (30–80) | 5 (1–10) | 4.1 | 28 (25–30) | 12 | 0.09 |
| Biceps brachii | 4.53 (3.45–4.93) | 4.27 | 75 (68–77) | 19 (11–41) | 22.6 | 28 (20–41) | 68 | 0.53 |
| Brachialis | 1.36 (0.65–2.70) | 1.28 | 72 (60–89) | 30 (19–40) | 4.2 | 11 (0–30) | 13 | 0.10 |
| Brachiocephalicus | 7.08 (3.59–8.75) | 6.68 | 167 (123–205) | 151 (115–186) | 4.4 | 0 | 13 | 0.10 |
| Deltod (acromial head) | 0.73 (0.45–0.94) | 0.69 | 35 (21–41) | 8 (5–9) | 9.2 | 33 (18–40) | 28 | 0.22 |
| Deltoid (scapular head) | 1.51 (1.26–2.10) | 1.42 | 69 (63–80) | 15 (8–25) | 9.5 | 8 (0–22) | 29 | 0.23 |
| Extensor carpi radialis | 1.54 (0.98–1.97) | 1.45 | 62 (40–112) | 24 (10–33) | 7.5 | 9 (0–26) | 23 | 0.18 |
| Extensor carpi ulnaris | 0.16 (0.12–0.18) | 0.15 | 52 (13–50) | 3 (1–5) | 4.9 | 27 (20–33) | 15 | 0.11 |
| Extensor digitorum communis | 1.10 (0.70–1.63) | 1.03 | 46 (40–51) | 14 (11–18) | 7.4 | 12 (0–33) | 22 | 0.17 |
| Extensor digitorum lateralis (quarti) | 0.33 (0.13–0.52) | 0.31 | 53 (33–68) | 7 (5–10) | 3.7 | 18 (0–27) | 11 | 0.09 |
| (quinti) | 0.29 (0.21–0.36) | 0.27 | 55 (44–69) | 7 (6–7) | 3.9 | 28 (25–30) | 11 | 0.09 |
| Flexor carpi radialis | 0.45 (0.35–0.52) | 0.43 | 55 (50–63) | 7 (3–10) | 6.0 | 27 (20–34) | 18 | 0.14 |
| Flexor carpi ulnaris | 1.27 (0.89–1.80) | 1.21 | 75 (72–78) | 35 (18–55) | 34.5 | 35 (23–43) | 10 | 0.81 |
| Flexor digitorum profundus (humeral head) | 1.44 (0.70–2.47) | 1.35 | 79 (63–89) | 7 (3–14) | 18.3 | 31 (21–37) | 55 | 0.43 |
| (middle head) | 0.49 (0.15–1.10) | 0.46 | 58 (39–86) | 10 (8–12) | 4.8 | 26 (20–30) | 14 | 0.11 |
| (radial head) | 1.27 (0.26–2.30) | 1.20 | 76 (60–97) | 13 (6–29) | 9.5 | 27 (19–34) | 28 | 0.23 |
| (ulnar head) | 0.62 (0.50–0.79) | 0.59 | 58 (47–76) | 13 (8–26) | 4.5 | 23 (10–37) | 13 | 0.10 |
| Flexor digitorum superficialis | 2.04 (1.65–2.38) | 1.92 | 87 (80–93) | 6 (4–8) | 32.8 | 31 (25–34) | 98 | 0.78 |
| Infraspinatus | 8.17 (3.77–10.46) | 7.71 | 81 (56–94) | 21 (12–42) | 36.6 | 28 (10–37) | 110 | 0.87 |
| Latissimus dorsi | 13.9 (10.3–16.0) | 13.1 | 17 (15–19) | 139 (117–152) | 9.4 | 0 | 28 | 0.22 |
| Pectoralis descedens | 6.64 (5.53–7.50) | 6.26 | 147 (135–107) | 12 (10–13) | 5.2 | 0 | 16 | 0.13 |
| Pectoralis profundus | 6.94 (4.40–8.56) | 6.54 | 73 (60–83) | 45 (42–48) | 14.6 | 0 | 44 | 0.34 |
| Pectoralis transvs. | 15.9 (10.3–23.7) | 15.1 | 143 (110–175) | 123 (114–135) | 12.3 | 0 | 37 | 0.29 |
| Rhomboideus (cervicis) | 4.71 (3.01–8.57) | 4.45 | 96 (63–130) | 63 (52–87) | 7.0 | 0 | 21 | 0.17 |
| (thoracis) | 6.24 (3.33–11.05) | 5.09 | 74 (53–98) | 57 (44–72) | 8.6 | 0 | 26 | 0.21 |
| Subclavius | 5.20 (3.50–6.51) | 4.91 | 145 (111–171) | 108 (95–123) | 4.5 | 0 | 14 | 0.10 |
| Subscapularis | 7.88 (6.66–10.30) | 7.43 | 68 (53–79) | 10 (7–12) | 76.9 | 33 (27–40) | 231 | 1.81 |
| Supraspinatus | 13.3 (10.5–15.0) | 12.6 | 83 (72–90) | 35 (16–73) | 36.1 | 28 (20–36) | 108 | 0.85 |
| Serratus ventralis (cervicis) | 7.89 (3.93–14.0) | 7.44 | 92 (66–134) | 67 (55–79) | 11.1 | 0 | 33 | 0.26 |
| (thoracis) | 9.10 (6.43–12.2) | 8.59 | 79 (68–86) | 63 (51–74) | 13.7 | 0 | 41 | 0.32 |
| Triceps brachii (lateral head) | 8.24 (6.70–9.14) | 7.77 | 82 (69–101) | 29 (21–41) | 26.5 | 27 (14–40) | 79 | 0.63 |
| (long head) | 16.5 (12.3–19.8) | 15.6 | 94 (80–102) | 20 (14–27) | 79.6 | 34 (12–47) | 239 | 1.89 |
| (medial head) | 2.32 (1.71–2.70) | 2.19 | 81 (73–87) | 19 (11–42) | 11.3 | 26 (0–42) | 34 | 0.26 |
| Teres major | 9.51 (8.00–11.3) | 8.98 | 89 (76–99) | 52 (29–76) | 17.2 | 0 | 52 | 0.41 |
| Teres minor | 1.09 (0.23–4.30) | 1.04 | 27 (19–39) | 18 (5–62) | 5.9 | 24 (20–30) | 18 | 0.14 |
| Trapezius (cervicis) | 5.63 (1.62–10.4) | 5.35 | 90 (55–145) | 60 (44–88) | 8.9 | 0 | 27 | 0.21 |
| (thoracis) | 4.51 (3.25–8.35) | 4.26 | 105 (68–120) | 66 (37–87) | 6.5 | 0 | 19 | 0.15 |
Tendon
All tendon data are provided in Table 3. The only discernible proximal limb tendons were those of Biceps brachii and Triceps brachii, which were short with large cross-sectional areas (CSAs). Most tendons were in the distal limb and were comparatively longer than those in the proximal limb, with small CSAs. The tendon with the largest CSA in the distal limb was that of the Flexor digitorum profundus (FDP; 0.55 ± 0.15 mm2), which was also by far the heaviest tendon in the thoracic limb.
Table 3.
Tendon data: mass, volume, and resting length, and estimated cross-sectional area (CSA), stress, strain, and length change of selected thoracic limb tendons. Parameters calculated using methods described in the text. Data are means, values in parentheses indicate range (n = 7)
| Muscle–tendon unit | Mass (g) | Volume (mm3) | Rest length (mm) | CSA (mm2) | Stress (MPa) | Strain (%) | Length change (mm) |
|---|---|---|---|---|---|---|---|
| Biceps brachii | 0.05 (0.03–0.1) | 4.7 | 13 (12–13) | 0.3 | 27 | 1.8 | 0.2 |
| Extensor carpi radialis | 0.06 (0.01–0.12) | 5.6 | 115 (70–146) | 0.1 | 46 | 3.1 | 0.2 |
| Extensor carpi ulnaris | 0.03 (0.01–0.05) | 2.5 | 50 (32–66) | 0.1 | 54 | 3.6 | 1.5 |
| Extensor digitorum communis | 0.16 (0.1–0.31) | 15.4 | 95 (48–108) | 0.1 | 20 | 1.3 | 1.3 |
| Extensor digitorum lateralis | 0.04 (0.01–0.06) | 3.6 | 76 (50–104) | 0.1 | 62 | 4.1 | 3.5 |
| Flexor carpi radialis | 0.03 (0.01–0.06) | 2.4 | 46 (33–59) | 0.1 | 62 | 4.1 | 1.9 |
| Flexor carpi ulnaris | 0.08 (0.06–0.10) | 7.5 | 35 (32–46) | 0.2 | 51 | 3.4 | 1.1 |
| Flexor digitorum profundus | 0.50 (0.30–0.62) | 47.0 | 76 (50–93) | 0.6 | 15 | 1.0 | 0.8 |
| Flexor digitorum superficialis | 0.18 (0.10–0.52) | 17.3 | 42 (20–50) | 0.3 | 56 | 3.7 | 4.2 |
| Triceps brachii Long head | 0.08 (0.06–0.10) | 7.5 | 7 (3–13) | 0.1 | 27 | 1.8 | 0.2 |
| Lateral head | 0.07 (0.01–0.20) | 6.9 | 9 (5–12) | 0.1 | 33 | 2.2 | 0.2 |
Muscle architecture
Distribution of fascicle lengths (Table 2) showed a distinct trend. Proximal muscles, in particular the extrinsic muscles, had longer fascicles, and the distal muscles relatively short fascicles. Fascicle lengths varied both between hares and within a muscle (hence ten measurements were taken from a variety of regions within the muscle belly). However, differences between regions within a muscle have not been quantified, as it was often difficult to obtain more than six measures for fascicle length, due to the small size of hare muscles in comparison with larger species which have been studied (Payne et al. 2005a; Smith et al. 2006); hence, it was not feasible to divide muscles into distinct regions.
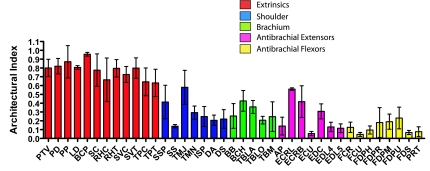
Longest fascicles were seen in brachiocephalicus (BCP) (151 ± 5 mm) and LD (135 ± 14 mm). The pectoral muscles and Subclavius also had long fascicles. The smaller distal limb muscles had the shortest fascicles, notably ECU and flexor carpi ulnaris (FCU) (both 3 ± 2 mm), and flexor digitorum superficialis (FDS) (6 ± 2 mm). The architectural index (AI) was calculated as the ratio between fascicle length and muscle belly length. This gives a true indication of the length of the fascicles in relation to the size of the muscle (Fig. 2). All extrinsic muscles, including those mentioned above, and Serratus ventralis (SVC, SVT), Rhomboideus (RHC, RHV) and Trapezius (TPC, TPV) had AIs of greater than 0.6. Other muscles with reasonably high AIs included Teres major and minor (TMN, TMJ; 0.57) and Extensor carpi radialis (ECR; 0.49). Lowest AIs were in the distal limb; in particular, ECU, FCU (both 0.05) and FDS (0.07) were exceptionally low, indicating a high degree of pennation.
Fig. 2.
Archituctural index (AI; fascicle length divided by muscle length) – for hare thoracic limb muscles. Abbreviations are given in Table 1. All values are means ± SD (n = 7).
Fibre pennation angles in the hare thoracic limb ranged from 0 to 47° (Table 2). All extrinsic muscles were parallel fibred. Of the other muscles, only TMJ had parallel fascicles. The remaining muscles had varying degrees of pennation, the highest being in FCU (35 ± 7°) and the long head of Triceps brachii (TBLO; 34 ± 11°). The acromial head of the Deltoid muscle (DA), Subscapularis (SS), FDS and Humeral head of Flexor digitorum profundus (DDFH) were also highly pennate.
Moment arms
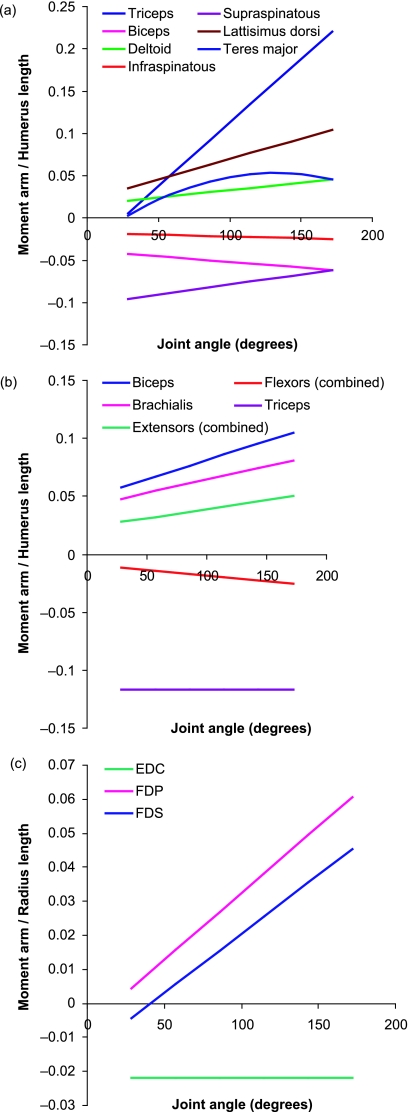
Moment arms were not measured for all muscles. Muscles that, as a consequence of their position within the limb, could not act in flexion or extension were neglected, as were moment arms for flexors and extensors of the digits. In addition, muscle–tendon units that were positioned very closely to one another, with the muscles appearing to follow the same line of action, were measured as one. Moment arms were scaled by limb segment length [to the radius length for muscles acting at the wrist joint, and to the humerus length for those at the shoulder and elbow (Payne et al. 2006; Williams et al. 2007)]. Segment lengths are given in Table 4. Equations for the relationships of tendon travel against joint angle are provided in Table 5. Moment arm curves are provided in Fig. 3, and are presented so that muscles acting to flex a joint have positive values whilst those that act to extend the joint in question have a negative value. The sign, however, is unimportant and absolute values will be referred to here. Maximum mean moment arms and joint moments of force, with the joint angles (within physiologically realistic range) at which these occur, and muscle fascicle length/moment arm (MFL : MA) ratios are given in Table 6.
Table 4.
Segment lengths used to calculate scaled moment arm values
| Hare no. | Humerus length (mm) | Radius length (mm) |
|---|---|---|
| 1 | 112 | 97 |
| 2 | 115 | 102 |
| 3 | 110 | 102 |
| 4 | 115 | 105 |
Table 5.
Equations of the trend lines fitted to the plots of tendon excursion against joint angle of flexion in the hare thoracic limb. Equations and R2 values are means, n = 4
| Muscle–tendon Unit | Joint of action | Equation | R2 |
|---|---|---|---|
| Biceps brachii | Elbow | y = 1.59x2 + 4.84x − 1.32 | 0.98 |
| Shoulder | y = −0.375x2 − 3.90x + 20.5 | 0.96 | |
| Brachialis | Elbow | y = 1.72x2 + 2.64x + 2.46 | 0.95 |
| Deltoid | Shoulder | y = 0.712x2 + 1.48x + 3.16 | 0.83 |
| Extensor digitorum communis | Wrist | y = −2.46x + 28.5 | 0.91 |
| Elbow Extensors (combined) | Elbow | y = 0.89x2 + 2.40x + 20.4 | 0.92 |
| Flexor digitorum profundus | Wrist | y = 1.26x2 − 0.77x − 12.5 | 0.89 |
| Flexor digitorum superficialis | Wrist | y = 1.11x2 − 1.61x + 19.3 | 0.93 |
| Elbow Flexors (combined) | Elbow | y = −0.55x2 − 0.95x − 12.2 | 0.92 |
| Infraspinatus | Shoulder | y = −1.19x2 − 1.78x + 13.0 | 0.89 |
| Latissimus dorsi | Shoulder | y = 2.79x2 + 2.17x + 8.61 | 0.89 |
| Supraspinatus | Shoulder | y = 1.34x2 − 10.2x + 33.6 | 0.95 |
| Teres major | Shoulder | y = 0.67x3 + 1.92x2 + 0.06x + 14.6 | 0.90 |
| Triceps (combined) | Elbow | y = −11.7x + 35.2 | 0.99 |
| Shoulder | y = 4.30x2 − 3.71x + 7.34 | 0.98 |
Fig. 3.
Mean moment arms (scaled to humerus or radius length, see Methods described in text; mean humerus and radius lengths are 113 and 102 mm, respectively; individual segment lengths are given in Table 4) for selected thoracic limb muscles across the full range of physiologically relevant joint positions. n = 4. (a) Muscles acting at the shoulder joint; (b) muscles acting at the elbow joint; (c) muscles acting at the carpal joint. A joint angle of 180° indicates the joint is straight.
Table 6.
Maximum moment arms, joint moments of force and MFL:TLC ratios for thoracic limb muscles
| Muscle–tendon unit | Joint of action | Joint angle of maximum moment arm (i.e. max flexion/extension) | Mean maximum muscle moment arm (mm) | Maximum joint moment of force (N.cm) | Muscle fascicle length: moment arm ratio |
|---|---|---|---|---|---|
| Triceps brachii | Shoulder | Max extension | 22.1 (17.7–31.1) | 777.9 | 1.0 |
| Elbow | All | 11.7 (5.2–14.0) | 411.8 | 2.0 | |
| Biceps brachii | Shoulder | Max extension | 6.1 (5.7–6.6) | 41.5 | 3.1 |
| Elbow | Max extension | 10.6 (7.6–13.6) | 72.1 | 1.8 | |
| Deltoid | Shoulder | Max extension | 4.7 (1.7–16.1) | 13.2 | 1.7 |
| Infraspinatus | Shoulder | Max extension | 2.5 (2.0–6.4) | 27.5 | 8.4 |
| Supraspinatus | Shoulder | Max flexion | 9.6 (7.1–11.7) | 103.7 | 3.6 |
| Latissimus dorsi | Shoulder | Max extension | 10.6 (2.4–18.7) | 29.7 | 13.1 |
| Teres major | Shoulder | Max extension | 5.2 (4.0–12.8) | 27.0 | 10.0 |
| Brachialis | Elbow | Max extension | 8.2 (7.0–9.6) | 10.7 | 3.7 |
| Extensors (combined) | Elbow | Max extension | 5.1 (3.5–5.9) | 30.6 | 2.2 |
| Flexors (combined) | Elbow | Max extension | 2.6 (1.1–4.0) | 59.0 | 5.0 |
| Extensor digitorum communis | Wrist | All | 2.5 (1.9–3.5) | 5.50 | 5.6 |
| Flexor digitorum profundus | Wrist | Max extension | 6.8 (5.2–7.6) | 74.8 | 1.6 |
| Flexor digitorum superficialis | Wrist | Max extension | 5.1 (3.8–5.7) | 50.0 | 1.2 |
The moment arm at the shoulder (Fig. 3a) of ISP was linear in shape and remained constant with changing joint angle. Those of Triceps brachii, BB, DA, and LD (all linear) all increased with joint extension, with maximum moment arms reached at full joint extension. The converse was true for the moment arm of SSP (also linear), which decreased with joint extension, its maximum moment arm being in full joint flexion. The moment arm at the shoulder of TMJ (parabolic) increased with joint extension, with the maximum moment arm occurring at, or near, full joint flexion. At the elbow joint (Fig. 3b), moment arms of all muscles, with the exception of Triceps brachii, were linear, and increased with joint extension. Maximum moment arms were reached in full joint extension. The moment arm at the elbow joint of Triceps, however, remained constant throughout joint motion. At the wrist (carpal) joint (Fig. 3c), the moment arm of EDC remained constant throughout joint movement, whilst those of FDP and FDS increased linearly with joint extension.
Discussion
Gross anatomy
The thoracic limb of the hare was small with surprisingly short distal limb tendons (in comparison with the forelimb of other cursors such as the horse). In small non-cursorial animals (including rabbits), the limb and trunk are connected by the sterno-clavicular, acromio-clavicular and coraco-clavicular articulations. The hare does not have a clavicle, rather a small, calcified remnant within the extrinsic musculature (see Table 1). The thoracic limb is hence attached to the trunk via a synsarcosis (the scapula is joined to the trunk only via muscle, rather than bones), a feature also present in other cursors such as horses, and that frees the motion of the scapula on the trunk, effectively increasing limb length during locomotion (and hence allowing a longer stride length, and lower stride frequency at a given speed). Extrinsic musculature attaches the thoracic limb to the trunk and as a result makes up the large part of the mass of the thoracic limb of the hare (49%). The anatomy of the hare thoracic limb differs from the horse (Brown et al. Pandy, 2003a; Payne et al. 2005b) in one main respect – it has four rather than three heads of the FDP muscle (humeral, radial, middle and ulnar). Other than this difference, the gross muscular anatomy of the thoracic limb bore similarities to that of larger cursorial species.
Functional distribution of muscle within the thoracic limb
Total thoracic limb locomotor muscle mass (both limbs, given symmetry is assumed) accounted for 9.24 ± 0.54% of total body mass. This amounts to only 26% of the total available locomotor muscle or approximately 35% of body mass, given that the total hind limb contribution is double this at 16.32 ± 0.88%, and spinal muscle mass is 8.9 ± 1.4% body mass (Williams et al. 2007).
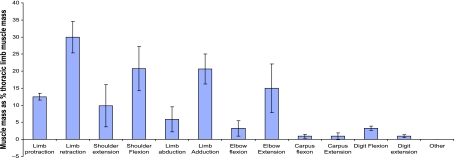
The functional distribution of muscle within the thoracic limb can be seen in Fig. 4 (note that percentages do not add to 100% given that some muscles may perform more than one role). Extrinsic muscles account for a large amount of the thoracic limb musculature, and hence 30% of the functional thoracic limb volume can be considered capable of limb retraction and 13% capable of limb protraction. In addition, 20% of muscle is available for limb adduction. That such a large amount of the limb mass of the hare is capable of these functions is not surprising given that not only must extrinsic muscles withstand the ground reaction force, and hence have to stabilise the limb, but they must also be able to move the limb/trunk during the swing stance phase of locomotion. Studies in horses suggest that limb retraction, at least at low speeds, is largely an active process (Clayton et al. 2000), whilst elastic mechanisms are likely to be more important in limb protraction (Wilson et al. 2003). This may be a reason for the larger proportion of muscle that can retract the limb in the hare compared with protraction if this is the case as in other cursorial quadrupeds. Alternatively, Triceps brachii accounts for the majority of ‘limb retractor’ volume, and it is possible that this muscle has an important role in stability rather than retraction (see below). The large amount of muscle capable of limb adduction is not surprising either given that stabilising the limb is probably very important when attached to the trunk only by musculature. In addition, for an animal that has to turn rapidly at high speed in order to escape predation, it might be functionally advantageous to have a large amount of muscle mass acting to stabilise the limb.
Fig. 4.
Distribution of muscle within the thoracic limb, grouped by function. Data are means ± SD (n = 7).
At the shoulder joint, 10% of thoracic limb muscle mass is capable of joint extension and 21% of flexion. This large functional division, and volume biased towards flexion of the shoulder joint, suggests that shoulder flexion is important in the hare and could suggest that the forelimb assists the pelvic limb in propulsion. However, this contrasts with current generalised thinking on quadrupedal locomotion, which has shown that the thoracic limbs mainly play a supportive role and decelerate the centre of mass (Payne et al. 2005a; Smith et al. 2006). Of course, differences exist in the locomotor strategies, anatomy and lifestyle of hares and other cursors. For example, hares are able to saltate at low speeds in addition to high-speed galloping, where other cursors might walk, trot or pace. It is possible that saltation requires the forelimb to assist the hind limb in its acceleratory role. The hare has been observed to locomote bipedally (Grange, 1932), and therefore perhaps to assume the forelimb acts similarly in all quadrupeds is not reasonable, and conceivably the role of the forelimb could be far less than would be expected. Unlike many cursors, the hare will use its forelimbs for more than simply locomotor functions – grooming, feeding and most significantly digging will all be necessary. Digging would presumably require large shoulder flexor and elbow extensor muscles, and this also seems a reasonable explanation for the apparent differences in the distribution of shoulder musculature in hares compared with other quadrupedal cursors.
At the elbow joint, differences in flexor vs. extensor muscle mass are also apparent, with only 3% of thoracic limb mass available for elbow flexion in comparison with 15% for extension. The massive Triceps brachii (TBLA, TBLO, TBM) muscle accounts for the majority (97%) of this elbow extensor mass, being the largest non-extrinsic muscle in the thoracic limb. Triceps has been suggested as a major limb retractor in the horse (Watson, 2004). In addition, however, the long head is antagonistic to biceps brachii by resisting shoulder extension during the stance phase. The long head also consists of predominantly type IIa muscle fibres, suggesting a dynamic role in the limb (Ryan et al. 1992). By contrast, the medial head has been suggested to act as an antigravity muscle, contributing to the postural maintenance of the forelimb by preventing flexion at the elbow joint during passive stance (Watson, 2004). It may, in addition, act to decelerate and absorb some of the kinetic energy of the limb at the end of the swing phase by resisting elbow flexion (Tokuriki et al. 1989; Tokuriki, 1999; Preedy, 1998). The medial head of triceps contains almost exclusively type I muscle fibres, indicating a more postural role in the limb (Ryan et al. 1992). Thus, the medial head of triceps may have an important role in stiffening the limb prior to foot on, and during foot contact to provide a rigid strut to land on and vault over during the stance phase (thus is important in resisting gravity), whilst the long and lateral heads may have major roles in decelerating and retracting the limb.
Biceps brachii (BB) in the horse has a large internal tendon, which has been thought to contribute to the role of the Biceps muscle as a ‘catapult’, enabling fast, largely passive limb protraction (Wilson et al. 2003). An internal tendon was present in the hare BB, although this was not exceptionally large. Given the small (intrinsic) functional volume capable of elbow flexion (BB being the major muscle here), and also as BB forms only 25% of the total volume of muscle capable of extending the shoulder, two possibilities arise. Either this elastic mechanism is also important in the hare and hence elbow flexor muscle mass does not need to be substantial (this seems unlikely given the apparent lack of tendon/elastic tissue/fascia surrounding the biceps muscle), or other muscles such as BR and SS (∼4 and 7% thoracic limb mass, respectively), which have until recently been suggested as potential forelimb protractors in the horse, are more important in limb protraction here than BB. Given the large size of these muscles in the hare, perhaps BB has a lesser role in limb protraction in this species, and hence BB and subsequently the functional volume of muscle available for elbow flexion is small. If the thoracic limb, as seems likely, acts as a strut during locomotion, it may be that elbow flexion is unnecessary except during the early part of the swing phase. If other limb protractors at this time are active as well, then it may not be necessary for the hare to have a large elbow flexor volume. Alternatively, the relatively small mass of the hare distal forelimb may allow elbow flexion via a very small flexor volume.
More distally, very small functional volumes compared with those higher in the limb, are available for carpal and digital flexion and extension (Carpus, 1% and 1%; Digits, 3% and 1%, respectively). This highlights, as in the pelvic limb of the hare (Williams et al. 2007), the replacement of distal muscle volume with tendon, and hence a proximal to distal reduction in muscle mass. This adaptation has been described many times before in various cursorial animals (Heglund et al. 1974; Taylor et al. 1974; Alexander & Vernon, 1975; Alexander & Bennet-Clark, 1977; Ker et al. 1989; Biewener, 1998; Brown et al. 2003b; Pasi & Carrier, 2003; Payne et al. 2005a; Smith et al. 2006) as a mechanism by which high-speed animals reduce the effects of rotational inertia on the swinging limb.
Similar amounts of muscle are available for flexion and extension at the carpus. The carpal muscles are likely to flex the joint during the swing phase of the stride, and to extend this joint during stance. In the digits, flexor functional volume exceeds extensor volume three-fold. Digital flexion is likely to be important in tasks such as digging. The digital flexors may, however, perform other functions such as damping vibrations in the limb (Wilson et al. 2001), antigravity roles or storing elastic energy. So whilst it is useful to some extent to study the functional distribution of muscle mass within the limb to give an overall picture of the functions of muscle groups at joints, further information on how individual muscles are used and their roles during locomotion is necessary. This information can be gained by assessing the architecture of each muscle tendon unit.
Muscle architecture
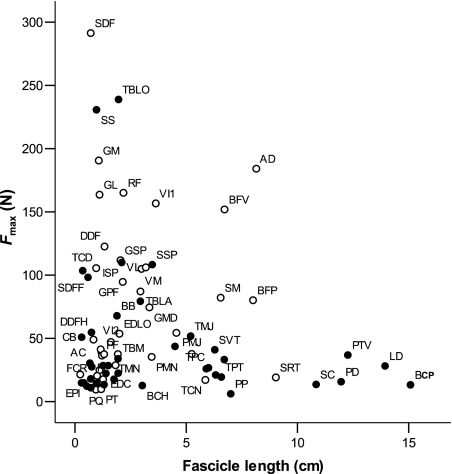
All extrinsic muscles had high AIs – indicating long muscle fascicles (Fig. 2) orientated in parallel – and had no external tendon. Muscles with many sarcomeres orientated in series with one another are capable of producing a greater length change than those with few located in series, and hence have a higher velocity of contraction. These long fascicles also allow the muscle to generate force over a wide range of motion. Hence, long fascicles are indicative of a limb moving function. Thus, muscles that are positioned ideally for limb protraction (BCP) and retraction (LD, PP) have the architecture to suggest that they have an important role in range of limb movement. The maximum isometric force that a muscle can generate (Fmax) was calculated (see Methods). When plotted against fascicle length for thoracic limb muscles, a representation is created of the relative role of muscles in locomotion (Fig. 5). All extrinsic muscles are located toward the bottom right of this plot, as they have relatively small PCSAs yet long fascicles. Any muscle in this area of the graph is specialised for velocity of contraction and range of motion. Figure 5 also depicts pelvic limb muscles. Notice the absence of any pelvic limb muscles in the bottom right area. Most long fibred muscles in the pelvic limb are of high volume, and hence are specialised more for power production (these muscles can be found towards the middle of the plot).
Fig. 5.
Estimated maximum isometric force generating capacity vs. Fascicle length for thoracic (solid) and pelvic limb (open) muscles. Abbreviations are given in Table 1.
Whilst in the pelvic limb AI decreased distally, and muscles acting at the stifle had higher AIs than tarsus and subsequently digital muscles (Williams et al. 2007), no such pattern was visible in the forelimb. Low AIs were seen for many muscles in the thoracic limb other than the extrinsic muscles, TMJ and TMN. This indicates that many muscles in the hare thoracic limb have an architecture that would suggest that they are adapted towards developing force economically. The degree of force, however, that they are able to generate depends upon the PCSA of the muscle, which is dependent on not only fascicle length but also muscle volume. Given this, TBLO and SS muscles both have large PCSAs and are hence able to generate large amounts of force (Fig. 5). In fact, both are able to generate similar amounts of force to some of the high force-producing, distally situated, pelvic limb muscles, i.e. Flexor digitorum superficialis and Gastrocnemius. This is interesting given that both TBLO and SS are located proximally in the thoracic limb. SS is located medially to the scapula, and is responsible for limb adduction. It must therefore resist large forces during turning, especially whilst the hare is manoeuvring at high speeds. The architecture of SS suggests that it is not involved in controlling large positional changes of the limb, as its short fascicles limit range of motion. Thus, the high force-generating capacity of SS may simply be required to perform the roles of controlling and stabilising the limb, and maintaining proximity of the limb to the rib cage.
TBLO is a shoulder flexor and elbow extensor and may play a role in limb retraction. It has the largest volume of all the thoracic limb muscles (15.6 ± 3.5 cm3) and a large PCSA, and thus it is able to generate large amounts of force. Its short-fibred structure means that it is not suited to fast speeds of contraction and high power production, and so is unlikely to have a dynamic role. It also has a short tendon of insertion that is unlikely to reach strains near those of the more distal limb tendons (Table 3), and so potential for elastic energy storage appears limited. This presents somewhat of a paradox as to why such a large muscle, which seems not to play an active role in joint motion or in energy storage and release, would need to generate so much force. It may be that this is another adaptation for digging, but it seems unusual given that with parallel fascicles for example, the muscle would be capable of producing a greater velocity of contraction and range of movement. TBLO must therefore have another function, possibly as a supporting role within the limb. It has been proposed that the medial head of triceps in the horse has a stabilising role during the stance phase (Watson, 2004), whilst long and lateral heads (which are long fibred) have a dynamic role in elbow extension and decelerating the limb. In fact, all three heads are pennate in the hare, and Triceps brachii as a whole was calculated here to be capable of producing 350 N, 50% more force than the vast Adductor and Biceps femoris muscles, and even superior to the highest force-producing muscle of FDS in the pelvic limb (Williams et al. 2007). If, as suggested previously, the thoracic limb of the hare acts as a ‘strut’, and is important in supporting and deflecting the body during high-speed locomotion, it is possible that such high forces are necessary in order to resist flexion of the elbow during stance (or during periods when the hindlimbs are off the floor, such as when kicking, or during intraspecific displays). Another potential role for the Triceps muscle is that of ‘dynamic control’, enabling controlled joint movements. More information will be gained about possible functions of TBLO once its effective moment arm has been considered, given that the moment arm of a muscle transfers applied forces to rotational movements of joints. This will be discussed later.
Other muscles capable of generating large amounts of force are SSP and ISP. Both muscles are in effect antagonists of one another, the SSP acting to extend the shoulder whilst the ISP can flex this joint. SSP is larger in mass than ISP; however, the shorter fascicles and more pennate architecture of ISP mean that its PCSA is similar to that of the SSP. Both are therefore able to generate surprisingly similar amounts of force (both 110 N). Given that neither has a long tendon of insertion, it again seems likely that both have a supporting role in the thoracic limb, and in particular could be essential in maintaining stability of the shoulder joint, particularly during stance.
More distally, the similar PCSAs are seen in the FDS and FDP muscles, meaning that they are both capable of similar amounts of force (100 and 110 N, respectively). The humeral head of FDP in the hare has very short (3–14 mm) fascicles, as does the middle head (8–12 mm). The radial and ulnar heads, however, have longer fascicles (6–29 and 8–26 mm, respectively). AI was very low in the humeral head (0.09), indicating short pennate fascicles, whilst all three other heads had higher AIs (0.17, 0.17 and 0.22), indicating longer fascicles. Thus, the middle, radial and ulnar heads of FDP are likely to be involved in digital flexion during the swing phase of gait and other activities such as digging, whilst the humeral head is unlikely to be involved in digital flexion given its very short fascicles. This is also the case for FDS (AI of 0.07). It is likely therefore that FDS and FDPH function similarly, perhaps in roles such as vibration damping (Wilson et al. 2001), but also in storage and release of elastic energy. The lower force-generating capacities in FDS and FDP in the thoracic compared with pelvic limb may highlight functional differences between the limbs. These thoracic limb tendons may not be required to resist such large forces if the forelimb is used primarily as a support, deflecting the body during running. Hence the need for elastic energy storage might be less than in the pelvic limb, if the latter is actively being used for propulsion.
Tendons
The principal function of tendons is to attach muscle to bone; however, in the distal limb in particular, they function to reduce distal limb inertia. In addition, some are capable of elastic energy storage. Brown et al. (2003) reported that in horses, the highest scope for elastic energy storage was in the thoracic, as opposed to pelvic limb tendons, with the superficial and deep digital flexor tendons being described as the most compliant. Payne et al. (2005a) suggested that the equine thoracic limb SDF tendon in particular could generate high forces whilst allowing the tendon to undergo extension, and thus storing elastic energy. The highest estimated stresses and strains of hare thoracic limb tendons were not in the digital flexor tendons, as might be expected, but in ECR and FCU (Table 2). These, however, were still small, reaching strains of around 3.5% when compared with the strains estimated in pelvic limb tendons (7.9% in the Gastrocnemius; Williams et al. 2007). FDS and FDP were estimated to achieve strains of only 3.7 and 1.0%, respectively, values nearer those seen in the majority of pelvic limb tendons.
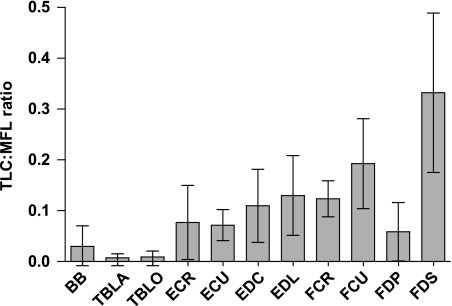
Tendon elongation under maximum isometric force from the muscle was calculated and its ratio to muscle fascicle length (TLC:MFL ratio) considered (Fig. 6). The TLC:MFL ratio gives an index of the relative importance of muscle vs. tendon length changes and strain energy storage capabilities at maximum load. High TLC:MFL ratio values are characteristic of compliant MTUs (those functioning with a high component of passive length change). All major distal limb tendons in the hare pelvic limb had TLC:MFL ratios greater than 0.4 [above which the tendon can be said to be capable of elastic energy storage (Pollock & Shadwick, 1994)]. MTUs with a low TLC:MFL ratio are optimised for actively undergoing maximum length change. All hare thoracic limb tendons had low (below 0.2) TLC:MFL ratios (Fig. 6), suggesting that these MTUs do not function with a high component of passive length change. They are therefore unlikely to be important in acting as biological ‘springs’ as thoracic limb tendons do in the horse. Thoracic limb tendons in the hare tended to be shorter than in the pelvic limb, and muscle mass appeared relatively more distally located in the thoracic limb (Fig. 4). These findings suggest that the forelimb in the hare may not be important in terms of energy storage during the stance phase of locomotion. It may in fact be important for the thoracic limb to remain ‘stiff’ during locomotion if, as has been suggested previously in this paper, it acts as a strut during high-speed running. It is most often large-bodied cursorial animals that are seen to exploit elastic energy storage in tendons. Perhaps there is a body mass below which it is not possible to put enough weight through these tendons for elastic energy storage to be effective. Or conceivably it may also require a more generalised structure to enable other non-locomotor activities.
Fig. 6.
Tendon length change (length change elicited at muscle maximum isometric force) to muscle fascicle length ratio (TLC:MFL) for hare thoracic limb tendons. Values are means ± SD (n = 7).
Moment arms
The highest maximum moment arm (Table 6) was observed in Triceps brachii when the shoulder joint was in full extension. However, in full joint flexion, the moment arm was exceptionally small. Thus, considerable change takes place in the moment arm and thus leverage of Triceps throughout joint motion. Triceps is capable of generating considerable forces (Table 2). High-force muscles can often have small moment arms to ensure that they can produce a large range of joint motion for a given unit of muscle contraction (Payne et al. 2006). This is true for Triceps when the shoulder is in flexed positions, suggesting this muscle acts to cause increased flexion of the shoulder joint at low joint angles such as during the swing phase of the stride. In more extended positions, however, this is not the case, thus suggesting range of motion is no longer important. More so, perhaps this is to create a large joint moment at the shoulder, potentially to initiate shoulder flexion or forelimb retraction. The muscle fascicle length/moment arm ratio (MFL:MA) was calculated (Table 6). A high value for this ratio indicated a muscle that is able to move a joint through large ranges of motion. Triceps has the lowest ratio for all thoracic limb muscles measured, suggesting range of motion is not important. It may be that its primary function is to initiate rather than fully carry out shoulder flexion. The high forces it is capable of producing would also support this suggestion. In addition, the long head of triceps has an effect at both the elbow and the shoulder joints, and thus a large moment of force applied to both joints during the swing phase (particularly during late swing, where the shoulder would be at its most extended, and the moment arm of triceps at its greatest) may act to decelerate the swinging limb.
The moment arm for Triceps at the elbow joint was also relatively large. At this joint the moment arm remained constant throughout joint movement. The large maximum moment arm value suggests range of motion again is not of primary importance here (corroborated by its low MFL:MA ratio) and that generation of a large joint moment is more essential. We have already suggested from observations of muscle architecture that Triceps acts to stabilise rather than actively extend the elbow joint. The large moment arm of Triceps at this joint thus supports this. It is hence more than likely that the role of Triceps at the elbow joint in the hare is to support and prevent collapse/flexion of the elbow joint during stance, thus allowing the hare to use its thoracic limb in a ‘strut-like’ manner during locomotion.
The moment arms of Deltoid and LD muscles (shoulder flexors) both increased with joint extension. A muscle may be able to generate a larger moment of force at a joint at a particular instant of the stride cycle if it has a larger moment arm at this point. This indicates that, as for Triceps, Deltoid and LD are also likely to be involved in initial flexion of the shoulder (although the moment of force that Triceps is able to develop suggests that it is the major contributor to this function). The smaller maximum moment arms of LD, which also has an extremely high MFL:MA ratio, may mean that it is more concerned with operating over a range of joint motion.
The moment arm of SSP (shoulder extensor) increased with joint flexion, suggesting that it may play a crucial role in initiating limb protraction. In the horse, BB is thought to be the major thoracic limb protractor (Wilson et al. 2003); however, the moment arm of BB at the shoulder is small, and maximum during full joint extension. Hence BB in the hare probably does not initiate limb protraction, and SSP is a more likely candidate to fulfil this role (given also the large value for estimated joint moment of force; Table 6). At the elbow joint, the MA of BB (elbow flexor) increased with extension, and was reasonably large in full joint extension. Thus, BB may initiate elbow flexion, and if it has a role in limb protraction it is more likely to be at this joint (where it is also capable of a larger joint moment than at the shoulder; Table 6).
The moment arm curve of ISP suggests that it may play a role in shoulder flexion (when in fact its position in the limb is on the lateral–posterior aspect of the shoulder joint, and from topographical examination, would be classed as a shoulder extensor). This may mean therefore that measuring its moment arm in the flexion–extension plane may not be entirely representative of its function and that the role of ISP is predominantly in limb abduction. A similar case occurred for the moment arm curve of TMJ, which was positive during more flexed joint positions, but negative in more extended postures. Measuring the moment arm for this muscle in a single plane may again not truly represent its action.
More distally, small moment arms were seen for all muscles at the carpal joint. Greatest changes in moment arms throughout joint motion were seen in the digital flexors, which had larger MAs in carpal extension. Along with this they have relatively high MFL:MA ratios. Thus, they may have a role in initiating carpal flexion in addition to their role in flexing the digits.
Conclusion
The architecture and moment arms of the thoracic limb muscles of the hare have been quantified. In addition, functional distribution of muscle mass within the thoracic limb has been established. The thoracic limb of the hare consists largely of extrinsic musculature with long parallel fascicles, able to generate force over a wide range of muscle excursion and joint motion. A large shoulder flexor/elbow extensor muscle mass is present, in particular Triceps brachii. The pennate nature of this muscle suggests it has an important role in stabilising the elbow joint during stance, whilst moment arm curves suggest that it may also play an important role in initiating shoulder flexion. In addition, SSP and ISP are capable of generating high forces, potentially in order to stabilise the shoulder joint during the stance phase of locomotion. SSP may also play an important role in forelimb protraction. The SS muscle was also capable of surprisingly high forces, suggesting that the hare must be able to withstand/produce high forces during activities such as turning. Distally, tendons were relatively short, showing little potential for elastic energy storage when compared with both their pelvic limb counterparts and their equivalents in the horse thoracic limb. Thus, a ‘stiffer’ thoracic limb may be beneficial in terms of supporting and deflecting the body during high-speed running. This more distal distribution of limb mass is also likely to be important in maintaining a generalised structure of the thoracic limb to facilitate non-locomotor activities.
Acknowledgments
We would like to express sincere thanks to Erica Gummery of the University of Nottingham for providing the line drawing in Fig. 1. We would also like to thank John Hutchinson, Charlotte Miller, Nicola Smith and two anonymous reviewers for helpful comments at various stages of the manuscript preparation. S.B.W. was funded by a Royal Veterinary College studentship. A.M.W. is holder of a BBSRC research development fellowship and a Royal Society Wolfson Research Merit Award.
References
- Alexander RM, Vernon A. Mechanics of hopping by kangaroos (Macropodidae) J Zool. 1975;177:265–303. [Google Scholar]
- Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature. 1977;265:114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Asher R, Meng J, Wible J, et al. Stem Lagomorpha and the antiquity of Glires. Science. 2005;307:1091–1094. doi: 10.1126/science.1107808. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Muscle–tendon stresses and elastic energy storage during locomotion in the horse. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Brown N, Kawcak CE, McIlwraith CW, Pandy M. Architectural properties of distal forelimb muscles in horses, Equus caballus. J Morph. 2003a;258:106–114. doi: 10.1002/jmor.10113. [DOI] [PubMed] [Google Scholar]
- Brown NA, Pandy MG, Kawcak CE, McIlwraith CW. Force- and moment-generating capacities of muscles in the distal forelimb of the horse. J Anat. 2003b;203:101–113. doi: 10.1046/j.1469-7580.2003.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton HM, Hodson E, Lanovaz JL. The forelimb in walking horses: 2. Net joint moments and joint powers. Equine Vet J. 2000;32:295–300. doi: 10.2746/042516400777032174. [DOI] [PubMed] [Google Scholar]
- Garland T. The relation between maximum running speed and body mass in terrestrial mammals. J Zool Lond. 1983;199:157–170. [Google Scholar]
- Grange WB. Observations on the Snowshoe Hare, Lepus americanums phaeonotus Allen. J Mammol. 1932;13:1–19. [Google Scholar]
- Halanych K, Demboski JR, van Vuuren BJ, Klein DR, Cook JA. Cytochrome b phylogeny af North American hares and jackrabbits (Lepus, Lagomorpha) and the effects of saturation in outgroup taxa. Mol Phylogenetics Evol. 1999;11:213–221. doi: 10.1006/mpev.1998.0581. [DOI] [PubMed] [Google Scholar]
- Heglund NC, Taylor CR, McMahon TA. Scaling stride frequency and gait to animal size: mice to horses. Science. 1974;186:1112–1113. doi: 10.1126/science.186.4169.1112. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Hurley JP. Energy of the oscillating legs of a fast-moving cheetah, pronghorn, jackrabbit, and elephant. J Morph. 1985;184:23–31. doi: 10.1002/jmor.1051840103. [DOI] [PubMed] [Google Scholar]
- Ker RF, Bennett MB, Alexander RM, Kester RC. Foot strike and the properties of the human heel pad. Proc Inst Mech Eng [H] 1989;203:191–196. doi: 10.1243/PIME_PROC_1989_203_038_01. [DOI] [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC. Distribution of ground reaction forces of the concurrently loaded limbs of the Dutch Warmblood horse at the normal walk. Equine Vet J. 1988a;20:209–213. doi: 10.1111/j.2042-3306.1988.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC. Evaluation of equine locomotion during different degrees of experimentally induced lameness. II: Distribution of ground reaction force patterns of the concurrently loaded limbs. Equine Vet J Suppl. 1988b;20:107–112. doi: 10.1111/j.2042-3306.1988.tb04656.x. [DOI] [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC, Van Osch GJ, Van den Bogert AJ. Ground reaction force patterns of Dutch warmblood horses at normal trot. Equine Vet J. 1993;25:134–137. doi: 10.1111/j.2042-3306.1993.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Pasi BM, Carrier DR. Functional trade-offs in the limb muscles of dogs selected for running vs. fighting. J Evol Biol. 2003;16:324–332. doi: 10.1046/j.1420-9101.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, Smith NC, Wilson AM. Functional specialisation of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005a;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Veenman P, Wilson AM. The role of the extrinsic thoracic limb muscles in equine locomotion. J Anat. 2005b;206:193–204. doi: 10.1111/j.1469-7580.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, Savage R, Vereecke E, Gunther M, Thorpe S, D’Aout K. Morphological analysis of the hindlimb in apes and humans. Part II: Moment arms. J Anat. 2006;208:725–742. doi: 10.1111/j.1469-7580.2006.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE. Allometry of muscle, tendon and elastic energy storage capacity in mammals. Am J Physiol. 1994;266:1022–1031. doi: 10.1152/ajpregu.1994.266.3.R1022. [DOI] [PubMed] [Google Scholar]
- Preedy D. Analysis of horse forelimb muscle activity (EMG) at walk and trot. 1998. PhD thesis. Department of Anatomy, University of Bristol. [Google Scholar]
- Ryan JM, Cobb MA, Hermanson JW. Elbow extensor muscles of the horse: postural and dynamic implications. Acta Anat (Basel) 1992;144:71–79. doi: 10.1159/000147288. [DOI] [PubMed] [Google Scholar]
- Smith NC, Wilson AM, Jespers K, Payne RC. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus) J Anat. 2006;209:765–780. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CR, Shkolnik A, Dmi’el R, Baharav D, Borut A. Running in cheetahs, gazelles, and goats: energy cost and limb configuration. Am J Physiol. 1974;227:848–850. doi: 10.1152/ajplegacy.1974.227.4.848. [DOI] [PubMed] [Google Scholar]
- Tokuriki M, Aoki O, Niki Y, Kurakawa Y, Hataya M, Kita T. Electromyographic activity of cubital joint muscles in horses during locomotion. Am J Vet Res. 1989;50:950–957. [PubMed] [Google Scholar]
- Tokuriki M. EMG activity of the muscles of the neck and forelimbs during different forms of locomotion. Equine Vet J Suppl. 1999;30:231–234. doi: 10.1111/j.2042-3306.1999.tb05224.x. [DOI] [PubMed] [Google Scholar]
- Usherwood JR, Wilson AM. Biomechanics: no force limit on greyhound sprint speed. Nature. 2005;438:753–754. doi: 10.1038/438753a. [DOI] [PubMed] [Google Scholar]
- Watson JC. Muscle function and control in the equine forelimb. 2004. PhD thesis. University of London. [Google Scholar]
- Williams SB, Payne R, Wilson A. Functional specialisation of the pelvic limb of the hare (Lepus europeus) J Anat. 2007;210:472–490. doi: 10.1111/j.1469-7580.2007.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, McGuigan MP, Su A, van Den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–899. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Watson JC, Lichtwark GA. A catapult action for rapid limb protraction. Nature. 2003;421:35–36. doi: 10.1038/421035a. [DOI] [PubMed] [Google Scholar]