Abstract
The age of emergence of the first molar (M1) is a developmental event correlated with many variables of primate life history, such as adult brain size. The evolution of human life history is characterized by the inclusion of childhood, which takes place between weaning and M1 emergence. Children still depend on adults for nutrition due to their small digestive system and their immature brains. By contrast, juveniles are not dependent because of M1 emergence, which enables shifting to adult type diet, and attainment of nearly adult brain size. In this study, developmental connections between M1 emergence and growth of cranial components were explored in two ways in order to understand the developmental basis of their evolutionary connections: (1) differences in growth trajectories of cranial components with respect to M1 emergence and (2) differences between individuals with and without fully emerged M1. Growth of anteroneural, midneural, posteroneural, otic, optic, respiratory, masticatory and alveolar cranial components was analysed in human skulls of individuals aged 0–20 years and in an adult reference skull. Volumetric indices were calculated to estimate size. Two subsamples were selected in order to focus on the transition between deciduous and permanent dentition: those with full deciduous dentition and before M1 reaches the occlusal plane; and those who present M1 in full emergence and no other cheek-tooth at the occlusal plane. The principal results were as follows. (1) Trajectories fitted using the whole sample are characterized by an inflection point that takes place before M1 emergence for neural components and around M1 emergence for facial components. (2) Associations between growth and age tend to be strong in those with full deciduous dentition, and weak in those who present M1 in full emergence. (3) Individuals who present M1 in full emergence are larger than those with full deciduous dentition. (4) Growth of components linked to the central nervous system is not linear until M1 emergence. Individuals who present M1 in full emergence are only larger than individuals with full deciduous dentition by 4–5% of adult size. (5) The alveolar component does not show increments between full deciduous dentition and M1 emergence. (6) When volumetric indices were standardized by age, the growth trajectories of individuals with full deciduous dentition and of those with M1 were not decoupled. In general terms, M1 emergence does not show a strong association with growth of the components that may explain differences in life histories. However, the main changes in neural and alveolar components occur in the first 3 years of life, which may be developmentally connected with M1 crown formation.
Keywords: cranial components, developmental connections, M1
Introduction
Craniofacial growth
Craniofacial morphology is the result of several epigenetic factors, such as inductions among cells and tissues, hormones, mechanical loads, and bioelectrical and biophysical events, acting at different structural levels (e.g. cells, tissues, organs) (Atchley & Hall, 1991; Herring, 1993; Vogl et al. 1993; Moss, 1997a,b,c; Lieberman et al. 2004; Buschang & Hinton, 2005), for instance by interactions between immediately adjacent tissues during growth and interactions of cells within a unit with the rest of the organism and with the environment. The functional matrix hypothesis (Moss & Young, 1960; Moss, 1973, 1997a,b,c) was one of the most important theories to explain craniofacial growth on the basis of interactions between adjacent tissues (Enlow & Hans, 1996; Lieberman et al. 2004). This hypothesis states that cranial shape reflects its primary functions, namely support and protection of the related functional tissues and spaces (Moss & Young, 1960). It proposes that the rate and direction of bone and cartilage growth are not regulated by means of their own genetic controls. Instead, bone is epigenetically modified by the growth of the functional matrix associated with it (Moss, 1973, 1997c). Each function of the head, such as digestion or vision, is performed by a functional cranial component consisting of both a functional matrix and a skeletal unit (Moss, 1973). All soft tissues, organs and cavities necessary for carrying out a function comprise the functional matrix. The assemblage of hard tissues (bone and cartilage) and other associated soft tissues (tendons and ligaments) that give biomechanical support to the functional matrix constitutes the skeletal unit. At least two major semi-independent modules or components can be recognized in the skull: neurocranium and face. These two components, and even different minor components within them, differ in their growth patterns and in the age of attainment of adult size (e.g. Enlow & Hans, 1996; Smith, 1996; Humphrey, 1998; Miller & German, 1999; VandeBerg et al. 2004a; Sardi & Ramírez Rozzi, 2005). Growth of the neurocranium is affected by brain growth (Delattre, 1951; Moss & Young, 1960; Moss, 1973; Michejda, 1975; Sirianni, 1985; Hartwig, 1995), whereas the face is affected by the development of airways, teeth and the associated muscular loadings (Enlow & Hans, 1996; Herring, 1993). Cranial growth occurs mainly at sutures and synchondroses (Enlow & Hans, 1996; Opperman et al. 2005); bone remodelling also accounts for part of the changes in size and shape. Neural structures are more advanced with respect to adult size than the facial structures (Buschang et al. 1983; Enlow & Hans, 1996; VandeBerg et al. 2004a). Buschang et al. (1983) observed that the craniofacial maturity intergrades between the neural and the general patterns of growth; the neural pattern is characterized by a steep increase of relative growth that occurs prenatally, followed by a rapid deceleration, whereas the general pattern is represented by a sigmoid curve with greater relative growth during infancy and adolescence (Buschang & Hinton, 2005). Sardi & Ramírez Rozzi (2005) described growth trajectories of eight functional cranial components and found that neural and optic components show changes in growth rates around 3 years of age whereas the respiratory, masticatory and otic components show changes in growth rates around 5 years of age, and the alveolar component shows a decrease of growth rate around age 3 years and a reacceleration after 14 years of age.
Dental development and its associations with life-history variables
Dental development can be characterized by two variables: chronology and sequence of tooth formation and emergence. In humans, the sequence of emergence of some teeth, such as P3 and P4, shows very small variation among individuals (Smith & Garn, 1987) and because this feature is under greater genetic control than other skeletal variables, it is the most accurate estimator of chronological age (Meindl & Russel, 1998; Hoppa & Fitzgerald, 1999; Konigsberg & Holman, 1999). By contrast, important variability has been reported in the chronology of tooth emergence, mainly in M3 (i.e. Garn & Moorrees, 1951; Garn et al. 1973; Lavelle, 1975), and in the relative calcification of teeth (Fanning & Moorrees, 1969; Tompkins, 1996). Dental emergence, in particular the emergence of permanent molars, offers an excellent way to gauge the growth of individuals because it is linked to the attainment of distinct somatic and reproductive growth stages (Liversidge et al. 1993). It has been used to delimit age classes in studies of unknown-age individuals (Corner & Richtsmeier, 1991, 1992, 1993) and to establish reference stages in interspecific comparisons (Schultz, 1960; Swindler, 1985; Smith, 1989; Smith et al. 1994; Bastir & Rosas, 2004).
The age of M1 emergence has been proposed to be strongly correlated with life-history variables in interspecific comparisons. Life history has been defined as the strategy of organisms for energy allocation during their lifespan (Bogin, 1999), and it includes variables referred to sequences of physiological, morphological and behavioural change, such as lifespan, age at weaning and fertility.
Human life history is divided into infancy, childhood, juvenility, adolescence and adulthood (Bogin, 1997, 1999). Ontogenetically, emergence of the deciduous dentition occurs during infancy; weaning indicates the end of this period and the onset of childhood. According to Bogin (1997, 1999), childhood is an ontogenetic period that evolved in humans. During childhood, humans are not capable of eating an adult type diet because both the deciduous teeth and their digestive system are small, while the brain grows rapidly to attain most of its adult size; for these reasons, children are still dependent on older people for feeding and protection (Bogin, 1997). Humans progress into juvenility due to M1 emergence and to the completion of brain growth (Bogin, 1997). In this sense, emergence of the permanent dentition represents a milestone with ecological implications, as it allows independence from parents for nutrition (Smith, 1991; Bogin, 1999), associated with enhanced performance of neurological and motor skills. In primates, Smith (1989, 1992) has compared the age of M1 emergence with many reproductive variables and found strong positive correlations. Similar results were found with respect to the age of eruption of other permanent teeth and brain weight in adult primates (Smith et al. 1994; Godfrey et al. 2001). These close associations among life-history variables led to the suggestion that M1 emergence is an important developmental milestone in primate life history (Bogin, 1999).
The purpose of this study was to explore structural changes of cranial components with respect to M1 emergence throughout the postnatal ontogeny of humans. The null hypothesis to be tested indicates that there are connections between cranial growth and M1 emergence at the individual level, as can be observed at the evolutionary level. According to Cheverud (1996), developmental integration or connection occurs when morphological elements interact during their formation or are directed by a common external source, such as epigenetic interactions.
Developmental connections between cranial components and M1 emergence were explored in two ways. On the one hand, growth trajectories of different cranial components with respect to M1 emergence were observed. If M1 emergence is delayed in humans and if it is associated with brain growth, then a concomitant delay in the growth of those cranial components linked to the central nervous system would be expected; significant growth of the cranial components associated with digestion, which would prepare individuals for processing and consuming an adult type diet, would also be expected. On the other hand, size differences were observed between individuals with and without M1 in full emergence. If M1 emergence is connected with cranial growth, then individuals showing M1 emergence would be expected to differ in size from those individuals that do not show M1.
Materials and methods
We measured a sample of 228 human skulls of known age at death, with ages ranging from 0 to 20 years (Table 1). The sample comprised 165 individuals of Portuguese origin, housed at the University of Coimbra (Portugal) and 63 individuals of French origin housed at the Musée de l’Homme, Paris (France). Another 121 human skulls (63 males and 58 females) from the Portuguese collection, with ages at death between 21 and 39 years, and showing closure of the spheno-occipital synchondrosis and complete permanent dentition, were added as adult references. Sex was known for the adult sample and for most of the subadults; those of unknown sex were mostly among individuals of 0–5 years of age (Table 1).
Table 1.
Frequencies of skulls by sex and age
| Age (years) | Unknown sex | Females | Males | Total |
|---|---|---|---|---|
| 0 | 30 | 0 | 0 | 30 |
| 1 | 2 | 0 | 0 | 2 |
| 2 | 5 | 2 | 1 | 8 |
| 3 | 0 | 1 | 1 | 2 |
| 4 | 4 | 1 | 1 | 6 |
| 5 | 3 | 0 | 0 | 3 |
| 6 | 0 | 0 | 1 | 1 |
| 7 | 2 | 6 | 3 | 11 |
| 8 | 3 | 5 | 5 | 13 |
| 9 | 1 | 2 | 2 | 5 |
| 10 | 2 | 6 | 3 | 11 |
| 11 | 1 | 5 | 4 | 10 |
| 12 | 0 | 9 | 3 | 12 |
| 13 | 0 | 2 | 5 | 7 |
| 14 | 4 | 5 | 2 | 11 |
| 15 | 2 | 8 | 10 | 20 |
| 16 | 0 | 6 | 7 | 13 |
| 17 | 1 | 14 | 8 | 23 |
| 18 | 1 | 10 | 9 | 20 |
| 19 | 0 | 6 | 5 | 11 |
| 20 | 0 | 4 | 5 | 9 |
| Total | 61 | 92 | 75 | 228 |
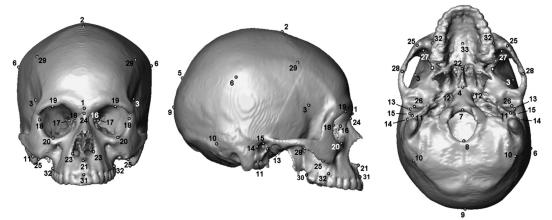
Components were delimited in the skull according to previous studies (Pucciarelli et al. 1990; Dressino & Pucciarelli, 1999; González-José et al. 2005; Ramírez Rozzi et al. 2005; Sardi & Ramírez Rozzi, 2005; Sardi et al. 2006) (Fig. 1, Table 2), on the basis of the functional matrix hypothesis (Moss & Young, 1960; Moss, 1973). The biological basis of components is related to specific functional matrices (Table 2). The neurocranium was divided into four components: anteroneural, midneural, posteroneural and otic. Another four components were established in the face: optic, respiratory, masticatory and alveolar.
Fig. 1.
Landmarks used for measurements recorded. Otic, optic and masticatory components were measured on the left side of the skull.
Table 2.
Functional matrix of cranial components and measurements. Length (L), breadth (B) and height (H) are orthogonal with respect to each other and were measured in skulls orientated according to the Frankfort plane
| Component | Functional matrix | Measurement |
|---|---|---|
| anteroneural | neural structures related to the anterior cranial fossa (mainly anterior lobes) and frontal sinus | L: Glabella (1) – Bregma (2) |
| B: Pterion (3) – Pterion (3) | ||
| H: Bregma (2) – Vomerobasilar (4) | ||
| midneural | neural structures related to middle and part of posterior cranial fossae and the most part of the brain hemispheres | L: Bregma (2) – Lambda (5) |
| B: Eurion (6) – Eurion (6) | ||
| H: Basion (7) – Bregma (2) | ||
| posteroneural | cerebellum | L: Opisthion (8) – Opisthocranion (9) |
| B: Asterion (10) – Asterion (10) | ||
| H: Lambda (9) – Opisthion (8) | ||
| otic | cavities and structures for hearing and equilibrium included in the petrosal and tympanic bones | L: inferior border of the tympanic bone (11) – inner extreme of petrosal bone (12) |
| B: external auditory meatus breadth (13–14) | ||
| H: external auditory meatus height (11–15) | ||
| optic | ocular globe and orbital muscles | L: Dacrion (16) – optic foramen (17) |
| B: Dacrion (16) – Ectoconquium (18) | ||
| H: Supraorbitary (19) – Infraorbitary (20) | ||
| respiratory | cavity for respiration and olfaction | L: Subspinale (21) – posterior nasal spine (22) |
| B: Alare left (23) – Alare right (23) | ||
| H: Nasion (24) – Subspinale (21) | ||
| masticatory | temporal and part of masseter muscles | L: Zygomaxillare (25) – posterior border of the glenoid cavity (26) |
| B: anterior sulcus of the sphenotemporal crest (27) – lowest point of the zygo temporal suture (28) | ||
| H: lowest border of the zygo temporal suture (28) – inferior temporal line at the coronal intersection (29) | ||
| alveolar | teeth and tissues of the oral cavity | L: Prosthion (31) – posterior limit of the maxillary alveolar arch (30) |
| B: maximum breath of the exterior alveolar border (32–32) | ||
| H: midsagital palatal depth (33) at point where alveolar breadth (32) was taken |
Landmarks were digitized with Microscribe (Fig. 1) and inter-landmark distances were calculated: length, breadth and height of each component (Table 2). Volumetric indices (VI), representing the geometric mean of the three dimensions, were constructed to estimate size variation, expressed in dimensionless units, as follows
The emergence of maxillary dentition was recorded and individuals were assigned to dental classes according to tooth emergence. Individuals with deciduous dentition are those in which M1 has not yet reached the occlusal plane (n = 52), with an age span between 0 and 8 years. Individuals with permanent dentition are considered those who present at least the M1 in full emergence (n = 176), with an age span between 5 and 20 years. Individuals who represent the upper limit of the first class and the inferior limit of the second class were differentiated. Within the deciduous dentition class, individuals with complete deciduous dentition in full emergence (FDD) and with M1 not reaching the occlusal plane were selected (n = 16); their ages ranged between 2 and 8 years. Among individuals with permanent dentition, those with full emergence of M1 and no other cheek-tooth at the occlusal plane (EM1) were selected (n = 34), with ages between 5 and 11 years.
Growth trajectories of cranial components were assessed by adjusting volumetric indices to chronological age using the non-parametric smoothing spline. Non-parametric methods are preferable for the description of growth because the curve is estimated without the constraint of fitting a particular shape (Simonoff, 1996). The range of the x variable is divided into segments and then y values are adjusted using parametric regression within each segment; thus, fittings of y in any given segment are independent of fittings in adjacent segments. The smoothing spline requires the definition of the smoothing parameter λ, which establishes the trade-off between smoothness and variance (Simonoff, 1996). A small λ produces low smoothness and high variance; conversely, a high λ produces better smoothness with lower variance; the linear regression is the expression of an extremely high λ. In this study, many different values of λ were explored. Finally, λ = 10 was chosen by visual inspection because curves did not vary with smaller λ values. In order to determine the distribution of major dental classes in relation to chronological age and volumetric indices, ellipses with 95% confidence limits were calculated for each major dental class. Analyses were performed pooling individuals of both sexes, including those of unknown sex that correspond to the most critical growth period (Table 1). Sexual dimorphism in growth patterns may exist, but it is not so pronounced at pre-adolescent stages, even in the most dimorphic structures (Sperber, 2001). Moreover, Guihard-Costa & Ramírez Rozzi (2004) have demonstrated that the inflection point of brain and neurocranial growth occurs at the same age in both males and females.
FDD and EM1 individuals were analysed in order to examine growth trajectories across the transition between deciduous and permanent dentition. Data were log-transformed to enhance variance stability and linearity of the distribution. Pearson's correlation coefficients and regression equations were calculated from volumetric indices (y) against age (x), to test the hypothesis that the slope is significantly different from 0.
The proportion of adult size of volumetric indices was calculated in FDD and EM1 individuals, using the adult sample as reference. This represents a means by which to measure relative increments between the two stages and the degree of advancement with respect to adult size.
In order to test differences between FDD and EM1 individuals, percentage of differences between means (PDM) of volumetric indices was calculated. Homoscedasticity was assessed using Levene's test. The hypothesis that differences between FDD and EM1 means equal 0 was tested by means of paired t and Wilcoxon tests for homoscedastic and heteroscedastic samples, respectively.
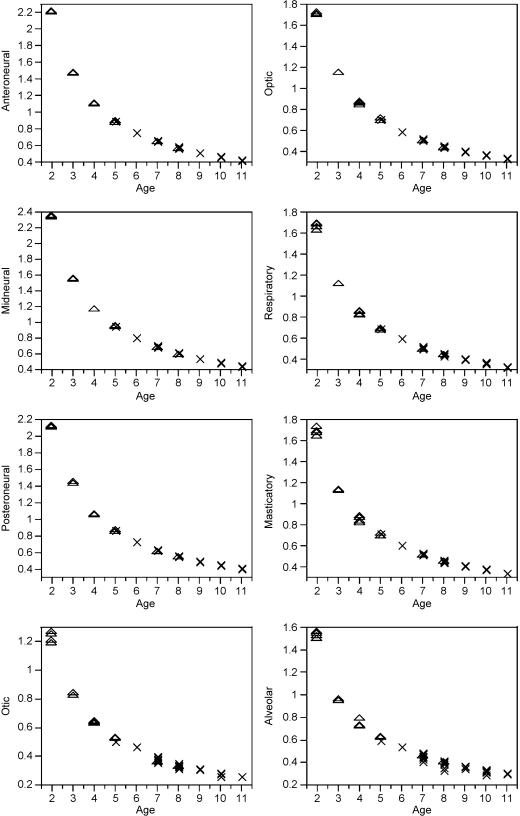
Size differences may be dependent on age differences between the two groups, i.e. EM1 individuals may be larger than individuals without M1 just because they are older, but the small sample size for the most critical ages (around M1 emergence) does not allow the comparison between individuals of different dental stages at the same age. However, distributions of log-transformed volumetric indices for FDD and EM1 individuals were standardized by age (log VI/age) for comparison. Log VI/age values (y) were plotted against age (x). If craniofacial growth is a function of age, it is expected that EM1 and FDD individuals will show similar distributions.
Statistical analyses were performed using the Jump 5.0.1 package.
Results
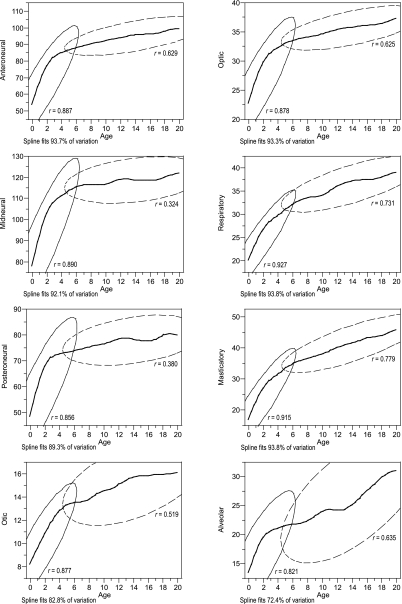
Figure 2 shows the growth trajectories of each component obtained with the smoothing spline. Adjustments explained a highly significant proportion of variation (r2). Growth trajectories revealed high growth rates up to ages 3–5 years, followed by an inflection point, which was in turn followed by lower growth rates. The alveolar component showed a different trajectory, with two moments of greater growth rate, during ages 0–4 and after age 14 years. Ellipses, representing the distribution of individuals before and after the full emergence of M1, overlapped near the inflection point of component growth trajectories (Fig. 2). The trajectories indicated that individuals with deciduous dentition have a higher growth rate whereas individuals with permanent dentition have a lower growth rate.
Fig. 2.
Smoothing spline of volumetric indices against chronological age. Ellipses represent 95% of the distribution of individuals with deciduous dentition (solid line) and with permanent dentition (dashed line). The Pearson's correlation coefficients (r) are indicated for both dental classes.
Correlations and linear equations calculated from log-transformed volumetric indices (Table 3) indicated that FDD individuals show significant and highly significant age-related changes for most of the components. The posteroneural and alveolar components had non-significant slopes; this may indicate that greatest growth rates occur before the acquisition of the full deciduous dentition for the posteroneural component and after 14 years of age for the alveolar component (Fig. 2). EM1 individuals showed significant and non-significant age-related changes, with the exception of the anteroneural component which continued to present highly significant increments. Table 4 shows the proportion of adult size in FDD and EM1 individuals. The anteroneural, midneural, posteroneural and optic components were the most advanced, while the masticatory and alveolar components were the most retarded at both FDD and EM1 stages. Both the masticatory and the respiratory components showed the greatest increments between these dental stages.
Table 3.
Pearson's correlation coefficients and linear regression equations for log VIs against age in FDD and EM1 individuals
| FDD | EM1 | |||||
|---|---|---|---|---|---|---|
| Component | r | Slope | Intercept | r | Slope | Intercept |
| anteroneural | 0.763** | 0.015** | 4.38 | 0.457** | 0.013** | 4.39 |
| midneural | 0.668* | 0.014* | 4.65 | 0.150 | 0.003 | 4.73 |
| posteroneural | 0.507 | 0.015 | 4.21 | 0.166 | 0.004 | 4.28 |
| otic | 0.666** | 0.027** | 2.44 | 0.392* | 0.027* | 2.41 |
| optic | 0.653** | 0.018** | 3.39 | 0.373* | 0.010* | 3.45 |
| respiratory | 0.754** | 0.034** | 3.26 | 0.279 | 0.010 | 3.42 |
| masticatory | 0.681** | 0.041** | 3.28 | 0.432* | 0.016* | 3.46 |
| alveolar | 0.484 | 0.036 | 2.92 | 0.282 | 0.035 | 2.80 |
P < 0.05
P < 0.01.
Table 4.
Proportion of adult size in volumetric indices
| FDD | EM1 | |||||
|---|---|---|---|---|---|---|
| Component | x ± SD | Lower | Upper | x ± SD | Lower | Upper |
| anteroneural | 0.85 ± 0.03 | 0.83 | 0.87 | 0.90 ± 0.04 | 0.89 | 0.91 |
| midneural | 0.91 ± 0.03 | 0.89 | 0.93 | 0.95 ± 0.03 | 0.94 | 0.96 |
| posteroneural | 0.88 ± 0.04 | 0.86 | 0.91 | 0.92 ± 0.04 | 0.91 | 0.94 |
| otic | 0.77 ± 0.05 | 0.75 | 0.80 | 0.85 ± 0.09 | 0.82 | 0.88 |
| optic | 0.86 ± 0.04 | 0.84 | 0.88 | 0.92 ± 0.03 | 0.91 | 0.93 |
| respiratory | 0.75 ± 0.06 | 0.72 | 0.78 | 0.84 ± 0.04 | 0.83 | 0.86 |
| masticatory | 0.69 ± 0.07 | 0.65 | 0.72 | 0.80 ± 0.04 | 0.79 | 0.82 |
| alveolar | 0.69 ± 0.08 | 0.64 | 0.73 | 0.71 ± 0.12 | 0.67 | 0.75 |
Table 5 shows descriptive statistics of volumetric indices studied for FDD and EM1 individuals. Student's t and Wilcoxon tests indicated that EM1 individuals are larger than FDD individuals in all components, except the alveolar component (Table 5). The PDM values indicated that the masticatory, respiratory and otic components have the greatest differences between both dental stages. Figure 3 shows the distribution of age-standardized log-volumetric indices. The trajectories followed by FDD and EM1 individuals were identical; thus, no shift between the distributions at both dental stages was observed (Fig. 3).
Table 5.
Means (x), standard deviations (SD), and percentage differences between means (PDM) of volumetric indices. Levene (F), Student's (t) and Wilcoxon (approximate z) tests in log VI
| Component | FDD x ± SD | EM1 x ± SD | PDM† | Levene's F | FDDx − EM1x |
|---|---|---|---|---|---|
| anteroneural | 85.33 ± 3.20 | 90.25 ± 3.89 | 1.18 | 0.22 | t = −3.90** |
| midneural | 111.02 ± 4.19 | 116.43 ± 3.82 | 0.69 | 1.11 | t = −4.07** |
| posteroneural | 71.81 ± 3.71 | 75.14 ± 3.10 | 1.03 | 2.01 | t = −3.20** |
| otic | 12.77 ± 0.87 | 14.06 ± 1.45 | 3.72 | 5.10* | z = −2.81** |
| optic | 32.00 ± 1.56 | 34.23 ± 1.33 | 1.91 | 0.53 | t = −5.02** |
| respiratory | 29.77 ± 2.39 | 33.51 ± 1.82 | 3.41 | 2.08 | t = −5.80** |
| masticatory | 31.38 ± 3.31 | 36.63 ± 1.94 | 4.62 | 19.16** | z = −4.41** |
| alveolar | 21.54 ± 2.72 | 22.29 ± 3.74 | 0.94 | 0.97 | t = −0.54 |
Calculated as (FDDx − EM1x)/FDDx. 100 in log-volumetric indices.
P < 0.05
P < 0.01.
Fig. 3.
Biplot of the log-transformed volumetric indices scaled to age (log VI/age) and chronological age in FDD (triangles) and in EM1 (crosses) individuals.
Discussion and conclusions
Age-related changes in cranial components have been previously described (Sardi & Ramírez Rozzi, 2005). Growth trajectories were characterized by higher growth rates in individuals under ages 3–5 years compared with those of individuals after age 5 years (Sardi & Ramírez Rozzi, 2005). Cranial components were classified into three groups according to differences among trajectories, mainly changes of growth rates (inflection points) and proportion of adult size: (1) anteroneural, midneural, posteroneural and optic; (2) respiratory, masticatory, and otic; and (3) alveolar trajectory. The coordinate variation among neural and facial components may express the modular organization of the craniofacial skeleton (Schilling & Thorogood, 2000), normally attributed to functional and developmental constraints (Cheverud, 1996). The similarity in growth patterns among components of groups (1) and (2) can be explained mainly by common embryological origins of these components, rather than by their participation in a common function or with their localization in the neurocranium or the face (Sardi & Ramírez Rozzi, 2005).
In this study, growth trajectories were described with respect to a developmental event, namely M1 emergence. According to Fig. 2, ellipses overlap the inflection point in growth trajectories, which would mean that changes in trajectories are quite coincident with the transition from deciduous to permanent dentition. Associations between growth and age are strong before the full emergence of M1, and they are weaker for most components after M1 emergence (Table 3). However, the anteroneural, posteroneural and alveolar components continue to show similar slopes during both dental stages, highly significant for the first component and non-significant for the others (Table 3). The respiratory component undergoes the most important age-related changes, showing highly significant correlations in FDD and non-significant correlations in EM1 individuals (Table 3).
If age of M1 emergence is developmentally connected with brain size, then size increments in neural components would be expected up to M1 emergence. Results of this study confirm that the neural components of EM1 individuals present much of their adult size; however, growth of the neural components is not linear up to the emergence of M1. Components linked to the central nervous system (anteroneural, midneural, posteroneural) and the optic component show an inflection point located earlier across ontogeny, long before M1 emergence (Fig. 2). Moreover, estimations of adult size indicate that FDD individuals have attained around 88% of adult size for the anteroneural, midneural and posteroneural components, and at least 83% of adult size considering the minimum value of the anteroneural component (Table 4). Little growth occurs in neural components (4–5%) from FDD to EM1 stages (Table 4). Guihard-Costa & Ramírez Rozzi (2004) also found an inflection point for brain and skull growth in living humans around 2–3 years of age. This means that growth of the central nervous system is more important during the first 3 years of life, which are the period of emergence of deciduous dentition and also the period of M1 crown calcification (Liversidge et al. 1993; Reid et al. 1998). It is possible that, as stated by Bogin (1997, 1999), the brain gains most of its adult size in weight up to the moment of M1 emergence, but according to the results of the present study, delay in M1 emergence does not translate as delayed growth of neural components or maintenance of high growth rates. It is possible that neurological performance up to M1 emergence is associated with histological and maturational changes (Thompson et al. 2000; Nagy et al. 2004).
After M1 emergence, only the anteroneural component maintains a growth pattern associated with age (Table 3) and shows a lesser proportion of adult size than midneural and posteroneural components (Table 4). The prolongation of growth of the anteroneural component may result in expansion of the frontal sinus, which presents a facial growth pattern rather than a neural one (Moss & Young, 1960), and pulls the outer table of the frontal bone forward (Lieberman et al. 2000b, 2004) also into old age (Sperber, 2001). By contrast, the growth pattern and proportion of adult size of the optic component is similar to those of neural components (Fig. 2, Table 4), even if some bones of the optic cavity are part of the face.
The respiratory and masticatory components belong to somatic systems. Expansion of most of their bones depends on sutural growth, mainly due to extrinsic forces (Opperman et al. 2005). Changes in growth trajectories take place around 5 years of age (Fig. 2). The respiratory component is the first portion of the respiratory system; its size may be linked in part to body size (Enlow & Hans, 1996) and its growth is to some degree dependent on changes in the anterior cranial base (Enlow & Hans, 1996; Lieberman et al. 2000a,b; Sperber, 2001; Bastir et al. 2004; Bastir & Rosas, 2006). By contrast, growth of the masticatory component is associated with growth of the masticatory muscles and is thus subject to hormonal influences (e.g. growth hormone; Vogl et al. 1993; VandeBerg et al. 2004b). Regarding M1 emergence, these components show the greatest size changes between FDD and EM1 stages, which would be associated with somatic growth (Bogin, 1997, 1999), even though more than 10% of their adult size is achieved during later ontogenetic stages (Table 4).
The otic trajectory is similar to the respiratory and masticatory trajectories. This is difficult to interpret given that the otic component includes different structures: the petrosal bone, which contains structures derived from the first and second pharyngeal arches, and the tympanic bone, which is derived from the pharyngeal arches (Sperber, 2001). The similarities with the masticatory and respiratory components may be due to associations of basicranial structures with the face (Enlow & Hans, 1996; Kemaloglu et al. 2000; Lieberman et al. 2000a,b; Bastir et al. 2004; Bastir & Rosas, 2006).
The alveolar component follows an S-shaped pattern, different from the other components derived from the pharyngeal arches, with highest growth rates up to 3 years old and after 14 years old (Fig. 2). As the alveolar component supports the teeth, and the mouth forms the first part of the digestive system, it could be expected to be the most affected by the emergence of M1, in order to enable children to consume an adult type diet (Smith, 1991; Bogin, 1999). However, in the transition between both dental stages, this component does not show either size changes associated with age (Table 3) or differences between FDD and EM1 means (Table 5). It is very probable that the space for M1 placement becomes available during crown formation, which starts before birth and finishes around age 2.5–3 years (Liversidge et al. 1993; Reid et al. 1998). The remaining growth of the alveolar component in later ontogeny (Fig. 2) may be linked to the formation of M2 and M3 (Sperber, 2001), as Boughner & Dean (2004) observed for the mandibular length in Pan and Papio.
Significant size differences between FDD and EM1 individuals (Table 4) indicate that considerable craniofacial changes take place during the transition from deciduous to permanent dentitions. Most components, except the alveolar component, are significantly larger in EM1 individuals (Table 4). However, when trajectories are standardized by age, EM1 individuals show the same growth pattern as FDD individuals (Fig. 3), thus indicating that there is no shift in growth that can be attributed to M1 emergence during the ontogenetic period spanned by both samples. This means that differences between both dental stages are likely to be due to the cumulative age-related increments rather than to the actual emergence of M1.
Based on life-history studies, M1 emergence is often considered to be a developmental event significantly associated with many variables. However, M1 emergence does not seem to be strongly connected with structural craniofacial changes across ontogeny at the individual level. Moreover, if developmental integration exists between M1 emergence and growth of neural and alveolar components, it is not mediated by processes that involve temporal continuity. Thus, the null hypothesis is rejected. Nevertheless, it must be taken into account that the M1 crown of humans grows and accommodates within the alveolus around age 2.5 years (Reid et al. 1998), much earlier than the M1 full emergence. That is, M1 crown formation may be developmentally associated to some extent with craniofacial growth by some causal mechanism that would produce this temporal association of events. By contrast, M1 emergence is a developmentally quite independent event.
Acknowledgments
We wish to thank the Editor and reviewers for corrections and observations that helped us to rework some of our arguments; Valeria Bernal, Paula González and Ivan Pérez for commentaries made on early versions of the manuscript; Fernando Ventrice, for technical assistance; and Phillipe Mennecier, Eugenia Cunha and Sofia Wasterlain for access to collections under their care. This work was made possible by a Fondation Fyssen Grant held by M.L.S.
References
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev Camb Philos Soc. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Comparative ontogeny in humans and chimpanzees: similarities, differences and paradoxes in postnatal growth and development of the skull. Ann Anat. 2004;186:503–509. doi: 10.1016/S0940-9602(04)80096-7. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A, Kuroe K. Petrosal orientation and mandibular ramus breadth: evidence for an integrated petroso-mandibular developmental unit. Am J Phys Anthropol. 2004;123:340–350. doi: 10.1002/ajpa.10313. [DOI] [PubMed] [Google Scholar]
- Bastir M, Rosas A. Correlated variation between the lateral basicranium and the face: a geometric morphometric study in different human groups. Arch Oral Biol. 2006 doi: 10.1016/j.archoralbio.2006.03.009. in press. [DOI] [PubMed] [Google Scholar]
- Bogin B. Evolutionary hypotheses for human childhood. Ybk Phys Anthropol. 1997;40:63–89. [Google Scholar]
- Bogin B. Patterns of Human Growth. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Boughner JC, Dean MC. Does space in the jaw influence the timing of molar crown initiation? A model using baboons (Papio anubis) and great apes (Pan troglodytes, Pan paniscus) J Hum Evol. 2004;46:255–277. doi: 10.1016/j.jhevol.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Buschang PH, Baume RM, Nass G. A craniofacial growth maturity gradient for males and females between 4 and 16 years of age. Am J Phys Anthropol. 1983;61:373–381. doi: 10.1002/ajpa.1330610312. [DOI] [PubMed] [Google Scholar]
- Buschang PH, Hinton RJ. A gradient of potential for modifying craniofacial growth. Semin Orthod. 2005;11:219–226. [Google Scholar]
- Cheverud JM. Developmental integration and the evolution of pleiotropy. Am Zool. 1996;36:44–50. [Google Scholar]
- Corner BD, Richtsmeier JT. Morphometric analysis of craniofacial growth in Cebus apella. Am J Phys Anthropol. 1991;84:323–342. doi: 10.1002/ajpa.1330840308. [DOI] [PubMed] [Google Scholar]
- Corner BD, Richtsmeier JT. Cranial growth in the squirrel monkey Saimiri sciureus: a quantitative analysis using three dimensional coordinate data. Am J Phys Anthropol. 1992;87:67–82. doi: 10.1002/ajpa.1330870107. [DOI] [PubMed] [Google Scholar]
- Corner BD, Richtsmeier JT. Cranial growth and growth dimorphism in Ateles geoffroyi. Am J Phys Anthropol. 1993;92:371–394. doi: 10.1002/ajpa.1330920308. [DOI] [PubMed] [Google Scholar]
- Delattre A. Du Crâne Animal au Crâne Humain. Paris: Masson; 1951. [Google Scholar]
- Dressino V, Pucciarelli HM. Growth of functional cranial components in Saimiri sciureus boliviensis (Cebidae): a longitudinal study. Growth Dev Aging. 1999;63:111–127. [PubMed] [Google Scholar]
- Enlow DH, Hans MG. Crecimiento Facial. Mexico DF: McGraw-Hill Interamericana; 1996. [Google Scholar]
- Fanning E, Moorrees C. A comparison of permanent mandibular molar formation in Australian Aborigines and Caucasoids. Arch Oral Biol. 1969;14:999–1006. doi: 10.1016/0003-9969(69)90069-7. [DOI] [PubMed] [Google Scholar]
- Garn SM, Moorrees C. Stature, body-build, and tooth emergence in Aleutian Aleut children. Child Dev. 1951;22:261–270. [PubMed] [Google Scholar]
- Garn SM, Sandusky ST, Nagy JM, Trowbridge FL. Negro-Caucasoid differences in permanent tooth emergence at a constant income level. Arch Oral Biol. 1973;18:609–615. doi: 10.1016/0003-9969(73)90099-x. [DOI] [PubMed] [Google Scholar]
- Godfrey LR, Samonds KE, Jungers WL, Sutherland MR. Teeth, brains, and primate life history. Am J Phys Anthropol. 2001;114:192–214. doi: 10.1002/1096-8644(200103)114:3<192::AID-AJPA1020>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- González-José R, Ramírez Rozzi F, Sardi M, Martínez-Abadías N, Hernández M, Pucciarelli HM. Functional-cranial approach to the influence of economic strategy on skull morphology. Am J Phys Anthropol. 2005;128:757–771. doi: 10.1002/ajpa.20161. [DOI] [PubMed] [Google Scholar]
- Guihard-Costa AM, Ramírez Rozzi F. Growth of the human brain and skull slows down at about 2.5 years old. CR Palevol. 2004;3:397–402. [Google Scholar]
- Hartwig NC. Effect of life history on the squirrel monkey (Platyrrhini, Saimiri) cranium. Am J Phys Anthropol. 1995;97:435–449. doi: 10.1002/ajpa.1330970409. [DOI] [PubMed] [Google Scholar]
- Herring SW. Formation of the vertebrate face: epigenetic and functional influences. Am Zool. 1993;33:472–483. [Google Scholar]
- Hoppa RD, Fitzgerald CM. From head to toe: integrating studies from bones and teeth in biological anthropology. In: Hoppa R, Fitzgerald CM, editors. Human Growth in the PastStudies from Bones and Teeth. Cambridge: Cambridge University Press; 1999. pp. 1–31. [Google Scholar]
- Humphrey LT. Growth patterns in the modern human skeleton. Am J Phys Anthropol. 1998;105:57–72. doi: 10.1002/(SICI)1096-8644(199801)105:1<57::AID-AJPA6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kemaloglu YK, Kobayashi T, Nakajima T. Associations between the Eustachian tube and craniofacial skeleton. Int J Pediatr Otorhinolaryngol. 2000;53:195–205. doi: 10.1016/s0165-5876(00)82007-8. [DOI] [PubMed] [Google Scholar]
- Konigsberg L, Holman D. Estimation of age at death from dental emergence and implications for studies of prehistoric somatic growth. In: Hoppa R, Fitzgerald CM, editors. Human Growth in the PastStudies from Bones and Teeth. Cambridge: Cambridge University Press; 1999. pp. 264–289. [Google Scholar]
- Lavelle C. A note on the variation in the timing of deciduous tooth eruption. J Dent. 1975;3:267–270. doi: 10.1016/0300-5712(75)90033-0. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Pearson OM, Mowbray KM. Basicranial influence on overall cranial shape. J Hum Evol. 2000a;38:291–315. doi: 10.1006/jhev.1999.0335. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function, and integration. Ybk Phys Anthropol. 2000b;43:117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Krovitz GE, McBratney-Owen B. Testing hypotheses about tinkering in the fossil record: the case of the human skull. J Exper Zool. 2004;302:284–301. doi: 10.1002/jez.b.21004. [DOI] [PubMed] [Google Scholar]
- Liversidge HM, Dean MC, Molleson TI. Increasing human tooth length between birth and 5.4 years. Am J Phys Anthropol. 1993;90:307–313. doi: 10.1002/ajpa.1330900305. [DOI] [PubMed] [Google Scholar]
- Meindl RS, Russel KF. Recent advances in method and theory in paleodemography. Ann Rev Anthropol. 1998;27:375–399. [Google Scholar]
- Michejda M. Ontogenetic growth changes of the skull base in four genera of nonhuman primates. Acta Anat. 1975;91:110–117. doi: 10.1159/000144376. [DOI] [PubMed] [Google Scholar]
- Miller JP, German RZ. Protein malnutrition affects the growth trajectories of the craniofacial skeleton in rats. J Nutr. 1999;129:2061–2069. doi: 10.1093/jn/129.11.2061. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–291. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Moss ML. A functional cranial analysis of primate craniofacial growth. Symp IVth Int Congr Primat. 1973;3:191–208. [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 2. The role of an osseous connected cellular network. Am J Orthod Dentof Orthop. 1997a;112:221–226. doi: 10.1016/s0889-5406(97)70249-x. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 3. The genomic thesis. Am J Orthod Dentof Orthop. 1997b;112:338–342. doi: 10.1016/S0889-5406(97)70265-8. [DOI] [PubMed] [Google Scholar]
- Moss ML. The functional matrix hypothesis revisited. 4. The epigenetic antithesis and the resolving synthesis. Am J Orthod Dentofac Orthop. 1997c;112:410–417. doi: 10.1016/s0889-5406(97)70049-0. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Gakunga PT, Carlson DS. Genetic factors influencing morphogenesis and growth of sutures and synchondroses in the craniofacial complex. Semin Orthod. 2005;11:199–208. [Google Scholar]
- Pucciarelli HM, Dressino V, Niveiro MH. Changes in skull components of the squirrel monkey evoked by growth and nutrition: an experimental study. Am J Phys Anthropol. 1990;81:535–543. doi: 10.1002/ajpa.1330810409. [DOI] [PubMed] [Google Scholar]
- Ramírez Rozzi FV, González-José R, Pucciarelli HM. Cranial growth in normal and low-protein-fed Saimiri. An environmental heterochrony. J Hum Evol. 2005;49:515–535. doi: 10.1016/j.jhevol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Beynon AD, Ramírez Rozzi F. Histological reconstruction of dental development in four individuals from a medieval site in Picardie, France. J Hum Evol. 1998;35:463–477. doi: 10.1006/jhev.1998.0233. [DOI] [PubMed] [Google Scholar]
- Sardi ML, Ramírez Rozzi FV. A cross-sectional study of human craniofacial growth. Ann Hum Biol. 2005;32:390–396. doi: 10.1080/03014460400027441. [DOI] [PubMed] [Google Scholar]
- Sardi ML, Novellino PS, Pucciarelli HM. Craniofacial morphology in the Argentine center-west: consequences of the transition to food production. Am J Phys Anthropol. 2006;130:333–343. doi: 10.1002/ajpa.20379. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Thorogood PV. Development and evolution of the vertebrate skull. In: O’Higgins P, Cohn M, editors. Development, Growth and EvolutionImplications for the Study of the Hominid Skeleton. London: Academic Press; 2000. pp. 57–83. [Google Scholar]
- Schultz AH. Age changes in primates and their modification in man. In: Tanner JM, editor. Human Growth. Oxford: Pergamon; 1960. pp. 1–20. [Google Scholar]
- Simonoff JS. Smoothing Methods in Statistics. New York: Springer; 1996. [Google Scholar]
- Sirianni JE. Nonhuman primates as models for human craniofacial growth. In: Watts ES, editor. Nonhuman Primate Models for Human Growth and Development. New York: Alan R. Liss, Inc.; 1985. pp. 95–124. [Google Scholar]
- Smith BH, Garn SM. Polymorphisms in eruption sequence of permanent teeth in American children. Am J Phys Anthropol. 1987;74:289–303. doi: 10.1002/ajpa.1330740303. [DOI] [PubMed] [Google Scholar]
- Smith BH. Growth and development and its significance for early hominid behaviour. Ossa. 1989;14:63–96. [Google Scholar]
- Smith BH. Dental development and the evolution of life history in Hominidæ. Am J Phys Anthropol. 1991;86:157–174. [Google Scholar]
- Smith BH. Life history and the evolution of human maturation. Evol Anthropol. 1992;1:134–142. [Google Scholar]
- Smith BH, Crummet TL, Brandt KL. Ages of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Ybk Phys Anthropol. 1994;37:177–231. [Google Scholar]
- Smith KK. Integration of craniofacial structures during development in Mammals. Am Zool. 1996;36:70–79. [Google Scholar]
- Sperber GH. Craniofacial Development. Hamilton, Ontario: BC Decker Inc.; 2001. [Google Scholar]
- Swindler DR. Nonhuman primate dental development and its relationship to human dental development. In: Watts ES, editor. Nonhuman Primate Models for Human Growth and Development. New York: Alan R. Liss; 1985. pp. 67–94. [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Tompkins RL. Human population variability in relative dental development. Am J Phys Anthropol. 1996;99:79–102. doi: 10.1002/(SICI)1096-8644(199601)99:1<79::AID-AJPA5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- VandeBerg JR, Buschang PH, Hinton RJ. Absolute and relative growth of the rat craniofacial skeleton. Arch Oral Biol. 2004a;49:477–484. doi: 10.1016/j.archoralbio.2003.12.007. [DOI] [PubMed] [Google Scholar]
- VandeBerg JR, Buschang PH, Hinton RJ. Craniofacial growth in growth hormone-deficient rats. Anat Rec Part A. 2004b;278A:561–570. doi: 10.1002/ar.a.20051. [DOI] [PubMed] [Google Scholar]
- Vogl C, Atchley WC, Cowley DE, Crenshaw P, Murray JD, Pomp D. The epigenetic influence of growth hormone on skeletal development. Growth Dev Aging. 1993;57:163–182. [PubMed] [Google Scholar]