Abstract
The pacemaker of the biological clock, the suprachiasmatic nucleus (SCN) of the hypothalamus, was studied in intact male rats to determine its immunoreactivity to glial fibrillary acidic protein (GFAP), a specific marker of astrocytes. Animals were kept under 12-h light–dark cycles in synchrony with day–night periods. Immunohistochemical reactions were carried out at midday and late at night in both winter (January) and summer (July). In winter, GFAP immunoreactivity was found to be low during the day and high at night. The findings were reversed in summer, when GFAP immunoreactivity was high during the day and low at night. Increased GFAP immunoreactivity appeared in the form of an abundance of thick immunopositive fibres rather than of cell bodies. This was interpreted as a hypertrophy of pre-existing astrocytes due to alternating photic stimulation conveyed by retinofugal fibres to the SCN.
The observed seasonal reversal in the direction of GFAP oscillations raises the possibility that a circannual timer exists outside the SCN.
Keywords: biological clock, GFAP, rat
Introduction
For many years, extensive studies of astrocyte–neuron interactions have been carried out in a number of brain areas (for references see Vernadakis & Fedoroff, 1984; Hertz, 2004; Kettenmann & Ransom, 2005). Special attention has been paid to the role played by astroglia in various types of neural plasticity. Changes that damage neurons and evoke a subsequent glial reaction (Cavanagh, 1970; Janeczko, 1989; Hajós et al. 1990; Zilles et al. 1991; Hajós & Csillag, 1995) and glial alterations that trigger a neuronal response can be induced experimentally (Reier, 1984). However, the assessment of glia–neuron interactions occurring in the intact brain during function appears to be more difficult. Recently, direct evidence was provided for the involvement of astroglia in synaptic plasticity coupled to normal function (Hirrlinger et al. 2004), similar to the previously described changes in various pathological conditions (Lindsay, 1984).
In previous studies of glia–neuron interaction (Hajós et al. 1999, 2000) we dealt with general metabolic influences, such as altered gonadal hormonal states, on the reactivity of astroglia. In the present study, we chose as an experimental paradigm the biological clock, the retinal input of which includes well-defined neuronal pathways.
It is well established that the pacemaker of the biological clock that generates circadian cycles is the suprachiasmatic nucleus of the hypothalamus (SCN; for references see Klein et al. 1991), which in addition to its endogenously generated rhythm (Hakim et al. 1991) receives important modulatory input from the retina (Pittendrigh, 1993). Two pathways have been identified as conveying photic stimuli from the retina to the SCN: (1) a direct retinohypothalamic connection (Speh & Moore, 1993) and (2) an indirect connection that begins with reticulogeniculate fibres terminating in the intergeniculate leaflet (IGL) of the lateral geniculate nucleus (Morin & Pace, 2002). IGL neurons then project to the SCN (Card & Moore, 1989; Botchkina & Morin, 1995). It has been shown that glutamate is released with cyclical intensity from the endings of input fibres to the SCN, and consequent neuronal activity was thought to be responsible for further cyclical changes (Lavialle & Serviere, 1995). This implies that retinal impulses generated by light–dark cycles are transmitted via a neuronal pathway onto the biological rhythm generator cells in the SCN. It is claimed that astrocytes contribute to the mechanism by the reactive uptake of cyclically elevated extracellular glutamate (Lavialle et al. 2001; Piet et al. 2004).
To understand more about the involvement of astrocytes in the function of the biological clock, we studied immunoreactivity of glial fibrillary acidic protein (GFAP-ir) in the SCN during 12-h light–dark cycles. Investigations were also carried out at the circannual level, i.e. in winter and in summer. In order to exclude the influence of fluctuations in gonadal steroid levels (Fernandez-Galaz et al. 1999; Hajós et al. 1999, 2000), we used intact males. Our principal aim was to study the participation of astrocytes in neuronal plasticity occurring during daily light–dark cycles within the rhythm generator of the biological clock.
Materials and methods
Intact male Wistar rats (250 g body weight) were kept on standard laboratory diet with free access to water, under 12-h light–dark cycles. Experimental procedures and perfusion of the animals with the fixative were carried out under deep anaesthesia. All manipulations were performed in compliance with international standards and the regulations of the Hungarian Animal Protection Act.
Animals were studied in winter (January) and summer (July), during daytime (11:00–12:00 h) and at night (23:00–24:00 h). The light period lasted from 06:00 to 18:00 h to mimic (at least approximately) the natural illumination phases. In some cases, illumination was prolonged for 4 h at the expense of the dark period. Four animals were examined in each group.
As fixative, a mixture of paraformaldehyde and picric acid (4% each in aqueous solution) was perfused through the aorta. Brains were removed from the skull and kept in the same fixative for 2 h. Coronal discs containing the entire SCN were then prepared with a Vibroslice (Campden Instruments, London, UK) at the level between the mammillary bodies and the optic chiasma (distance from bregma: −0.26 to −1.40; Paxinos & Watson, 1986). For study of the IGL, discs were excised between coordinates −3.80 and −5.20 to include the region of the lateral geniculate body. From the coronal discs, serial 50-µm-thick vibratome sections were cut and processed floating for GFAP immmunostaining. This was introduced by 10 min of treatment with 3% H2O2 to suppress endogenous peroxidase activity. Sections were then immersed for 2 h in 10% normal goat serum to inhibit non-specific immunoreaction. Both treatments were performed at room temperature. Monoclonal anti-GFAP serum (Boehringer, Mannheim, Germany) was applied at 1 : 5000 dilution. Incubation with the antiserum was carried out for 36 h at 4 °C under vigorous shaking. As second antibody, biotinylated rabbit anti-mouse IgG was used at a dilution of 1 : 100 for 5 h, followed by the avidin–biotin–peroxidase complex (Vectastain, 1 : 100). The immune reaction was visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB-T). Sections were mounted on glass slides and coverslipped for light microscopy.
Control incubations were carried out either with the omission of the primary antiserum, the sections being processed with peroxidase conjugates only, or with preabsorbed antiserum. In these cases no immunostaining occurred.
To assess the exact territory of the SCN for measurements, some vibratome slices were embedded flat in paraffin and cut into 10-µm serial sections for staining with Nissl's cresyl violet. In these preparations, the number of astrocytes was also counted under an eyepiece graticule.
Surface densitometry was applied to pictures taken with a Nikon Coolpix 4500 digital camera (Nikon Inc., Tokyo, Japan). Images were converted to 8-bit colour-depth greyscales. Analysis was carried out with Scion Image (Scion Inc., Frederick, MD, USA) macros developed by us. Identical areas were selected in the images over both the territory of the nuclei and the non-nuclear reference field. Immunoreactions in digitized greyscale images appear as high-density (dark) areas whereas negative fields have lower greyscale density values. According to this greyscale distribution, immunopositive territories can be selected by their greyscale range. With our macros this process could be performed automatically. Intensity of the immunoreaction was expressed as the number of pixels covering the immunostained structures within a standard area. These values were compared with those obtained in immunonegative areas of similar size taken from the area immediately surrounding the SCN.
Results
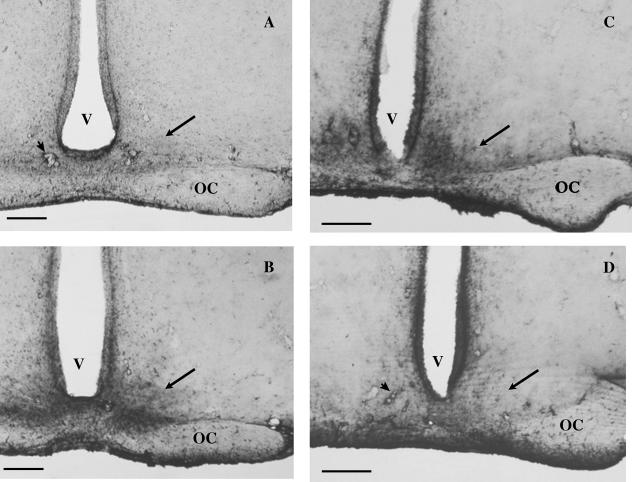
When intact males were studied in winter, their SCN showed low GFAP-ir in the light period (Fig. 1A). Cell bodies were not stained, and immunoprecipitate was found only around blood vessels. In the dark period (Fig. 1B) intense GFAP-ir was observed. The extent of the reactive area corresponded to the entire territory of the SCN. This was verified by projecting the nuclear borders delineated in the Nissl-stained preparations on immunohistochemical preparations. Nevertheless, the reaction was more pronounced in the ventromedial region of the SCN. No difference in overall immunoreactivity was encountered between the rostral and caudal parts of the nucleus as scanned through between coordinates −0.92 and −1.40.
Fig. 1.
GFAP-ir in the SCN. (A,B) GFAP-ir in the SCN in winter during the day and at night. In winter, during the light period (day, A), GFAP-ir of astrocytes is weak in the SCN (arrow). Note the prominent immunostaining of pericapillary glial sheaths (arrowhead). In the dark period (night, B) the reaction is intense in the SCN (arrow). (C,D) GFAP-ir in the SCN in summer during the day and at night. In summer, during the light period (day, C), GFAP-ir of the SCN (arrow) is intense, whereas in the dark period (night, D) it is weak. OC, optic chiasma; V, third ventricle. Scale bar = 350 µm.
In summer, the circadian fluctuation of GFAP reaction in the SCN showed an opposite direction to that observed during the winter period. Accordingly, intense GFAP-ir was observed in the light period, whereas low reactivity was seen in the dark period (Fig. 1C,D).
A 4-h prolongation of the light period within the 24-h cycle brought about no appreciable difference.
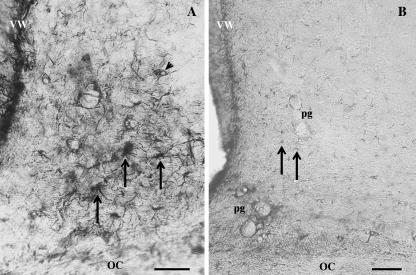
In all cases of intensely stained SCNs, the bulk of the reaction decorated long and thick astrocyte processes meandering throughout the territory of the nucleus (Fig. 2A). Along their course, many of them came into contact with capillaries, and some of them surrounded capillaries fully. Relatively few cell bodies were seen distributed randomly in the SCN. The number of immunoreactive cell bodies was broadly similar (15–17 per section) to that of the astrocytes counted in Nissl-stained preparations. This number did not change under different illumination conditions. Some astrocytes in the optic chiasma adjacent to the nucleus were also immunostained but they were not evaluated because the fibres of the chiasma clearly indicated the ventral border of the nucleus.
Fig. 2.
The pattern of strong and weak GFAP-ir in the SCN. High-power micrographs of the GFAP-ir in the SCN exemplify the intense and weak types of reactions. In the intensely immunoreactive SCN (A), thick, immunoprecipitated astrocyte processes enmesh the nucleus. Cell bodies (arrows) are also strongly stained. The cell nucleus (arrowhead) is occasionally seen as an area devoid of reaction precipitate. In the weakly stained SCN (B), faintly stained fragments of astrocytes (arrows) are found. Perivascular glia (pg) show a strong reaction. OC, optic chiasma; VW, ventricular wall. Scale bar = 30 µm.
In the weakly stained SCNs (Fig. 2B), only a few short segments of immunopositive processes were encountered.
The IGL of the lateral geniculate body displayed an invariably high GFAP-ir, in light or dark conditions, regardless of the season. Pericapillary glia showed no fluctuations under the above conditions.
For surface densitometry, the area of the SCN was determined in pixels in sections stained with Nissl's cresyl violet at ×270 magnification. Densitometry was performed consistently in areas composed of a similar number of pixels when digitized at the same magnification. To assess background density, matching fields were measured in the immunonegative area surrounding the SCN. The method expressed densitometric values in number of pixels covering immunopositive structures. Whereas the background value was in the range of 60–80 pixels, the strongly and weakly reacting SCNs contained 10 141 (SD ± 1090; n = 4) and 2891 (SD ± 224; n = 4) pixels, respectively. Thus, the reaction/background ratio for the strongly reacting SCN was 169, whereas for the weakly reacting SCN this value was 36.
Discussion
Several findings support the existence of illumination-dependent oscillations in astroglial activity in the rodent SCN, but observations are not unequivocal (Elliott & Nunez, 1994; Moriya et al. 2000). Conclusions relevant to circadian astroglial activation are vague or contradictory as to both the exact nature of the change (increase or decrease) and the exact nature of the condition (light or dark) that induces the change. For example, when revealing daily changes, Lavialle & Serviere (1995) talked about a transition of GFAP-ir from a network pattern to an isolated cell pattern that could not be interpreted in quantitative terms. By contrast, in a further study of the same group (Lavialle et al. 2001), quantitative changes were obtained in GFAP-ir expression, but under extreme experimental conditions such as enucleation or rearing animals in darkness or under constant light. Moreover, the ability of astrocytes to generate circadian rhythm has so far been shown only in cell culture (Prolo et al. 2005). Our experimental conditions and our findings are most similar to those of Harley et al. (2001), who relied on daily light–dark cycles and reported on a densitometrically verified increase in GFAP-ir in the dark phase. This is in agreement with our observations in the winter period.
GFAP-ir, when pronounced, was found to be strongest in the ventromedial part of the nucleus. This does not overlap with the suggested functional division of the SCN into ventrolateral and dorsomedial parts (Noguchi et al. 2004; Sumova & Illnerova, 2005). Nor could we identify differences in the rostrocaudal direction within the nucleus as reported for gene expression rhythms by Hazlerigg et al. (2005). Therefore, it seems that glioarchitectonics has its own pattern.
The most unexpected finding of the present work was the seasonal reversal in the direction of circadian changes of GFAP-ir. Accordingly, in winter GFAP-ir was low during the day (light period) and high at night (dark period), whereas in summer this was reversed. We were unable to find a similar observation in the literature. Therefore, we assume that ambiguities regarding the nature of oscillations of GFAP-ir as a function of day–night cycles may be due partly to having disregarded the season when experiments were carried out. Although this offers a possible explanation for the divergence of findings, the significance of the observed seasonal reciprocity remains unknown.
In this study, light–dark periods were adjusted to approximate the cycles of natural illumination. It is interesting to note that short-term deviations from natural conditions, e.g. an extension of the light period for a few hours (at the expense of the dark period) caused no changes in GFAP-ir either in winter or in summer. This suggests that the seasonal type of the circadian GFAP-ir cycle is firmly imprinted in the SCN.
In females, the fluctuations of gonadal hormones were shown to affect the function of the biological clock (Fernandez-Galaz et al. 1999). Our previous findings also suggested that in both males and females gonadal steroids may alter GFAP-ir in astrocytes outside the main hypothalamic endocrine regulatory centres (Hajós et al. 1999, 2000). Recently, Satriotomo et al. (2004) provided evidence for astrocyte activation in the male SCN by excess testosterone. Although the rat is not a seasonal breeder (Hoffman, 1981), and hormonal cycles are regular, we found it more reliable to use intact males to minimize hormonal interferences. Further experiments are required to reveal eventual alterations of circadian GFAP-ir in the SCN related to the sexual cycle of females.
The fact that an increase in GFAP-ir appeared in the form of an accumulation of intensely immunostained thick fibres, rather than of cell bodies, argues for hypertrophy rather than proliferation of the activated astrocytes. This is consistent with our earlier findings suggesting a primary role of hypertrophy over proliferation in cases of astroglial activation (Hajós et al. 1993). As our present observations were made during light–dark cycles, we regard the resulting astroglial hypertrophy as a reaction triggered by natural stimuli through a neuronal pathway. Thus, we corroborate the view of Lavialle & Serviere (1995) that GFAP-ir may serve as an indicator of circadian neuronal plasticity in the SCN.
A possible explanation for the astroglial reaction is that there is an uptake of excess glutamate (and possibly of other transmitters) by SCN astrocytes (Lavialle et al. 2001) released upon stimulation by the nerve endings of retinofugal fibres (Speh & Moore, 1993; Botchkina & Morin, 1995). With regard to the cyclicity of astroglial changes in the SCN, the action of cyclically released and bound melatonin should be taken into consideration. Accordingly, melatonin may scavenge free radicals in the SCN, thereby acting as a neuroprotective agent (Reiter, 1998). Melatonin release and/or binding has been found to be higher in winter (Holloway et al. 1985; Rom-Bugoslavskaja & Bondarenko, 1987), which may underlie seasonal differences in astroglial reactivities of the SCN. At the circadian level it has been observed that melatonin production and release is enhanced in the dark, parallel to the reduction in number of pineal photoreceptor-like structures such as synaptic rods and ribbons. In the studies of Rudeen et al. (1975) and Hoffmann (1981) the activity of N-acetyltransferase, a rate-limiting enzyme of melatonin synthesis, was found to be increased 60-fold at night in the rat. All these findings suggest a photosensitive interaction between pineal and SCN.
To our present knowledge, retinal input to the SCN is either direct or reaches the nucleus through the IGL, a thin borderline area between the dorsal and ventral subdivisions of the lateral geniculate nucleus. Compared with its surrounding, the IGL is prominent, with an intense GFAP-ir (Morin et al. 1989). Because in pilot experiments with the IGL the observed high GFAP-ir was found to be insensitive to 12-h dark–light periods, we suggest that the direct retinohypothalamic input to the SCN is the one that can be instrumental in producing circadian astroglial oscillations. In addition to circadian rhythm control, seasonal timekeeping may also be a task of the SCN (Sumova et al. 2004), but the existence of a seasonal timer distinct from the circadian photoperiod mechanism as proposed by Lincoln et al. (2005) cannot be dismissed. This may encourage a search for an extrahypothalamic site responsible for the seasonally different astroglial GFAP-IRs in the SCN.
Conclusion
This paper has provided a description of the light-dependent fluctuations of GFAP-ir in the SCN, the rhythm generator area of the biological clock. In addition to the light–dark oscillations, we were able to demonstrate seasonal differences that offer an explanation for the ambiguities found in the literature concerning the participation of astrocytes in biological rhythms. The results suggest that a seasonal timer operates either in the SCN or at another photosensitive site.
Acknowledgments
This work was supported by grant no. T037805 of the Hungarian Science Research Fund (OTKA).
References
- Botchkina GI, Morin LP. Specialised neuronal and glial contributions to development of the hamster lateral geniculate complex and circadian visual system. JNeurosci. 1995;15:190–201. doi: 10.1523/JNEUROSCI.15-01-00190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Moore RY. Organization of lateral geniculate-hypothalamic connections in the rat. J Comp Neurol. 1989;284:135–147. doi: 10.1002/cne.902840110. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. The proliferation of astrocytes around a needle wound in the rat brain. J Anat. 1970;106:471–487. [PMC free article] [PubMed] [Google Scholar]
- Elliott AS, Nunez AA. An ultrastructural study on somal appositions in the suprachiasmatic nucleus and anterior hypothalamus of the rat. Brain Res. 1994;662:278–282. doi: 10.1016/0006-8993(94)90826-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Martinez Munoz R, Villanua MA, Garcia-Segura LM. Diurnal oscillation in glial fibrillary acidic protein in a perisuprachiasmatic area and its relationship to the luteinizing hormone surge in the female rat. Neuroendocrinology. 1999;70:368–376. doi: 10.1159/000054498. [DOI] [PubMed] [Google Scholar]
- Hajós F, Kálmán M, Zilles K, Schleicher A, Sótonyi P. Remote astrocytic response as demonstrated by GFAP immunohistochemistry in the visual cortex of DLG-lesioned rats. Glia. 1990;3:301–310. doi: 10.1002/glia.440030410. [DOI] [PubMed] [Google Scholar]
- Hajós F, Gerics B, Turai É. Astroglial reaction following Wallerian degeneration in the rat visual cortex: proliferation or hypertrophy? Neurobiology. 1993;1:123–131. [PubMed] [Google Scholar]
- Hajós F, Csillag A. The remote astroglial response (RAR): a holistic approach for evaluating the effects of lesions of the central nervous system. Neurochem Res. 1995;20:571–577. doi: 10.1007/BF01694538. [DOI] [PubMed] [Google Scholar]
- Hajós F, Halasy K, Gerics B, Szalay F. Glial fibrillary acidic protein (GFAP)-immunoreactivity is reduced by castration in the interpeduncular nucleus of male rats. NeuroReport. 1999;10:2229–2233. [PubMed] [Google Scholar]
- Hajós F, Halasy K, Gerics B, Szalay F, Michaloudi E, Papadopoulos GC. Ovarian cyle-related changes of glial fibrillary acidic protein (GFAP) immunoreactivity in the rat interpeduncular nucleus. Brain Res. 2000;862:43–48. doi: 10.1016/s0006-8993(00)02065-5. [DOI] [PubMed] [Google Scholar]
- Hakim H, DeBernardo AP, Silver R. Circadian locomotor rhythms, but not photoperiodic responses, survive surgical isolation of the CNS in hamsters. J Biol Rhythms. 1991;6:97–113. doi: 10.1177/074873049100600201. [DOI] [PubMed] [Google Scholar]
- Harley CW, Farrell RC, Rusak B. Daily variation in the distribution of glycogen phosphorylase in the suprachiasmatic nucleus of Syrian hamsters. J Comp Neurol. 2001;435:249–258. doi: 10.1002/cne.1206. [DOI] [PubMed] [Google Scholar]
- Hazlerigg DG, Ebling FJ, Johnston JD. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr Biol. 2005;5:449–450. doi: 10.1016/j.cub.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Hertz L. Non-neural cells in the nervous system: function and dysfunction I–III. Adv Mol Cell Biol. 2004.
- Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Photoperiodic function of the mammalian pineal organ. In: Oksche A, Pévet P, editors. The Pineal OrganPhotobiology – Biochronometry – Endocrinology. Amsterdam: Elsevier/North-Holland Biomedical Press; 1981. pp. 123–128. [Google Scholar]
- Holloway WR, Grota LJ, Brown GM. Immunohistochemical assessment of melatonin binding in the pineal gland. J Pineal Res. 1985;2:235–251. doi: 10.1111/j.1600-079x.1985.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Janeczko K. Spatiotemporal patterns of astroglial proliferatio in rat brain injured at the postmitotic stage of postnatal development: a combined immunocytochemical and autoradiographic study. Brain Res. 1989;485:236–243. doi: 10.1016/0006-8993(89)90566-0. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. Neuroglia. Oxford: Oxford University Press; 2005. [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic NucleusThe Mind's Clock. New York: Oxford University Press; 1991. [Google Scholar]
- Lavialle M, Serviere J. Developmental study in the circadian clock of the golden hamster: a putative role of astrocytes. Dev Brain Res. 1995;86:275–282. doi: 10.1016/0165-3806(95)00039-g. [DOI] [PubMed] [Google Scholar]
- Lavialle M, Begue A, Papillon C, Vilaplana J. Modifications of retinal afferent activity induce changes in astroglial plasticity in the hamster circadian clock. Glia. 2001;34:88–100. doi: 10.1002/glia.1044. [DOI] [PubMed] [Google Scholar]
- Lincoln GA, Johnston JD, Andersson H, Wagner G, Hazlerigg DG. Photorefractoriness in mammals: dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology. 2005;146:3782–3790. doi: 10.1210/en.2005-0132. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Reactive gliosis. In: Vernadakis A, Fedoroff S, editors. Astrocytes. Vol. 2. London: Academic Press; 1984. pp. 231–262. [Google Scholar]
- Morin LP, Johnson RF, Moore RY. Two brain nuclei controlling circadian rhythms are identified by GFAP immunoreactivity in hamsters and rats. Neurosci Lett. 1989;99:55–60. doi: 10.1016/0304-3940(89)90264-4. [DOI] [PubMed] [Google Scholar]
- Morin LP, Pace L. The intergeniculate leaflet, but not the visual midbrain, mediates hamster circadian rhythm in response to constant light. J Biol Rhythms. 2002;17:217–226. doi: 10.1177/07430402017003005. [DOI] [PubMed] [Google Scholar]
- Moriya T, Yoshinobu Y, Kouzu Y, et al. Involvement of glial fibrillary acidic protein (GFAP) expressed in astroglial cells in circadian rhythm under constant lighting conditions in mice. J Neurosci Res. 2000;60:212–218. doi: 10.1002/(SICI)1097-4547(20000415)60:2<212::AID-JNR10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Watanabe K, Ogura A, Yamaoka S. The clock in the dorsal suprachiasmatic nucleus runs faster that that in the ventral. Eur J Neurosci. 2004;20:3199–3202. doi: 10.1111/j.1460-9568.2004.03784.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sidney: Academic Press; 1986. [Google Scholar]
- Piet R, Poulain DA, Oliet SH. Contribution of astrocytes to synaptic transmission in the rat supraoptic nucleus. Neurochem Int. 2004;45:251–257. doi: 10.1016/j.neuint.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Ann Rev Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci. 2005;25:404–408. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reier PJ. Gliosis following CNS injury: the anatomy of astrocytic scars and their influence on axonal elongation. In: Vernadakis A, Fedoroff S, editors. Astrocytes. Vol. 2. London: Academic Press; 1984. pp. 263–324. [Google Scholar]
- Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol. 1998;56:359–384. doi: 10.1016/s0301-0082(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Rom-Bugoslavskaja ES, Bondarenko L. Seasonal pecularities of thyroine influence on the way of serotonin metabolism in the pineal gland of the rat. Acta Physiol Hung. 1987;70:397–401. [PubMed] [Google Scholar]
- Rudeen PK, Reiter RJ, Vaughan MK. Pineal serotonin-N-acetyltransferase activity in four mammalian species. Neurosci Lett. 1975;1:225–229. [Google Scholar]
- Satriotomo I, Miki T, Gonzalez D, et al. Excessive testosterone treatment and castration induce reactive astrocytes and fos immunoreactivity in suprachiasmatic nucleus of mice. Brain Res. 2004;1020:130–139. doi: 10.1016/j.brainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Speh JC, Moore RY. Retinohypothalamic tract development in the hamster and rat. Dev Brain Res. 1993;76:171–181. doi: 10.1016/0165-3806(93)90205-o. [DOI] [PubMed] [Google Scholar]
- Sumova A, Bendova Z, Sladek M, Kovacikova Z, Illnerova H. Seasonal molecular timekeeping with the rat circadian clock. Physiol Res. 2004;53(Suppl. 1):167–176. [PubMed] [Google Scholar]
- Sumova A, Illnerova H. Effect of photic stimuli disturbing overt circadian rhythms on the ventrolateral and dorsomedial SCN rhythmicity. Brain Res. 2005;1048:161–169. doi: 10.1016/j.brainres.2005.04.061. [DOI] [PubMed] [Google Scholar]
- Zilles K, Hajós F, Kálmán M, Schleicher A. Mapping of glial fibrillary acidic protein immunorecativity in the rat forebrain by computerized image analysis. J Comp Neurol. 1991;308:340–355. doi: 10.1002/cne.903080303. [DOI] [PubMed] [Google Scholar]
- Vernadakis A, Fedoroff S. Astrocytes. Vol. 2. London: Academic Press; 1984. [Google Scholar]