Abstract
The notochord constitutes the main axial support during the embryonic and larval stages, and the arrangement of collagen fibrils within the notochord sheath is assumed to play a decisive role in determining its functional properties as a fibre-wound hydrostatic skeleton. We have found that during early ontogeny in Atlantic salmon stepwise changes occur in the configuration of the collagen fibre-winding of the notochord sheath. The sheath consists of a basal lamina, a layer of type II collagen, and an elastica externa that delimits the notochord; and these constituents are secreted in a specific order. Initially, the collagen fibrils are circumferentially arranged perpendicular to the longitudinal axis, and this specific spatial fibril configuration is maintained until hatching when the collagen becomes reorganized into distinct layers or lamellae. Within each lamella, fibrils are parallel to each other, forming helices around the longitudinal axis of the notochord, with a tangent angle of 75–80° to the cranio-caudal axis. The helical geometry shifts between adjacent lamellae, forming enantiomorphous left- and right-handed coils, respectively, thus enforcing the sheath. The observed changes in the fibre-winding configuration may reflect adaptation of the notochord to functional demands related to stage in ontogeny. When the vertebral bodies initially form as chordacentra, the collagen lamellae of the sheath in the vertebral region are fixed by the deposition of minerals; in the intervertebral region, however, they represent a pre-adaptation providing torsional stability to the intervertebral joint. Hence, these modifications of the sheath transform the notochord per se into a functional vertebral column. The elastica externa, encasing the notochord, has serrated surfaces, connected inward to the type II collagen of the sheath, and outward to type I collagen of the mesenchymal connective tissue surrounding the notochord. In a similar manner, the collagen matrix of the neural and haemal arch cartilages is tightly anchored to the outward surface of the elastic membrane. Hence, the elastic membrane may serve as an interface between the notochord and the adjacent structures, with an essential function related to transmission of tensile forces from the musculature. The interconnection between the notochord and the myosepta is discussed in relation to function and to evolution of the arches and the vertebra. Contrary to current understanding, this study also shows that notochord vacuolization does not result in an increased elongation of the embryo, which agrees with the circular arrangement of type II collagen that probably only enables a restricted increase in girth upon vacuolization, not aiding elongation. As the vacuolization occurs during the egg stage, this type of collagen disposition, in combination with an elastica externa, also probably facilitates flexibility and curling of the embryo.
Keywords: Atlantic salmon, chordacentrum, hydroskeleton, notochord sheath, notochord
Introduction
The notochord of the early vertebrate embryo produces secreted factors that provide positional and fate information for a broad variety of adjacent tissues (for a review, see Stemple, 2005). The notochord is also a midline structural element that serves as an axial skeleton during embryonic and larval stages, and even in adults of some primitive fish groups; in most clades, it is functionally, and in part structurally, replaced by the backbone. Although the role of the notochord in the formation of the backbone is not fully understood, notochord excision in chick embryos and in amphibian larvae results in the formation of an unsegmented gutter-shaped rod of vertebral cartilage, indicating that the notochord plays a role in segmental patterning (Kitchin, 1949; Holtzer, 1952; Holtzer & Detwiler, 1953; Strudel, 1955; Hall, 1977; Keynes & Stern, 1988; for a review, see Hall, 2005). In teleost species, such as the Atlantic salmon and the zebrafish, the notochord nucleates vertebral development by forming the initial structural elements of the vertebral body, the mineralized chordacentrum, and the intervertebral joint (Grotmol et al. 2003, 2005; Fleming et al. 2004; Nordvik et al. 2005). In the adult teleost, a functional notochord persists; in addition to interconnecting the vertebrae, it runs through the vertebral body, filling the amphicoelous concavities at the articular ends and holding the vertebrae apart. The notochord thus plays multiple roles in supporting the movements of the backbone.
In the early gastrula, the notochord arises from the dorsal organizer that forms the chordamesoderm. Orchestrated cell movements occur within this cell layer, where the planar cell polarity pathway has been implicated (see review by Tada, 2005). Medio-laterally aligned cells intercalate and converge towards the dorsal midline, resulting in longitudinal elongation of the tissue, and thus the embryo (Adams et al. 1990; Glickman et al. 2003). This convergent extension, in combination with cell division, gives rise to the notochord as a single-cell-diameter file of disc-shaped cells, the chordoblasts (Boeke, 1908; Pasteels, 1958; Hausen & Riebesell, 1991; Kimmel et al. 1995). These continue to divide, and start to deposit a basal lamina and other extracellular matrix components that eventually form an acellular sheath delimiting the notochord, a morphogenetic process in which secretion of laminin by the chordoblasts is essential (Parsons et al. 2002; Stemple, 2005). From the initial file of chordoblasts, an additional cell type, the chordocyte, differentiates, and inflates a spacious intracellular vacuole, which requires coatomer complex (Coutinho et al. 2004).
Concomitant with chordocyte differentiation, profound changes in notochord architecture take place. The chordocytes accumulate within the core, and are surrounded by a monolayer of epitheloid non-vacuolated chordoblasts, often designated the notochord epithelium. The morphology of the chordoblasts ranges between species from thin squamous to high cylindrical. Chordoblasts retain their germinal properties, and continue to secrete the basal lamina and the notochord sheath, sustaining further notochord growth. The external surface of the sheath comprises of lamellar elastin (Bruns & Gross, 1970; Nordvik et al. 2005), formed as a continuous layer, the elastica externa, which encases a much thicker collagenous layer in which type II collagen fibrils and aggrecan constitute the bulk of the matrix (Linsenmayer et al. 1973; Sandell, 1994; Domowicz et al. 1995; Gotz et al. 1995; Ng et al. 1997; Hayes et al. 2001).
Functionally, the notochord comprises a fibre-wound hydrostatic skeleton in which turgor rigidity is dependent on combined effects of colloid osmotic pressure within the vacuoles of the chordocytes, and the sheath wall, which restricts expansion; the inflation may aid both elongation and straightening of the embryo (Adams et al. 1990; Koehl et al. 2000). The mechanical properties of the notochord may be compared with those of an elastic rod, combining longitudinal compressive stiffness, lateral flexibility and elastic recoil, in which the matrix composition, and the arrangement of collagen fibrils in opposing spiralling helical patterns within the sheath, is assumed to be a structural prerequisite (Koehl et al. 2000).
In primitive actinopterygians and sarcopterygians, an unconstricted notochord may persist and contribute to the axial skeleton throughout life. Conversely, in amniote embryos the notochord is transitory, and a vertebral column is formed prior to exposure to the external environment. However, in teleosts such as the Atlantic salmon, an intermediate scenario is found, where the notochord, combined with neural and haemal cartilages (arcualia), constitutes the axial skeleton during the period of early ontogeny, encompassing both embryonic and free-living larval stages. During these stages of development, significant transitions occur both in body posture and in locomotor activity, placing different functional demands on the notochord. Salmon eggs are buried in the riverbed gravel immediately after spawning; the embryo grows curved around the yolk-sac and, just prior to hatching, the body length is equivalent to the inner circumference of the egg. The body axis straightens at hatching, and the free-living yolk sac larvae (alevins) remain stationary in the gravel for about one month (7 °C), preparing for increased activity. Once the yolk has been consumed, at the beginning of the fry stage, the salmon emerge from the gravel and start swimming vigorously in order to seek food and escape predation (Dill, 1977). Preceding and during this initial period of increasing activity, the vertebrae appear within the notochord sheath as mineralized rings, the chordacentra; here, the notochord generates both the segmental pattern and the molecules that facilitate the mineralization process, such as alkaline phosphatase (Grotmol et al. 2003, 2005; Fleming et al. 2004).
This ontogenetic sequence may place changing functional demands on the axial skeleton that may reflect those having driven the evolution of axial skeletal structures. The aim of our study in salmon was therefore to elucidate the relationships between locomotory behaviour and the structural development of the notochord, with the chordacentra and associated arches. Because of its large embryos and larvae with a large notochord, in addition to a slow early stage of development, the salmon facilitates detailed examination of developmental events that, in other species, may be closely related in time.
Materials and methods
Stock maintenance and sampling
Eggs, larvae and juveniles of the Atlantic salmon (Salmo salar L.) were collected from a local commercial hatchery, where they had been held in a flow-through system. At the hatchery the water temperature was kept at 8.0 °C throughout the egg stage, and after hatching, when half of the yolk had been consumed, the temperature was raised to 8.5 °C. At start-feeding the temperature was raised to 15.0 °C linearly over a period of 2 days. The fish were fed a commercial feed ad libitum. Developmental stages were classified by day degrees (day°), which are defined as the sum of daily mean ambient water temperatures (°C) for each day of development. Hatching occurred at around 500 day°, while first feeding commenced towards the end of the yolk-sac period, at approximately 870 day° (Fig. 1). Developmental stages up to 1500 day° were analysed sequentially. Samples of 20 fish were collected at intervals of 2 days and brought to the laboratory in bags of oxygenated water. Before the preparative procedures, the fish were anaesthetized with 5% benzocaine dissolved in water.
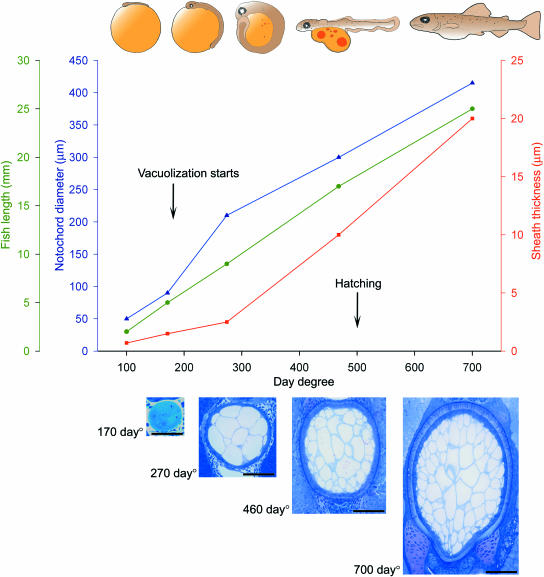
Fig 1.
Illustrations, graphs and microscopic images that show embryonic and larval growth of Atlantic salmon, in relation to the development of the notochord. Scale bars: 50 µm.
Histology
Specimens for methacrylate embedding were fixed by immersion in a mixture of 10 mL 10% formaldehyde (fresh from paraformaldehyde), 10 mL 25% glutaraldehyde, 20 mL 0.2 m cacodylate buffer and 60 mL PBS, and the pH adjusted to 7.35. The larger specimens were decalcified in buffered formic acid for 5–7 days, depending on size. The decalcified specimens were rinsed in PBS and dehydrated in ethanol (50, 70 and 96%), before being embedded in Technovit 7100 (Heraeus Kulzer GmbH & Co, Germany). Sections 1–2 µm in thickness were stained with toluidine blue.
Digital micrographs were acquired with a ProgRes C14 camera (Jenoptik GmbH, Jena, Germany) on an Olympus Vanox AHBT3 microscope employing both bright-field and DIC optics (Olympus, Tokyo, Japan), and processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, California, USA).
Transmission electron microscopy (TEM)
Specimens for TEM were fixed and decalcified in the same way as the tissues embedded in methacrylate. They were then rinsed in PBS and in dH2O, before post-fixation in 1% OsO4. The specimens were dehydrated in ethanol (70, 90 and 100%) and embedded in Epon 812 (Fluka Chemie AG, Switzerland). Ultrathin sections were placed on grids, contrasted with uranyl acetate and lead citrate, and viewed in a Jeol 1011 transmission electron microscope.
Scanning electron microscopy (SEM)
For SEM studies of the notochord, the specimens were fixed according to the same procedure as for light microscopy. Specimens were carefully sectioned with a razor blade, rinsed in buffer and post-fixed in 1% OsO4. All specimens were then dehydrated in an acetone series, critical-point dried, and studied in a Jeol 6400 conventional SEM and a Zeiss Supra 55VP field-emission SEM.
Morphometry
The total body length, the diameter of the notochord and the thickness of the notochord sheath were measured, and the mean values were calculated for the following developmental stages: 81, 101, 171, 274, 468 and 694 day°. The diameter of the notochord and the thickness of the sheath were measured in a region just caudal to the abdominal cavity on histological sections by employing AnalySISD software (Soft Imaging System GmbH, Münster, Germany). The morphology of the notochord in the period of inflation was reconstructed from serial transverse sections from head to tail bud. The number of collagen layers within the sheath was counted on histological sections using DIC optics. Mean collagen fibre angles within the layers were estimated from measurements performed on FESEM images.
Results
Vacuolization of the notochord occurs within the egg, and does not cause straightening or elongation of the embryo
In the early stages (88–170 day°), the notochord comprised a single-cell file of disc-shaped chordoblasts, and during this period the individual layers of the sheath were formed. Vacuolization of the notochord occurred between 170 and 275 day°, this process resulting in the formation of a core of chordocytes surrounded by a simple layer of chordoblasts, which together with the sheath comprised a hydroskeleton. During vacuolization of the notochord, the diameter increased at a higher rate than during subsequent development (Fig. 1). The vacuolization started in the occipital end of the notochord, and proceeded caudad with a linear increase in notochord girth along the axis (Fig. 2). The presumptive stretching of the sheath during vacuolization resulted in a small increase in thickness during this event. After vacuolization, until the chordacentra formed, both the notochord diameter and the sheath thickness increased linearly. During the periods of notochord vacuolization and subsequent development, the embryo grew linearly in length. Confined within the spherical egg, the embryo did not straighten, but maintained a constant curved posture (Fig. 1).
Fig 2.
Schematic drawing of the notochord during inflation based on serial transverse and longitudinal sections. The process of chordocyte vacuolation proceeds at a constant pace from the occipital region (to the left) towards the tail bud, where the chordoblasts retain a discoid shape until the embryo is fully elongated. The girth of the notochord increases linearly along the cranio-caudal axis. Developmental stage: 200 day°.
The basal lamina is the first matrix to be secreted by the chordoblasts, followed by elastin and collagen fibrils
During the initial phase of the single-cell file stage (Fig. 3A,B) a basal lamina that covered the whole surface of the cell file was the first extracellular structure to be formed. It comprised an inner electron-lucent and an outer electron-dense region, the latter composed of a network of fine filaments. The total thickness of the basal lamina, which was about 100 nm, remained constant. Subsequently, an electron-dense material resembling elastic fibres was secreted in a thin layer external to the basal lamina (100 day°) (Fig. 3C). As the last of the three layers of the sheath to appear, thin collagen fibrils, with a banding pattern and a diameter of ∼15 nm, were deposited between the basal lamina and the elastica externa (Fig. 3D).
Fig 3.
Early stages in the morphogenesis of the notochord with emphasis on the sheath. (A) Methacrylate-embedded tissue stained with toluidine blue. Longitudinal section of the single-cell file stage, just before the onset of vacuolization. Cranial to the left, dorsal side up. Developmental stage: 170 day°. (B–F) TEM images. (B) Transverse section of single-cell file stage. The sheath is indicated by arrowheads. The discoid chordoblasts appear to form an ideal structure to dispose collagen fibrils circumferentially into the sheath. Developmental stage: 88 day°. (C) Initial stage of sheath development at the single-cell file stage. The outer layer (el) probably represents an early stage of morphogenesis of the elastica externa, which here consists of electron-dense foci, presumptive elastin and a lattice of microfibrils. This layer is located external to the basal lamina (arrow). The cytoplasm of the chordoblast reflects high protein production. Inset: higher magnification of part of a polyribosome aggregation. Developmental stage: 88 day°. (D) Transverse section showing a few type II collagen-like fibrils (arrow), running parallel to the plane of section. The fibrils are located between the basal lamina and the outward layer of elastin and microfibrils (el). Inset: higher magnification of elastin that is cross-linked at regular intervals. Developmental stage: 100 day° (E) Longitudinal section of the sheath, where the evenly disposed collagen fibrils appear in cross-section, reflecting a circumferential arrangement. Outward the collagen (col) is covered by a network of elastin (el). Developmental stage: 170 day°. (F) Transverse section of the sheath where the collagen fibrils (col) are parallel to the plane of section, thus running circumferentially. Note the growth in thickness of the collagenous layer and the increased density of the elastica externa (el) when compared with E. Basal lamina is indicated by an arrow. Developmental stage: 220 day°. (G) FESEM image that shows the elastica externa (el). The membrane has been split into two, revealing its homogeneous structure presumed to be made up of tightly packed elastin. Single fibrils can be discerned on the split surface and along the edge of the torn membrane (top inset). The elastica externa stains black with Verhoff's method (bottom inset) Developmental stage: 170 day°. Scale bars; (A,B) 50 µm; (C) 0.5 µm; (C insert) 0.2 µm; (D) 0.1 µm; (E,G) 1 µm; (G insert) 2 µm.
Initially the collagen has a circumferential configuration
The total increase in thickness of the notochord sheath was mainly due to growth of the collagenous layer (Fig. 3D–F) that had a matrix with evenly disposed collagen fibrils (Fig. 3E,F). Collagen secretion started during the single-cell-diameter column stage (100 day°), when single fibrils accumulated between the previously deposited elastica externa and the basal lamina (Fig. 3D). The collagen had a circumferential arrangement along the periphery of the discoid chordoblasts, which appear to form an ideal structure to dispose collagen fibrils circumferentially into the sheath. The collagen fibrils thus formed circles, or partial loops, that were orientated perpendicular to the cranio-caudal axis (Fig. 3E,F). This specific spatial fibril architecture was present until hatching, which occurred at approximately 500 day°.
Elastica externa accumulates gradually to form a continuous and homogeneous outer membrane
Initially, the elastin-like material was organized as an even layer of electron-dense foci that were deposited in a scaffold of microfibrils (Fig. 3C,D). As the membrane grew in thickness, the electron-dense aggregates gradually increased in size to form a continuous homogeneous outer membrane (Fig. 3G). During the period until the elastica externa attained a continuous lamellar structure, the material had a high electron density, and when the elastic membrane was fully formed the electron density decreased to that typical for elastic fibres and lamellar elastin (Fig. 4A). Both surfaces of the membrane were serrated and interconnected outwardly to the thick collagen fibrils of the mesenchymal connective tissue surrounding the sheath, and inwardly to the thin collagen fibrils of the sheath (Fig. 4A–C). At all stages the membrane had an even thickness in all regions, and when mineralization of the chordacentra started, the thickness was approximately 1 µm.
Fig 4.
The connection between the elastica externa and adjacent collagen. (A) TEM image from a longitudinal section of the elastica externa (el), showing the serrated outward and inward surfaces of the membrane that provides a large surface for attachment of collagen. On the external surface (top of image) larger type I collagen fibrils (col I) of the connective tissue surrounding the notochord attach, while on the inward surface the type II collagen (col II) of the sheath inserts. Note the homogeneous structure of the elastica externa, where neither single elastin fibres nor microfibrillar projections can be discerned. Developmental stage: 780 day°. (B,C) FESEM images. (B) The outward serrated surface of the elastica externa (el), where connective tissue type I collagen has been torn away. Developmental stage: 780 day°. (C) Oblique cut through the elastica externa (el) and surrounding collagen layers. Note the insertion of type II collagen fibrils (col II) of the sheath and type I collagen fibres (col I) of the surrounding connective tissue (to the right). Developmental stage: 780 day°. Scale bars (A) 0.5 µm; (B,C) 2 µm.
A shift in the collagen-winding architecture occurs after hatching
During the first phase of the alevin stage (500–630 day°), in the period from hatching to chordacentra formation, a shift in the spatial arrangement of fibrils in the collagenous layer of the sheath took place. From an initial arrangement of concentric loops around the notochord, the collagen fibrils became reorganized into distinct layers or lamellae (Fig. 5A–G). Within each lamella fibrils were parallel to each other, forming helices or coils around the longitudinal axis of the notochord, with a tangent angle of 75–80° to the cranio-caudal axis (Fig. 5E,F). The helical geometry shifted between adjacent lamellae, forming enantiomorphous left- and right-handed coils, respectively. When the chordacentra had formed, towards the end of the alevin stage, a series of six lamellae could usually be discerned (Fig. 5A, C, D).
Fig 5.
Lamellar arrangement of the collagenous layer of the notochord sheath. (A) Methacrylate-embedded tissue stained with toluidine blue viewed using DIC optics. This type of illumination reveals, through refraction, the lamellar structure of the sheath, which appears as an alternating pattern of blue and red. This phenomenon could not be observed in the transverse plane, which is probably due to the angle of the collagen winding, where the collagen fibres are cut at highly different angles in the transverse vs. longitudinal plane. The collagen lamellae are embedded in a chordacentrum (cc) and radiate into the intervertebral region of the sheath (iv). Usually six lamellae may be discerned. The arrow indicates the border between the chordacentrum and the intervertebral region. Vertebral bone (vb). Developmental stage: 1050 day°. (B–F) FESEM images. (B) Transverse section of the sheath revealing its lamellar structure. Chordoblasts (cb) appear in the lower left corner, while the elastica externa (el) delimitates the sheath, and is seen towards the upper right corner. Developmental stage: 1050 day°. (C) Transverse section showing the lamellae. Elastica externa (el) and chordoblasts (cb). Developmental stage: 1050 day°. (D) Transverse section revealing the lamellar structure of the sheath. Developmental stage: 1050 day°. (E) External surface of the notochord where collagen fibrils of the outer lamella are visible through a fissure that has appeared during tissue processing, revealing the fibre-winding angle of approximately 75–80° relative to the cranial–caudal axis. Inset: higher magnification beneath the fissure. el: elastica interna. Developmental stage: 1050 day°. (F) Surface of the elastica externa (el). The collagen fibrils of the outer lamella are visible through a rupture of the membrane. Developmental stage: 1050 day°. (G) TEM image showing different fibre angles within the collagen layer of the sheath. Developmental stage: 1050 day°. Scale bars (A,B) 40 µm; (C) 50 µm; (D–F) 1 µm; (E inset) 0.25 µm; (G) 1 µm.
The lamellae are embedded in the chordacentra and form the major part of the intervertebral ligament
In the period between 630 and 700 day°, segmentally arranged mineralized chordacentra appeared as demarcated compartments in the outer half of the sheath. The process of mineralization started within the collagenous layer, immediately beneath the elastica externa, as described by Grotmol et al. (2003). Gradually, after formation of the initial mineralized compartment, mineralization proceeded inwardly, at a slower pace, finally to include the whole thickness of the collagenous layer; all the collagen lamellae thus became embedded in the chordacentra (Fig. 5A). In the intervertebral region the individual collagen lamellae, and thus the overall sheath, increased considerably in thickness, forming a ligament in which the collagen fibrils of successive lamellae crossed the joint obliquely in opposite directions, interconnecting adjacent chordacentra (Fig. 5A).
The chordacentra developed asymmetrically relative to the myosepta
The chordacentra formed caudal to the myoseptal midline (Fig. 6A). As they increased their cranio-caudal length, the myoseptum became aligned with the cranial portion of the chordacentra.
Fig 6.
Relationship between the myosepta and the notochord sheath. (A,C–F) Methacrylate-embedded tissue stained with toluidine blue. (A) A horizontal section of the notochord with adjacent myotomes (mt). The myosepta (arrowhead) are thickest at the intersection with the notochord, where they radiate and gradually decrease in thickness towards the ventral branch of the spinal nerves (sn), and end (arrows). The chordacentra (cc) are formed where the myosepta meet the notochord. Interestingly, note that the chordacentra are asymmetrically positioned in relation to the myosepta, aligned with the caudal radiation of the myosepta. This is illustrated by a line drawn through a myoseptum and its position relative to the adjacent chordacentrum, which is coloured. iv: inter-vertebral ligament. Developmental stage: 725 day°. (B) Whole mount stained with Alcian blue showing the paired arcualia (neural and haemal arch cartilages). The arcualia are situated directly on the elastica externa, which is seen as a dark line delimiting the notochord. Developmental stage: 410 day°. (C–E) Formation of the arcualia. (C) Mesenchymal condensations where the myosept meet the notochord. Developmental stage: 410 day°. (D) The mesenchymal cells develop chondroblast-like morphology, and initiate secretion of an extracellular matrix. Developmental stage: 390 day°. (E) The arcualium is formed in hyaline cartilage. Developmental stage: 460 day°. (F) Relationship between the proximal part of the arcualian cartilage and the notochord. The chondroblasts rest directly on the elastica externa. Both the arculalia and the part of the notochord adjacent to the cartilage are surrounded by the connective tissue of the myoseptum. Developmental stage: 560 day°. (G,H) TEM images of the border between the cartilage matrix (cm) of the arcualia (top of image) and the elastica externa (el) of the notochord. Developmental stage: 760 day°. (G) The type II collagen of the cartilage inserts directly on the elastica externa. (H) Detail showing the serrated surface of the elastic membrane where it connects to the matrix of the cartilage. Scale bars (A) 100 µm; (B) 300 µm; (C,D) 15 µm; (E) 10 µm; (F) 10 µm; (G) 1 µm; (H) 0.5 µm.
The notochord is surrounded by the mesenchymal connective tissue of the myosepta, wherein the arcualian cartilages develop
Surrounding the notochord, hence outward of the elastica externa, a connective tissue containing fibroblasts developed (Fig. 6A). The fibres were typical of type I collagen, with diameters in the range 0.1–0.6 µm, and were anchored to the outward serrated surface of the elastic membrane (Fig. 4A). The connective tissue was thickest and densest in the intermyotomal region, where it formed a continuum with the myosepta, while tapering towards the mid-myotomal region (Fig. 6A). Within the vertical axis of the myosepta, the dorsal and ventral pairs of arcualia (neural and haemal arch rudiments) were formed (Fig. 6B) through mesenchymal condensations that developed into hyaline cartilage (Fig. 6C–E).
The arcualia were situated directly on the dorso- and ventro-lateral surface of the elastica externa of the notochord sheath (Fig. 6B,F); and the cartilage collagen, at the proximal ends of the arcualia, was closely interconnected with the serrated outward surface of the elastic membrane (Fig. 6G,H) in a similar manner as the thick collagen of the myoseptum.
For clarity, the main stages of the development of the notochord sheath are summarized in Fig. 7.
Fig 7.
Schematic illustrations of the development of the notochord sheath. In all drawings the cellular core is in light grey, the basal lamina grey and the elastic membrane blue. (A) The structure of the sheath with circumferential collagen fibres (in red), as seen during the embryonic stage. (B) After hatching the configuration of collagen becomes lamellar (in pink and turquoise). (C) Fry stage, after the chordacentra have been formed. The lamellae embedded within the chordacentra are shown in darker shades. Intervertebral regions are shown in lighter shades. For clarity, the thickness of the sheath is significantly exaggerated.
Discussion
This survey of the morphogenesis of the salmon notochord shows that the notochord itself develops into a functional vertebral column through stepwise enforcement of its sheath. The results raise intriguing and fundamental questions on structure–function relationships, and may elucidate the evolution of the axial skeleton as discussed below.
Elastica externa: a force transmitter?
In an array of vertebrates the collagen of the notochord sheath has been shown mainly to be of type II (Bruns & Gross, 1970; Eikenberry et al. 1984; Brodsky et al. 1994; Sandell, 1994; Domowicz et al. 1995; Golz et al. 1995; Ng et al. 1997; Hayes et al. 2001), and the phenotype of the thin fibres present in salmon complies with that of this collagen type (Kielty & Grant, 2002). The elastica externa (Cole, 1905; Goodrich, 1930), with its membranous morphology and staining properties, complies with that of lamellar elastin, for instance as found in the internal elastic lamina of arteries (Cross & Mercer, 1993). During the presumptive elastogenesis in salmon, the electron-dense aggregates probably represent elastin surrounded by other proteins, such as fibrillin and fibulin, which are major constituents of microfibrils. At an early stage of notochord development in several vertebrates, both fibrillin and fibulin have been found to accumulate around the notochord (Visconti et al. 2003; Skoglund et al. 2006); these findings, however, have not been related to the morphogenesis of the elastica externa. Furthermore, in zebrafish, the protein calymmin is transiently expressed by the notochord from 13 to 24 h post-fertilization, and has been detected in an outer compartment of the notochord sheath (Cerdà et al. 2002), a layer with the same morphological features as the elastica externa observed here at an early stage in salmon (see Cerdà et al. 2002, fig. 6). Thus, we suggest that fibrillin, fibulin and calymmin are probably proteins associated with the elastica externa during morphogenesis, and may have functions related to assembly of elastic fibres, i.e. cross-linking of elastin. It is also interesting that the Xenopus notochord expresses, during early development, a lysyl oxidase (Geach & Dale, 2005), an enzyme that catalyses covalent cross-linking of collagen and elastic fibres in the extracellular matrix (Kagan & Li, 2003). Hence, this enzyme may play a role in linking type I and type II collagen to the outward and inward serrated surfaces of the elastica externa, respectively, which may facilitate force transmission between myosepta and notochord.
Is the morphogenesis of the sheath dependent on an intact basal lamina?
The basal lamina of the salmon notochord showed a typical morphology. In accordance with current interpretations, the inner lucent region is mainly made up of integrins, some of which bind laminin that spans the lucent region. Laminin also forms a major constituent of the filamentous electron-dense region, together with type IV collagen, entactin and an array of heparin sulphate proteoglycans (Hassell et al. 1980; Yurchenco & Ruben, 1987; Durkin et al. 1988; Mann et al. 1989; Gupta et al. 1997; Yasothornsrikul et al. 1997). In images from zebrafish wild-type embryos of 28 h post-fertilization, the three initial layers of the notochord sheath are present, but these have not been delineated individually (Parsons et al. 2002; Stemple, 2005). The sheath, as a whole, is described as a ‘perinotochordal basement membrane’, and our interpretation of this structure is that it is equivalent to that seen in salmon. Thus, the inner layer observed in the zebrafish represents the basal lamina, the middle layer represents the collagenous portion of the sheath and the outer layer represents elastin. In zebrafish laminin mutants, sleepy and grumpy, formation of the basal lamina is disrupted, and this seems to result in disorganization of the collagenous and elastic layers. Despite probable secretion of both type II collagen and elastin, the absence of a functional basal lamina leads to disordered accumulation of these compounds. Normal sheath organization therefore seems to depend on laminin, and, in extension, an intact basal lamina. Although the zebrafish coatomer mutants sneezy, happy and dopey appear to secrete a normal basal lamina, these mutants have a phenotype where the collagen and elastin is disorganized, similar to that of the laminin mutants (Coutinho et al. 2004; Stemple, 2005). This may reflect failure in the intracellular processing of type II collagen and elastin, so that these are not functional when secreted, resulting in disruption of sheath formation.
Does enforcement of the sheath reflect the evolution of the locomotorsystem?
Selective pressure towards improved swimming performance has resulted in evolution of the teleost locomotor system that includes a large segmented musculature with complex anatomical architecture, attached to an axial skeleton, which has attained specialized structures to provide multisegmental force transmission and resist the tensile and compressive forces generated by muscle contractions (Gemballa et al. 2003a,b; Donley et al. 2004). The ontogenetic development of the axial skeleton in salmon may shed light upon its evolution. A persistent notochord is a primitive character found in early gnathostomes (Janvier, 1996) and during ontogenesis, the salmon larva passes through a similar developmental stage, where the notochord constitutes the sole axial support. Early in this phase, the elastica externa, due to probable cross-linking with the collagen on both sides, may act as an interface for transmission of tensile forces between the collagen of myosepta and notochord, so that myotomal contractions generate bending moments. The radiation of the collagen fibres of the myosepta in the vicinity of the notochord provides a broad region of insertion to a peri-myoseptal segment of the notochord (see Fig. 7A), which may be essential to prevent kinking. In addition, this medial muscular attachment, as seen in the salmon larva, has similarities to that seen in, for instance, lamprey (Vogel & Gemballa, 2000), and may also reflect a primitive vertebrate condition.
In salmon, the growth of the notochord, and the parallel increase in sheath thickness and formation of collagen lamellae, before the chordacentra appear as the initial part of the vertebrae, probably reflects a series of evolutionary events promoting the ability of the notochord to transfer muscle forces. The persistence of the notochord as the sole axial skeleton ceases early in ontogeny, and vertebrae are formed as further enforcement. The appearance of chordacentra in salmon (Grotmol et al. 2003) as mineralized segmental enforcements of the notochord sheath, and subsequent deposition of vertebral bone around them, probably reflects an evolutionary transition that proved selectively advantageous compared with enlargement of a persistent notochord alone. To achieve nucleation of vertebra by the forming of chordacentra, segmental activity within the notochord probably has evolved (Fleming et al. 2004); indeed, in salmon, this may be reflected by the segmentally arranged notochord cells (chordoblasts) that express ALP, and thus with osteoblast-like properties, which seem to initiate the mineralization within the sheath (Grotmol et al. 2005). The process of vertebral development, initially orchestrated by the notochord, may thus be looked upon as a result of an evolutionary process that has provided dispersion and stabilization of tensile forces from the myosepta acting along the cranio-caudal axis of the notochord.
Interestingly, the chordacentra of salmon are not situated symmetrically relative to the myosepta, but take a more caudal position so that the myosepta traverse their cranial part. This offset positioning of the vertebra supports the notion that the segmentation of the vertebral column is derived from an embryonic metameric activity within the notochord, as mentioned above, rather than from a symmetrical division and de novo fusion of the somite halves, as implicit in the ‘resegmentation theory’ (Christ et al. 2000, 2004). In the adult teleost vertebra, the myoseptum attaches on the cranial end of the vertebral body, and our results indicate that this localization of the myoseptum is already established when the chordacentra form, coinciding with the ontogenetic appearance of W-shaped myomeres.
Does the notochord seek help from the outside before locking itself in?
In teleosts, the barrier imposed by the chordacentrum prevents further expansion of the notochord and its sheath (Nordvik et al. 2005). The capacity of notochord cells to produce extracellular matrix that contributes to growth and reinforcement of vertebrae may therefore be restricted. Further growth of the axial skeleton is achieved by recruitment of cells from the adjacent somite-derived sclerotomes, and in salmon these start depositing vertebral bone around the notochord shortly after chordacentrum formation (Grotmol et al. 2003, 2005).
The initial secretion of sonic hedgehog (SHH) from the notochord is essential for the survival of the early-stage somite (Johnson et al. 1994; Marti et al. 1995; Chiang et al. 1996; Teillet et al. 1998); and, because somites are more primitive structures than vertebrae, this may have been one of the initial roles of SHH secreted by the notochord, itself already evolved before the emergence of the vertebrates. Intriguingly, in extant vertebrates such as zebrafish, chick and mice, which represent crown groups of the actinopterygian and sarcopterygian lineages, SHH together with Noggin synergize in sclerotome induction by activation of Pax1, which is a key event, triggering sclerotome differentiation (for a review, see Monsoro-Burq, 2005). This process occurs later in somitogenesis, thus probably representing a more advanced character than that related to somite survival. This ability of SHH to induce the sclerotomes may have evolved after, or in parallel with, the still occult metameric genetic signalling pathways within the notochord that position the chordacentra/vertebrae along the cranio-caudal axis, adjacent to the myosepta. Hence, the somites have constituted a pre-adaptation from where cells could be recruited in a near-symmetrical manner around the myosepta, giving the advantage of external reinforcement of the chordacentrum with cartilage/bone. In addition, the type II collagen, as found in the notochord sheath, may promote chondrogenesis in adjacent mesoderm (for a review, see Hall, 2005), and this may also be interpreted as a result of an evolutionary process favouring reinforcement of the sheath by depositing more of this collagen type to make way for the cartilaginous endoskeleton.
Did type II collagen originate as an epithelial protein – not as the structural protein of cartilage?
Cartilaginous extracellular matrix with type II collagen is thought to be chordate-specific, although tissue types with similar histological morphology are found in several invertebrates (Cole & Hall, 2004a,b). In chordates, a number of epithelial/epitheloid tissues also secrete this collagen. The notochord may be considered as an epithelial structure with a thick sheath derived from a basement membrane; similarly, the neuroepithelium of the neural tube secretes basement membrane-associated type II collagen (Cohen & Hay, 1971). Type II collagen and SHH from both these epithelial tissues contribute to the induction of vertebral cartilage from the somitic mesoderm (for a review, see Hall, 2005); and here, taking into account the epithelial nature of the secretor, the role of type II collagen may be considered as a component of an epithelial–mesenchymal interaction promoting chondrogenesis. Interestingly, a number of other epithelia also secrete type II collagen to adjacent basement membrane-associated extracellular matrix; in early embryogenesis, type II collagen is transiently secreted by ectodermal cells within the otic (Van De Water & Galinovic-Schwartz, 1987; Goodyear & Richardson, 2002) and optic vesicles (Newsome et al. 1976), by the brain vesicles and olfactory conchi, in addition to epithelia of the visceral arches (Wood et al. 1991). Some of these loci are sites of epithelial–mesenchymal interactions believed to generate component parts of the chondrocranium; here, as for the notochord and the neural tube, epithelially derived type II collagen appears in advance of overt chondrogenesis in the mesenchyme, and may also here play an inductive role (Wood et al. 1991). In a similar manner, epithelia of the inner ear secrete type II collagen, and disruption of this expression adversely affects the morphogenesis of the inner ear (Van De Water & Galinovic-Schwartz, 1987). Furthermore, epithelial production of type II collagen is maintained, for instance by retina and lens into the vitreous body (Swann et al. 1972; Newsome et al. 1976), and by epithelia of the inner ear (Goodyear & Richardson, 2002). In cephalochordates, an acellular matrix, where type II collagen constitutes the main structural protein, supports the pharyngeal arches; the collagen is secreted by the pharyngeal epithelium (Rychel et al. 2005), cells that may also secrete type II collagen during embryogenesis in amniotes, as mentioned above (Wood et al. 1991).
In chordates, as indicated, a number of epithelia are sites of type II collagen secretion; hence, it may be that this molecule originated as an epithelial protein with a variety of structural functions, and later in evolution, the molecule at these sites attained inductive properties through epithelial–mesenchymal interactions, e.g. related to vertebral and craniofacial chondrogenesis (Wood et al. 1991).
Type II collagen is absent from cartilages of the extant agnathans (Wright et al. 1983; Robson et al. 2000), but nevertheless present in their notochords (Eikenberry et al. 1984; Welsch et al. 1998), which may imply that type II collagen became the predominant structural protein of hyaline cartilage first with the emergence of the gnathostomes. In addition, the notochord expresses many genes that are regarded as characteristic of cartilage, such as those that encode type IX and X collagen, aggregan, Sox9 and chondromodulin (Linsenmayer et al. 1986; Domowicz et al. 1995; Ng et al. 1997; Zhao et al. 1997; Dietz et al. 1999; Sachdev et al. 2001). These, together with type II collagen, may have appeared early in chordate evolution, possibly associated with basement membrane-derived structures such as the notochord sheath rather than hyaline cartilage.
The lamellae: an enforcement of the sheath after hatching, but also a pre-adaptation giving torsional stability to the intervertebral joint
In the period from hatching to chordacentra formation, a novel and highly ordered architecture appears, made up of multiple lamellae of helically wound collagen with a fibre angle of approximately 75° relative to the long axis. Concomitant with the reconfiguration of the sheath, its thickness increases substantially, and it is unclear whether, during this process, the initial circumferentially orientated collagen becomes displaced to form a thin layer along the periphery or whether it is reorganized.
What is the significance of this profound shift in fibre configuration? At hatching, the body axis straightens and increased swimming activity occurs. Hence, the reconfiguration may be an integral part of this process, where increased flexural stiffness and elastic recoil may be advantageous in creating thrust during swimming. Moreover, with the increased compressive load imposed by the axial muscles, the notochord needs to resist kinking and buckling, while simultaneously promoting the sinusoidal conformation that is associated with undulatory swimming. Although not elucidated in detailed functional terms, the lamellar cross-helical architecture of the sheath probably contributes to the above-mentioned notochordal properties.
The segment of the notochord sheath in the mid-myotomal region may have constituted a pre-adaptation for the intervertebral joint. In salmon, when the vertebrae initially form as chordacentra (Grotmol et al. 2003), the collagen lamellae of the sheath in the peri-myoseptal regions are fixed by the deposition of minerals; in the mid-myotomal region, however, they persist and form a major component of the intervertebral ligament. Thus, in a similar manner to the annulus fibrosus of mammals, the collagen fibrils of the lamella form overlapping transversing bands, obliquely crossing the joint in opposite directions, thus stabilizing intervertebral torsion by preventing rotation.
The arcualia: sesamoids within the myoseptum that transmit force from the vertical axis upon the notochord?
In salmon, the two pairs of arcualia are inserted directly on the elastica externa of the notochord sheath, a condition also seen in primitive vertebrates, such as lamprey. The arcualia, to which the myosepta attach along their entire vertical length, may be viewed as evolutionary devices within the vertical line of the myoseptum to transmit load from a steadily enlarging mass of muscle towards the notochord, and thereby along the horizontal midline axis of the animal. Their initial appearance in pairs within the myosepta, not joining apically, and their tight interconnection with the notochord, probably reflect their initial function as force transmitters, rather than protective structures for the neural tube. Thus, the arcualium may be regarded as a cartilage that is formed in a tendinous structure – the myoseptum – conferring mechanical advantage like a sesamoid evolved in response to selection for increased swimming speed, acceleration and manoeuvrability.
How does the configuration of fibre winding relate to functional properties upon notochord inflation?
Current views of the functional anatomy of the notochord are based on two main sources: one dealing with the structure and function of the Xenopus notochord (Adams et al. 1990); and a second study, based on the results of the former, that employed physical inflatable models to mimic notochord hydroskeletal properties experimentally (Koehl et al. 2000).
Adams et al. (1990) dissected whole notochords for observation in SEM and, in our experience, when doing so, part of the mesenchymal connective tissue surrounding the notochord will remain on the surface, as the collagen of the connective tissue is tightly anchored to the elastica externa. Although probably having other functions, these fibres may easily be interpreted as an integral part of the sheath, hence determining notochord hydroskeletal properties. Using the above-mentioned approach, Adams et al. (1990) have probably misattributed this layer of type I collagen as belonging to the sheath. The collagen being of type I is substantiated by the measured fibre diameters (0.2–0.4 µm), which are within the range of this type of collagen, far exceeding the diameter of type II collagen fibrils of notochords. Both in the amphibians (Waddington & Perry, 1962; Bruns & Gross, 1970) and in salmon, as described herein, the diameter of the collagen fibrils within the sheath has been measured to be ∼15 nm. Moreover, the fibres studied by Adams et al. (1990) formed a cluttered non-ordered meshwork, with fibres running in all angles with a calculated mean fibre angle of 54° relative to the cranial–caudal axis (as shown in their fig. 5). This clearly deviates from the highly organized concentric fibre arrangement within the notochord sheath, as earlier observed in amphibians (Waddington & Perry, 1962; Bruns & Gross, 1970), and now in salmon, by employing TEM. These TEM studies have also shown that the notochord sheath of amphibians consists of three layers: a basement membrane, circumferentially oriented collagen fibrils and outwardly an elastic membrane, a description that complies with that of the embryonic notochord of salmon as reported herein. Furthermore, as in salmon, these studies show the presence of a mesenchymal connective tissue, which comprises a layer of thicker fibres – presumably type I collagen – that connects to the notochord, and is continuous with the connective tissue of the myosepta. Hence, the type I collagen layer with a mean fibre angle of 54°, as observed by Adams et al. (1990), is unlikely to be a part of the notochord per se, and may thus be of no relevance to the mechanical properties of the notochord. As the type I collagen layer is continuous with the myosepta, we suggest that its probable function is to provide attachment to the notochord, and, through tightening and slacking, to allow efficient transmission of the oscillating power output from the myotomes during undulatory locomotion, as previously discussed.
Have studies employing artificial inflatable models indicated the functional properties of the notochord?
Based on the results of Adams et al. (1990), artificial physical inflatable curved models of the notochord, enforced by cross-helically wound inextensible fibres with initial fibre angles of between 34 and 63°, have been employed to mimic notochord mechanical properties (Koehl et al. 2000). This study concluded that a fibre angle of 54°, as observed by Adams et al. (1990), possessed favourable mechanical properties, among others permitting elongation and straightening when pressurized, in addition to resisting buckling. All of the simulated fibre angles are out of range compared with the type II collagen winding observed within the notochord sheath either of salmon (90° within the egg and 75° in the lava), described herein, or in amphibians (90°) (Waddington & Perry, 1962; Bruns & Gross, 1970). In addition, the models do not take into account the composite structure of the sheath, with both inextensible collagen and a highly distensible elastica externa. Probably, the elastin membrane, which inwardly is tightly linked to the layer of concentric type II collagen, has major functions related to flexural stiffness and the prevention of kinking and buckling. We argue therefore that the notochord simulations performed by Koehl et al. (2000) are of limited biological relevance, and that a re-examination of the functional anatomy of embryonic notochords is warranted.
Is elongation and straightening of the notochord upon inflation favourable?
The notochord model with a 54° fibre-winding angle straightens and elongates upon inflation (Koehl et al. 2000), but are these properties favourable to an embryo confined in an egg with a near linear longitudinal growth during the inflation period? Our measurement of embryonic growth in salmon clearly shows that longitudinal growth is not increased in the period when the notochord inflates. This complies with the circular arrangement of type II collagen, which probably only enables a restricted increase in girth upon inflation, not aiding elongation. Hence, during growth the notochord constitutes a supportive axis for the surrounding tissues, and probably, in a synchronized manner, elongates through growth at the same pace as the adjacent tissues. Moreover, in teleosts such as the Atlantic salmon, as in amphibian embryos, which all have spherical eggs, the embryo remains in a constant curved posture along the inner spherical surface of the egg wall. In salmon, for instance, the tip of the tail reaches the snout at hatching. Hence, no straightening occurs during these stages in vivo; indeed, to prevent excessive pressure between the embryo and the surrounding egg wall (zona radiata), it is essential that the notochord is not prone to straightening, but remains highly flexible when inflated. The concentric configuration of collagen, overlaid by an elastic membrane, may ensure this flexibility, simultaneously constituting a structural element that withstands longitudinal compression. In addition, the flexural stiffness of the notochord may be modulated independently during embryogenesis by regulation of osmotic pressure within the chordocyte vacuole, a mechanism that has yet to be studied. Finally, because it may be argued that elongation of the notochord upon inflation would physically induce changes in fibre angle, as is the case when setting a coil spring, the fact that no alteration in the fibre-winding pattern is observed in the salmon notochord after inflation substantiates the notion that inflation per se does not cause elongation.
In contrast to species that hatch in water, land-living vertebrates such as mammals and birds need skeletons with the ability to bear weight upon birth or hatching. Hence, vertebral development occurs during embryogenesis, and in this process partial embryonic straightening may be a prerequisite. The spacious oval egg of birds, and intrauterine development in mammals, render straightening possible, but whether notochord structure in these species is adapted to promote straightening, as compared with notochords of species with spherical eggs, remains to be elucidated.
Acknowledgments
This work was funded by the Research Council of Norway. We gratefully acknowledge the expert help of EWOS AS at Lønningdal, Norway, in rearing the salmon. Expert technical assistance was provided by T. Cieplinska and N. Ellingsen.
References
- Adams DS, Keller R, Koehl AR. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- Boeke J. Das Geldrollenstadium der Vertebraten-Chorda und des Skelettes der Mundcirren von Branchiostoma lanceolatum, und seine cytomechanische Bedeutung. Anat Anz. 1908;33:541–556. 574–580. [Google Scholar]
- Brodsky B, Bel, Bruno KC, Hardt TA, Eikenberry EF. Collagen fibril structure in lamprey. J Mol Biol. 1994;243:38–47. doi: 10.1006/jmbi.1994.1628. [DOI] [PubMed] [Google Scholar]
- Bruns RR, Gross J. Studies on the tadpole tail. I. Structure and organization of the notochord and its covering layers in Rana catesbeiana. Am J Anat. 1970;128:193–224. doi: 10.1002/aja.1001280206. [DOI] [PubMed] [Google Scholar]
- Cerdà J, Gründ C, Franke WW, Brand M. Molecular characterization of Calymmin, a novel notochord sheath-associated extracellular matrix protein in the zebrafish embryo. Dev Dyn. 2002;224:200–209. doi: 10.1002/dvdy.10101. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, et al. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Wilting J. The development of the avian vertebral column. Anat Embryol. 2000;202:179–194. doi: 10.1007/s004290000114. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M. Formation and differentiation of the avian sclerotome. Anat Embryol. 2004;208:333–350. doi: 10.1007/s00429-004-0408-z. [DOI] [PubMed] [Google Scholar]
- Cohen AM, Hay ED. Secretion of collagen by embryonic neuroepithelium at the time of spinal cord–somite interaction. Devel Biol. 1971;26:578–605. doi: 10.1016/0012-1606(71)90142-4. [DOI] [PubMed] [Google Scholar]
- Cole AG, Hall BK. The nature and significance of invertebrate cartilages revisited: distribution and histology of cartilages and cartilage-like tissues within the Metazoa. Zoology. 2004a;107:261–273. doi: 10.1016/j.zool.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Cole AG, Hall BK. Cartilage is a metazoan tissue; integrating data from nonvertebrate sources. Acta Zool. 2004b;85:69–80. [Google Scholar]
- Cole FJ. A monograph on the general morphology of the myxinoid fishes, based on a study of Myxine. I. The anatomy of the skeleton. Trans Roy Soc Edinb. 1905;41:749–788. [Google Scholar]
- Coutinho P, Parsons MJ, Thomas KA, et al. Differential requirements for COPI transport during vertebrate early development. Dev Cell. 2004;7:547–558. doi: 10.1016/j.devcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Cross PC, Mercer KL. Cell and Tissue Ultrastructurea Functional Perspective. New York: W.H. Freeman; 1993. [Google Scholar]
- Dietz UH, Ziegelmeier G, Bittner K, Bruckner P, Balling R. Spatio-temporal distribution of chondromodulin-I mRNA in the chicken embryo: expression during cartilage development and formation of the heart and eye. Dev Dyn. 1999;216:233–243. doi: 10.1002/(SICI)1097-0177(199911)216:3<233::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dill PA. Development of behaviour in alevins of Atlantic salmon (Salmo salar) and rainbow trout (S. gairdneri) Anim Behav. 1977;25:116–121. [Google Scholar]
- Domowicz M, Li H, Hennig A, Henry J, Vertel BM, Schwartz NB. The biochemically and immunologically distinct CSPG of notochord is a product of the aggrecan gene. Dev Biol. 1995;172:655–664. doi: 10.1006/dbio.1995.1312. [DOI] [PubMed] [Google Scholar]
- Donley JM, Sepulveda CA, Konstantinidis P, Gemballa S, Shadwick RE. Convergent evolution in mechanical design of lamnid sharks and tunas. Nature. 2004;429:61–65. doi: 10.1038/nature02435. [DOI] [PubMed] [Google Scholar]
- Durkin ME, Chakravarti S, Baros BB, Liu SH, Friedman RL, Chung AE. Amino acid sequence and domain structure of entactin. Homology with epidermal growth factor precursor and low density lipoprotein receptor. J Cell Biol. 1988;107:2749–2756. doi: 10.1083/jcb.107.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenberry EF, Childs B, Sheren SB, Parry DA, Craig AS. Crystalline fibrils structure of type II collagen in lamprey notochord sheath. J Mol Biol. 1984;176:261–277. doi: 10.1016/0022-2836(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Fleming A, Keynes RJ, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–880. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- Geach TJ, Dale L. Members of the lysyl oxidase family are expressed during the development of the frog Xenopus laevis. Differentiation. 2005;73:414–424. doi: 10.1111/j.1432-0436.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- Gemballa S, Ebmayer L, Hagen K, et al. Evolutionary transformations of myoseptal tendons in gnathostomes. Proc Roy Soc Lond B. 2003a;270:1229–1235. doi: 10.1098/rspb.2003.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemballa S, Weitbrecht GW, Sànchez-Villagra MR. The myosepta in Branchiostoma lanceolatum (Cephalochordata): 3D reconstruction and microanatomy. Zoomorphology. 2003b;122:169–179. [Google Scholar]
- Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–887. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates. London: Macmillan; 1930. [Google Scholar]
- Goodyear RJ, Richardson GP. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J Neurobiol. 2002;52:212–227. doi: 10.1002/neu.10097. [DOI] [PubMed] [Google Scholar]
- Gotz W, Osmers R, Herken R. Localisation of extracellular matrix components in the embryonic human notochord and axial mesenchyme. J Anat. 1995;186:111–121. [PMC free article] [PubMed] [Google Scholar]
- Grotmol S, Kryvi H, Nordvik K, Totland GK. Notochord segmentation may lay the pathway for the development of the vertebral bodies of the Atlantic salmon Salmo salar. Anat Embryol. 2003;207:263–272. doi: 10.1007/s00429-003-0349-y. [DOI] [PubMed] [Google Scholar]
- Grotmol S, Kryvi H, Nordvik K, Totland GK. A segmental pattern of alkaline phosphatase (ALP) activity within the notochord coincides with the initial formation of the vertebral bodies. J Anat. 2005;206:427–436. doi: 10.1111/j.1469-7580.2005.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MC, Graham PL, Kramer JM. Characterization of alpha (IV) collagen mutations in Caenohabditis elegans and the effects of alpha1 and alpha2 (IV) mutations on type IV collagen distribution. J Cell Biol. 1997;137:1185–1196. doi: 10.1083/jcb.137.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK. Chondrogenesis of the somitic mesoderm. Adv Anat Embryol Cell Biol. 1977;53:1–50. [PubMed] [Google Scholar]
- Hall BK. Bone and CartilageDevelopmental and Evolutionary Skeletal Biology. Amsterdam: Elsevier/Academic Press; 2005. [Google Scholar]
- Hassell JR, Robey PG, Barrach HJ, Wilczek J, Rennard SI, Martin GR. Isolation of a heparin sulphate-containing proteoglycans from basement membrane. Proc Natl Acad Sci USA. 1980;77:4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausen P, Riebesell M. The Early Development of Xenopus Laevisan Atlas of the Histology. Berlin: Springer; 1991. [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in the development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Holtzer H. An experimental analysis of the development of the spinal column. II. The dispensability of the notochord. J Exp Zool. 1952;121:573–591. [Google Scholar]
- Holtzer H, Detwiler SR. An experimental analysis of the development of the spinal column. III. Induction of skeletogenous cells. J Exp Zool. 1953;123:335–366. [Google Scholar]
- Janvier P. Early Vertebrates. Oxford: Clarendon Press; 1996. [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of sonic hedgehog alters dorsal–ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Mechanisms of vertebrate segmentation. Development. 1988;103:413–429. doi: 10.1242/dev.103.3.413. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Grant ME. Connective Tissue and its Heritable DisordersMolecular, Genetic, and Medical Aspects. New York: Wiley-Liss; 2002. The collagen family: structure, assembly, and organization in the extracellular matrix; pp. 159–221. [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kitchin IC. Effects of notochordectomy in Amblystoma mexicanum. J Exp Zool. 1949;112:393–415. doi: 10.1002/jez.1401120303. [DOI] [PubMed] [Google Scholar]
- Koehl MAR, Quillin KJ, Pell CA. Mechanical design of fiber-wound hydraulic skeletons: the stiffening and straightening of embryonic notochords. Am Zool. 2000;40:28–41. [Google Scholar]
- Linsenmayer TF, Trelstad RL, Gross J. The collagen of chick embryonic notochord. Biochem Biophys Res Commun. 1973;53:39–45. doi: 10.1016/0006-291x(73)91397-1. [DOI] [PubMed] [Google Scholar]
- Linsenmayer TF, Gibney E, Schmid TM. Segmental appearance of type X collagen in the developing avian notochord. Dev Biol. 1986;113:467–473. doi: 10.1016/0012-1606(86)90182-x. [DOI] [PubMed] [Google Scholar]
- Mann K, Deutzmann R, Aumailley M, et al. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J. 1989;8:65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A-H. Sclerotome development and morphogenesis: when experimental embryology meets genetics. Int J Dev Biol. 2005;49:301–308. doi: 10.1387/ijdb.041953am. [DOI] [PubMed] [Google Scholar]
- Newsome DA, Linsenmayer TF, Trelstad RL. Vitreous body collagen. J Cell Biol. 1976;71:59–67. doi: 10.1083/jcb.71.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, et al. SOX9 Binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Nordvik K, Kryvi H, Totland GK, Grotmol S. The salmon vertebral body develops through mineralization of two preformed tissues that are encompassed by two layers of bone. J Anat. 2005;206:103–114. doi: 10.1111/j.1469-7580.2005.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Pollard SM, Saude L, Feldman B, Coutinho Hirst EMA, Stemple DL. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–3146. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- Pasteels J. Développement des agnathes. In: Grassé P, editor. Traité de Zoologie. Paris: Masson et Cie; 1958. pp. 106–144. 13, [Google Scholar]
- Robson P, Wright GM, Keeley FW. Distinct non-collagen based cartilages comprising the endoskeleton of the Atlantic hagfish, Myxine glutinosa. Anat Embryol. 2000;202:281–290. doi: 10.1007/s004290000113. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Smith SE, Shimamoto HT, Swalla BJ. Evolution and development of the chordates: collagen and pharyngeal cartilage. Mol Biol Evol. 2005;23:541–549. doi: 10.1093/molbev/msj055. [DOI] [PubMed] [Google Scholar]
- Sachdev SW, Dietz UH, Oshima Y, et al. Sequence analysis of zebrafish chondromodulin-1 and expression profile in the notochord and chondrogenic regions during cartilage morphogenesis. Mech Dev. 2001;105:157–162. doi: 10.1016/s0925-4773(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Sandell LJ. In situ expression of collagen and proteoglycan genes in notochord and during skeletal development and growth. Microsc Res Techn. 1994;28:470–482. doi: 10.1002/jemt.1070280603. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Dzamba B, Coffman CR, Harris WA, Keller R. Xenopus fibrillin is expressed in the organizer and is the earliest component of matrix at the developing notochord–somite boundary. Dev Dyn. 2006;235:1974–1983. doi: 10.1002/dvdy.20818. [DOI] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Strudel G. L’action morphogène du tube nerveux et de la corde sur la différenciation des vertèbres et des muscles vertebraux chez l’embryon de poulet. Arch Anat Micr Morph Exp. 1955;44:209–235. [PubMed] [Google Scholar]
- Swann DA, Constable IJ, Harper E. Vitreous structure. III. Composition of bovine vitreous collagen. Invest Ophthalmol. 1972;11:735–738. [PubMed] [Google Scholar]
- Tada M. Notochord morphogenesis: a prickly subject for Ascidians. Curr Biol. 2005;15:14–16. doi: 10.1016/j.cub.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Teillet MA, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM. Sonic hedgehog is required for survival of both myogenic and chondrogenic somatic linages. Development. 1998;125:2019–2030. doi: 10.1242/dev.125.11.2019. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Galinovic-Schwartz V. Collagen type II in the otic extracellular matrix effect on inner ear development. Hearing Res. 1987;30:39–47. doi: 10.1016/0378-5955(87)90181-x. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Barth JL, Keeley FW, Little CD. Codistribution analysis of elastin and related fibrillar proteins in early vertebrate development. Matrix Biol. 2003;22:109–121. doi: 10.1016/s0945-053x(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Vogel F, Gemballa S. Locomotory design of ‘cyclostome’ fishes: spatial arrangement and architecture of myosepta and lamellae. Acta Zool. 2000;81:267–283. [Google Scholar]
- Waddington CH, Perry MM. The ultrastructure of the developing urodele notochord. Proc Roy Soc B. 1962;156:459–482. [Google Scholar]
- Welsch U, Chiba A, Honma Y. The notochord. In: Jørgensen JM, Lomholt JP, Weber RE, Malte H, editors. The Biology of Hagfishes. London: Chapman & Hall; 1998. pp. 145–159. [Google Scholar]
- Wood A, Ashhurst DE, Corbett A, Thorogood P. The transient expression of type II collagen at tissue interfaces during mammalian craniofacial development. Development. 1991;111:955–968. doi: 10.1242/dev.111.4.955. [DOI] [PubMed] [Google Scholar]
- Wright GM, Keeley FW, Youson JH. Lamprin: a new vertebrate protein comprising the major structural protein of adult lamprey cartilage. Experientia. 1983;39:495–497. [Google Scholar]
- Yasothornsrikul S, Davis WL, Cramer G, Kimbrell SA, Dearolf CR. Viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997;198:17–25. doi: 10.1016/s0378-1119(97)00274-6. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Ruben GC. Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol. 1987;105:2559–2568. doi: 10.1083/jcb.105.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Eberspaecher H, Lefebvre V, Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. doi: 10.1002/(SICI)1097-0177(199708)209:4<377::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]