Abstract
Using cleared-and-stained whole mounts and computer-aided three-dimensional reconstructions made from serial histological sections, we studied the development of the pectoral girdle in Discoglossus pictus, an extant member of an ancient frog lineage, represented for example by Eodiscoglossus from the Middle Jurassic to Early Cretaceous periods in Europe. Basic developmental features were compared with those of extinct Temnospondyli, considered to be the most probable anuran ancestors, and with Triadobatrachus, an early Triassic proanuran. In the endochondral girdle, the separate scapula and coracoid of Discoglossus and other anurans (completed by suprascapular and procoracoid cartilages) evolved from the compact scapulocoracoid of temnospondyls by paedomorphosis. In parallel, the dermal ossifications of the girdle were reduced to a small clavicle and cleithrum. The overall reduction in ossification of the anuran pectoral girdle supports the hypothesis of a paedomorphic origin for Anura. The almost simultaneous appearance of dermal and endochondral ossifications may be explained by the accumulation of developmental events during a short, distinct metamorphosis (which did not occur in neotenic temnospondyls living permanently in water). The sternal elements seem to be neomorphs for the most part, which help to cushion the shock of landing in jumping anurans but which also evolved as functional substitutes (insertion area for the pectoralis muscles) of the temnospondyl interclavicle.
Keywords: Anura, development, evolution, heterochrony, pectoral girdle
Introduction
Anurans are one of three clades of modern amphibians (i.e. anurans, caudates and apodans, often grouped under the name ‘Lissamphibia’), which are known by their peculiar, saltatory locomotion. Like caudates, they are contemporary survivors of the late Permian and Triassic members of the amphibian group Temnospondyli (although the modern amphibian orders, in a strict sense, belong among Temnospondyli, the term ‘temnospondyls’ is often employed in its traditional, informal sense as shorthand for extinct ‘non-lissamphibian’ temnospondyls). Comparisons of the anuran skeletal structure with that of Permo-Triassic temnospondyls (roughly dated to 300–250 Ma) revealed that it most closely recalls some neotenic, i.e. permanently water-dwelling Dissorophoidea or Capitosauroidea, which did not undergo metamorphosis into a terrestrial adult. This explains why the origin of anurans is meaningfully explained as a result of abbreviated somatogenesis.
The pectoral girdle of anurans (Fig. 1A) retains nearly all the skeletal elements of early tetrapods. However, as with the general trend in all tetrapods, the dermal part is strongly reduced. The interclavicle was a large median element connecting both halves of the girdle in early tetrapods and their piscine ancestors (Jarvik, 1980, 1996; Clack, 2000). This was preserved in extinct temnospondyls, sometimes with a posterior extension that presumably developed in connection with the pectoralis (i.e. adductor) musculature (Romer, 1947). Variation in the size and proportions of the temnospondyl interclavicle occurred even within species and was obviously ontogenetic (Warren & Snell, 1991). However, this dermal element was lost in anurans and the pectoralis muscles became attached to new, median elements of endochondral origin. The clavicles and cleithra were still relatively broad, thin plates in extinct temnospondyls, whereas in anurans the clavicles are slender and elongated, and the cleithra are represented by vestigial dermal ossification adjoining the suprascapular cartilage.
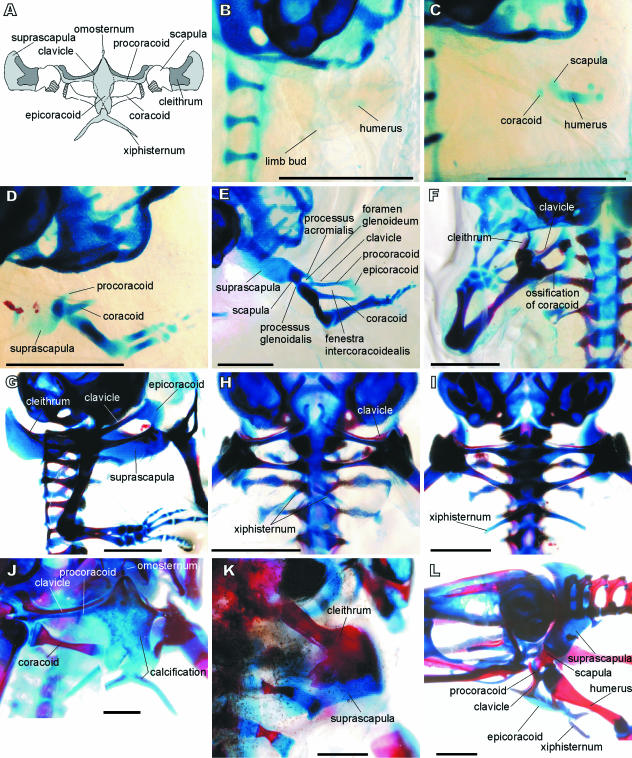
Fig. 1.
Discoglossus pictus. (A) Pectoral girdle of adult in ventral view, with scapula, suprascapula and cleithrum deflected. Dermal ossifications in dark grey, cartilages in light grey, glenoid shaded. (B) Stage 52. (C) Stage 53. (D) Stage 56. (E) Stage 57. (F) Stage 58. (G) Stage 59. (H) Stage 60. (I) Stage 66. (J) Adult (snout–vent length 2.5 cm). (K) Adult (snout–vent length 3 cm). (L) Subadult (snout–vent length 1.5 cm). Note the ossification progress in E–G and extension of the epicoracoid from the procoracoid in E. B, C, K in dorsal view; F, H–J in ventral view; G in right lateral view; L in left lateral view. Scale bars = 1 mm.
In the most primitive tetrapods, the endochondral part of the shoulder girdle was a single element termed the scapulocoracoid. In the osteolepid ancestors of tetrapods, this bone was fused to the inner surface of the cleithrum but was the only element contributing to the glenoid in which the pectoral fin articulated (Jarvik, 1980; Clack, 2000). However, in the earliest tetrapods (Ichthyostega, Acanthostega), the scapulocoracoid became partly exposed in lateral aspect due to reduction of the cleithrum (Jarvik, 1996; Clack, 2000). In extinct temnospondyls there was apparently only one centre of ossification in the endochondral part of the girdle (Warren & Snell, 1991) but in some anthracosaurs (Seymouria) there was a second, ventral, ossification centre. In addition, the presence of a separate coracoid ossification in the nectridean genus Diplocaulus may suggest that the scapulocoracoid was already divided into two parts in some early tetrapods (Romer, 1947). In all anurans, the scapula and coracoid are the two principal ossifications of the girdle, and they are well separated from each other.
As the interclavicle was lost in the course of the temnospondyl–anuran transition (Roček & Rage, 2000), the pectoralis muscles became attached to thin but extensive overlapping cartilages attached to the medial end of each coracoid (a condition termed ‘arciferal’; Kaplan, 2004). No sternum has ever been reported in primitive tetrapods, but it may be assumed that some cartilaginous structures serving for the insertion of pectoral muscles were present between the two halves of the girdle. These structures may have been similar to the sternal elements of recent caudates and anurans (e.g. Engler, 1929; Francis, 1934; Hoffman, 1936), and they may have expanded into the area previously occupied by the interclavicle. Whatever their size and morphological appearance, these postulated cartilaginous sternal elements in temnospondyls did not articulate with the ribs, so the girdle was separate from the axial skeleton and attached only by muscles. This was no doubt an important precondition for the landing phase of the frog jump. The reduction of the ribs in anurans was probably not a direct consequence of saltatory locomotion, but was a prerequisite for jumping, as may be judged from the markedly reduced (or underdeveloped) ribs in Triadobatrachus, a proanuran amphibian from the early Triassic (some 250 Ma), which was not yet capable of jumping (Rage & Roček, 1989).
The pectoral girdle of the only specimen of Triadobatrachus is badly crushed and intermingled with fragments of the lower jaw, making interpretation difficult. Rage & Roček (1989) believed it could have been structurally similar to that of a primitive frog type, which means that the scapula and coracoid were separated as in anurans. However, Borsuk-Białynicka & Evans (2002) were of a different opinion and suggested that Triadobatrachus still possessed a single scapulocoracoid, as was the case with ancestral temnospondyls. This interpretation was supported by the association of frog-like ilia from the Lower Triassic of Poland, described as Czatkobatrachus (Evans & Borsuk-Białynicka, 1998), with disarticulated scapulocoracoids of approximately corresponding size.
It is now generally agreed that anurans evolved from their temnospondyl ancestors by shortening their somatic development (hypomorphosis). Paedomorphic changes are primarily manifested in the cranial structures (Roček & Rage, 2000), but they occur in the postcranial skeleton as well. Among these changes, small size with retention of juvenile proportions, shortening of the ossification sequence and reduction or loss of dermal bones are the most significant. Miniaturization is already recognized in some phylogenetic lineages of extinct temnospondyls (Milner, 1988; Schoch, 1995), but a shortening of the ossification sequence that culminated in the loss of some dermal bones apparently occurred later, during the temnospondyl–anuran transition, as evidenced from comparisons of complete ossification sequences, e.g. in branchiosaurs (Boy, 1974) and the simplified anuran skeletal structure. The shortening of the ossification sequence in anurans not only eliminates those elements that appeared in the late larval or post-metamorphic stages of branchiosaurs (e.g. ectopterygoid, prefrontal, postfrontal, postorbital, jugal, interclavicle; Shishkin, 1973; Smirnov, 1995, 1999), but also arrests ossification at an earlier level (cartilage or even membrane), before it is complete (e.g. sclerotic ring, braincase, otic capsule). Arrested ossification is particularly conspicuous in caudates (especially neotenic caudates) where the dermal part of the shoulder girdle (including clavicles and cleithra) is entirely lost and the endochondral part ossifies only as a tiny scapula, with all other parts remaining cartilaginous (Hoffman, 1936). Similar consequences of arrested ossification of the scapulocoracoid may be supposed in temnospondyls; the scapulocoracoid of Palaeozoic taxa was well ossified but it became progressively smaller and less well ossified in Mesozoic taxa (Warren & Snell, 1991). It may be supposed that if the scapulocoracoid of temnospondyls developed from two ossification centres that coalesced with one another in adults, then arrested ossification in anurans could result in the retention of two elements separated by cartilage, as is the case in the anuran otic capsule in which the prootic and opisthotic are isolated by a strip of cartilage.
The differences between the extinct temnospondyl and anuran shoulder girdles raise the question of the transformation between the two and, consequently, the homology of their parts. Two different interpretations of the shoulder girdle in Triadobatrachus (Rage & Roček, 1989; Borsuk-Białynicka & Evans, 2002) and disarticulated scapulocoracoids associated with Czatkobatrachus (Borsuk-Białynicka & Evans, 2002) obscure a key period in this transformation. Because palaeontological evidence is still under discussion, one may turn to the developmental morphology of the shoulder girdle in recent anurans, and try to recognize primitive features in their early development. Discoglossus was chosen as it is one of the most primitive recent anurans (i.e. the extant offshoot of the discoglossoids which are recorded as early as the middle Jurassic, i.e. more than 160 million years ago; Evans et al. 1990). The aim of this study was to assess to what degree, if any, primitive temnospondyl features are preserved in the development of the shoulder girdle of Discoglossus, and whether it might be possible to make any inferences about the evolutionary sequence of changes between the temnospondyl and anuran types of shoulder girdle.
Materials and methods
We examined 87 captive-born individuals of painted frog Discoglossus pictus. This developmental series was bred in the laboratory of the Department of Zoology, University of South Bohemia, České Budějovice. The specimens were staged according to the table of normal development of Xenopus laevis (Nieuwkoop & Faber, 1967); stages are abbreviated as NF in the text. Each larval and metamorphic stage (NF 50–66) was represented by at least three, but usually five, individuals, and this series was supplemented with five subadult and four adult specimens. The specimens were all cleared and double-stained for bone and cartilage following the method of Wassersug (1976), with slight modifications. Seven additional individuals (stages 54–55, 55–56, 57–58, 59, 61, 63–64 and 66) were studied on three-dimensional (3D) models developed from histological sections using 3D Studio MAX 5.0, and smoothing with Corel Photo-Paint 8.0 and Corel Draw 8.0. Osteological terminology follows that of Gaupp (1896) and Duellman & Trueb (1994). Photographs were taken using a Nikon SMZ 1500 binocular microscope fitted with a Nikon DXM 1200 digital camera. Illustrations were made from photographs using Microsoft Office Power Point.
For comparison, we also used a developmental series of fire-bellied toad Bombina bombina (130 specimens covering the period of NF stages 50–66, plus six adults), yellow-bellied toad B. variegata (98 specimens covering the period of NF stages 50–66, plus eight adults), African clawed toad Xenopus laevis (37 specimens covering the period of NF stages 53–66, plus three adults), common spadefoot Pelobates fuscus (88 specimens in NF stages 50–65, plus one adult), common toad Bufo bufo (53 specimens covering the period of NF stages 50–66, plus five adults) and agile frog Rana dalmatina (64 specimens covering the period of NF stages 50–66, plus five adults). The material is deposited in the collections of the Department of Zoology, Charles University in Prague and the Department of Zoology, University of South Bohemia in České Budějovice.
Results
The forelimb buds are recognizable in NF stage 51 as oval condensations of connective tissue, located on the dorsal side, posterolateral to the otic capsule. The earliest chondrification centre is that of the humerus and appears in stage 52, when the forelimb bud protrudes from the outline of the body as a slightly bent paddle (Fig. 1B). The pectoral girdle appears shortly afterwards, in stage 53 (Fig. 1C), from two chondrification centres near the proximal end of the humerus; the rudiment of the coracoid is oval and located ventrally, whereas that of the scapula is circular, and located dorsally. Shortly afterwards, during stages 54–55, another chondrification centre appears, clearly separated from the others and elongated in shape; this is the primordium of the procoracoid. The procoracoid then joins the lateral end of the coracoid (intumescentia glenoidalis). By stage 56, the three elements (i.e. procoracoid, coracoid and scapula) have fused to one other. At the same time, the suprascapula appears as a thin cartilage connected to the dorsal edge (margo suprascapularis) of the scapula (Fig. 1D).
After NF stage 57, chondrification extends so that the scapula, procoracoid and coracoid gradually achieve their ultimate shape, preformed in cartilage. Two separate connections between the scapula and coracoid are formed at stage 57 (Fig. 1E). The first is a thin curved bridge connecting the pars acromialis of the scapula with the anterolateral end of the coracoid (intumescentia glenoidalis). This connection (processus acromialis) forms the anterior margin of the glenoid foramen (foramen glenoideum). The posterior margin of the glenoid foramen is formed by an outgrowth of the scapula (processus glenoidalis) that is attached to the posterior margin of the coracoid intumescentia glenoidalis. The glenoid foramen persists throughout life. Simultaneously (still in stage 57), the medial end of the procoracoid extends posteriorly to join the medial end of the coracoid (pars epicoracoidealis). This connection, for the most part produced by the procoracoid, is called the epicoracoid (cartilago epicoracoidea). The posterior margin of the procoracoid, the epicoracoid, and the anterior margin of the coracoid (margo fenestralis) form the border of the fenestra intercoracoidealis. In later development, the epicoracoid cartilage extends medially, attains its final shape and comes into contact with its counterpart from the opposite side (Figs 1H and 2A). The epicoracoids begin to overlap one another at the beginning of metamorphosis (stage 60), and the overlap increases during metamorphosis (stages 61–66), although they are joined only by a connective tissue.
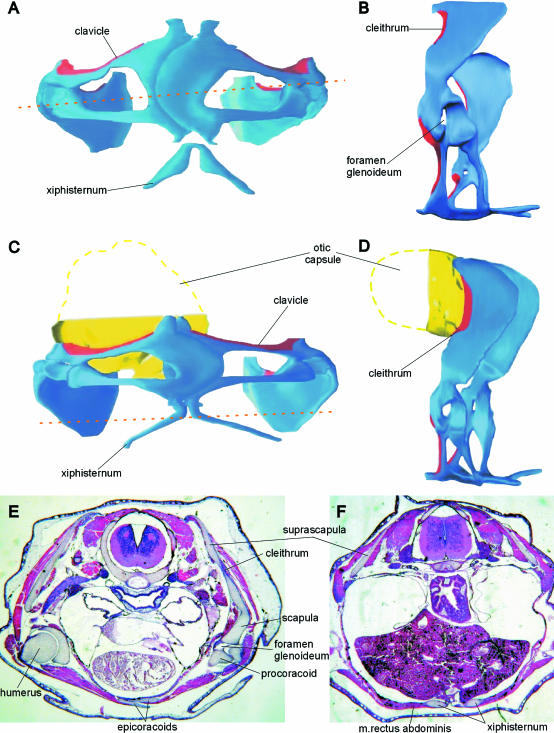
Fig. 2.
Discoglossus pictus. (A,B) Three-dimensional model of pectoral girdle in stage 61. (C,D) Three-dimensional model of pectoral girdle in stage 66. (E) Frontal section at the level indicated by arrows in A. (F) Frontal section at stage 66 at the level indicated by arrows in C. In the models, dermal bones are in red; endochondral elements in blue. A, C in ventral view; B, D, left lateral view.
Ossification begins at stages 57–58 (Fig. 1E,F), the dermal elements before the endochondral elements. The clavicle develops along the anterior surface of the procoracoid and the cleithrum appears along the anterior margin of the suprascapula (at stage 57). Later (at stage 58), one ossification centre is visible in the centre of the coracoid, with another close to the anterior margin of the scapula. In the course of further development (stages 59–60), the clavicle extends laterally to overlap both the procoracoid and the ventral part of the scapula (its pars acromialis). The medial part of the cleithrum extends posteriorly over the dorsal surface of the suprascapula.
Concomitant with the beginning of ossification (stages 57–58), the glenoid cavity attains its final shape as a result of development of its constituent elements; consequently, the foramen glenoideum becomes reduced in size. A pair of cartilaginous rods (the early constituents of the xiphisternum) appears on the ventral side, posterior to the epicoracoids (Fig. 1H). They grow in size and soon they join to form the xiphisternum. Shortly afterwards, they become connected to the epicoracoids by connective tissue; this connection chondrifies in advanced metamorphosis (stages 63–64; Figs 1I and 2C). The xiphisternum then grows posteriorly. When metamorphosis is complete (NF stage 66<), a small independent cartilage appears anterior to the medial tips of both procoracoids (and/or clavicles). This is the rudiment of the unpaired prezonal sternal element termed the omosternum (Fig. 1J). Later this becomes a thin cartilaginous rod that is moderately swollen at its anterior end.
At the end of metamorphosis (NF stage 66), the pectoral girdle (like other parts of the skeleton) is only feebly ossified (Fig. 1I). Ossification of both endochondral and dermal elements continues throughout post-metamorphic development. The cleithrum grows in size and ultimately covers a large portion of the anterior and dorsal surface of the suprascapula (Figs 1K and 2D). The clavicle overlaps the glenoid end of the procoracoid. Even in fully mature adults, some calcified areas may be recognized within the epicoracoid, suprascapula and xiphisternum (Fig. 1J).
Discussion
Discoglossus is a modern survivor of the ancient discoglossoid phylogenetic lineage, the earliest representatives of which are known from as early as the middle Jurassic (Evans et al. 1990), more than 160 million years ago. Development of the pectoral girdle in this genus might be expected to reflect evolutionary transformations that occurred during the transition from pre-anuran temnospondyl stages to anurans. Similarly, Triadobatrachus, which is the only articulated fossil representative of this transition, should fit into this sequence.
It is generally agreed that heterochrony, specifically paedomorphosis, is the principal phenomenon associated with the origin of modern amphibians (‘lissamphibians’) (Boy & Sues, 2000; Roček & Rage, 2000, and references therein). Paedomorphosis may be defined as the retention of subadult ancestral features in the adult descendant form. This means that the structure of the anuran pectoral girdle (except for additional sternal elements considered to be neomorphs; see below) should correspond to that of an earlier developmental stage in extinct temnospondyls; in other words, the anuran pectoral girdle is an ‘underdeveloped’ temnospondyl girdle (the ‘hypomorphosis’ of Alberch et al. 1979). Therefore, attempts to derive features in the pectoral girdle of adult anurans from that of adult temnospondyls are meaningless unless they too belong to paedomorphic lineages. However, differences in the morphology of homologous structures may be used to infer the degree of evolutionary change that occurred during the temnospondyl–anuran transition.
It is apparent that the general developmental pattern of the pectoral girdle is the same in all anurans, disregarding their evolutionary status. The earliest rudiments of endochondral elements are the scapula and coracoid, which are soon joined by the procoracoid. The same observation was made in Discoglossus sardus (Púgener & Maglia, 1997), as well as in Rana pipiens (Shearman, 2005). Some authors did not recognize the procoracoid as an independent chondrification centre (De Vos, 1938; Hall & Larsen, 1998; Baleeva, 2001) and considered its earliest rudiment to be a part of the developing scapula. Dermal ossification of the pectoral girdle (clavicle and cleithrum) in Discoglossus begins almost simultaneously with the appearance of endochondral ossification centres within the scapula and coracoid. It is also worth noting that in discoglossids (Discoglossus, Bombina) dermal ossification begins earlier (e.g. Púgener & Maglia, 1997; Maglia & Púgener, 1998; our personal observations) than in more derived taxa, such as Bufo and Rana (Shearman, 2005; our personal observations).
In typical adult temnospondyls (exemplified by Dendrerpeton from the late Carboniferous; see Holmes, 2000), the main part of the pectoral girdle consists of the large dermal elements (interclavicle, clavicles and cleithra). The scapulocoracoid is unitary and bears a glenoid; its scapular portion is pierced by a supraglenoid foramen located dorsal to the glenoid, and the coracoid plate is pierced by a supracoracoid foramen.
In the earliest known temnospondyl larvae, the dermal skeleton of the pectoral girdle (interclavicle, clavicles and cleithra) was fully formed whereas only a small portion of the supraglenoid region of the scapulocoracoid was ossified (Boy, 1974; Schoch, 1992; Boy & Sues, 2000). Even in adult individuals of some Branchiosauridae (which were neotenic) the scapulocoracoid was incompletely ossified. This means that dermal ossification still dominated in their pectoral girdle and markedly preceded endochondral ossification. By contrast, the scapulocoracoid was already subject to hypomorphosis in some neotenic temnospondyls. Its scapular part ossified separately from the coracoid part, so that they can be considered independent elements, and markedly preceded the coracoid in developmental sequence (Boy, 1974, table 1). It should be noted that, as in contemporary anurans, the humerus of larval temnospondyls began development before all parts of the pectoral girdle.
The pectoral girdle of Triadobatrachus is poorly preserved and intermingled with elements of the lower jaw and some unidentifiable skeletal fragments. Rage & Roček (1986) originally believed that the comparatively robust element might be the scapulocoracoid, but later (Rage & Roček, 1989) re-interpreted the skeletal parts within this area as a separate scapula and coracoid. Borsuk-Białynicka & Evans (2002) proposed a different interpretation, which requires displacement and rotation of some elements. They concluded that Triadobatrachus still possesed a unitary scapulocoracoid (see Fig. 3), the coracoid portion of which was broken at the level of the supraglenoid foramen and shifted posteriorly. It seems there is nothing to contradict this interpretation. From this interpretation it may be inferred that there was some area of weakened ossification ventromedial to the glenoid (in the region of the future epicoracoid), or that this area was formed only by a cartilage. On the other hand, the supraglenoid portion of the scapulocoracoid suggests that the scapular portion was still better ossified than in true frogs, whether or not the coracoid and procoracoid were separated by an unossified, i.e. cartilaginous, part. If reduction of dermal ossification occurred in proportion to the degree of endochondral ossification of the scapulocoracoid, then the scapulocoracoid of Triadobatrachus should be less ossified considering the size reduction of the clavicle and complete loss of the interclavicle (no interclavicle was found in Triadobatrachus).
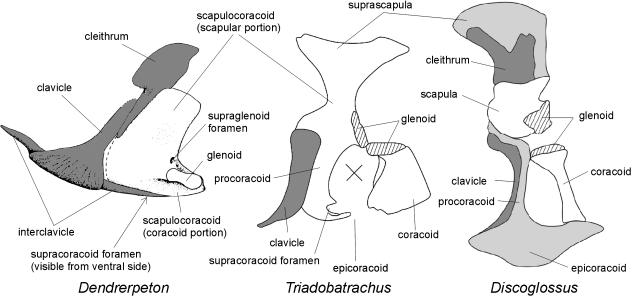
Fig. 3.
Possible sequence in transformation of the pectoral girdle from typical adult temnospondyl (Dendrerpeton) to primitive anuran (Discoglossus), via pro-anuran stage (Triadobatrachus), in left lateral view. The cross in Triadobatrachus represents missing material. Dermal ossification in dark-grey, cartilages (if illustrated) in light grey. Not to scale. Dendrerpeton after Holmes et al. (1998), from Holmes (2000); Triadobatrachus from Borsuk-Białynicka & Evans (2002).
More important in this context is the fact that the endochondral part of the pectoral girdle of Triadobatrachus was more extensively ossified than in the adult anurans. This means that in the course of evolution from the pro-anuran stage represented by Triadobatrachus to the most primitive anurans (represented by the early Jurassic Prosalirus, Jenkins & Shubin, 1998, and middle Jurassic Eodiscoglossus, Evans et al. 1990), the structure and development of which may be investigated in contemporary Discoglossus, ossification was gradually arrested at earlier developmental stages so that their adults are ossified to a lesser degree. This arrested ossification is manifested by the fact that large portions of the original scapulocoracoid remain cartilaginous in the anurans (e.g. epicoracoid, procoracoid) or even membraneous (fenestra intercoracoidealis).
It therefore seems that the scapulocoracoid, which was a large, unitary element (probably completed by a suprascapular cartilage) in adult temnospondyls, was arrested in its development in anurans at the level of two separate ossifications (as evidenced by temnospondyl larvae). The anteromedial portion (termed the procoracoid) remained cartilaginous; that part which is called the epicoracoid is a ventral extension of the procoracoid. The scapulocoracoid of Triadobatrachus probably represents a stage in which the ventromedial area of the coracoid portion became a thin and fragile plate of bone or possibly remained cartilaginous. In the anurans, this area either remains membraneous (fenestra intercoracoidealis) or is represented by a cartilage that appears late in development by extension of the procoracoid (epicoracoid). The entire evolutionary sequence of the scapulocoracoid may thus be characterized as having occurred through increasing hypoossification until the final stage (in anurans) corresponds to the early developmental stage of temnospondyl larvae.
In the course of evolution from pre-anuran stages to anurans, the hypoossification of the pectoral girdle may be related to paedomorphosis (see above). The general mechanism by which the scapulocoracoid was divided into a separate scapula and coracoid may be inferred from branchiosaurs. They are the only group of extinct temnospondyl amphibians in which the development and ossification sequence is sufficiently known. At the same time, there is a general consensus that the ancestors of modern amphibians should be sought among the extinct Temnospondyli (see Roček & Rage, 2000 for a review of literature, and Schoch & Carroll, 2003 for more recent data). Branchiosauridae were neotenic so they did not undergo metamorphosis, usually defined as a loss of gill-mediated respiration and acquirement of pulmonary respiration (with all accompanying transformations of the skeletal and non-skeletal structures). This is why their development was gradual, with no concentration of developmental changes over a short period of time that can be termed metamorphosis. Consequently, their skeletal elements (including those of the pectoral girdle) appear gradually and continuously throughout post-embryonic development. Their clavicle appears first, later followed by the cleithrum and interclavicle; the scapula appears in the middle of larval development, whereas the coracoid was found only in most advanced individuals (Boy, 1974; Schoch, 1992). In some other groups of extinct Temnospondyli related to anuran ancestry (e.g. Capitosauridae and Brachyopidae, which extend to the Early Triassic), development was similar and various features observable in anuran development (especially of the skull; see Shishkin, 1973) can be found in their adult anatomy. All were permanent water-dwellers dependent largely on gill respiration.
In contrast to extinct neotenic temnospondyls (and, to a lesser degree, also to caudate amphibians), anurans undergo metamorphosis, which is a short, distinct period of anatomical transformations that are associated with transition from larvae living in water to land-dwelling adults. Metamorphosis is abbreviated and the developmental events associated with metamorphosis are concentrated accordingly (Roček & Van Dijk, 2006). Sharp differences between the metamorphosis of anuran tadpoles and caudate larvae, and the continuous development of neotenic temnospondyls depend on the fact that tadpoles have different types of jaws (which is why dermal bones appear later in development: after metamorphosis larval jaws associated with herbivory are replaced by the adult, predatory jaw apparatus) and a substantial part of the larval body (tail) is resorbed (necrobiotic metamorphosis). The first aspect of anuran metamorphosis, the retardation of dermal ossification due to jaw transformation, may be related to the appearance of the extremities and associated structures, such as the pectoral girdle. This is the most plausible explanation for the contraction of developmental events, including the appearance of the pectoral girdle elements, into a very limited period of time, so that they occur almost simultaneously, not chronologically separated over a long time span, as in ancestral forms. However, several basic features (such as the appearance of the humerus before all elements of the pectoral girdle) are retained in anuran development.
The median elements of the anuran pectoral girdle in Discoglossus, and their comparatively late appearance (in post-metamorphic development) deserve some comment. The omosternum appears as an unpaired cartilaginous rod and remains cartilaginous throughout life. This type of omosternum also occurs in Bombina orientalis (Maglia & Púgener, 1998), B. maxima, and aged individuals of B. bombina and B. variegata (P.H., personal observations). The xiphisternum is made up of paired cartilaginous (calcified in old individuals) elements and is embedded in the m. rectus abdominis (Fig. 2F). Both prezonal and postzonal sternal elements arise as chondrifications within the median muscular septum and in myosepta of m. rectus abdominis (De Villiers, 1922), as a functional response to the need for fixation of the glenoids. This region is still incomplete in discoglossids, but is massive and fully functional in advanced anurans, and may be partially ossified. The sternal elements (except for the epicoracoids) may be considered neomorphs that, seen from a purely functional standpoint, serve for shock absorption in the landing phase of the jump (Emerson, 1983, 1984). However, in the context of evolutionary morphology, they provide a functional substitute for the interclavicle of anuran ancestors because they have taken over the role of providing an insertion surface for the pectoralis muscles, which were undoubtedly present in the earliest tetrapods.
Conclusions
The pectoral girdle of Discoglossus pictus appears as three separate chondrification centres (rudiments of the scapula, coracoid and procoracoid), which are preceded by that of the humerus. Both halves of the girdle are widely separated from one another. These rudiments of endochondral bones are soon followed by rudiments of those parts that remain cartilaginous in the adult (epicoracoid and suprascapula). The epicoracoid is just the posteromedial part of the procoracoid.
The endochondral part of the pectoral girdle, in which only scapula and coracoid ossify, is no doubt derived from the scapulocoracoid of neotenic temnospondyl amphibians. The separate scapular and coracoid portions found in neotenic branchiosaurids provide evidence for hypomorphosis (hypoossification) of the scapulocoracoid in the anuran lineage.
Dermal bones (clavicle and cleithrum) appear almost simultaneously with the earliest rudiments of endochondral ossification. This abbreviated ossification sequence is probably a consequence of the accumulation of developmental events during the short distinct anuran metamorphosis, whereas in the absence of metamorphosis, the ossification sequence of ancestral neotenic temnospondyls extends over a long period of time.
The medial elements of the girdle (omosternum, xiphisternum) arise as intramuscular chondrifications (ossifications in more advanced anurans) in the course of post-metamorphic development, when both halves of the girdle come into contact. They are neomorphs that both help to cushion the shock of landing in jumping frogs and provide functional substitutes for the interclavicle of temnospondyl ancestors (insertion area for pectoralis muscle).
Acknowledgments
Thanks are due to Jenny Narraway, Hubrecht Laboratory, Utrecht, and Peter Giere, Institut für Systematische Zoologie, Museum für Naturkunde, Berlin, for the loan of sectioned material of Discoglossus under their care. We express our gratitude to Susan Evans, University College, London, and two anonymous referees, for their useful comments on the manuscript. This research was made possible by grant AVOZ30130516 to the Geological Institute, Academy of Sciences of the Czech Republic.
References
- Alberch P, Gould SJ, Oster GF, Wake DB. Size and shape in ontogeny and phylogeny. Paleobiology. 1979;5:296–317. [Google Scholar]
- Baleeva NV. Formation of the scapular part of the pectoral girdle in anuran larvae. Russ J Herpetol. 2001;8:195–204. [Google Scholar]
- Borsuk-Białynicka M, Evans SE. The scapulocoracoid of an early Triassic stem-frog from Poland. Acta Palaeontol Pol. 2002;47:79–96. [Google Scholar]
- Boy JA. Die Larven der rhachitomen Amphibien (Amphibia: Temnospondyli; Karbon – Trias) Paläont Z. 1974;48:236–268. [Google Scholar]
- Boy JA, Sues H-D. Branchiosaurs: larvae, metamorphosis and heterochrony in temnospondyls and seymouriamorphs. In: Heatwole H, Carroll E, editors. Amphibian Biology, Vol. 4.– Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1150–1197. [Google Scholar]
- Clack JA. The origin of tetrapods. In: Heatwole H, Carroll E, editors. Amphibian Biology, Vol. 4. – Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 979–1029. [Google Scholar]
- De Villiers CGS. Neue Beobachtungen über den Bau und die Entwickelung des Brustschluterapparates bei den Anuren, insbesondere bei Bombinator. Acta Zool. 1922;3:1–73. [Google Scholar]
- De Vos CM. The zonal and sternal skeleton of the Leiopelmidae (Anura) Anat Anz. 1938;87:54–81. [Google Scholar]
- Duellman WE, Trueb L. Biology of Amphibians. New York: McGraw-Hill Co; 1994. [Google Scholar]
- Emerson SB. Functional analysis of frog pectoral girdles. I. The epicoracoid cartilages. J Zool. 1983;201:293–308. [Google Scholar]
- Emerson SB. Morphological variation in frog pectoral girdles: testing alternatives to a traditional adaptive explanation. Evolution. 1984;38:376–388. doi: 10.1111/j.1558-5646.1984.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Engler E. Untersuchungen zur Anatomie und Entwicklungsgeschichte des Brustschulterapparates der Urodelen. Acta Zool Stockholm. 1929;10:144–229. [Google Scholar]
- Evans SE, Milner AR, Musset F. A discoglossid frog from the Middle Jurassic of England. Palaeontology. 1990;33:299–311. [Google Scholar]
- Evans SE, Borsuk-Białynicka M. A stem-group frog from the Early Triassic of Poland. Acta Palaeontol Pol. 1998;43:573–580. [Google Scholar]
- Francis ETB. The Anatomy of the Salamander. Oxford: Clarendon Press; 1934. [Google Scholar]
- Gaupp E. Anatomie des Frosches. Erste Abtheilung – Lehre vom Skelet und vom Muskelsystem. Braunschweig: Friedrich Vieweg und Sohn; 1896. [Google Scholar]
- Hall SA, Larsen JH. Postembryonic ontogeny of the Spadefoot toad, Scaphiopus intermontanus (Anura: Pelobatoidea): skeletal morphology. J Morph. 1998;238:179–244. doi: 10.1002/(SICI)1097-4687(199811)238:2<179::AID-JMOR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hoffman AC. Die anatomie van die skouergordels en die ontwickeling van die sternum by die urodele –Cryptobranchus alleghaniensis en Necturus maculatus. Soölogiese Navorsing, Nasionale Museum Bloemfontein. 1936;1(5):33–50. [Google Scholar]
- Holmes R, Carroll RL, Reisz RR. The first articulated skeleton of Dendrerpeton acadianum (Temnospondyli, Dendrerpetontidae) from the Lower Pennsylvanian locality of Joggins, Nova Scotia, and a review of its relationships. J Vert Paleont. 1998;18:64–79. [Google Scholar]
- Holmes R. Palaeozoic temnospondyls. In: Heatwole H, Carroll E, editors. Amphibian Biology, Vol. 4.–Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1081–1120. [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates. London: Academic Press; 1980. [Google Scholar]
- Jarvik E. The Devonian tetrapod Ichthyostega. Fossils Strata. 1996;40:1–213. [Google Scholar]
- Jenkins FA, Jr, Shubin NH. Prosalirus bitis and the anuran caudopelvic mechanism. J Vert Paleont. 1998;18:495–510. [Google Scholar]
- Kaplan M. Evaluation and redefinition of the states of anuran pectoral girdle architecture. Herpetologica. 2004;60:84–97. [Google Scholar]
- Maglia AM, Púgener LA. Skeletal development and adult osteology of Bombina orientalis (Anura: Bombinatoridae) Herpetologica. 1998;54:344–363. [Google Scholar]
- Milner A. Benton MJ, editor. The relationships and origin of living amphibians. The Phylogeny and Classification of the Tetrapods, 1 – Amphibians, Reptiles, Birds. 1988. pp. 59–102. Systematics Association Special Volume no. 35A.
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishing Co; 1967. [Google Scholar]
- Púgener LA, Maglia AM. Osteology and skeletal development of Discoglossus sardus (Anura: Discoglossidae) J Morph. 1997;233:267–286. doi: 10.1002/(SICI)1097-4687(199709)233:3<267::AID-JMOR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rage J-C, Roček Z. Triadobatrachus revisited. In: Roček Z, editor. Studies in Herpetology. Prague: Charles University Publishers House; 1986. pp. 255–258. [Google Scholar]
- Rage J-C, Roček Z. Redescription of Triadobatrachus massinoti (Piveteau, 1936) an anuran amphibian from the early Triassic. Palaeontogr A. 1989;206:1–16. [Google Scholar]
- Roček Z, Rage J-C. Anatomical transformations in transition from temnospondyl to proanuran stages. In: Heatwole H, Carroll E, editors. Amphibian Biology, Vol. 4.–Paleontology. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1276–1284. [Google Scholar]
- Roček Z, Van Dijk E. Patterns of larval development in Mesozoic pipid frogs. Acta Paleont Pol. 2006;51:111–126. [Google Scholar]
- Romer AS. Review of the Labyrinthodontia. Bull Mus Comp Zool. 1947;99:1–368. [Google Scholar]
- Schoch RR. Comparative ontogeny of Early Permian branchiosaurid amphibians from southwestern Germany. Palaeontogr Abt A. 1992;222:43–83. [Google Scholar]
- Schoch RR. Heterochrony in the development of the amphibian head. In: McNamara KJ, editor. Evolutionary Change and Heterochrony. New York: John Wiley & Sons; 1995. pp. 107–124. [Google Scholar]
- Schoch RR, Carroll RL. Ontogenetic evidence for the Paleozoic ancestry of salamanders. Evol Dev. 2003;5:314–324. doi: 10.1046/j.1525-142x.2003.03038.x. [DOI] [PubMed] [Google Scholar]
- Shearman RM. Growth of the pectoral girdle of the leopard frog, Rana pipiens (Anura: Ranidae) J Morph. 2005;264:94–104. doi: 10.1002/jmor.10322. [DOI] [PubMed] [Google Scholar]
- Shishkin MA. The Morphology of the Early Amphibia and Some Problems of the Lower Tetrapod Evolution. Moscow: Nauka Publishers.; 1973. [Google Scholar]
- Smirnov SV. Extra bones in the Pelobates skull as evidence of the paedomorphic origin in the anurans. Zh Obshch Biol. 1995;56:317–328. [Google Scholar]
- Smirnov SV. Paedomorphic origin of the anurans: a new approach to prove it. Russian J Herpetol. 1999;6:118–124. [Google Scholar]
- Warren A, Snell N. The postcranial skeleton of Mesozoic temnospondyl amphibians: a review. Alcheringa. 1991;15:43–64. [Google Scholar]
- Wassersug RJ. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technol. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]