Abstract
Free and elongating (DNA-bound) forms of RNA polymerase II were separated from yeast. Most cellular polymerase II was found in the elongating fraction, which contained all enzyme phosphorylated on the C-terminal domain and none of the 15-subunit mediator of transcriptional regulation. These and other findings suggest that mediator enters and leaves initiation complexes during every round of transcription, in a process that may be coupled to C-terminal domain phosphorylation.
Keywords: RNA polymerase II, C-terminal domain, yeast
There is mounting biochemical and genetic evidence for a role of the mediator complex in RNA polymerase II transcription control. Mediator was first revealed by an indirect assay, based on relief of activator inhibition (“squelching”) in vitro (1). The subsequent development of a direct assay, in which mediator was required for transcriptional activation, provided a basis for fractionation (2). Mediator was resolved to homogeneity as a complex of more than a dozen polypeptides, including products of some genes found in previous genetic screens, such as Srb2, -4, -5, and -6, and Gal11 (3). The presence of Srb proteins, as well as evidence for the association of mediator with the C-terminal domain (CTD) of RNA polymerase II, demonstrated a relationship to an Srb–polymerase II complex (4), isolated by similar procedures. Most recently, mediator has also been shown to contain products of the SIN4, RGR1, and ROX3 genes (5, 6), which were identified in previous genetic screens for negative regulatory proteins, indicating a role of mediator in repression as well as activation of transcription.
The analysis of mediator–polymerase interaction presented here was motivated by a paradox. The abundance of Srb proteins is about 6% that of polymerase subunits in whole-cell extracts, suggesting a role of the Srb–polymerase complex at a limited number of yeast promoters (7). In partially purified preparations, however, the level of mediator is about half that of polymerase, pointing to a more general role (3). It occurred to us that the paradox would be resolved if the majority of polymerases in a cell were engaged in transcription, devoid of mediator, and lost during fractionation, because of being bound to DNA and discarded with insoluble material. According to this idea, mediator–polymerase interaction would be limited to initiation complexes and would be disrupted in the transition to RNA chain elongation. As a test of the idea, we investigated the stability of mediator–polymerase interaction and the presence of mediator in transcription elongation complexes.
MATERIALS AND METHODS
Proteins.
RNA polymerase II holoenzyme, comprising 12-subunit core RNA polymerase II, 15-subunit mediator, and three-subunit TFIIF, was purified as described (5). Hexahistidine-tagged core RNA polymerase II was from strain DB1 (8).
Separation of Free and DNA-Bound RNA Polymerase II.
Whole-cell extract was prepared from a strain expressing an influenza hemagglutinin epitope-tagged form of the largest RNA polymerase II subunit (9) as described (10), except with the addition of 10 mM sodium fluoride, 10 mM potassium phosphate, and 5 mM potassium pyrophosphate to inhibit protein phosphatases. The potassium acetate concentration was adjusted to 550 mM, and DNA was precipitated by the addition of 0.7% polyethyleneimine (PEI) and sedimentation at 14,000 rpm for 5 min at 4°C in a microcentrifuge. Supernatant and pellet were analyzed by SDS/PAGE and immunoblotting.
Immunoblot Analysis.
Immunoblotting was performed as described (11). Secondary antibodies were goat anti-rabbit or mouse alkaline phosphatase conjugates (Bio-Rad) and horseradish peroxidase conjugates [for enhanced chemiluminescence (ECL), Amersham]. Quantitation of ECL was performed on a PhosphorImager (Molecular Dynamics).
RESULTS
Dynamic Mediator–Polymerase Interaction.
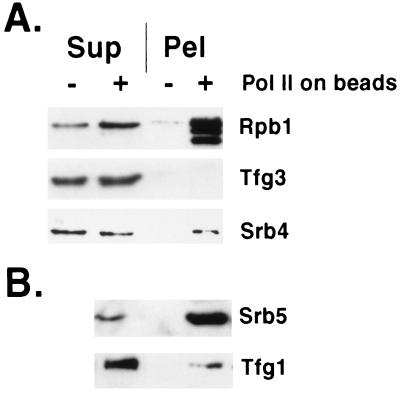
Mediator is capable of exchange between polymerase molecules, as shown by transfer from free holoenzyme (mediator–core polymerase complex) to hexahistidine-tagged core polymerase bound to Ni-agarose beads. Mediator was detected by immunoblotting with antibodies against Srb4 and Srb5. Transfer to the bead-bound polymerase was about 50% complete after 10 min (Fig. 1A, Srb4) and more extensive following 20 min of incubation (Fig. 1B, Srb5). Similar results were obtained with antibodies against the additional mediator proteins Srb2 and Med6 (not shown). Specificity was demonstrated by a lack of transfer to beads without polymerase.
Figure 1.
Mediator exchange between RNA polymerases. Exchange was for 10 min (A) or 20 min (B, separate experiment from A) by slow rotation at room temperature between free RNA polymerase II holoenzyme (0.1–0.5 μg) and hexahistidine-tagged core RNA polymerase II bound to Ni2+-agarose beads (+) or beads with no bound polymerase (−). Following sedimentation in a microcentrifuge for 2 min at 5,000 rpm, supernatant (Sup) and beads (Pel) were analyzed by SDS/PAGE (10% gel) and immunoblotting with antibodies against the proteins indicated. The Rpb1 subunit of the polymerase bound to beads was partly N-terminally degraded, resulting in a double band, which served to identify the core polymerase. Binding of core polymerase (25–50 μg in 40–150 μl) to beads (Qiagen) was in 40 mM Hepes, pH 7.6/20% glycerol/5 mM 2-mercaptoethanol/0.25 mg/ml bovine serum albumin/protease inhibitors (12) by slow rotation for 45 min at 4°C. Following several washes by centrifugation and resuspension in 200 mM Hepes, pH 7.6/20% glycerol/5 mM 2-mercaptoethanol/0.25 mg/ml bovine serum albumin/0.2% Tween-20/0.01% Nonidet P-40/10 mM imidazole/protease inhibitors, beads were resuspended in 100 μl of the same buffer. Exchange was performed with 10 μl of beads (2–5 μg of bound polymerase) in 40 mM Hepes, pH 7.6/150 mM potassium acetate/5 mM magnesium acetate/10% glycerol/5 mM 2-mercaptoethanol/0.1 mg/ml bovine serum albumin/0.06% Tween-20/0.003% Nonidet P-40/3 mM imidazole/protease inhibitors.
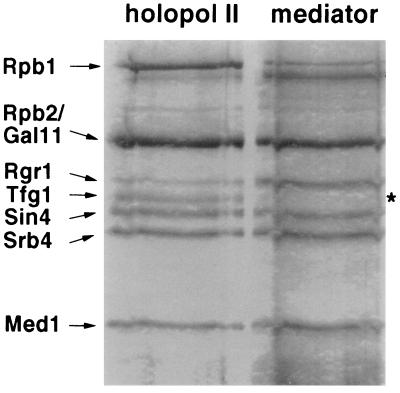
Specificity was further demonstrated by a lack of transfer of TFIIF, detected by immunoblotting with antibodies against the largest (Fig. 1B, Tfg1) and smallest (Fig. 1A, Tfg3) subunits. A difference in behavior between mediator and TFIIF was also shown by SDS/PAGE of proteins displaced from the polymerase CTD by anti-CTD antibodies (Fig. 2). All mediator proteins were recovered in the displaced fraction, whereas TFIIF remained entirely associated with the polymerase.
Figure 2.
Displacement of mediator but not TFIIF from RNA polymerase II holoenzyme by anti-CTD antibodies. Holoenzyme (100 μg) was incubated with 8WG16 monoclonal antibodies immobilized on protein A–Sepharose beads in 20 mM Tris⋅acetate, pH 7.8/20% glycerol/200 mM potassium acetate/0.01% Nonidet P-40/0.1 mM EDTA, as described (13) for 4 h at 4°C. The beads were removed by sedimentation in a microcentrifuge for 5 min at 5,000 rpm, and starting material (holopol II) and supernatant proteins (mediator) were compared by SDS/PAGE and silver staining. The top portion of a 10% gel is shown. Protein components of the holoenzyme preparation are indicated on the left. In addition to the Tfg1 subunit of TFIIF, the two smaller Tfg2 and Tfg3 subunits failed to appear in the supernatant (mediator) fraction, as did the subunits of RNA polymerase II.
Additional evidence of a dynamic mediator–polymerase interaction came from measurements of CTD phosphorylation by TFIIH. Phosphorylation is stimulated 30- to 50-fold by mediator (3), and in a mixture of equal quantities of holoenzyme and core polymerase, the amount of phosphorylation was twice that obtained with holoenzyme alone (not shown). The mediator component of the holoenzyme was evidently capable of stimulating phosphorylation of the core polymerase CTD, in all likelihood by exchange between CTDs.
Lack of Mediator Associated with Elongating RNA Polymerase II.
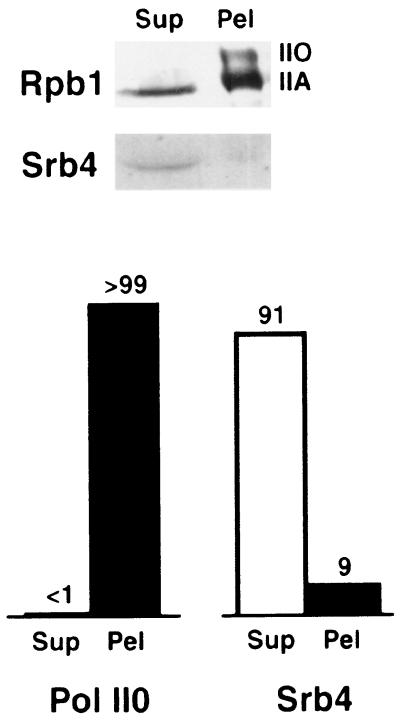
Having demonstrated the facile release of mediator from RNA polymerase II, we investigated the hypothesis that the enzyme engaged in transcription is devoid of mediator. Yeast whole-cell extract was prepared by bead-beating, which converts chromatin to soluble fragments (14), and was fractionated by PEI precipitation. A high ionic strength was employed to prevent nonspecific interactions. Previous work had shown that mediator–polymerase and transcription–elongation complexes are stable under these conditions. Free polymerase was recovered from the PEI supernatant, while DNA-bound enzyme was concentrated in the pellet. On the basis of SDS/PAGE and immunoblotting with antibody against the largest polymerase subunit (Rpb1), we estimate that about 30% of the enzyme was in the supernatant and 70% was in the pellet (Fig. 3).
Figure 3.
Components of free and DNA-bound RNA polymerase II complexes. Following separation as described, free (Sup) and DNA-bound (Pel) fractions were analyzed by SDS/PAGE (6% gel) and immunoblotting to reveal the largest polymerase subunit Rpb1 (12CA5 antibody) and the mediator component Srb4. Relative amounts of phosphorylated Rpb1 (Pol II0) and Srb4 in the free and DNA-bound fractions are indicated above the bars in the graph at the bottom. The relative amounts of phosphorylated (II0) and unphosphorylated (IIA) Rpb1 varied from one preparation to another, possibly due to phosphatase action, but the phosphorylated form was always found exclusively in the DNA-bound fraction.
Two observations confirmed the identification of polymerase in the PEI pellet as enzyme engaged in transcription. First, immunoblots of protein in the pellet revealed two bands due to Rpb1, one comigrating with Rpb1 of the free enzyme in the supernatant, and an additional, slower migrating band (Fig. 3). Such an additional band was previously attributed to hyperphosphorylation of the CTD (15, 16), believed to occur in the transition from transcription initiation to elongation (17).
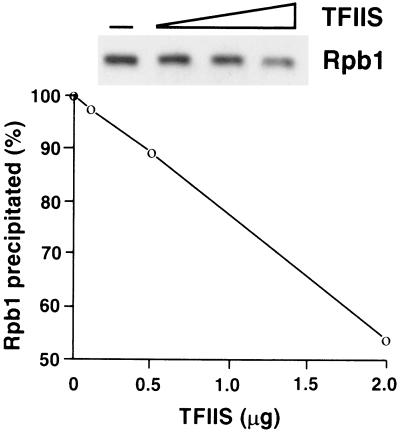
The second line of evidence for transcribing polymerase in the PEI pellet came from the effect of TFIIS, an elongation factor that facilitates polymerase passage through pause sites. TFIIS is believed to reverse RNA polymerase II action, causing degradation of the nascent transcript and retrograde movement of the enzyme on template DNA. In the absence of nucleoside triphosphates to support resumption of transcription, the transcript is degraded completely and polymerase is released (18). Incubation of whole-cell extracts with TFIIS diminished the precipitation of polymerase by PEI in proportion to the amount of TFIIS added (Fig. 4). Precipitation of polymerase was similarly affected by treatment with DNase I (data not shown), further attesting to the association of enzyme in the PEI pellet with DNA.
Figure 4.
Effect of TFIIS on recovery of RNA polymerase II in DNA-bound fraction. Free and DNA-bound RNA polymerase II complexes were separated as described, except that the whole-cell extract (50 μl, 20–30 mg protein/ml) was treated with TFIIS (gift from A. M. Edwards, McMaster University, Hamilton, Ontario, Canada) in the amounts indicated in a volume of 100 μl containing 50 mM Tris⋅Cl, pH 7.5/60 mM ammonium sulfate/10 mM magnesium chloride/10% glycerol/10 μM zinc sulfate for 30 min at 30°C, followed by chilling in ice, adjustment of potassium acetate concentration, and PEI precipitation. The amount of Rpb1 in the PEI pellet was determined by SDS/PAGE (10% gel) and immunoblotting. Phosphorylated and unphosphorylated forms of Rpb1 were not resolved in this percentage gel.
The clean separation of free and transcribing polymerases between PEI supernatant and pellet enabled us to investigate the association of mediator with the two forms of the enzyme. Mediator was detected by immunoblotting with anti-Srb4 antibodies, as described above. Srb4 was found almost entirely in the PEI supernatant (Fig. 3). We conclude that mediator is absent from transcribing polymerase.
DISCUSSION
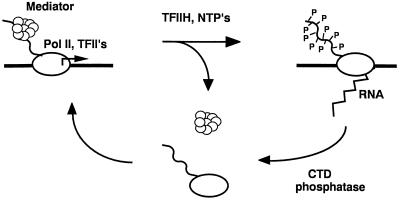
The data presented here substantiate the hypothesis that most RNA polymerase II in a yeast cell is engaged in transcription, and that the transcribing polymerase lacks associated mediator. A picture emerges of a mediator cycle accompanying every round of transcription (Fig. 5). Mediator is presumed to enter an initiation complex with polymerase, since it is found associated with the free enzyme. In the course of initiation, mediator plays multiple roles in the activation and repression of transcription (3, 5, 7). Mediator is released at the end of initiation or early in RNA chain elongation, as shown by its absence from the transcribing polymerase. Reassociation of mediator with polymerase completes the cycle.
Figure 5.
Proposed mediator cycle. RNA polymerase II (Pol II), associated with mediator and general initiation factors (TFII’s), interacts with DNA (thick horizontal line) at the start site of transcription (arrow above line). TFIIH phosphorylates the CTD (wavy line), concomitant with mediator release and the initiation of transcription. Dephosphorylation of the CTD and rebinding of mediator complete the cycle. NTP’s, nucleoside triphosphates.
The proposed mediator cycle parallels the previously described CTD phosphorylation cycle (17). In the presence of mediator, during transcription initiation, the CTD is unphosphorylated, whereas in the absence of mediator, during RNA chain elongation, the CTD is hyperphosphorylated (Fig. 5). The possibility arises that the mediator cycle is driven by CTD phosphorylation. For example, phosphorylation might interfere with mediator binding to the CTD. Our attempts to demonstrate such an effect, by exchange (as described above) between mediator bound to immobilized RNA polymerase and either unphosphorylated or hyperphosphorylated free enzyme, have so far been inconclusive. Such exchange may be catalyzed by additional protein factors in vivo, and dependence on the phosphorylation state of the CTD may be conferred by these factors.
Specificity of exchange was demonstrated here by a difference in behavior between mediator and TFIIF. More rapid exchange of mediator than of TFIIF is consistent with the idea that only mediator is released in the transition from transcription initiation to elongation. TFIIF plays functional roles in both initiation and elongation, and so might be expected to persist in elongation complexes (18). The differential release of mediator is, however, inconsistent with our previous evidence for mediator–TFIIF interaction (3). Our evidence was indirect, based on association and dissociation of mediator and TFIIF from polymerase II under similar conditions, and we have since isolated a mediator–polymerase complex (“holoenzyme”) free of TFIIF (19). For these reasons, we now regard mediator and TFIIF as distinct and possibly unrelated assemblies.
Our finding that most RNA polymerases in a yeast cell are engaged in transcription is of both fundamental and practical significance. It suggests an efficient use of the cellular supply of RNA polymerase II to attain the maximal rate of mRNA synthesis. It also explains our inability to detect the hyperphosphorylated form of polymerase in previous preparations of the yeast enzyme, as the transcribing polymerase was discarded with DNA and cell debris following high-speed centrifugation. The isolation of transcribing polymerase may reveal other important differences from the free enzyme in the future.
Acknowledgments
Work in the Kornberg laboratory was supported by National Institutes of Health Grant GM36659.
ABBREVIATIONS
- CTD
C-terminal domain
- PEI
polyethyleneimine
References
- 1.Kelleher R J, III, Flanagan P M, Kornberg R D. Cell. 1990;61:1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan P M, Kelleher R J, III, Sayre M H, Tschochner H, Kornberg R D. Nature (London) 1991;350:436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson C M, Koleske A J, Chao D M, Young R A. Cell. 1993;73:1361–1375. doi: 10.1016/0092-8674(93)90362-t. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Bjorklund S, Jiang Y W, Kim Y-J, Lane W S, Stillman D J, Kornberg R D. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsson C M, Myers L C, Li Y, Redd M J, Erdjument-Bromage H, Tempst P, Kornberg R D. J Biol Chem. 1996;272:48–50. doi: 10.1074/jbc.272.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Koleske A J, Young R A. Nature (London) 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 8.Bushnell D A, Bamdad C, Kornberg R D. J Biol Chem. 1996;271:20170–20174. doi: 10.1074/jbc.271.33.20170. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Kornberg R D. Proc Natl Acad Sci USA. 1994;91:2362–2366. doi: 10.1073/pnas.91.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svejstrup J Q, Feaver W J, LaPointe J, Kornberg R D. J Biol Chem. 1994;269:28044–28048. [PubMed] [Google Scholar]
- 11.Chasman D I, Kornberg R D. Mol Cell Biol. 1990;10:2916–2923. doi: 10.1128/mcb.10.6.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan P M, Kelleher R J, III, Feaver W J, Lue N F, LaPointe J W, Kornberg R D. J Biol Chem. 1990;265:11105–11107. [PubMed] [Google Scholar]
- 13.Cairns B R, Kim Y J, Sayre M H, Laurent B C, Kornberg R D. Proc Natl Acad Sci USA. 1994;91:1950–1954. doi: 10.1073/pnas.91.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorch Y, Kornberg R D. Proc Natl Acad Sci USA. 1994;91:11032–11034. doi: 10.1073/pnas.91.23.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feaver W J, Gileadi O, Li Y, Kornberg R D. Cell. 1991;67:1223–1330. doi: 10.1016/0092-8674(91)90298-d. [DOI] [PubMed] [Google Scholar]
- 16.Valay J-G, Simon M, Dubois M-F, Bensaude O, Facca C, Faye G. J Mol Biol. 1995;249:535–544. doi: 10.1006/jmbi.1995.0316. [DOI] [PubMed] [Google Scholar]
- 17.Dahmus M E. Prog Nucleic Acid Res Mol Biol. 1994;48:143–179. doi: 10.1016/s0079-6603(08)60855-7. [DOI] [PubMed] [Google Scholar]
- 18.Reines D, Conaway J W, Conaway R C. Trends Biochem Sci. 1996;21:351–355. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Bjorklund S, Kim Y-J, Kornberg R D. Methods Enzymol. 1996;273:172–176. doi: 10.1016/s0076-6879(96)73017-3. [DOI] [PubMed] [Google Scholar]