Abstract
This study demonstrated that there is a pathway from the zona incerta to the thalamic reticular nucleus. Injections of horseradish peroxidase or Fluorogold were made, using stereotaxic coordinates, into the rostral, intermediate or caudal regions of the thalamic reticular nucleus of adult Sprague–Dawley rats. The results show that the different regions of the thalamic reticular nucleus have distinct patterns of connections with the sectors of the zona incerta. In terms of the relative strength of the connections, injections made into the rostral regions of the thalamic reticular nucleus showed the highest number of labelled cells within the rostral and ventral sectors of the zona incerta; injections made into the intermediate regions of the thalamic reticular nucleus showed labelled cells in the dorsal and ventral sectors; while injections to the caudal regions of the thalamic reticular nucleus showed only a few labelled cells in the caudal sector of the zona incerta. Previous studies have shown that the zona incerta projects to the higher order thalamic nuclei but not first order thalamic nuclei. The labelling observed in the present study may represent collaterals of zona incerta to higher order thalamic nuclei projections.
Keywords: connections, thalamic reticular nucleus, zona incerta
Introduction
The main currently defined inputs to the thalamic reticular nucleus (TRN) are collaterals from the excitatory branches of thalamocortical and corticothalamic axons, and the primary outputs are inhibitory GABAergic axons to the dorsal thalamus (Jones, 1975; Steriade et al. 1984; Ohara & Lieberman, 1985; De Biasi et al. 1986). Previous studies have shown that TRN connections with the thalamus and the cortex are topographically organized (Carman et al. 1964; Jones, 1975; Crabtree & Killackey, 1989; Crabtree, 1992, 1996) so that, for example, the caudal TRN is connected to the visual and auditory cortex and the lateral and medial geniculate nuclei of the thalamus, and the intermediate TRN has connections with the somatosensory cortex and the ventrobasal thalamic nucleus, whereas the rostral TRN is connected to the motor and limbic centres (Carman et al. 1964; Jones, 1975; Conley & Diamond, 1990; Crabtree, 1992, 1996; Lozsádi, 1994, 1995; Guillery et al. 1998). Although the precise role of the TRN is still under debate, defining the nature of the inputs to the nucleus will prove essential for understanding its functions. It has been postulated that the TRN acts to mediate selective attention, in particular to gate dorsal thalamic input to the cerebral cortex (Crick, 1984; Jones, 1985; Guillery & Harting, 2003; Sherman & Guillery, 2006). Furthermore, it has been suggested that the TRN is involved in modulation of thalamic and/or cortical neuronal firing patterns and in generation of oscillatory activity responsible for cortical spindles during the early stages of sleep (Steriade & Deschenes, 1984; Steriade et al. 1986, 1987).
The zona incerta (ZI) forms one of the cytoarchitectonically and neurochemically most diverse cell groups within the thalamus (Romanowski et al. 1985; Kim et al. 1992; May et al. 1997; Kolmac et al. 1998). Four distinct sectors are recognized: rostral, dorsal, ventral and caudal (Nicolelis et al. 1992; Kolmac et al. 1998; Kolmac & Mitrofanis, 1999; Power et al. 1999). Each of these sectors has distinct connections with other regions of the central nervous system. The precise role of the ZI, like that of the TRN, is not clear. However, it has been implicated in a great variety of different functions ranging from sociosexual behaviour (Edwards & Isaacs, 1991) to aspects of visual, nociceptive and somatosensory processing (Legg, 1979; Shammah-Lagnado et al. 1985; Ficalora & Mize, 1989; Kim et al. 1992; Gonzalez-Lima et al. 1993; Lechner et al. 1993; Hermanson et al. 1995; Nicolelis et al. 1995; see also Morgenson et al. 1985; May et al. 1997). Recent accounts of its connections to the ventral thalamus are of particular interest here, and may relate to the broad range of functions in which the ZI has been implicated (Ricardo, 1981; Watanabe & Kawana, 1982; Power et al. 1999).
Recent studies have shown that there are two groups of inhibitory afferents entering major thalamic nuclei from adjacent diencephalic centres. Those from the TRN have long been recognized (Jones, 1985), whereas those from the ZI have been revealed only recently (Kolmac & Mitrofanis, 1999; Barthõ et al. 2002). The present study shows evidence that there are axons that connect these two sources of inhibitory pathways.
The aim of the present study was to examine connections between the TRN and the ZI that have not been described previously, and to explore how sectors of the ZI are connected to the differerent regions of the TRN.
Materials and methods
Sprague–Dawley albino rats weighing 250–350 g were housed in Plexiglass cages with a 12-h light/dark cycle in a temperature-controlled room (20 ± 3 °C). They were fed with a standard laboratory rat chow and tap water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Marmara University.
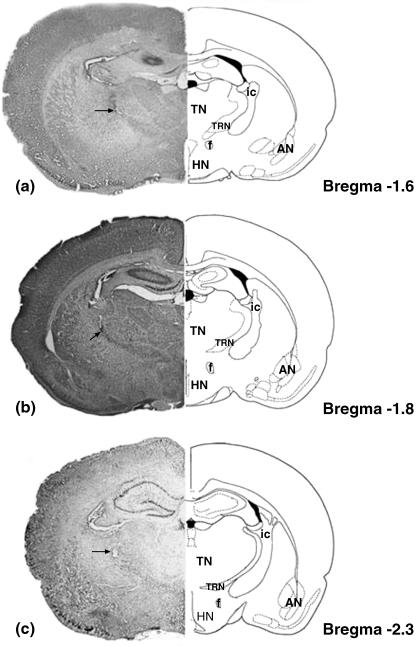
Rats were anaesthetized with ketamine (50 mg kg−1, i.p.) and chlorpromazine (1 mg kg−1, i.p.). The animals were placed with their heads in a sterotaxic frame (Model 51600, Stoelting, USA), the scalp was incised longitudinally and the skull was exposed between lambda and bregma. A small hole was drilled in the skull at a position appropriate for a unilateral injection of horseradish peroxidase (HRP) or fluorogold into the TRN. A glass micropipette (tip diameter 10–18 µm) containing the HRP or fluorogold solution was lowered into one of three (rostral, intermediate or caudal) regions of the right TRN on the basis of the rat brain atlas of Paxinos & Watson (1998). The coordinates were as follows: rostral TRN (n = 8), 1.3–1.6 mm posterior and 2.2–2.6 mm lateral to bregma and 5.4–6.6 mm ventral to the surface of the skull; intermediate TRN (n = 6), 1.8–2.1 mm posterior and 2.2–2.6 mm lateral to bregma, 5.4–6.6 mm ventral to the surface of the skull; caudal TRN (n = 3), 2.2–3.1 mm posterior and 2.2–2.6 mm lateral to bregma, 5.4–6.6 mm ventral to the surface of the skull (Fig. 1a–c).
Fig. 1.
A representative section of the injection site and its corresponding schematic illustration for the (a) rostral, (b) intermediate and (c) caudal thalamic reticular nucleus. Arrow indicates the injection site. T, thalamic nuclei; HN, hypothalamic nuclei; AN, amygdaloid nuclei; TRN, thalamic reticular nuclei; ic, internal capsule; f, fornix.
The tip of the micropipette was filled with air (20 nL) to avoid diffusion of HRP or fluorogold to unwanted areas as the pipette was lowered to the TRN. A Hamilton microsyringe connected through a cannula to an infusion pump (Kd Scientific, USA) was used to inject 50 nL of HRP or fluorogold into the TRN over a period of 20 s. Following the injection, the pipette remained at the target for 1.5 h to avoid loss of tracer during removal of the pipette.
After 2–3 days survival for HRP and 3–4 days survival for fluorogold, the animals were deeply anaesthetized with urethane (1.2 g kg−1 i.p.) and perfused transcardially with 500 mL of saline solution, followed by 2.5% of paraformaldehyde and 1.25% glutaraldehdyde in 0.1 m phosphate buffer (500 mL). Brains were removed and post-fixed for 24 h at 4 °C. Coronal sections (50 µm) were cut on a cryostat (Microm, Germany). Brains with HRP were collected in phosphate buffer (pH 7.2) and were then treated with tetramethylbenzidine as described by Mesulam (1978). Sections of the brains with fluorogold were placed on glass slides, covered with DPX and examined under a fluorescence microscope. Three sections from each case were selected and the number of perikarya per section was counted and the average numbers were calculated (Table 1). We used two tracers to ensure that our results were not limited by the transport capabilities of one tracer alone.
Table 1.
Average number of retrogradely labelled cells within the sectors of the zona incerta (ZI) after an injection of HRP or fluorogold into the rostral, intermediate and caudal regions of the thalamic reticular nucleus (TRN)
| Av. no. labelled cells rZI | Av. no. labelled cells vZI | Av. no. labelled cells dZI | Av. no. labelled cells cZI | |||||
|---|---|---|---|---|---|---|---|---|
| HRP | FG | HRP | FG | HRP | FG | HRP | FG | |
| Rostral TRN (n = 8) | 15.13 ± 2.03 | 45.25 ± 2.12 | 7.63 ± 2.39 | 20.63 ± 3.66 | – | – | – | – |
| Intermediate TRN (n = 6) | 12.83 ± 2.71 | 29.33 ± 3.88 | 7.83 ± 1.94 | 18.33 ± 4.32 | – | – | ||
| Caudal TRN (n = 3) | – | – | – | – | – | – | 5.33 ± 1.53 | 11.33 ± 0.58 |
Results
Our principal aim was to examine the general pattern of retrograde labelling in the ZI subsequent to injections made into the rostral, intermediate or caudal regions of the TRN. The injections of HRP or fluorogold resulted in injection sites, and only injections limited to the target regions (the rostral, intermediate or caudal TRN) were considered in this study. The accuracy and location of the resultant injections were determined by staining sections with thionin to verify the location of the injection. Both HRP and fluorogold injections into the different regions of the TRN produced consistent retrograde labelling patterns in the ZI. In the present study the ZI projections were evaluated in terms of the four sectors of the ZI: rostral (rZI), dorsal (dZI), ventral (vZI) and caudal (cZI), as defined by Nicolelis et al. (1992, 1995).
There were major differences in the distribution of retrogradely labelled cells within the ZI subsequent to injections made into the rostral, intermediate or caudal regions of the TRN. Injections into the rostral region of the TRN showed labelled cells in the rZI and vZI sectors (Fig. 2), whereas injections into the intermediate region of the TRN showed labelled cells in the dZI and vZI sectors (Fig. 3). Injections into the caudal region of the TRN showed only very few labelled cells in the cZI sector of the ZI (Fig. 4). The average number of HRP- and fluorogold-labelled cells was counted subsequent to rostral, intermediate and caudal TRN injections. The number of fluorogold-labelled cells was always greater than the number of HRP-labelled cells for all three regions of the TRN (Table 1).
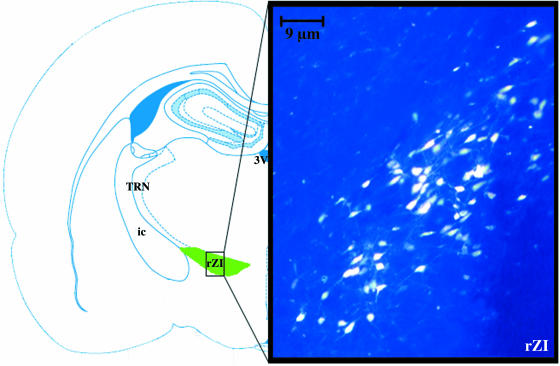
Fig. 2.
Injection into the rostral region of the thalamic reticular nucleus showed numerous fluorogold-labelled cells within the rostral sector of the zona incerta. The shaded area on the schematic diagram indicates the sectors of the zona incerta. ×400.
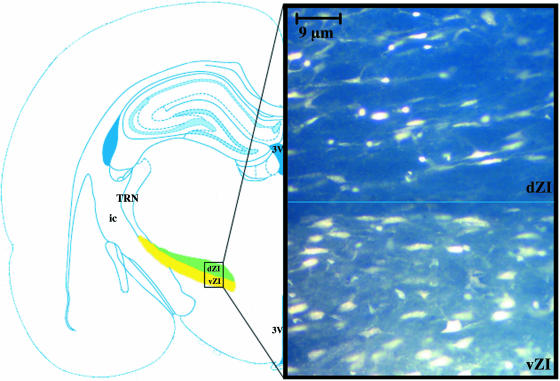
Fig. 3.
Injection into the intermediate region of the thalamic reticular nucleus showed fluorogold-labelled cells within the ventral and dorsal sectors of the zona incerta. The shaded area on the schematic diagram indicates the sectors of the zona incerta. ×400.
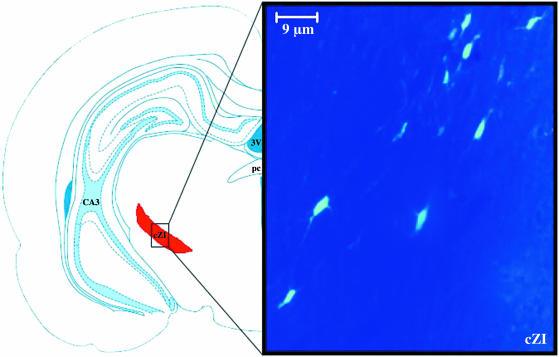
Fig. 4.
Injection into the caudal region of the thalamic reticular nucleus showed few fluorogold-labelled cells within the caudal sector of the zona incerta. The shaded area on the schematic diagram indicates the sectors of the zona incerta. ×400.
Discussion
Our results show that retrograde tracer injections into different regions of the TRN produce different patterns of labelling in the different subdivisions of the ZI. In terms of the numbers of labelled cells, injections made into the rostral TRN showed the most labelled cells in the rZI and vZI sectors, whereas injections made into the intermediate TRN showed labelled cells primarily in the dZI and vZI sectors, and only a few labelled cells in the cZI were observed subsequent to injections into the caudal TRN (Figs 2–4). Furthermore, consistent labelling was also present in the subthalamic nucleus subsequent to injections into the rostral TRN but not the intermediate or caudal regions of the TRN.
Technical considerations
The possibility that some of the labelled cells observed within the ZI may have been due to the spread of the tracer from regions adjacent to the TRN or to uptake by fibres of passage must be considered. Contamination by diffusion of the tracer to the neighbouring anterior structures would have included the ventral anterior (VA) thalamic nucleus for rostral TRN injections and the ventral posterolateral (VPL) nuclei for the intermediate and caudal TRN injections. Posterior contamination would have involved the internal capsule. Power et al. (1999) showed that neither the VA nor the VPL thalamic nuclei have any distinct connections from the ZI. Futhermore, no connections of the ZI to the internal capsule have been described in previous studies. The possibility that the tracer may have diffused during the injection procedure as the canula traversed the cortex also has to be excluded. Because the tip of the pipette was filled with air to avoid this problem, and given that all of the experiments involved passage through the somatosensory cortex but labelling in the ZI varied in accordance with the part of the TRN that was injected (rostral, intermediate or caudal), a cortical source of the label can be excluded. In addition, although cortical projections to the ZI have been described, no evidence of ZI connections to the cortex have been detected (Mitrofanis & Mikuletic, 1999).
Recent studies have shown pathways from the ZI to higher order thalamic relays including the intralaminar nuclei (Lévesque et al. 1996; Kolmac & Mitrofanis, 1997; Mitrofanis & Mikuletic, 1999; Barthõ et al. 2002). These pathways are inhibitory (GABAergic) and are likely to serve as modulators of thalamic transmission (Sherman & Guillery, 1998, 2006). In view of the suggestion made by Sherman and Guillery that modulators of thalamic activity can be expected to send branches (collaterals) to the TRN, the present observations can be seen as support for this suggestion. However, it has to be recognized that an earlier study of axons passing from the ZI to the thalamus (Barthõ et al. 2002) showed no axons that could be traced to the TRN. The only way in which the clearly distinct patterns of retrograde labelling seen in the ZI after injections into the different regions of the TRN can be reconciled with these earlier observations is to suggest that the reticular branches were significantly finer than the relatively thick incertothalamic axons shown by Barthõ et al. (2002).
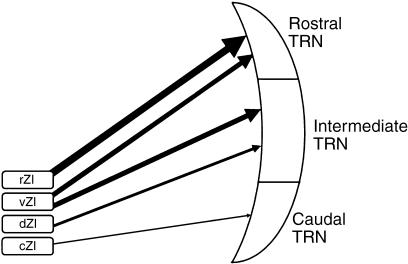
Mitrofanis & Mikuletic (1999) described widespread cortical inputs to the ZI arising from layer V but not from layer VI. They showed these layer V axons in a summary schema as branches of motor fibres descending to the brainstem and spinal cord. Although details of such connections are not currently available, this input to the ZI can reasonably be regarded as relating to corticofugal motor pathways. This view of the ZI as primarily concerned with motor pathways is also supported by the observation of Mitrofanis & Mikuletic (1999, their fig. 10) that most of the corticofugal inputs to the ZI come from the frontal and limbic areas, with fewer inputs coming from parietal cortex, and almost none from the visual or auditory cortex. This pattern of cortical connections is in striking accord with our observations on the pathway from the ZI to the TRN, which show that the rostral regions of TRN, linked to frontal and limbic cortex, receive the strongest connections whereas the caudal regions, linked to auditory and visual cortex, receive almost no incertal inputs. The intermediate regions of the TRN are also intermediate in the strengh of incertal inputs, again matching the corticoincertal pathway described by Mitrofanis & Mikuletic (1999) (Fig. 5).
Fig. 5.
Schematic summary of the observations, showing the connections of zona incerta with the TRN. The thickness of the neuronal output from the sectors of the zona incerta is proportional to the connection density.
The pattern of afferents to the thalamus from the ZI, illustrated by Barthõ et al. (2002), is in accord with the pattern of connections summarized by Power et al. (1999), who stressed the fact that the incertal axons supply the higher order relays but not the first-order relays. Furthermore, when the higher order relays are considered, the observations illustrated by Barthõ et al. (2002) indicate that higher order relays related to the caudal parts of the the TRN (lateral posterior nucleus and pulvinar) receive few or no incertal afferents whereas the higher order afferents related to more rostral parts of the TRN receive more incertal afferents.
The dorsal thalamus is the gateway to the neocortex. All messages reaching the neocortex must pass through the thalamus and the mode of transmission to the cortex can be modified by the functional connections made between thalamocortical, corticothalamic, corticoreticular, thalamoreticular and reticulothalamic connections. These connections demonstrate the importance of the TRN in the control of thalamocortical transmission. The present results show that the ZI is in a position to influence the thalamocortical pathways not only through its direct connections (ZI–thalamic connection) demonstrated by Power et al. (1999) and Barthõ et al. (2002), but also through its TRN connections (ZI–TRN–thalamic connections). The present results also show that the ZI may play a particularly significant role in this mechanism for limbic and motor functions and to a lesser extent for somatosensory functions.
Acknowledgments
This project was supported by Marmara University Scientific Research Council (SAG-065/131102). We thank Professor Ray Guillery for his critical review and contributions to the preparation of the manuscript.
References
- Barthõ P, Freund TF, Acsády L. Selective Gabaergic innervation of the thalamic nuclei from zona incerta. Eur J Neurosci. 2002;16:999–1014. doi: 10.1046/j.1460-9568.2002.02157.x. [DOI] [PubMed] [Google Scholar]
- Carman JB, Cowan WM, Powell TPS. Cortical connexion of the thalamic reticular nucleus. J Anat. 1964;98:587–598. [PMC free article] [PubMed] [Google Scholar]
- Conley M, Diamond IT. Organization of the visual reticular thalamic nucleus in Galago. Eur J Neurosci. 1990;2:211–226. doi: 10.1111/j.1460-9568.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Crabtree JW, Killackey HP. The topographic organization and axis of projections within the visual sector of the rabbit's thalamic reticular nucleus. Eur J Neurosci. 1989;1:94–109. doi: 10.1111/j.1460-9568.1989.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Crabtree JW. The somatotopic organization within the rabbit's thalamic reticular nucleus. Eur J Neurosci. 1992;4:1343–1351. doi: 10.1111/j.1460-9568.1992.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Crabtree JW. Organization in the somatosensory sector of the cat's thalamic reticular nucleus. J Comp Neurol. 1996;336:207–222. doi: 10.1002/(SICI)1096-9861(19960304)366:2<207::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi A, Frassoni C, Spreafico R. GABA immunoreactivity in the thalamic reticular nucleus of the rat. A light and electron microscopical study. Brain Res. 1986;339:143–147. doi: 10.1016/0006-8993(86)90608-6. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Isaacs S. The zona incerta lesions: effects on copulation, partner preference and other socio-sexual behaviours. Behav Brain Res. 1991;44:145–150. doi: 10.1016/s0166-4328(05)80019-1. [DOI] [PubMed] [Google Scholar]
- Ficalora AS, Mize RR. The neurons of the substantia nigra and the zona incerta which project to the cat superior colliculus are GABA immunoreactive: a double label study using GABA immunocytochemistry and lectin retrograde transport. Neuroscience. 1989;29:567–581. doi: 10.1016/0306-4522(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Helmstetter FJ, Agudo J. Functional mapping of the rat brain during drinking behaviour: a fluorodeoxyglucose study. Physiol Behav. 1993;54:605–612. doi: 10.1016/0031-9384(93)90256-f. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: advancing views over half of century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- Hermanson O, Hallbeck M, Blomqvist A. Proenkephalinergic mRNA-expressing neurones in the rat thalamus. Neuroreport. 1995;6:833–836. doi: 10.1097/00001756-199504190-00002. [DOI] [PubMed] [Google Scholar]
- Jones EG. Some aspects of the organization of the thalamic reticular complex. J Comp Neurol. 1975;162:285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum; 1985. [Google Scholar]
- Kim U, Gregory E, Hall WC. Pathway from the zona incerta to the superior colliculus in the rat. J Comp Neurol. 1992;321:555–575. doi: 10.1002/cne.903210405. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Mitrofanis J. Organization of the reticular thalamic projections to the intralaminar and midline nuclei in the rat. J Comp Neurol. 1997;377:165–178. doi: 10.1002/(sici)1096-9861(19970113)377:2<165::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Power BD, Mitrofanis J. Patterns of connections between the zona incerta and brainstem in rats. J Comp Neurol. 1998;396:544–555. doi: 10.1002/(sici)1096-9861(19980713)396:4<544::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kolmac CI, Mitrofanis J. Distribution of various neurochemicals within zona incerta: an immunocytochemical and histochemical study. Anat Embryol. 1999;199:265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- Lechner J, Leah JD, Zimmermann M. Brainstem peptidergic neurones projecting to the medial and lateral thalamus and the zona incerta in the rat. Brain Res. 1993;603:47–56. doi: 10.1016/0006-8993(93)91298-7. [DOI] [PubMed] [Google Scholar]
- Legg CR. Visual discrimination after lesions in the zona incerta or lateral terminal nucleus of accessory optic tract. Brain Res. 1979;177:461–478. doi: 10.1016/0006-8993(79)90464-5. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Gagnon S, Parent A, Deschénes M. Axonal arborisations of cortico-striatal and cortica-thalamic fibers arising from the second somatosensory area in the rat. Cerebral Cortex. 1996;6:759–770. doi: 10.1093/cercor/6.6.759. [DOI] [PubMed] [Google Scholar]
- Lozsádi DA. Organization of cortical afferents to the rostral, limbic sectors of the rat reticular nucleus. J Comp Neurol. 1994;341:520–533. doi: 10.1002/cne.903410408. [DOI] [PubMed] [Google Scholar]
- Lozsádi DA. Organization of connections between the thalamic reticular and the anterior thalamic nuclei in the rat. J Comp Neurol. 1995;358:233–246. doi: 10.1002/cne.903580206. [DOI] [PubMed] [Google Scholar]
- May PJ, Sun W, Halls WC. Reciprocal connections between the zona incerta and the pretectum and superior colliculus of the cat. Neuroscience. 1997;77:1091–1114. doi: 10.1016/s0306-4522(96)00535-0. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Tetramethylbenzidine for horseradish peroxidase neurochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Mikuletic L. Organization of the cortical projections to the zona incerta of the thalamus. J Comp Neurol. 1999;412:173–185. [PubMed] [Google Scholar]
- Morgenson GJ, Swanson LW, Wu M. Evidence that projections from substantia innominata to the zona incerta and mesencephalic locomotor region contribute to locomotor activity. Brain Res. 1985;334:65–76. doi: 10.1016/0006-8993(85)90568-2. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chapin JK, Lin RC. Somatotropic maps within the zona incerta relay parallel GABAergic somatosensory pathways to the neocortex, superior colliculus and brainstem. Brain Res. 1992;577:134–141. doi: 10.1016/0006-8993(92)90546-l. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Chopin JK, Lin RC. Development of direct GABAergic projections from the zona incerta to the somatosensory cortex of the rat. Neuroscience. 1995;65:609–631. doi: 10.1016/0306-4522(94)00493-o. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J Neurocytol. 1985;14:365–411. doi: 10.1007/BF01217752. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. pp. 8–74. [Google Scholar]
- Power BD, Kolmac CI, Mitrofanis J. Evidence for a large projection from the zona incerta to the dorsal thalamus. J Comp Neurol. 1999;404:554–565. [PubMed] [Google Scholar]
- Ricardo JA. Efferent connections of the subthalamic region in the rat. II. Zona incerta. Brain Res. 1981;214:43–60. doi: 10.1016/0006-8993(81)90437-6. [DOI] [PubMed] [Google Scholar]
- Romanowski CAJ, Mitchell IJ, Crossman AR. The organisation of the efferent projections of the zona incerta. J Anat. 1985;143:75–95. [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Negrao N, Ricardo JA. Afferent connections of the zona incerta: a horseradish peroxidase study in the rat. Neuroscience. 1985;15:109–134. doi: 10.1016/0306-4522(85)90127-7. [DOI] [PubMed] [Google Scholar]
- Sherman MS, Guillery RW. On the actions that the nerve cells can have on another: distinguishing ‘drivers’ from ‘modulators’. Proc Natl Acad Sci USA. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MS, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. 2. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Steriade M, Deschenes M. The thalamus as a oscillator. Brain Res Rev. 1984;8:1–62. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Parent A, Hada J. Thalamic projections of nucleus reticularis thalami in the cat: a study using retrograde transport of horseradish peroxidase and fluorescent tracers. J Comp Neurol. 1984;299:531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticularis thalami nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus and spinal cord in the rat: a study using autoradiographic and horseradish peroxidase methods. Neuroscience. 1982;7:2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]