Abstract
The role of Schmidt–Lanterman incisures (SLIs) within the myelin sheath remains the subject of much debate. Previous studies have shown a positive correlation between the number of SLIs per internode and internodal width for both normal and pathological myelin internodes. As chronic nerve compression (CNC) injury induces demyelination, we sought to evaluate if CNC injury altered the occurrence of SLIs using nerve-teasing techniques and light microscopy. Rigorous examination of the teased axons from nerves subjected to CNC injury for 1 month, 2 months or 8 months revealed that there is indeed a positive correlation between the number of SLIs per internode and the internodal width. However, unlike previous studies, the degree of positive correlation between these two parameters was greater in the internodes that had undergone remyelination in response to CNC injury as compared with the internodes from control nerves. These findings support the theory that SLIs are likely to assist in the metabolic processes of the myelin sheath, including growth and maintenance of the myelin sheath.
Keywords: chronic nerve compression injury, internode, myelin, Schmidt–Lanterman incisure
Introduction
Within the myelin sheath, there are regions where the myelin is not compacted, regions known as Schmidt–Lanterman incisures (SLI). SLIs have been the subject of several studies (Hiscoe, 1947; Sotnikov, 1965; Ghabriel & Allt, 1979, 1980a,b) and have been found to contain a variety of cellular structures normally found in the cytoplasm of the portion of the Schwann cell not contained within the myelin sheath. These structures include a single microtubule within each spiral, desmosome-like bands, actin filaments, mitochondria-like organelles and smooth endoplasmic reticulum (Blakemore, 1969; Singer, 1968; Landon & Hall, 1976). Furthermore, SLIs also contain a variety of cellular proteins, such as alkaline phosphatase, myelin-associated glycoprotein and P2 basic protein, as well as a variety of cytoskeletal proteins, such as connexin 32 (Pinner et al. 1964; Novikoff, 1967; Trapp et al. 1979; Schober et al. 1981; Xu et al. 2000). Although the actual function of SLIs remains unknown, the presence of these cellular components within SLIs has led to suggestions that SLIs are actively involved in metabolic processes of the myelin sheath and are vital to myelin function (Robertson, 1962; Singer & Salpeter, 1966; Singer, 1968; Hall & Williams, 1971; Singer et al. 1972; Rawlins, 1973).
Different conditions and diseases can alter the occurrence of SLIs. Hiscoe (1947) and Sotnikov (1965) showed that the number of incisures per internode increased as internodal width increased in developing, normal adult and regenerated myelinated axons. Similarly, Ghabriel and Allt showed that the number of incisures per internode was positively correlated with the internodal width for internodes that had undergone remyelination following injection of lysophosphatidyl choline into rat sural nerves (Ghabriel & Allt, 1980a). Yet there is currently little known about the occurrence of SLIs after commonly acquired disease states. One of the most common peripheral neuropathies, carpal tunnel syndrome, is a chronic nerve compression (CNC) injury. Previous work has shown that CNC injuries alter not only Schwann cell activity by inducing proliferation and apoptosis, but also induce demyelination (Gupta & Steward, 2003; Gupta et al. 2004). We sought to evaluate if CNC injury altered the occurrence of SLIs at different time points after injury.
Materials and methods
Animal models
Using a previously described model of CNC injury (Gupta & Steward, 2003), CNC injury was applied to male Sprague–Dawley rats (200–300 g; Charles River Laboratories, Wilmington, MA, USA) in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. Rats were anaesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg kg−1, i.p.; Abbott Laboratories, North Chicago, IL, USA). Sciatic nerves were mobilized by a gluteal-splitting incision, and a sterile 1-cm non-constrictive silastic tube (inner diameter 1.3 mm, outer diameter 2.0 mm; Baxter Healthcare, Deerfield, IL, USA) was atraumatically placed around the nerve. The contralateral sciatic nerves were mobilized and returned to the host bed to serve as a control. The wound was sutured closed in all layers.
Harvest and preparation of nerves
Nerve specimens were harvested at different postoperative time points: 1 month, 2 months and 8 months. Control and experimental nerve specimens were harvested from each animal. Each sciatic nerve was separated into its three main fascicles by removing the epineurium with the use of a dissecting microscope and ultra-fine forceps. The fascicles of each separate nerve were washed five times for 5 min each time in 0.05 m isotonic cacodylate buffer at pH 7.38. Each nerve separated into its fascicles was osmicated for 2 h using 1% osmium tetraoxide. After osmicating the nerves, each nerve was treated with 45, 66 and 100% glycerin, each for 24 h at 45 °C. After 24 h, each of the tubes containing the separate nerves was removed from the oven and stored at room temperature, covered with aluminium foil.
Nerve teasing and light microscopy
Once the nerves were properly prepared, 25 axons were separated from each nerve. The number of axons to be separated from each nerve was determined based on our previous nerve teasing study (Gupta et al. 2004). For each nerve, a fascicle was placed on a microscope slide along with a few drops of 100% glycerol. The fascicle was then separated proximal to distal, using ultra-fine forceps and a dissecting microscope, into smaller bundles of axons until individual axons could be separated. Five axons were separated out, proximal to distal, on each slide so that for each nerve sample, five slides, each containing five axons, were obtained. Each slide containing five axons was placed in a warm oven for several hours in order to ensure that the axons adhered to the slide. Each slide was then prepared for light microscopy. One drop of 100% glycerol was added on top of the axons on each slide, and then a coverslip was placed over the drop of glycerol and axons so that the glycerol spread out over the axons. Each axon was then observed under a 20× light microscope objective using an Olympus microscope, and digital photographs were taken of each section of each axon by using a 20× objective. The Olympus Microsuite computer program was used to measure internodal length and internodal width. Using these images, the number of SLIs was also calculated per internode.
Statistical analysis
It has previously been shown that CNC produces demyelination, and that the portions of the axon that undergo remyelination consist of internodes that have shorter internodal lengths and more narrow internodal widths (Gupta et al. 2004). Based on those findings, data regarding the number of SLIs per internode, internodal length and internodal width were separated into three categories: internodes from control nerve samples, internodes consisting of normal myelin not affected by CNC from nerves that were subjected to CNC, and internodes consisting of new myelin following remyelination of portions of axons affected by CNC from nerves subjected to CNC. In order to identify remyelinated internodes, two criteria that were utilized by Ghabriel and Allt for identifying remyelinated internodes were used: (1) there had to be a transition from thick, unaffected myelin to thin remyelinated myelin, and (2) the remyelinated internodes had to be significantly shorter than normal unaffected internodes (Ghabriel & Allt, 1980b). Previous studies that have quantified SLIs have revealed that there is much variation in the occurrence of SLIs per internode, and that the best way to analyse the occurrence of SLIs is with scatter plots and trend-lines (Ghabriel & Allt, 1979, 1980; Cooper & Kidman, 1984). Using the Microsoft Excel computer program, scatter plots were constructed comparing the number of SLIs per internode to internodal length and the number of SLIs per internode to internodal width for each of the three categories. Scatter plots were constructed in this manner for the 1-month, 2-month and 8-month nerve samples. For each scatter plot, trend-lines were constructed and the slope of each trend-line was calculated. In addition, Pearson's coefficient and confidence intervals (at the 95% level) were calculated.
Results
Morphology
Examination of the internodes within axons from control nerves revealed that within each axon, the internodal length and width were quite similar between each internode. By contrast, axons from nerves that had undergone CNC injury had a very different pattern. The occurrence of demyelination was apparent, as evidenced by the presence of internodes with internodal lengths and internodal widths similar to those of respective control internodes, and different internodes with internodal lengths and internodal widths that were significantly decreased from those of the respective control internodes. Table 1 shows the ranges of internodal length and internodal width for internodes from normal nerve samples and for internodes from remyelinated nerve samples. Examination of the appearance of SLIs revealed that in both normal and remyelinated internodes, SLIs appeared as oblique segments on both sides of the internode (Fig. 1). Morphological examination of the placement of SLIs revealed that most SLIs are located within the internode region, and only occasionally are SLIs found in the paranodal regions for both normal and remyelinated internodes. This finding is consistent with previous studies, which also showed incisures being less frequent in the paranodal region relative to the rest of the internode for normal fibres (Webster, 1965; Reynolds & Heath, 1995). For remyelinated axons, studies have shown that although the length between the node of Ranvier and the adjacent SLI is decreased, the occurrence of paranodal SLIs is still less frequent compared with the rest of the internode (Hiscoe, 1947; Ghabriel & Allt, 1980b).
Table 1.
Measurement ranges (µm) for internodal length and internodal width from the 1-, 2- and 8-month time points for internodes from normal nerve samples and internodes from remyelinated nerve samples
| Internode lengths | Internode width | |||
|---|---|---|---|---|
| Post-operative time-point | Normal | Remyelinated | Normal | Remyelinated |
| 1 | 440–1720 | 280–1000 | 7.4–14.8 | 5.5–10.2 |
| 2 | 810–1805 | 200–980 | 10.4–22.3 | 5.2–10.8 |
| 8 | 850–1800 | 155–750 | 9.8–18.1 | 5.9–12.1 |
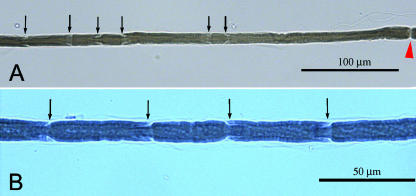
Fig. 1.
(A,B) Images of Schmidt–Lanterman incisures (SLIs) within an internode in an axon from a control nerve (arrows indicate SLIs, arrowhead indicates node of Ranvier).
Occurrence of SLIs
Demyelination occurred in axons from nerves subjected to CNC injury. Axons from CNC-injured nerves had regions with internodes with internodal lengths and internodal widths that were decreased from those of normal internodes from control samples, and also had internodes with internodal lengths and internodal widths that were similar to those of normal internodes from control samples. Trend-lines constructed from the scatter plots comparing the number of SLIs per internode to internodal length, along with Pearson correlation coefficients, revealed no consistently significant relationship between these two parameters (Table 2). Analysis of the scatter plots comparing the number of SLIs per internode and internodal width in control internodes and internodes unaffected by CNC injury revealed that no significant correlation exists between these two parameters for the 1-month, 2-month and 8-month time points. However, analysis of the scatter plots comparing the number of SLIs per internode and internodal width did reveal a significant positive correlation between these two parameters for internodes within segments of axon that had undergone remyelination in response to CNC injury and had clearly demonstrable segments that consisted of new myelin (Figs 2–5; Table 3).
Table 2.
Slopes of trend-lines, Pearson correlation coefficients (r) and 95% confidence intervals of r from scatter plots comparing the number of Schmidt–Lanterman incisures per internode to internodal length for the 1-, 2- and 8-month time points
| Time point | Sample | Slope | Pearson correlation coefficient (r) | 95% CI |
|---|---|---|---|---|
| 1 month | Control | 0.003 | 0.106 | (0.007, 0.205) |
| CNC nerve internodes consisting of normal myelin | 0.013 | 0.261 | (0.158, 0.376) | |
| CNC nerve internodes consisting of new myelin following remyelination | 0 | −0.030 | (−0.336, 0.276) | |
| 2 months | Control | 0 | 0.012 | (−0.047, 0.179) |
| CNC nerve internodes consisting of normal myelin | 0.002 | 0.052 | (−0.084, 0.188) | |
| CNC nerve internodes consisting of new myelin following remyelination | 0.006 | 0.287 | (0.115, 0.475) | |
| 8 months | Control | 0.008 | 0.245 | (0.146, 0.354) |
| CNC nerve internodes consisting of normal myelin | 0.009 | 0.218 | (0.078, 0.366) | |
| CNC nerve internodes consisting of new myelin following remyelination | 0.019 | 0.238 | (0.108, 0.378) |
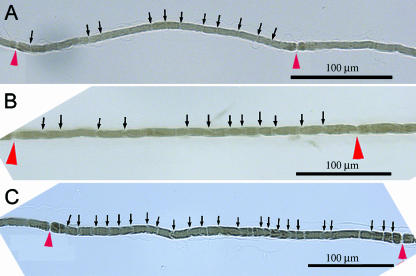
Fig. 2.
Image of Schmidt–Lanterman incisures (SLIs) within a remyelinated internode at 20× objective following chronic nerve compression for (A) 1 month, (B) 2 months, (C) 8 months (arrows indicate SLIs, arrowheads indicate nodes of Ranvier).
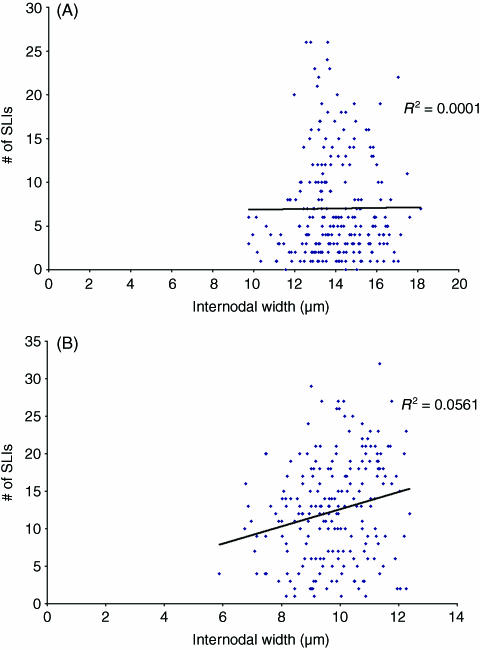
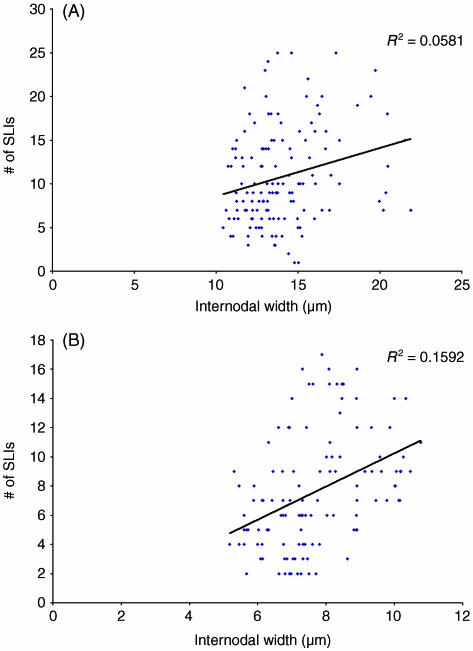
Fig. 5.
Comparison of the number of Schmidt–Lanterman incisures per internode and internodal width at the 8-month time point for (A) internodes from control nerves and (B) internodes from segments of axons affected by chronic nerve compression, consisting of new myelin.
Table 3.
Slopes of trend-lines, Pearson correlation coefficients (r) and 95% confidence intervals of r from scatter plots comparing the number of Schmidt–Lanterman incisures per internode to internodal width for the 1-, 2- and 8-month time points
| Time point | Sample | Slope | Pearson correlation coefficient (r) | 95% CI |
|---|---|---|---|---|
| 1 month | Control | 0.195 | 0.040 | (−0.059, 0.139) |
| CNC nerve internodes consisting of normal myelin | 0.032 | 0.069 | (−0.039, 0.177) | |
| CNC nerve internodes consisting of new myelin following remyelination | 1.464 | 0.263 | (−0.049, 0.587) | |
| 2 months | Control | 0.550 | 0.241 | (0.079, 0.413) |
| CNC nerve internodes consisting of normal myelin | 0.420 | 0.137 | (0.002, 0.274) | |
| CNC nerve internodes consisting of new myelin following remyelination | 1.138 | 0.399 | (0.241, 0.605) | |
| 8 months | Control | 0.037 | 0.010 | (−0.134, 0.154) |
| CNC nerve internodes consisting of normal myelin | −0.793 | 0.052 | (−0.196, 0.092) | |
| CNC nerve internodes consisting of new myelin following remyelination | 1.158 | 0.237 | (0.105, 0.377) |
Fig. 3.
Comparison of the number of Schmidt–Lanterman incisures per internode and internodal width at the 1-month time point for (A) internodes from control nerves and (B) internodes from segments of axons affected by chronic nerve compression, consisting of new myelin.
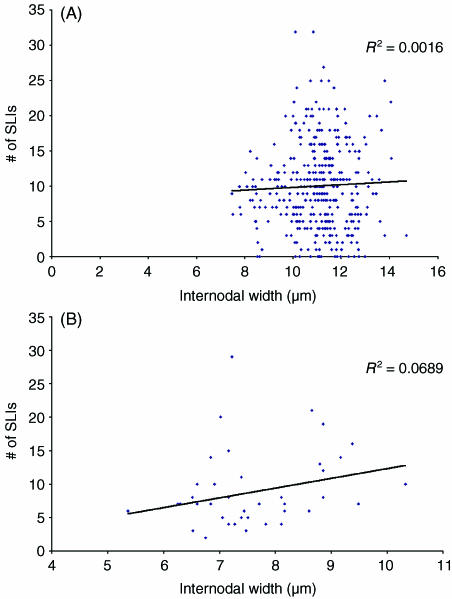
Fig. 4.
Comparison of the number of Schmidt–Lanterman incisures per internode and internodal width at the 2-month time point for (A) internodes from control nerves and (B) internodes from segments of axons affected by chronic nerve compression, consisting of new myelin.
Discussion
Previous studies have utilized a variety of techniques to induce neural pathology and subsequently evaluated their effect on the occurrence of SLIs. These techniques have included nerve transection (Reynolds & Heath, 1995), injection of demyelination-inducing compounds such as diphtheria toxin (Cooper & Kidman, 1984) and lysophosphatidyl choline (Ghabriel & Allt, 1980a), and nerve crush for 10 s (Ghabriel & Allt, 1979) to 30 s (Cooper & Kidman, 1984). Each of these studies showed that for internodes consisting of new myelin following the process of remyelination, there was a clear positive correlation between the number of SLIs per internode and internodal width. This study confirms a previous report that CNC injury induces demyelination, followed by remyelination (Gupta et al. 2004). The region affected by CNC injury produces remyelinated internodes that also possess a significant positive correlation between the number of SLIs per internode and internodal width, as evidenced by the increased positive slope and positive Pearson correlation coefficient. This positive correlation reveals that as the myelin sheath becomes thicker, the number of SLIs per internode increases. A thicker myelin sheath means that there is a greater amount of myelin to be metabolically supported, as well as a greater distance between the axon and the Schwann cell with potentially slower transfer speeds of molecules due to the increased size of the SLI. By increasing the number of SLIs per internode, the Schwann cell may ensure that there are an adequate number of pathways to allow communication and metabolic processes to occur between the Schwann cell and the axon.
Analysis of the data has shown that there is a slightly positive correlation between the number of SLIs per internode and internodal length for control internodes and remyelinated internodes. The only exception occurred with the remyelinated internodes from the 1-month time point. This exception most likely can be attributed to sampling error. However, the relationship between the number of SLIs per internode and the internodal length for the remaining control and remyelinated samples corresponds to similar findings obtained by Cooper & Kidman (1984), who examined the occurrence of SLIs in the sciatic nerves of hens. They showed that for normal adult internodes and remyelinated internodes either 100 or 200 days after diptheria toxin injection, there was a small positive correlation between the number of SLIs per internode and internodal length.
The more striking feature of this study relates to the correlation between the number of SLIs per internode and internodal width. Studies have shown that there tends to be a positive correlation between the number of SLIs per internode and internodal width for both normal internodes and remyelinating internodes (Hiscoe, 1947; Sotnikov, 1965; Ghabriel & Allt, 1980a). Similarly, this study has shown that a positive correlation does exist between these two parameters for normal internodes. However, the results of this study differ from previous studies in that the degree of positive correlation between the number of SLIs per internode and internodal width is greater for the remyelinating internodes. A possible explanation for this difference is that in previous studies the number of normal internodes sampled may have been too few. In the study of Ghabriel & Allt (1980) only 47 control internodes were examined. In this study, 392 internodes from the 1-month time point were examined, 138 internodes from the 2-month time point and 186 internodes from the 8-month time point. Based on results obtained from their studies, Ghabriel and Allt suggest that the occurrence of SLIs per internode may demonstrate greater variation in normal internodes. As such, sampling a larger number of internodes would lead to greater variation in the number of SLIs per internode, which would produce a less positive correlation between the number of SLIs per internode and internodal width (Ghabriel & Allt, 1979, 1980a). As we sampled a greater number of control internodes at each time point, this is the likely explanation for why the degree of positive correlation between the number of SLIs per internode and internodal width is less in this study than in previous studies.
An interesting feature regarding the increased positive correlation between the number of SLIs per internode and internodal width for remyelinating internodes is that the degree of positive correlation between these two parameters remains fairly consistent for samples harvested after 1, 2 and 8 months of CNC. This would indicate that the increased frequency of SLIs in remyelinating internodes is not a transient event. Ghabriel & Allt (1980a) suggested that a greater SLI frequency supports the theory that SLIs are involved in growth and maintenance of the myelin sheath as the synthesis of myelin during remyelination involves increased metabolic processes as compared with the metabolic processes of an already established myelin sheath. Our results would seem to indicate that the increased demands of the ‘new’ myelin within the remyelinated internodes are maintained with CNC injury at the later time points. Assuming that SLIs are crucial for growth and maintenance of the myelin sheath, the existence of increased SLI frequency at the later time points after CNC injury would seem to indicate that the remyelinated internodes never return to the baseline level of physiological functioning. As such, SLIs are providing some vital function after the remyelination process, and one must contemplate the processes with which these specialized regions of the myelin sheath are involved.
The idea that SLIs provide a functional role in the myelin sheath is supported by various other studies. Connexin 32 (Cx32) is a gap junction protein that has been localized to SLIs. When Cx32 is absent due to mutation, Charcot–Marie–Tooth disease ensues as a demyelinating peripheral neuropathy characterized by decreased nerve conduction velocities and thinly myelinated large fibres (Abrams et al. 2000). The localization of this crucial protein to the SLIs would support the notion that SLIs are actively involved in the proper function of myelinated axons. Abrams et al. (2000) suggest that Cx32 is likely to form channels between adjacent myelin loops at SLIs and thereby provide the much shorter diffusion pathway within the Schwann cell cytoplasm. Similarly, as insulin is known to have neurotrophic effects, the localization of insulin receptors to SLIs further suggests that SLIs are involved in metabolic activities of the internode (Sugimoto et al. 2000). Tricaud et al. (2005) demonstrated that when the expression of E-cadherin, an adhesion molecule that is a key component of Schwann cell adherens junctions, is disturbed, there is a complete loss of SLIs in the majority of affected cells. This loss of SLIs in response to disturbance of E-cadherin indicates that the Schwann cell deliberately inserts and maintains SLIs within the myelin sheath with the use of cytoskeletal proteins and provides further evidence as to the functional value of SLIs.
Various alternative suggestions have been made regarding the function of SLIs. One suggested function is that SLIs act as a pathway for substances to cross the myelin sheath in order to reach the axon (Singer & Salpeter, 1966; Singer, 1968; Hall & Williams, 1971; Singer et al. 1972; Rawlins, 1973). Robertson (1962) suggested that SLIs may function in the maintenance and metabolism of myelin. It has also been hypothesized that SLIs may provide a means to allow for changes in axoplasm volume and in fibre length caused by changes in body part positions, especially the limbs (Glees, 1943; Sulzmann, 1957). However, despite these hypotheses, the true function of SLIs in the myelin sheath remains unknown.
Nevertheless, previous studies have shown that SLIs apparently are crucial to the function of internodes, especially remyelinating internodes. This study supports this idea by demonstrating that those regions of the axon that have undergone remyelination have an increased SLI frequency and are likely to have increased metabolic needs. Based on this idea, one must contemplate the mechanism by which the remyelinating Schwann cell inserts SLIs into the new internode. Animals with a knockout gene mutation for myelin basic protein (MBP) showed an increase in the number of SLIs per internode if both copies of the MBP gene were knocked-out (Gould et al. 1995). Furthermore, it has been shown that when Schwann cells are subjected to shear stress in vitro, stress that mimics the mechanical stress that occurs with CNC injury, they down-regulate mRNA and protein expression of MBP and of myelin-associated glycoprotein (MAG) (Chafik et al. 2003; Gupta et al. 2005). MAG is a promyelinogenic marker in Schwann cells that is located in SLIs, as well as periaxonal cytoplasm and nodes of Ranvier (Xu et al. 2000). Taken together, these studies would indicate that the increased frequency of SLI insertion after CNC injury may involve the regulation of either the MBP or the MAG gene. In order to understand better the function of SLIs and the mechanism by which the remyelinating Schwann cell inserts a greater frequency of SLIs into the remyelinated internode, future studies examining gene expression within SLIs of normal and remyelinated internodes are needed.
Acknowledgments
This study was supported by NIH grants 5K08 NS02221 (R.G.) and 5R01 NS049203 (R.G.).
References
- Abrams CK, Oh S, Ri Y, Bargiello TA. Mutations in connexin 32: the molecular and biophysical bases for the X-linked form of Charcot–Marie–Tooth disease. Brain Res Rev. 2000;32:203–214. doi: 10.1016/s0165-0173(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Schmidt–Lantermann incisures in the central nervous system. J Ultrastruct Res. 1969;29:496–498. doi: 10.1016/s0022-5320(69)90069-0. [DOI] [PubMed] [Google Scholar]
- Chafik D, Bear D, Bui P, et al. Optimization of Schwann cell adhesion in response to shear stress in an in vitro model for peripheral nerve tissue engineering. Tissue Eng. 2003;9:233–241. doi: 10.1089/107632703764664701. [DOI] [PubMed] [Google Scholar]
- Cooper NA, Kidman AD. Quantitation of the Schmidt–Lanterman incisures in juvenile, adult, remyelinated and regenerated fibres of the chicken sciatic nerve. Acta Neuropathol (Berl) 1984;64:251–258. doi: 10.1007/BF00688116. [DOI] [PubMed] [Google Scholar]
- Ghabriel MN, Allt G. The role of Schmidt–Lanterman incisures in wallerian degeneration. I. A quantitative teased fibre study. Acta Neuropathol (Berl) 1979;48:83–93. [PubMed] [Google Scholar]
- Ghabriel MN, Allt G. Schmidt–Lanterman incisures. I. A quantitative teased fibre study of remyelinating peripheral nerve fibres. Acta Neuropathol (Berl) 1980a;52:85–95. doi: 10.1007/BF00688005. [DOI] [PubMed] [Google Scholar]
- Ghabriel MN, Allt G. The role of Schmidt–Lantermann incisures in remyelination. Fol Morph. 1980b;28:129–133. [PubMed] [Google Scholar]
- Glees P. Observations on the structure of the connective tissue sheath of cutaneous nerves. J Anat. 1943;77:153–159. [PMC free article] [PubMed] [Google Scholar]
- Gould RM, Byrd AL, Barbarese E. The number of Schmidt–Lanterman incisures is more than doubled in shiverer PNS myelin sheaths. J Neurocytol. 1995;24:85–98. doi: 10.1007/BF01181552. [DOI] [PubMed] [Google Scholar]
- Gupta R, Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol. 2003;461:174–186. doi: 10.1002/cne.10692. [DOI] [PubMed] [Google Scholar]
- Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187:500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gupta R, Truong L, Bear D, Chafik D, Modafferi E, Hung CT. Shear stress alters Schwann cell expression of myelin associated glycoprotein (MAG) and myelin basic protein (MBP) J Ortho Res. 2005;23:1232–1239. doi: 10.1016/j.orthres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Hall SM, Williams PL. The distribution of electron-dense tracers in the peripheral nerve fibres. J Cell Sci. 1971;6:767–791. doi: 10.1242/jcs.8.2.541. [DOI] [PubMed] [Google Scholar]
- Hiscoe HB. Distribution of nodes and incisures in normal and regenerated nerve fibers. Anat Rec. 1947;99:447–475. doi: 10.1002/ar.1090990404. [DOI] [PubMed] [Google Scholar]
- Landon DN, Hall SM. The myelinated nerve fibre. In: Landon DN, editor. The Periperhal Nerve. London: Chapman & Hall; 1976. pp. 1–105. [Google Scholar]
- Novikoff AB. Enzyme localisation and ultrastucture of neurons. In: Hyden H, editor. The Neuron. Amsterdam: Elsevier; 1967. pp. 255–318. [Google Scholar]
- Pinner B, Davison JF, Campbell JB. Alkaline phosphatase in peripheral nerves. Science. 1964;145:936–938. doi: 10.1126/science.145.3635.936. [DOI] [PubMed] [Google Scholar]
- Rawlins FA. A time-sequence autoradiographic study of the in vivo incorporation of (1,2,3H) cholesterol into peripheral nerve myelin. J Cell Biol. 1973;58:42–53. doi: 10.1083/jcb.58.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RJ, Heath JW. Patterns of morphological variation within myelin internodes of normal peripheral nerve: quantitative analysis by confocal microscopy. J Anat. 1995;187:369–378. [PMC free article] [PubMed] [Google Scholar]
- Robertson JD. The unit membrane of cells and mechanism of myelin formation. In: Korey SR, Pope A, Robins E, editors. Ultrastructure and Metabolism of the Nervous System. Baltimore: Williams & Wilkins; 1962. pp. 94–158. [Google Scholar]
- Schober R, Itoyama Y, Sternberger NH, et al. Immunocytochemical study of PO glycoprotein, P1 and P2 basic proteins, and myelin-associated glycoprotein (MAG) in lesions of idiopathic polyneuritis. Neuropath Appl Neurobiol. 1981;7:421–434. doi: 10.1111/j.1365-2990.1981.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Singer M, Salpeter MM. The transport of 3H–l–histidine through the Schwann and myelin sheath into the axon, including a reevaluation of myelin function. J Morph. 1966;120:281–316. doi: 10.1002/jmor.1051200305. [DOI] [PubMed] [Google Scholar]
- Singer M. Penetration of labeled amino acids into the peripheral nerve fibre from surrounding body fluids. In: Wolstenholme EW, O'Connor M, editors. Growth of the Nervous System. London: Churchill; 1968. pp. 200–215. [Google Scholar]
- Singer M, Krishnan N, Fyfe DA. Penetration of ruthenium red into peripheral nerve fibers. Anat Rec. 1972;173:375–390. doi: 10.1002/ar.1091730401. [DOI] [PubMed] [Google Scholar]
- Sotnikov OS. Structure of Schmidt–Lantermann incisures. Arkh Anat Gistol Embriol. 1965;43:31–42. (translated in Fed. Am Soc Exp Biol (1966), 25, T204–210) [PubMed] [Google Scholar]
- Sugimoto K, Murakawa Y, Zhang W, Xu G, Sima AAF. Insulin receptor in rat peripheral nerve: its localization and alternatively spliced isoforms. Diabetes Metab Res Rev. 2000;16:354–363. doi: 10.1002/1520-7560(200009/10)16:5<354::aid-dmrr149>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sulzmann R. Beiträge zur Morphologie der peripheren Nervenfser III. Mitteilung die Schmidt-Lantermansche inzisur und die Schwannsche Zelle. Z Zellforsch Mikrosk Anat. 1957;46:489–516. [PubMed] [Google Scholar]
- Trapp BD, McIntyre LJ, Quarles RH, Sternberger NH, Webster HDEF. Immunocytochemical localization of rat peripheral nervous system myelin proteins: P2 protein is not a component of all peripheral nervous system myelin sheaths. Proc Nat Acad Sci USA. 1979;76:3552–3556. doi: 10.1073/pnas.76.7.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricaud N, Perrin-Tricaud C, Brusés JL, Rutishauser U. Adherens junctions in myelinating Schwann cells stabilize Schmidt–Lanterman incisures via recruitment of p120 catenin to E-cadherin. J Neurosci. 2005;25:3259–3269. doi: 10.1523/JNEUROSCI.5168-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HDEF. The relationship between Schmidt–Lanterman incisures and myelin segmentation during Wallerian degeneration. Ann NY Acad Sci. 1965;122:29–38. doi: 10.1111/j.1749-6632.1965.tb20189.x. [DOI] [PubMed] [Google Scholar]
- Xu W, Manichella D, Jiang H, et al. Absence of P0 leads to the dysregulation of myelin gene expression and myelin morphogenesis. J Neurosci Res. 2000;60:714–724. doi: 10.1002/1097-4547(20000615)60:6<714::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]