Abstract
The aim of the present study was to discover how intergenerational undernutrition affects the growth of major and minor functional cranial components in two generations of rats. Control animals constituted the parental generation (P). The undernourished generations (F1 and F2) were fed 75% of the control diet. Animals were X-rayed every 10 days from 20 to 100 days of age. The length, width and height of the major (neurocranium and splanchnocranium) and minor (anterior-neural, middle-neural, posterior-neural, otic, respiratory, masticatory and alveolar) cranial components were measured on each radiograph. Volumetric indices were calculated to estimate size variations of these components. Data were processed using the Kruskal–Wallis and Kolmogorov–Smirnov tests for two samples. Impairment in splanchnocranial and neurocranial growth was found, the latter being more affected than the former in F1. Comparison between F2 and F1 animals showed cumulative effects of undernutrition in both major and minor components (anterior-neural, respiratory, masticatory and alveolar in males, and middle-neural and respiratory in females). Such differential effects on minor components may reflect a residual mechanical strain resulting from the linkage between components. This phenomenon was clearly observed in the neurocranium and could be understood as an adaptive response to the demands of the associated functional matrices.
Keywords: rats, skull growth, transgenerational undernutrition
Introduction
Intergenerational factors were defined by Emanuel (1986) as those factors, conditions, exposures and environments experienced by one generation that relate to the health, growth and development of the next. The existence of intergenerational factors has been reported in both humans (Emanuel et al. 1992; Stein & Lumey, 2000; Stein et al. 2004; Veena et al. 2004) and rats (Stewart et al. 1973; Zamenhof & van Marthens, 1978; Pessoa et al. 2000; Cesani et al. 2001; Pucciarelli et al. 2001). According to Emanuel (1997), humans who grow at below-average rates have small organs, primarily because of a reduced cytoplasmic/nuclear ratio and/or reduced cell number, resulting in long-term physiological consequences.
Chronic undernutrition is the most common type of malnutrition in human populations (Resnick & Morgane, 1984). Its effects may go beyond the generation under stress (Kenney & Barton, 1975). Therefore, nutritional deficiencies can be regarded as intergenerational factors. We recently reported that under certain experimental conditions, chronic generational undernutrition results in a cumulative growth deficit of the two major components of the skull: the neurocranium and face (Cesani et al. 2003). According to the functional cranial theory (Moss & Young, 1960; Moss, 1973), the mammalian skull comprises several discrete units called functional cranial components (FCCs). These FCCs vary during growth according to particular patterns, which in turn make up the general pattern of the entire skull. Each FCC is composed of a functional matrix (FM) and a skeletal unit (SKU). The FM includes all the elements (tissues, organs, functional spaces, etc.) necessary to perform a function. The SKUs support and protect specific FMs (Moss, 1973), reflecting the functional demands imposed by the growth of the related soft-tissue structures. In the rat, the neurocranium and splanchnocranium consist of minor components (anterior-neural, middle-neural, posterior-neural, otic, respiratory, masticatory and alveolar) that reflect specific functions. Modifications of major components may involve changes in all minor components or selected components. The aim of this study was to determine how intergenerational undernutrition affects the growth of major and minor cranial components in two generations of rats.
Materials and methods
Experimental groups
One hundred and twenty-five Wistar rats (Rattus norvegicus albinus) raised at the Bioterio of the Centro de Investigaciones en Genética Básica y Aplicada (CIGEBA, Facultad de Ciencias Veterinarias, UNLP) were maintained as an outbreed colony. The animals were kept free of pathogens and treated in compliance with standardized institutional guidelines. They were housed in solid stainless-steel cages. Room temperature ranged from 21 to 25 °C, and the photoperiod consisted of 12 h of light and 12 h of dark (lights on at 06:00 h). The animals were fed on a pelleted and sterilized commercial stock diet containing proteins (23%), carbohydrates (44%), lipids (11%), water (8%), fibre (5%), ash (5%), minerals (3%) and vitamin mixture (1%).
When the rats reached adulthood (70 days), they were mated overnight. Pregnant rats were isolated and fed ad libitum. At birth, pups were randomly assigned to one of two groups. (1) Control: the animals of the parental generation (P) received stock diet ad libitum from weaning (21 days old) to sampling (100 days old). (2) Undernourished: pregnant rats were submitted to nutritional restriction during gestation (75% of daily food intake of a control dam aged-matched, pair-feeding technique). Their offspring constituted the first filial (F1). Because it is well known that diet restriction during lactation substantially alters the mother's behaviour, the mothers ate the stock diet ad libitum and the ‘overcrowding method’ was adopted at this period (12 pups per litter instead of the usual eight) to ensure undernutrition. Overcrowding has been frequently employed in several studies to produce body growth retardation (Widdowson & McCance, 1963; Rajanna et al. 1984; Cesani et al. 2003). After weaning, the animals were fed on 75% of the food eaten by their control peers. F1 adult females were mated to give birth to the second filial (F2). The F2 animals received the same treatment as the F1 animals.
Measurements
Approximately 20 males and 20 females of each generation were chosen randomly from a larger group and X-rayed every 10 days from 20 to 100 days of age in order to obtain the longitudinal data of each animal (Table 1).
Table 1.
Samples and treatments
| Generation | Treatment | Males | Females | Total |
|---|---|---|---|---|
| Parental (P) | normal nutrition (control) | 20 | 21 | 41 |
| First filial (F1) | intergenerational undernutrition | 22 | 20 | 42 |
| Second filial (F2) | intergenerational undernutrition | 20 | 22 | 42 |
| Total | 62 | 63 | 125 |
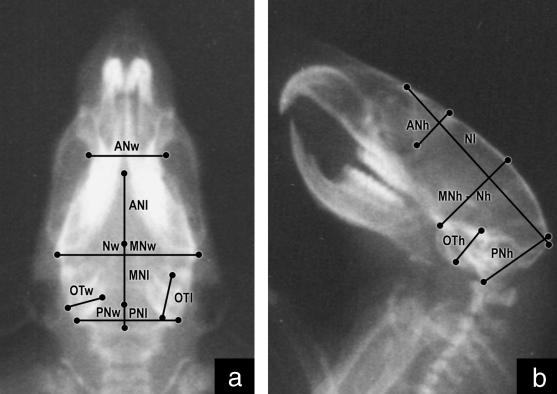
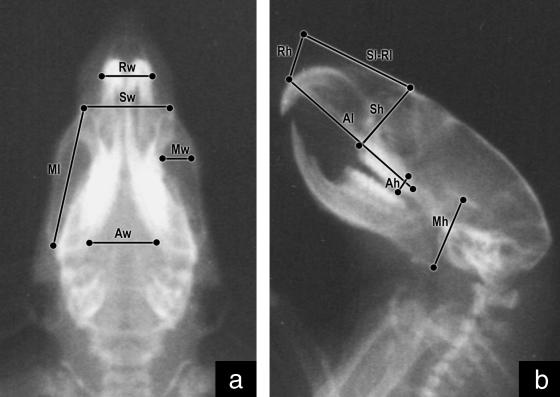
Light-ether anaesthesia was given during the procedure. Once the rats were sedated they were orientated with a cephalostat and radiographed in dorsal–ventral and lateral planes using a Siemens Heliophos 4 from the Servicio de Diagnóstico por Imágenes at 240 mA/125 kV. Shooths were regulated at 100 mA, 0.02 seg, 40–50 kW (according to the age of the animal). A 110-cm focus-film (AGFA Mamoray MR5-II, 18 × 24 cm) distance was used to reduce the magnification effect, calculated as MgC = Bx/Ax, where MgC is the magnification coefficient, Ax a variable measured on the 100th-day radiograph and Bx the same variable measured on the skull (Pucciarelli et al. 2001). The following measurements were taken on each radiograph using a Fowler Max-Cal Digitrix caliper (0.01 mm accuracy) (Figs 1 and 2):
Fig. 1.
Radiograph of a rat skull in dorsal–ventral (a) and lateral (b) planes showing neurocranial measurements.
Fig. 2.
Radiograph of a rat skull in dorsal–ventral (a) and lateral (b) planes showing splanchnocranial measurements.
Length, width and height of major components: neurocranium (Nl, Nw, Nh) and splanchnocranium (Sl, Sw, Sh).
Length, width and height of minor components of the neurocranium: anterior-neural (ANl, ANw, ANh), middle-neural (MNl, MNw, MNh), posterior-neural (PNl, PNw, PNh), otic (OTl, OTw, OTh); and of the splanchnocranium: respiratory (Rl, Rw, Rh), masticatory (Ml, Mw, Mh) and alveolar (Al, Aw, Ah).
Intraobserver repeatability was assessed by remeasuring 20 randomly selected cases per age (15% of the total sample). All measurements were made by one author (M.F.C.), which precluded interobserver differences. The Dahlberg statistic was used: √Σ d2/2n, where d2 are the quadratic differences between pairs of repeated measurements and n is the number of pairs of measurements. This statistic is expressed in millimetres and can be interpreted as the average disparity between the measurement sessions. Intraobserver error was less than 0.1 mm for all variables.
To estimate the size variations of major and minor components by age and sex, volumetric indices were calculated as follows (Cesani, 2004):
neurocranial index (NI) = 3√(Nl*Nw*Nh)
anterior-neural index (ANI) = 3√(ANl*ANw*ANh)
middle-neural index (MNI) = 3√(MNl*MNw*MNh)
posterior-neural index (PNI) = 3√(PNl*PNw*PNh)
otic index (OTI) = 3√(OTl*OTw*OTh)
splanchnocranial index (SI) = 3√(Sl*Sw*Sh)
respiratory index (RI) = 3√(Rl*Rw*Rh)
masticatory index (MI) = 3√(Ml*Mw*Mh)
alveolar index (AI) = 3√(Al*Aw*Ah)
The normality of distributions was assessed by the one-sample Kolmogorov–Smirnov test. This indicated that 36% of the variables were non-normal, compelling us to employ the non-parametric Kruskal–Wallis (K-W) test for the factors significance, and the two-sample Kolmogorov–Smirnov (K-S) test for the comparison between generations. Statistical procedures were performed by use of the Systat 7.0 and SPSS 7.5 programs.
Percentage differences between means (PDM) were calculated in order to obtain standardized differences between generations, according to the formula:
For instance, X1 = mean value of F1 and X2 = mean value of P. If we compare F1-P and PDMNI = −10, this indicates that NI in F1 is 10% smaller than in P. This standardization method has been frequently employed (see Cesani et al. 2003). In its current form, it reduces any difference to a percentage value, which cannot be affected by the magnitude of the variables or by the sign of the differences.
Results
Tables 2 and 3 show the means and standard deviations in males and females, respectively.
Table 2.
Means (M) and standard deviations (SD) in males
| 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (days) | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Generation P | ||||||||||||||||||
| NI | 14.5 | 0.2 | 15.3 | 0.9 | 16.0 | 0.4 | 16.8 | 0.4 | 17.2 | 0.4 | 17.7 | 0.4 | 18.0 | 0.4 | 18.5 | 0.4 | 18.8 | 0.5 |
| ANI | 7.3 | 0.2 | 7.9 | 0.2 | 8.9 | 0.1 | 9.7 | 0.1 | 9.7 | 0.1 | 10.0 | 0.1 | 10.1 | 0.1 | 10.1 | 0.1 | 10.4 | 0.1 |
| MNI | 8.7 | 0.2 | 9.8 | 0.3 | 10.2 | 0.3 | 10.8 | 0.2 | 11.0 | 0.2 | 11.2 | 0.2 | 11.4 | 0.2 | 11.6 | 0.2 | 11.9 | 0.3 |
| PNI | 7.5 | 0.1 | 7.7 | 0.1 | 8.1 | 0.1 | 8.3 | 0.1 | 8.4 | 0.1 | 8.4 | 0.1 | 8.4 | 0.1 | 8.5 | 0.1 | 8.7 | 0.1 |
| OTI | 5.3 | 0.1 | 5.5 | 0.1 | 5.9 | 0.0 | 6.2 | 0.2 | 6.3 | 0.2 | 6.4 | 0.2 | 6.4 | 0.2 | 6.4 | 0.2 | 6.6 | 0.2 |
| SI | 8.5 | 0.3 | 9.5 | 0.3 | 10.7 | 0.3 | 11.5 | 0.2 | 12.1 | 0.3 | 12.7 | 0.3 | 13.1 | 0.3 | 13.4 | 0.3 | 13.7 | 0.3 |
| RI | 6.1 | 0.2 | 6.8 | 0.2 | 7.7 | 0.2 | 8.3 | 0.2 | 8.7 | 0.2 | 9.1 | 0.2 | 9.3 | 0.2 | 9.6 | 0.2 | 9.9 | 0.2 |
| MI | 6.4 | 0.2 | 7.3 | 0.2 | 8.4 | 0.2 | 9.1 | 0.2 | 9.8 | 0.2 | 10.1 | 0.3 | 10.4 | 0.2 | 10.8 | 0.3 | 11.4 | 0.3 |
| AI | 6.6 | 0.3 | 7.3 | 0.3 | 8.1 | 0.2 | 8.5 | 0.2 | 8.9 | 0.3 | 9.2 | 0.3 | 9.6 | 0.3 | 9.9 | 0.3 | 10.0 | 0.3 |
| First filial F1 | ||||||||||||||||||
| NI | 14.1 | 0.2 | 14.7 | 0.2 | 15.2 | 0.2 | 15.6 | 0.2 | 16.0 | 0.2 | 16.3 | 0.2 | 16.6 | 0.2 | 16.9 | 0.2 | 17.0 | 0.3 |
| ANI | 6.4 | 0.2 | 7.4 | 0.2 | 7.6 | 0.2 | 7.8 | 0.2 | 8.6 | 0.1 | 8.8 | 0.1 | 9.3 | 0.1 | 9.5 | 0.1 | 9.6 | 0.1 |
| MNI | 8.9 | 0.1 | 9.2 | 0.2 | 9.4 | 0.2 | 9.6 | 0.2 | 9.9 | 0.3 | 10.1 | 0.2 | 10.3 | 0.1 | 10.4 | 0.2 | 10.7 | 0.3 |
| PNI | 7.1 | 0.1 | 7.1 | 0.1 | 7.2 | 0.1 | 7.3 | 0.1 | 7.7 | 0.1 | 7.7 | 0.1 | 7.8 | 0.3 | 8.0 | 0.3 | 7.9 | 1.8 |
| OTI | 4.5 | 0.1 | 4.9 | 0.2 | 5.0 | 0.2 | 5.0 | 0.2 | 5.2 | 0.3 | 5.2 | 0.3 | 5.4 | 0.2 | 5.5 | 0.2 | 5.7 | 0.2 |
| SI | 8.1 | 0.3 | 9.3 | 0.3 | 10.2 | 0.3 | 10.9 | 0.2 | 11.6 | 0.3 | 12.0 | 0.3 | 12.4 | 0.2 | 12.8 | 0.3 | 13.2 | 0.2 |
| RI | 6.0 | 0.2 | 6.7 | 0.2 | 7.4 | 0.1 | 7.9 | 0.1 | 8.3 | 0.1 | 8.7 | 0.1 | 8.9 | 0.1 | 9.2 | 0.2 | 9.5 | 0.1 |
| MI | 6.0 | 0.2 | 6.8 | 0.3 | 7.8 | 0.2 | 8.6 | 0.3 | 9.2 | 0.2 | 9.7 | 0.2 | 10.1 | 0.2 | 10.5 | 0.2 | 10.9 | 0.3 |
| AI | 6.2 | 0.2 | 7.1 | 0.2 | 7.7 | 0.1 | 8.2 | 0.1 | 8.5 | 0.1 | 8.7 | 0.1 | 9.0 | 0.1 | 9.2 | 0.2 | 9.5 | 0.2 |
| Second filial F2 | ||||||||||||||||||
| NI | 14.4 | 0.2 | 14.9 | 0.2 | 15.4 | 0.1 | 15.6 | 0.1 | 15.9 | 0.1 | 16.2 | 0.1 | 16.4 | 0.2 | 16.5 | 0.2 | 16.7 | 0.2 |
| ANI | 6.4 | 0.1 | 6.8 | 0.2 | 8.2 | 0.1 | 8.3 | 0.1 | 8.4 | 0.1 | 8.5 | 0.2 | 8.7 | 0.2 | 8.9 | 0.1 | 9.1 | 0.2 |
| MNI | 8.9 | 0.1 | 9.2 | 0.1 | 10.0 | 0.2 | 10.1 | 0.2 | 10.2 | 0.0 | 10.3 | 0.0 | 10.5 | 0.2 | 10.6 | 0.2 | 10.7 | 0.2 |
| PNI | 6.8 | 0.3 | 7.1 | 0.1 | 7.5 | 0.1 | 7.6 | 0.1 | 7.7 | 0.1 | 7.7 | 0.1 | 7.8 | 0.2 | 7.9 | 0.2 | 8.0 | 0.2 |
| OTI | 4.7 | 0.0 | 5.1 | 0.1 | 5.7 | 0.0 | 5.7 | 0.0 | 5.8 | 0.0 | 5.8 | 0.0 | 5.8 | 0.0 | 5.9 | 0.0 | 5.9 | 0.1 |
| SI | 8.0 | 0.3 | 9.2 | 0.2 | 10.0 | 0.3 | 10.7 | 0.3 | 11.4 | 0.2 | 11.8 | 0.2 | 12.1 | 0.1 | 12.4 | 0.2 | 12.9 | 0.3 |
| RI | 5.9 | 0.2 | 6.7 | 0.2 | 7.2 | 0.2 | 7.7 | 0.2 | 8.2 | 0.2 | 8.4 | 0.2 | 8.7 | 0.2 | 8.8 | 0.2 | 9.2 | 0.2 |
| MI | 6.1 | 0.3 | 6.7 | 0.3 | 7.7 | 0.4 | 8.4 | 0.2 | 9.0 | 0.3 | 9.4 | 0.2 | 9.8 | 0.2 | 10.0 | 0.2 | 10.5 | 0.3 |
| AI | 6.3 | 0.3 | 7.1 | 0.2 | 7.6 | 0.2 | 8.0 | 0.2 | 8.4 | 0.2 | 8.6 | 0.1 | 8.8 | 0.2 | 9.0 | 0.2 | 9.2 | 0.2 |
Table 3.
Means (M) and standard deviations (SD) in females
| 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (days) | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Generation P | ||||||||||||||||||
| NI | 14.5 | 0.1 | 15.3 | 0.2 | 16.0 | 0.3 | 16.7 | 0.2 | 17.2 | 0.3 | 17.6 | 0.3 | 17.9 | 0.3 | 18.3 | 0.3 | 18.6 | 0.4 |
| ANI | 7.2 | 0.2 | 7.9 | 0.2 | 8.8 | 0.1 | 9.5 | 0.1 | 9.6 | 0.1 | 9.9 | 0.1 | 9.9 | 0.1 | 9.9 | 0.1 | 10.3 | 0.1 |
| MNI | 8.6 | 0.1 | 9.6 | 0.2 | 10.1 | 0.2 | 10.4 | 0.2 | 10.6 | 0.2 | 10.8 | 0.2 | 11.0 | 0.2 | 11.1 | 0.1 | 11.5 | 0.2 |
| PNI | 7.5 | 0.1 | 7.8 | 0.1 | 8.1 | 0.1 | 8.3 | 0.1 | 8.4 | 0.1 | 8.4 | 0.1 | 8.5 | 0.1 | 8.5 | 0.1 | 8.6 | 0.1 |
| OTI | 5.4 | 0.1 | 5.6 | 0.1 | 5.7 | 0.1 | 6.0 | 0.1 | 6.0 | 0.1 | 6.1 | 0.1 | 6.1 | 0.1 | 6.1 | 0.0 | 6.4 | 0.0 |
| SI | 8.4 | 0.2 | 9.4 | 0.2 | 10.5 | 0.3 | 11.2 | 0.2 | 11.7 | 0.3 | 12.0 | 0.3 | 12.4 | 0.2 | 12.7 | 0.2 | 13.0 | 0.2 |
| RI | 6.1 | 0.2 | 6.7 | 0.2 | 7.5 | 0.2 | 7.9 | 0.1 | 8.3 | 0.2 | 8.5 | 0.2 | 8.7 | 0.2 | 8.9 | 0.2 | 9.1 | 0.1 |
| MI | 6.5 | 0.2 | 7.3 | 0.2 | 8.3 | 0.3 | 9.0 | 0.2 | 9.6 | 0.2 | 9.9 | 0.1 | 10.1 | 0.1 | 10.4 | 0.2 | 10.8 | 0.2 |
| AI | 6.4 | 0.3 | 7.3 | 0.2 | 7.8 | 0.2 | 8.3 | 0.2 | 8.5 | 0.2 | 8.8 | 0.2 | 8.9 | 0.2 | 9.1 | 0.2 | 9.3 | 0.2 |
| First filial F1 | ||||||||||||||||||
| NI | 14.0 | 0.1 | 14.5 | 0.2 | 14.9 | 0.2 | 15.4 | 0.2 | 15.8 | 0.3 | 16.1 | 0.3 | 16.4 | 0.2 | 16.7 | 0.2 | 16.9 | 0.3 |
| ANI | 6.2 | 0.1 | 6.3 | 0.2 | 6.6 | 0.2 | 6.8 | 0.2 | 7.2 | 0.1 | 7.3 | 0.2 | 7.7 | 0.2 | 7.9 | 0.3 | 8.5 | 0.2 |
| MNI | 8.8 | 0.1 | 9.0 | 0.1 | 9.1 | 0.0 | 9.3 | 0.2 | 9.6 | 0.2 | 10.2 | 0.2 | 10.3 | 0.2 | 10.4 | 0.2 | 10.6 | 0.2 |
| PNI | 7.0 | 0.1 | 7.0 | 0.1 | 7.1 | 0.1 | 7.2 | 0.1 | 7.3 | 0.1 | 7.3 | 0.1 | 7.4 | 0.1 | 7.5 | 0.1 | 7.7 | 0.1 |
| OTI | 4.8 | 0.1 | 4.8 | 0.2 | 4.8 | 0.1 | 4.8 | 0.2 | 4.9 | 0.2 | 4.9 | 0.1 | 5.0 | 0.1 | 5.2 | 0.0 | 5.3 | 0.0 |
| SI | 7.8 | 0.3 | 8.9 | 0.3 | 9.9 | 0.2 | 10.4 | 0.2 | 11.1 | 0.2 | 11.6 | 0.2 | 11.9 | 0.2 | 12.2 | 0.2 | 12.5 | 0.2 |
| RI | 5.8 | 0.2 | 6.5 | 0.3 | 7.1 | 0.2 | 7.6 | 0.2 | 8.1 | 0.2 | 8.4 | 0.2 | 8.6 | 0.1 | 8.8 | 0.0 | 9.0 | 0.1 |
| MI | 5.5 | 0.2 | 6.5 | 0.2 | 7.4 | 0.2 | 8.3 | 0.2 | 8.9 | 0.2 | 9.2 | 0.2 | 9.4 | 0.2 | 9.7 | 0.2 | 10.0 | 0.2 |
| AI | 6.4 | 0.2 | 7.0 | 0.3 | 7.6 | 0.2 | 8.0 | 0.2 | 8.4 | 0.5 | 8.5 | 0.1 | 8.7 | 0.2 | 8.8 | 0.2 | 9.0 | 0.0 |
| Second filial F2 | ||||||||||||||||||
| NI | 14.1 | 0.2 | 14.6 | 0.2 | 14.7 | 0.2 | 15.3 | 0.2 | 15.6 | 0.1 | 15.8 | 0.2 | 16.0 | 0.2 | 16.1 | 0.2 | 16.3 | 0.2 |
| ANI | 6.4 | 0.1 | 6.6 | 0.1 | 8.1 | 0.1 | 8.1 | 0.1 | 8.1 | 0.1 | 8.2 | 0.1 | 8.2 | 0.1 | 8.5 | 0.1 | 8.9 | 0.1 |
| MNI | 8.8 | 0.0 | 9.0 | 0.0 | 9.7 | 0.0 | 9.8 | 0.0 | 9.9 | 0.0 | 10.0 | 0.0 | 10.1 | 0.1 | 10.2 | 0.1 | 10.2 | 0.1 |
| PNI | 7.0 | 0.1 | 7.2 | 0.1 | 7.4 | 0.1 | 7.4 | 0.1 | 7.5 | 0.1 | 7.5 | 0.0 | 7.5 | 0.0 | 7.6 | 0.0 | 7.6 | 0.0 |
| OTI | 4.7 | 0.1 | 5.0 | 0.0 | 5.5 | 0.0 | 5.6 | 0.0 | 5.6 | 0.0 | 5.6 | 0.0 | 5.6 | 0.0 | 5.7 | 0.0 | 5.7 | 0.0 |
| SI | 8.0 | 0.2 | 9.0 | 0.2 | 10.2 | 0.2 | 10.6 | 0.2 | 11.1 | 0.1 | 11.4 | 0.2 | 11.7 | 0.2 | 12.1 | 0.2 | 12.3 | 0.1 |
| RI | 5.9 | 0.2 | 6.6 | 0.2 | 7.3 | 0.1 | 7.7 | 0.2 | 8.0 | 0.1 | 8.1 | 0.1 | 8.4 | 0.2 | 9.0 | 2.1 | 8.8 | 0.1 |
| MI | 6.3 | 0.2 | 6.9 | 0.2 | 7.7 | 0.2 | 8.4 | 0.2 | 8.9 | 0.2 | 9.1 | 0.2 | 9.5 | 0.3 | 9.7 | 0.2 | 10.1 | 0.2 |
| AI | 6.3 | 0.3 | 7.1 | 0.2 | 7.7 | 0.2 | 8.0 | 0.2 | 8.3 | 0.2 | 8.5 | 0.2 | 8.7 | 0.2 | 8.8 | 0.2 | 9.1 | 0.2 |
The Kruskal–Wallis test showed significant effects of age, sex and generation factors in all the indices, allowing us to make intergroup comparisons of each of the analysed factors (Table 4).
Table 4.
Kruskal–Wallis test for factors of significance on the dependent variables
| Age† | Sex‡ | Generation§ | ||||
|---|---|---|---|---|---|---|
| Indices | H | P | H | P | H | P |
| NI | 805.7 | ** | 17349.5 | ** | 224.1 | ** |
| ANI | 603.1 | ** | 19861.6 | ** | 339.4 | ** |
| MNI | 749.9 | ** | 18305.6 | ** | 207.5 | ** |
| PNI | 411.4 | ** | 19056.5 | ** | 521.6 | ** |
| OTI | 380.5 | ** | 18587.8 | ** | 584.9 | ** |
| SI | 1016.1 | ** | 182669.5 | ** | 40.8 | ** |
| RI | 1011.9 | ** | 18588.5 | ** | 31.3 | ** |
| MI | 1009.3 | ** | 17917.4 | ** | 49.2 | ** |
| AI | 991.2 | ** | 18069.7 | ** | 39.3 | ** |
P < 0.01.
Nine levels (20–30–40–50–60–70–80–90–100 days old)
two levels (males–females)
three levels (P, F1 and F2).
F1–P comparison
In males and females, the neurocranium and all of its minor components were significantly different between F1 and P. Significant differences in SI were found between males and females. However, differences in the minor components were dependent on the sex. In males, RI showed significant differences from 40 days of age, MI at 20–80 and 100 days, and AI at 20 and 40–100 days. In females, there were significant differences in RI at 20–50 and 90 days of age, MI at all ages, and AI at 50–70 and 90–100 days (Table 5).
Table 5.
Percentage differences between means (PDM) and two-samples Kolmogorov–Smirnov test (z) between F1and P in males and females
| 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (days) | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P |
| Neurocranium | |||||||||||||||||||||||||||
| NI | |||||||||||||||||||||||||||
| Males | −2.7 | 2.8 | * | −4.4 | 3.2 | * | −4.9 | 2.9 | * | −6.9 | 3.2 | * | −7.1 | 3.2 | * | −7.9 | 3.2 | * | −8.0 | 3.2 | * | −8.7 | 3.2 | * | −9.4 | 3.2 | * |
| Females | −3.7 | 3.2 | * | −5.6 | 3.2 | * | −6.8 | 3.2 | * | −8.1 | 3.2 | * | −8.0 | 3.2 | * | −8.4 | 3.2 | * | −8.2 | 3.2 | * | −8.7 | 3.2 | * | −8.9 | 3.2 | * |
| ANI | |||||||||||||||||||||||||||
| Males | −12.8 | 3.2 | * | −6.4 | 2.9 | * | −14.6 | 3.2 | * | −19.3 | 3.2 | * | −11.5 | 3.2 | * | −11.9 | 3.2 | * | −7.2 | 3.2 | * | −5.8 | 3.2 | * | −7.1 | 3.2 | * |
| Females | −13.6 | 3.2 | * | −19.4 | 3.2 | * | −24.8 | 3.2 | * | −28.5 | 3.2 | * | −25.1 | 3.2 | * | −26.5 | 3.2 | * | −22.0 | 3.2 | * | −20.4 | 3.2 | * | −17.8 | 3.2 | * |
| MNI | |||||||||||||||||||||||||||
| Males | 2.3 | 1.9 | * | −5.6 | 2.8 | * | −7.6 | 3.2 | * | −11.0 | 3.2 | * | −10.0 | 3.2 | * | −10.3 | 3.2 | * | −10.1 | 3.2 | * | −10.3 | 3.2 | * | −10.3 | 3.2 | * |
| Females | −3.0 | 2.9 | * | −6.4 | 3.2 | * | −9.7 | 3.2 | * | −10.7 | 3.2 | * | −10.1 | 3.2 | * | −5.8 | 3.2 | * | −5.6 | 2.7 | * | −6.3 | 3.1 | * | −8.0 | 3.2 | * |
| PNI | |||||||||||||||||||||||||||
| Males | −5.6 | 3.2 | * | −7.9 | 3.2 | * | −10.7 | 3.2 | * | −12.5 | 3.2 | * | −8.0 | 3.2 | * | −8.1 | 3.2 | * | −7.5 | 3.2 | * | −5.6 | 2.8 | * | −8.8 | 2.9 | * |
| Females | −7.6 | 3.2 | * | −9.9 | 3.2 | * | −12.7 | 3.2 | * | −13.6 | 3.2 | * | −13.6 | 3.2 | * | −13.7 | 3.2 | * | −12.4 | 3.2 | * | −11.3 | 3.2 | * | −11.5 | 3.2 | * |
| OTI | |||||||||||||||||||||||||||
| Males | −15.2 | 3.2 | * | −10.9 | 3.2 | * | −15.8 | 3.2 | * | −20.4 | 3.2 | * | −18.1 | 3.2 | * | −18.6 | 3.2 | * | −16.5 | 3.2 | * | −14.3 | 3.2 | * | −13.2 | 3.2 | * |
| Females | −12.4 | 3.2 | * | −13.3 | 3.2 | * | −16.1 | 3.2 | * | −18.7 | 3.2 | * | −19.2 | 3.2 | * | −19.9 | 3.2 | * | −18.4 | 3.2 | * | −14.9 | 3.2 | * | −16.7 | 3.2 | * |
| Splanchnocranium | |||||||||||||||||||||||||||
| SI | |||||||||||||||||||||||||||
| Males | −5.1 | 2.2 | * | −2.9 | 1.5 | −4.5 | 2.5 | * | −5.2 | 2.9 | * | −4.3 | 2.2 | * | −5.2 | 2.6 | * | −5.1 | 2.9 | * | −4.2 | 2.5 | * | −3.5 | 2.3 | * | |
| Females | −7.0 | 3.0 | * | −5.5 | 2.3 | * | −6.3 | 2.9 | * | −6.5 | 3.2 | * | −5.2 | 2.7 | * | −3.5 | 2.3 | * | −4.0 | 2.4 | * | −3.9 | 2.9 | * | −3.4 | 2.7 | * |
| RI | |||||||||||||||||||||||||||
| Males | −2.0 | 1.1 | −1.0 | 0.8 | −3.9 | 2.2 | * | −5.0 | 3.1 | * | −4.4 | 2.9 | * | −4.7 | 2.8 | * | −4.3 | 2.9 | * | −4.1 | 2.5 | * | −4.1 | 2.7 | * | ||
| Females | −4.0 | 2.0 | * | −3.1 | 1.9 | * | −4.9 | 2.4 | * | −3.5 | 1.9 | * | −2.2 | 1.5 | −1.5 | 1.5 | −2.1 | 1.7 | −2.1 | 2.1 | * | −1.1 | 1.5 | ||||
| MI | |||||||||||||||||||||||||||
| Males | −6.1 | 2.3 | * | −6.6 | 2.5 | * | −6.6 | 2.8 | * | −6.5 | 2.8 | * | −5.8 | 2.9 | * | −4.0 | 2.6 | * | −2.9 | 2.0 | * | −2.3 | 1.5 | −4.0 | 2.3 | * | |
| Females | −10.3 | 2.9 | * | −10.9 | 3.2 | * | −10.6 | 2.9 | * | −8.3 | 3.2 | * | −7.3 | 3.2 | * | −6.8 | 3.2 | * | −6.4 | 3.2 | * | −6.0 | 3.1 | * | −7.6 | 3.2 | * |
| AI | |||||||||||||||||||||||||||
| Males | −6.4 | 1.9 | * | −3.0 | 1.6 | −4.7 | 2.6 | * | −4.1 | 2.6 | * | −3.7 | 2.3 | * | −5.4 | 2.6 | * | −5.5 | 2.8 | * | −6.9 | 2.9 | * | −5.2 | 2.6 | * | |
| Females | 0.0 | 0.9 | −3.2 | 1.5 | −2.4 | 1.3 | −4.2 | 2.6 | * | −1.3 | 1.6 | * | −4.0 | 2.3 | * | −1.9 | 1.2 | −3.5 | 2.1 | * | −3.4 | 2.7 | * | ||||
PDM values were negative in all cases, indicating that F1 was smaller than P. For NI, these differences increased with age, whereas for SI and the minor components they were more uniform across the ages (Table 5).
F2–F1 comparison
In males, differences were found in NI at 20 and 90–100 days. Neural minor components showed differences at 30–100 (ANI), 40–70 and 90 (MNI), 40–50 (PNI), and 20 and 40–90 days of age (OTI). Splanchnocranial indices showed differences at 80–100 (SI), 70–100 (RI), 60 and 80–100 (MI), and 50–60 and 90–100 days of age (AI) (Table 6).
Table 6.
Percentage differences between means (PDM) and two-samples Kolmogorov–Smirnov test (z) between F2 and F1 in males and females
| 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (days) | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P | PDM | z | P |
| Neurocranium | |||||||||||||||||||||||||||
| NI | |||||||||||||||||||||||||||
| Males | 1.9 | 2.3 | * | 1.5 | 1.7 | 1.4 | 1.6 | 0.1 | 0.7 | −0.7 | 1.2 | −0.7 | 1.6 | −1.2 | 1.4 | −2.1 | 2.3 | * | −2.3 | 2.4 | * | ||||||
| Females | 0.7 | 1.2 | 0.9 | 1.1 | 0.2 | 0.8 | −0.5 | 0.7 | 1.7 | 1.9 | * | −1.9 | 2.1 | * | −2.7 | 2.5 | * | −3.2 | 2.8 | * | −3.9 | 3.1 | * | ||||
| ANI | |||||||||||||||||||||||||||
| Males | 0.3 | 1.3 | −8.1 | 2.8 | * | 7.4 | 3.2 | * | 6.0 | 2.9 | * | −1.7 | 1.8 | * | −3.9 | 2.6 | * | −7.1 | 3.2 | * | −6.3 | 3.2 | * | −5.5 | 3.2 | * | |
| Females | 3.6 | 2.5 | * | 3.5 | 2.1 | * | 21.9 | 3.2 | * | 19.4 | 3.2 | * | 13.8 | 3.2 | * | 12.4 | 3.2 | * | 6.0 | 3.2 | * | 7.4 | 2.8 | * | 4.9 | 2.9 | * |
| MNI | |||||||||||||||||||||||||||
| Males | −0.2 | 0.5 | −0.7 | 1.0 | 6.1 | 3.1 | * | 4.9 | 2.9 | * | 2.7 | 2.0 | * | 2.0 | 2.3 | * | 2.0 | 1.6 | 1.3 | 1.7 | * | −0.2 | 1.1 | ||||
| Females | 0.0 | 0.4 | 0.4 | 0.9 | 6.2 | 3.2 | * | 5.4 | 3.1 | * | 3.8 | 3.1 | * | −1.6 | 1.7 | * | −2.5 | 2.0 | * | −2.6 | 2.1 | * | −3.5 | 2.3 | * | ||
| PNI | |||||||||||||||||||||||||||
| Males | −3.7 | 1.5 | −0.4 | 1.0 | 4.7 | 3.2 | * | 4.7 | 3.2 | * | 0.3 | 1.2 | 0.3 | 1.0 | 0.0 | 1.2 | −1.5 | 1.2 | 1.4 | 1.7 | |||||||
| Females | 1.3 | 1.6 | 2.4 | 2.3 | * | 4.9 | 3.1 | * | 3.8 | 2.8 | * | 3.3 | 2.8 | * | 3.2 | 2.8 | * | 1.6 | 2.3 | * | 0.8 | 1.8 | * | −0.8 | 1.2 | ||
| OTI | |||||||||||||||||||||||||||
| Males | 3.5 | 2.2 | * | 3.3 | 1.5 | 13.9 | 3.2 | * | 14.7 | 3.2 | * | 11.8 | 3.2 | * | 11.5 | 3.2 | * | 8.4 | 2.8 | * | 6.2 | 2.8 | * | 3.1 | 1.7 | ||
| Females | −2.1 | 1.4 | 3.1 | 1.8 | * | 15.0 | 3.2 | * | 15.3 | 3.2 | * | 15.5 | 3.2 | * | 15.6 | 3.2 | * | 13.3 | 3.2 | * | 9.1 | 3.2 | * | 6.4 | 3.2 | * | |
| Splanchnocranium | |||||||||||||||||||||||||||
| SI | |||||||||||||||||||||||||||
| Males | −0.8 | 0.6 | −0.1 | 0.9 | −1.4 | 0.9 | −1.5 | 1.5 | −1.4 | 1.0 | −2.1 | 1.7 | −2.6 | 2.5 | * | −3.4 | 2.5 | * | −2.7 | 1.9 | * | ||||||
| Females | 2.5 | 1.4 | 1.4 | 1.2 | 3.1 | 2.0 | * | 1.9 | 1.5 | 0.5 | 1.4 | −1.6 | 1.5 | −1.6 | 1.2 | −0.5 | 1.0 | −1.6 | 2.0 | * | |||||||
| RI | |||||||||||||||||||||||||||
| Males | −2.0 | 1.2 | −0.1 | 0.4 | −1.6 | 1.2 | −1.8 | 1.2 | −1.9 | 1.4 | −3.1 | 2.4 | * | −2.8 | 2.3 | * | −3.7 | 2.6 | * | −3.0 | 2.1 | * | |||||
| Females | 1.5 | 0.9 | 2.2 | 1.5 | 2.5 | 1.4 | 0.3 | 0.4 | −1.4 | 1.1 | −2.9 | 2.3 | * | −2.1 | 1.6 | 3.0 | 1.8 | * | −2.7 | 2.5 | * | ||||||
| MI | |||||||||||||||||||||||||||
| Males | 0.3 | 0.9 | −1.5 | 0.7 | −1.2 | 1.1 | −1.4 | 0.9 | −2.2 | 1.8 | * | −3.0 | 1.7 | −3.1 | 2.1 | * | −5.0 | 2.8 | * | −4.3 | 2.4 | * | |||||
| Females | 3.2 | 1.5 | 5.8 | 2.2 | * | 3.9 | 2.2 | * | 1.1 | 0.9 | 0.1 | 0.5 | −0.7 | 0.8 | 0.1 | 0.5 | −0.3 | 0.6 | 1.1 | 0.9 | |||||||
| AI | |||||||||||||||||||||||||||
| Males | 1.9 | 0.9 | −0.6 | 0.8 | −1.4 | 1.5 | −2.6 | 2.3 | * | −2.0 | 1.9 | * | −1.6 | 1.4 | −2.0 | 1.5 | −2.8 | 2.0 | * | −3.1 | 1.9 | * | |||||
| Females | −1.9 | 1.3 | 1.3 | 1.1 | 0.8 | 1.0 | 0.9 | 0.8 | −1.5 | 0.8 | 0.0 | 0.5 | −0.5 | 0.9 | −0.2 | 1.2 | 0.3 | 0.6 | |||||||||
In females, differences between filial generations in NI were found at the older ages (60–100). By contrast, differences were noticed in ANI at all ages, MNI at 40–100, PNI at 30–90 and OTI at 30–100 days. In the splanchnocranium, SI showed differences at 40 and 100 days, RI at 70 and 90–100, and MI at 30–40 days. There were no differences in AI (Table 6).
Both negative and positive PDM values were found, indicating that F2 was smaller than F1 and vice versa. In males, PDMs were negative in NI and ANI and positive in the remaining neurocranial minor components. In the splanchnocranium, all the differences were negative. In females, PDM values were negative in NI and MNI (from 70 days of age) and positive in ANI, PNI and OTI. The splanchnocranium of F1 and F2 was similar (Table 6).
Discussion
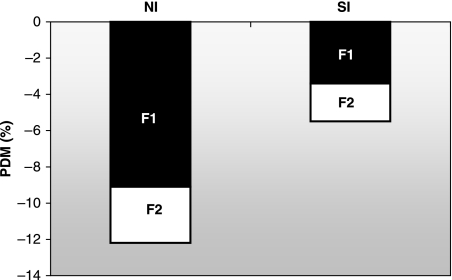
Intergenerational undernutrition modified both neurocranial and splanchnocranial growth. Previous studies have found facial components to be more strongly influenced by epigenetic factors than those of the neurocranium (Pucciarelli, 1981; Fields, 1991; Miller & German, 1999) and the latter tends to be more stable because of its functional relevance (Deter et al. 1995; Dressino & Pucciarelli, 1997; Oyhenart et al. 2003). This phenomenon may be explained by the growth pattern of the mammalian skull, in which facial structures mature slower than neurocranial structures (Moss & Baer, 1956). At birth, the neurocranium has already completed most of its growth, and the viscerocranium grows more rapidly (Clark & Smith, 1993). In fact, in a previous study we found that the first filial, undernourished at weaning, exhibited a greater growth retardation of the face than that of the neurocranium. However, in the second generation, pups of which were undernourished in utero and onwards, the neurocranium was relatively more affected than the face (Cesani et al. 2003). The relationship between the two major components (relative growth) showed significant growth retardation in F1 compared with P (Fig. 3). NI was significantly more reduced than SI (9.1 vs. 3.1%, respectively). This confirms that intrauterine stress may affect neurocranial growth in spite of its functional implications. By contrast, growth retardation increased along with undernutrition through the following generation (F2). However, such growth restriction was similar in both major components (NI, 3.4%; SI, 2.1%), suggesting a possible adaptive response to nutritional stress in F2 (Fig. 3).
Fig. 3.
Average growth retardation of the major functional cranial components in F1 (black bars) and F2 (white bars). Negative PDMs indicate that F1 and F2 < P.
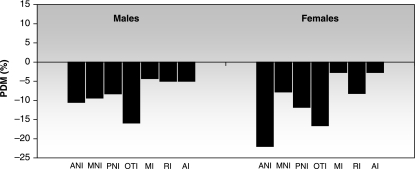
Nutritional deficit also evoked differential modifications among minor components. The anterior-neural and otic components were the most affected, and all the facial minor components were similarly retarded in males but not in females, which exhibited a strong reduction of the masticatory component (Fig. 4).
Fig. 4.
Average growth retardation of the minor functional cranial components: comparison between F1 and P. The bars are expressed as percentage differences between means (PDM). Negative PDMs indicate that F1 < P.
Studies in rats with prenatal and postnatal generational undernutrition have reported reduction of visual capacity (Galler, 1980), auditive dysfunctions (Stewart et al. 1973, 1975), disturbed neuronal development (Gundappa & Desiraju, 1988), and reduction in brain weight, DNA and brain proteins (Zamenhof et al. 1971; Resnick & Morgane, 1984). These findings can be related to the present results in the context of the functional cranial theory, in which modifications in the skeletal unit are explained as secondary growth responses to previous alterations of the functional matrix.
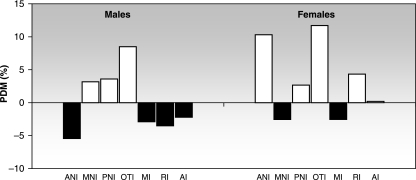
There is some disagreement about the cumulative effect of generational undernutrition on physical growth (Zamenhof & Van Marthens, 1978; Hoet et al. 1997; Pessoa et al. 2000; Orden et al. 2003; Rogers et al. 2003). Some authors support a progressive impairment of growth through generations. For example, Resnick & Morgane (1984) found that a protein restriction in the first generation becomes more severe in the second generation, based on brain weight at birth among other parameters. Other authors argue against this idea; Zamenhof & van Marthens (1978), who studied six generations of rats, did not find cumulative effects on growth. In our study, both neurocranial and splanchnocranial sizes were smaller in F2 than in F1, adding support to the cumulative hypothesis. However, such an effect was not uniform and varied between sexes and minor components. This cumulative effect in males was seen in ANI and all the splanchnocranial components, whereas MNI, PNI and OTI were larger in F2. In females, MNI and RI showed cumulative growth retardation, whereas the other components were larger in F2 (Fig. 5). These differential patterns, which result in modifications of cranial shape, suggest a kind of ‘carry over’ effect, i.e. a residual mechanical strain resulting from the linkage between components. This process gives experimental support to the well-known principle of functional interdependence among cranial components (van der Klaauw, 1948–52). This phenomenon, more clearly observed within the neurocranium, could be understood as an adaptive response to demands of the associated functional matrices.
Fig. 5.
Average growth retardation of the minor functional cranial components: comparison between F2 and F1. The bars are expressed as percentage differences between means (PDM). Negative PDMs (black bars) indicate that F2 < F1. Positive PDMs (white bars) indicate that F2 > F1.
In summary, intergenerational undernutrition would produce a cumulative effect on cranial growth. These results validate previous longitudinal studies and the application of the functional cranial theory, given that the expression of growth retardation is dependent on the age, sex and level of cranial discrimination employed.
Acknowledgments
This study was partially supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP 4714), Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT, PMT-PICT 0245) and Universidad Nacional de La Plata (UNLP).
References
- Cesani MF, Orden AB, Zucchi M, Oyhenart EE, Muñe MC, Pucciarelli HM. Influencia de la alimentación durante la lactancia sobre la desnutrición intergeneracional. Un estudio experimental. Rev Arg Antrop Biol. 2001;3:101–111. [Google Scholar]
- Cesani MF, Orden B, Zucchi M, Muñe MC, Oyhenart EE, Pucciarelli HM. Effect of undernutrition on the cranial growth of the rat. An intergenerational study. Cells Tissues Organs. 2003;174:129–135. doi: 10.1159/000071153. [DOI] [PubMed] [Google Scholar]
- Cesani MF. La Plata: Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata; 2004. Influencia de la subnutrición proteico-calórica transgeneracional sobre el crecimiento de la descendencia en la rata (Rattus norvegicus albinus var. Wistar). Un estudio de antropología biológica experimental. Tesis Doctoral. [Google Scholar]
- Clark CT, Smith KK. Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae) J Morphol. 1993;215:119–149. doi: 10.1002/jmor.1052150203. [DOI] [PubMed] [Google Scholar]
- Deter RL, Nazar R, Milner LL. Modified neonatal growth assessment score: a multivariate approach to the detection of intrauterine growth retardation in the neonate. Ultrasound Obstet Gynecol. 1995;6:400–410. doi: 10.1046/j.1469-0705.1995.06060400.x. [DOI] [PubMed] [Google Scholar]
- Dressino V, Pucciarelli HM. Cranial growth in Saimiri sciureus (Cebidae) and its alteration by nutritional factors: a longitudinal study. Am J Phys Anthropol. 1997;102:545–554. doi: 10.1002/(SICI)1096-8644(199704)102:4<545::AID-AJPA8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Emanuel I. Maternal health during childhood and later reproductive performance. Ann NY Acad Sci. 1986;477:27–39. doi: 10.1111/j.1749-6632.1986.tb40318.x. [DOI] [PubMed] [Google Scholar]
- Emanuel I, Filakti H, Alberman E, Evans SJW. Intergenerational studies of human birth weight from the 1958 birth cohort. 1. Evidence for a multigenerational effect. Br J Obstet Gynaecol. 1992;99:67–74. doi: 10.1111/j.1471-0528.1992.tb14396.x. [DOI] [PubMed] [Google Scholar]
- Emanuel I. Invited commentary: an assessment of maternal intergenerational factors in pregnancy outcome. Am J Epidemiol. 1997;146:820–825. doi: 10.1093/oxfordjournals.aje.a009199. [DOI] [PubMed] [Google Scholar]
- Fields HW. Craniofacial growth from infancy to adulthood. Pediatr Clin N Am. 1991;38:1053–1088. doi: 10.1016/s0031-3955(16)38189-5. [DOI] [PubMed] [Google Scholar]
- Galler JR. Home-orienting behavior in rat pups surviving postnatal or intergenerational malnutrition. Dev Psychobiol. 1980;13:563–572. doi: 10.1002/dev.420130602. [DOI] [PubMed] [Google Scholar]
- Gundappa G, Desiraju T. Deviation in brain development of F2 generation on caloric undernutrition and scope of their prevention by rehabilitation: alterations in dendritic spine production and pruning of pyramidal neurons of lower laminae of motor cortex and visual cortex. Brain Res. 1988;26:205–223. doi: 10.1016/0006-8993(88)90220-x. [DOI] [PubMed] [Google Scholar]
- Hoet JJ, Reusens B, Dahri S, El-Hajjaji H, Remacle C. Protein malnutrition during pregnancy in the rat has an intergenerational effect on the endocrine pancreas. Proceedings of 16th Int Cong Nutrition, Montreal, Canada. 1997;11:4–70. [Google Scholar]
- Kenney MA, Barton EB. Malnutrition and fetal development in two generations of rats. Nutr Rep Int. 1975;11:243–250. [Google Scholar]
- van der Klaauw CJ. Size and position of the functional components of the skull. Arch Neerl Zool. 1948–52;9:1–559. [Google Scholar]
- Miller JP, German RZ. Protein malnutrition affects the growth trajectories of the craniofacial skeleton in rats. J Nutr. 1999;129:2061–2069. doi: 10.1093/jn/129.11.2061. [DOI] [PubMed] [Google Scholar]
- Moss ML, Baer MJ. Differential growth of the rat skull. Growth. 1956;20:107–120. [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Moss ML. Symposium IVth International Congress of Primatology. Vol. 3. Basel: Karger; 1973. A functional cranial analysis of primate craniofacial growth; pp. 191–208. [Google Scholar]
- Orden AB, Pucciarelli HM, Cesani MF, et al. Variación craneofacial en ratas sometidas a subnutrición transgeneracional moderada. In: Aluja MP, Malgoza A, Nogués R, editors. Antropología y Biodiversidad. Barcelona: Bellaterra; 2003. pp. 423–429. [Google Scholar]
- Oyhenart EE, Orden B, Fucini MC, Muñe MC, Pucciarelli HM. Sexual dimorphism and postnatal growth of intrauterine growth retarded rats. Growth Dev Aging. 2003;67:73–83. [PubMed] [Google Scholar]
- Pessoa DC, Lago ES, Teodosio NR, Bion FM. Dietary proteins on reproductive performance in three consecutive generations of rats. Arch Latinoam Nutr. 2000;50:55–61. [PubMed] [Google Scholar]
- Pucciarelli HM. Growth of the functional components of the rat skull and its alteration by nutritional effects. Am J Phys Anthropol. 1981;56:33–41. doi: 10.1002/ajpa.1330560104. [DOI] [PubMed] [Google Scholar]
- Pucciarelli HM, Orden AB, Cesani MF, Oyhenart EE, Muñe MC, Zucchi M. Relative food intake of rats submitted to a moderate transgenerational undernutrition. Growth Dev Aging. 2001;65:83–93. [PubMed] [Google Scholar]
- Rajanna B, Mascarenhas C, Desiraju T. Experimental study on rats to find the usefulness of nutritional supplementation to undernourished offspring of parents undernourished life-long. Indian J Physiol Pharmacol. 1984;28:83–96. [PubMed] [Google Scholar]
- Resnick O, Morgane PJ. Generational effect of protein malnutrition in the rat. Brain Res. 1984;317:219–227. doi: 10.1016/0165-3806(84)90099-3. [DOI] [PubMed] [Google Scholar]
- Rogers EH, Hunter ES, Rosen MB, et al. Lack of evidence for intergenerational reproductive effects due to prenatal and postnatal undernutrition in the female CD-1 mouse. Reprod Toxicol. 2003;17:519–525. doi: 10.1016/s0890-6238(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Stein AD, Lumey LH. The relationship between maternal and offspring birth weight after maternal prenatal famine exposure: the Dutch famine birth cohort study. Hum Biol. 2000;72:641–654. [PubMed] [Google Scholar]
- Stein AD, Barnhat HX, Wang M, et al. Comparison of linear growth patterns in the first three years of life across two generations in Guatemala. Pediatrics. 2004;113:270–275. doi: 10.1542/peds.113.3.e270. [DOI] [PubMed] [Google Scholar]
- Stewart RJ, Preece RF, Sheppard HG. The long-term effects of marginal protein-energy deficiency. Proc Nutr Soc. 1973;32:102A–103A. [PubMed] [Google Scholar]
- Stewart RJ, Preece RF, Sheppard HG. Twelve generations of marginal protein deficiency. Br J Nutr. 1975;33:233–253. doi: 10.1079/bjn19750027. [DOI] [PubMed] [Google Scholar]
- Veena SR, Kumaran K, Swarnagowri MN, et al. Intergenerational effects on size at birth in South India. Paediatr Perinat Epidemiol. 2004;18:361–370. doi: 10.1111/j.1365-3016.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- Widdowson EM, McCance RA. The effect of finite periods of undernutrition at different ages on the composition and subsequent development on the rat. Proc Roy Soc B. 1963;158:329–342. doi: 10.1098/rspb.1963.0051. [DOI] [PubMed] [Google Scholar]
- Zamenhof S, van Marthens E, Grauel L. DNA (Cell Number) in neonatal brain: second generation (F2) alteration by maternal (F0) dietary protein restriction. Science. 1971;172:850–851. doi: 10.1126/science.172.3985.850. [DOI] [PubMed] [Google Scholar]
- Zamenhof S, van Marthens E. The effects of chronic undernutrition over generations on rat development. J Nutr. 1978;108:1719–1723. doi: 10.1093/jn/108.11.1719. [DOI] [PubMed] [Google Scholar]