Abstract
Improved techniques for pancreatic islet extraction can yield a reasonable number of transplantable cells. However, the isolation and purification process may damage the islets and impair their physiological functions. The aim of this study was to determine the effect of the isolation procedure on the structure of isolated islets and to correlate this with their functionality. Islets were isolated from rat pancreata and purified by Eurocollins-Ficoll discontinuous density gradient processing, and then processed for light microscopy, and scanning and transmission electron microscopy. Morphometric analysis was also performed. Islet functionality was determined by reversal of streptozotocin-induced diabetes and the intraperitoneal glucose tolerance test in a syngeneic rat model of pancreatic islet transplantation. Fragments of variable size and shape comprised a relatively large proportion (26%) of the isolated endocrine tissue. Isolated islets showed slight alterations of cell ultrastructure. Major damage (including breakage of the plasma membrane) and loss of cells were observed in the peripheral cells of the isolated islets. An equal mass of islet equivalent (IEq, islets with an average diameter of 150 µm), but with a different islet equivalent/islet number ratio, was transplanted in diabetic animals. When larger and more complete islets were transplanted (higher ratio), better function of the graft was observed by reversal of hyperglycaemia and response to the glucose tolerance test as compared with the functionality and response of smaller (fragmented) islets transplanted (lower ratio). Digestion, trauma and hypoxia during isolation are responsible for qualitative and quantitative changes of isolated islets. Alterations in normal secretory function after the transplant were related to lower islet equivalent/islet number ratio. The incomplete integrity of the islets may explain the failure of the fine glycaemic metabolic regulation.
Keywords: islets of Langerhans, isolation, structure, transplant, ultrastructure
Introduction
Pancreatic islet transplantation is currently one of the most attractive strategies for the treatment of type I diabetes. This field, with its research and clinical applications, has been particularly active during the last decade, when major experimental findings were applied to the clinical setting (Hering & Ricordi, 1999). However, pancreatic islet transplantation has not been satisfactory as a potential treatment for autoimmune diabetes (Bretzel et al. 1996; Hering & Ricordi, 1999). Consistent reversal of diabetes in 12 consecutive patients receiving pancreatic islet transplantation has been reported, with a follow-up of > 24 months (Shapiro et al. 2000; Ryan et al. 2001). This experience has reinvigorated the field and has led to several innovative approaches being proposed, e.g. the use of multiple islet donors and a novel immunosuppressive strategy (Edmonton protocol). Results from more recent clinical trials are still very encouraging (CITR, 2005), especially when compared with trials conducted in the early 1990s. However, islet transplantation still presents major limitations that restrict its clinical application. The total number of islets infused in each patient (in the Edmonton protocol obtained from multiple donors, from two to four) seems to be a key factor in its success. Their experience has shown that a ‘critical mass’ is necessary to reverse diabetes in humans. Islet proliferation, generation, preservation during the isolation procedure and engraftment are crucial areas for investigation in order to achieve the critical mass and improve clinical success (Ryan et al. 2005). Moreover, the use of multiple donors appears to be a major limitation contributing even more to the dramatic donor/recipient disparity.
The current techniques for human islet isolation are difficult to perform, but it is well established that a human pancreas can provide a large number of viable islets. Many unpredictable variables can influence the outcome of human islet isolation, jeopardizing both the quantity and the quality of islets; and in order to improve outcome in clinical pancreatic islet isolation and transplantation, various factors should be investigated thoroughly during the process of islet retrieval, in order to gain as much information as possible. Ideally, improving the quantity and quality of islet preparations should lead to a successful 1 : 1 ratio of donor and recipient.
There is some evidence that pancreatic islets cannot be considered as aspecific aggregates of cells. Each islet should be considered as a microorganism with a complex and definite organization, and only intact islet architecture enables normal endocrine function (Pipeleers et al. 1982; Hopcroft et al. 1985; Baca et al. 1990). Several studies have emphasized the importance of the interactions among the different cell types (Trimble et al. 1982; Jörns, 1994), and also between cells of the same type (Meda et al. 1980; Jörns, 1994), for optimal regulation of insular hormonal secretion and metabolic control.
Islet isolation and purification techniques have improved over the years (Ricordi & Rastellini, 1995; Linetsky et al. 1997), with the aim of enabling the largest and purest collection of viable islets to be obtained. Assessments of the number, volume and purity of isolated islets have been widely reported (Gray et al. 1984; Warnock et al. 1988; Delaby et al. 1989; Ricordi et al. 1990), but relatively few studies have focused on morphological investigations (Tahir et al. 1992; El-Naggar et al. 1993).
Comparing the data from allo- and auto-transplantation cases, it is clear that the immunological component is the most important factor in the failure of the establishment and maintenance of insulin independence (Rastellini et al. 1997; Hering & Ricordi, 1999). Nevertheless, the structural alterations that can affect both the entire islet and the single cell during the isolation and purification processes may be an important factor leading to the loss of the physiological secretory function.
The aim of this study was to detect the alterations affecting the structure and ultrastructure of isolated islets and to correlate them to functionality, in order to investigate the morphological basis related to potential post-transplant functional damage.
Materials and methods
Animals
Thirty-six male adult (∼12 weeks old) Lewis rats, weighing approximately 200 g, were used as pancreas (pancreatic islets) donors; six more animals of the same age were used as controls for the observation of intact islets within the native pancreas. Animals were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), housed in a standard animal facility, and provided with Purina rodent chow and tap water ad libitum.
Pancreas procurement
Under Metofane- (methoxyflurane, Pittman-Moore, Inc., Munderlein, IL, USA) induced anesthesia, a midline abdominal incision of the donor was performed. After cannulation of the pancreatic duct, cold (4 °C) collagenase (0.7 mg mL−1; Crescent Chemical Co., Inc., Hauppange, NY, USA) solution was injected. After adequate distention, the pancreas was harvested and stored on ice.
Islet isolation and purification
Islets were isolated by a modification of the automated method described by Ricordi & Rastellini (1995). Briefly, the harvested pancreata were loaded into a stainless steel digestion chamber and perfused with recirculating activated collagenase solution (flow rate 85 mL min−1). Microscopic examination of dithizone-stained samples obtained serially at 2-min intervals (starting at the 6th min of digestion) was used to monitor tissue digestion. With the appearance of dissociated intact islets, this process was stopped and the tissue was collected and washed (400g for 3 min). Islets were further purified by centrifugation (800 g for 10 min) on discontinuous density gradients (1.108, 1.096, 1.039). Washings after purification were performed at 800 g for 3 min and 400 g for 3 min.
Three isolations were performed for the entire study with 12 animals for each isolation.
Islet assessment
Islets were assessed in vitro for number, purity and viability by dithizone and trypan blue dye exclusion. Islet functionality was assessed by reversing chemically induced diabetes in vivo. Lewis rats (n = 6, ∼8 weeks of age) were rendered diabetic (blood glucose level > 300 mg dL−1 for three consecutive observations) by the use of Streptozotocin (STZ; Sigma, St Louis, MI, USA) injection (70 mg kg−1 i.v. on day −3). Approximately 1000 islets (IEq, islet equivalent: islets of an average diameter of 150 µm) were transplanted under the kidney capsule of each diabetic animal. The recipients' body weights and blood glucose levels were monitored daily. Transplanted islets were considered to have engrafted when blood glucose levels of < 200 mg dL−1 were attained and maintained.
In long-term normoglycaemic animals, islet graft functionality was assessed by intraperitoneal glucose tolerance tests (IPGTTs). Briefly, animals were fasted overnight, and following the detection of baseline blood glucose level, 2 g kg−1 body weight of glucose (in 0.5 mL of saline) was injected into the peritoneal cavity. Blood glucose level was then detected at 15, 30, 45, 60, 90 and 120 min after injection.
Light microscopy (LM)
The islets remaining from the transplantation were processed for morphological studies; 7266 IEq, corresponding to more than 5700 (real number) islets and coming from the three isolations, were used for LM and electron microscopy analysis.
Specimens from both isolated islets and native intact pancreata were fixed in 4% buffered formaldehyde solution for 24 h at room temperature, and routinely embedded in paraffin; 5-mm-thick sections were cut. Finally, specimens were stained with haematoxylin–eosin (HE), Masson's trichromic and Gomori's method for reticular fibres.
Some specimens were also embedded in (glycol-methacrylate) hydrophilic resin (Technovit® 7100, Heraeus Kulzer, Wehrheim, Germany) to obtain semithin sections. After fixation, specimens were dehydrated in alcohol; 2 h pre-infiltration (equal parts of ethanol 100% and resin) preceded the infiltration at room temperature for 24 h; after infiltration, the tissue was placed in embedding moulds containing embedding solution and hardener until the resin had polymerized (1 h at 37 °C); the embedding blocks were then mounted on holders; 1.5-mm semithin sections were cut with an LKB 2218 historange microtome and finally stained using HE.
Scanning electron microscopy (SEM)
The isolated tissue was pelleted by centrifugation and resuspended in 2.5% phosphate buffer (0.1 m, pH 7.4) glutaraldehyde solution for 2 h at 4 °C. A pellet of the isolated islets was obtained by a second centrifugation, and the supernatant (containing glutaraldheyde) was carefully removed. The tissue was washed with phosphate buffer (0.1 m), post-fixed in 1% OsO4 for 2 h at 4 °C, dehydrated and critical-point dried. Specimens were then glued onto stubs, covered with gold in an S150 (Edwards, London, UK) sputter coater and examined with a Hitachi S4000 field-emission (Hitachi Ltd, Tokyo, Japan) scanning electron microscope operating at 10 kV.
Transmission electron microscopy (TEM)
After the isolation and purification processes the final pellet was centrifuged and suspended in 2.5% phosphate buffer (0.1 m, pH 7.4) glutaraldehyde solution for 24 h at 4 °C. The islets were then repelleted, washed in 0.1 m phosphate buffer and post-fixed in 1% OsO4 for 2 h at 4 °C. Samples were then dehydrated through a graded ethanol series and propylene oxide and embedded in Epon 812 for 48 h. Semithin sections (1 mm thick) were cut with a glass knife on a Top Ultra 170 ultramicrotome (Pabish, Milan, Italy) and stained with methylene blue to select representative areas. Ultrathin sections were cut with a diamond knife, stained with uranyl acetate and lead citrate and observed by using a Zeiss EM9A (Carl Zeiss, Oberkochen, Germany) transmission electron microscope.
Morphometry
Histological sections were utilized for morphometric analysis. Up to 250 islet profiles were randomly selected from slides of isolated islet specimens. A similar number of native islets, randomly selected from sections of six different pancreata, were evaluated as controls.
The samples were observed using a Leitz DMR microscope. Images were captured by a C4742-95 Hamamatsu Digital Camera with resolution level up to 1280 × 1024, and the measurements were performed by means of a KS400 (Zeiss, Germany) image analysis system. The size threshold set was less than 0.5 µm with a 20× objective.
The major (A) and major at right angle (B) diameters were measured for each islet, both native and isolated, as measured in sections. From these data the profile diameter (d) of each islet was calculated using the equation  (49), where a and b are the semidiameters. Therefore, the mean profile diameter, the mean axial ratio of the profiles, the mean true diameter (D), the mean surface area and the size frequency distribution of the diameters were calculated. Assuming that the islets were spheroid in shape, the mean true diameter was calculated as described by Williams (1977).
(49), where a and b are the semidiameters. Therefore, the mean profile diameter, the mean axial ratio of the profiles, the mean true diameter (D), the mean surface area and the size frequency distribution of the diameters were calculated. Assuming that the islets were spheroid in shape, the mean true diameter was calculated as described by Williams (1977).
The results were presented as mean ± SE. Comparisons between the profile diameters of native and isolated islets were statistically evaluated using Student's t-test for unpaired values.
Subsequently, the mean surface area and the major and minor diameters of isolated islet fragments and cell cords within the pancreas were measured and compared statistically.
Results
Pancreatic islet isolations were performed successfully, yielding about 20% of the entire number of islets attributed to the native pancreas. Comparable numbers of islets, values for IEq and ratio (IEq/real islet number), purity and viability were obtained from the three isolations, as detailed in Table 1. Intact and fragmented islets were observed (dithizone staining) in the final fresh samples of the three isolations. The functionality of the islets in vivo was demonstrated by their capacity to reverse diabetes in rats within 4–5 days post-transplant and to maintain their euglycaemic status (Fig. 1). However, if the ratio was higher (1.6), normal functionality followed by long-term, stable, glycaemic control was achieved. Similar results were observed for the 1.2 ratio. When the ratio was lower (1.0), an immediate drop in blood glucose level was observed, with delayed control of hyperglycaemia in the following days before reaching stable but higher glycaemic levels as compared with the other two groups (average blood glucose: ratio 1.6 = 119 mg dL−1, ratio 1.2 = 126 mg dL−1, ratio 1.0 = 149 mg dL−1).
Table 1.
Assessment of isolated rat pancreatic islets
| Isolation no. | Islet no.* | IEq† | Ratio‡ | Purity (%)§ | Viability (%)¶ | Function** |
|---|---|---|---|---|---|---|
| 1 | 3500 | 3516 | 1.0 | 70 | 92 | 2/2 |
| 2 | 4500 | 5600 | 1.2 | 65 | 88 | 2/2 |
| 3 | 2500 | 4150 | 1.6 | 70 | 96 | 2/2 |
Number of islets counted on dithizone-stained fresh samples.
Number of islets counted on dithizone-stained fresh samples with regard to diameter (50–100, 100–150, 150–200, 200–250 µm) and averaged to a diameter of 150 µm.
Ratio IEq/islet number.
Dithizone-stained vs. unstained tissue.
By trypan blue dye exclusion.
In vivo reversibility of chemically induced diabetes (in rats) by transplantation of ∼1000 IEq, under the kidney capsule.
Fig 1.
Blood glucose level (mg dL−1) in rats following islet transplantation (2 months follow-up), with 1.0, 1.2 and 1.6 representing the different ratios between IEq and real islets (with 1.6 as the isolation with larger islets).
When islets were stimulated by glucose injection (IPGTT), an abnormal response was observed in all animals, with the worst response from the 1.2 and 1.0 ratio animals (Fig. 2).
Fig 2.
Intraperitoneal glucose tolerance test. The 1.6 ratio animals showed the best response, with lower levels of glycaemia at 45 min post glucose infusion.
Light microscopy
Samples observed under LM contained endocrine tissue containing both whole and fragmented islets. Furthermore, we observed clusters of exocrine tissue including acini and ducts, and soft tissue elements with residual vessels, digested collagen fibres and various residual cells forming an indistinct mass (Fig. 3). Except for digested collagen fibres, this material appeared apparently well preserved. To evaluate the extent of the purification, the ratio of islet/non-islet tissue was measured on resin-embedded semithin sections. The purity of the endocrine tissue was approximately 68% (Table 2).
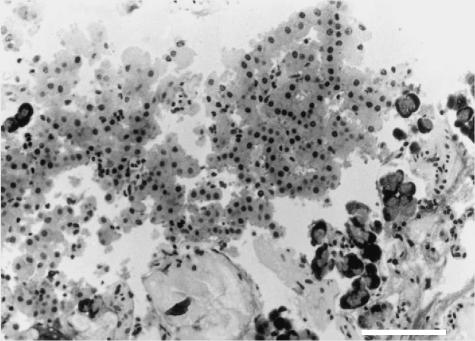
Fig 3.
LM, semithin section, H&E stain, 200× (scale bar = 100 µm). Endocrine tissue, comprising a large islet with an intact core and fragments at the periphery (left). Some residual exocrine acini (right side) and soft tissue containing digested collagen fibres (bottom) are also present.
Table 2.
Percentage of different tissue types after the isolation and purification process, as measured from the surface area from resin-embedded semithin sections. Data obtained from three different isolations
| Islets | 49.97% |
| Fragments of islets | 18.37% |
| Exocrine tissue | 14.10% |
| Soft tissue | 17.55% |
Most of the isolated islets were round or oval in shape, and of various sizes, similar to native islets. The thin connective capsule that surrounded in situ islets could be seen sporadically in isolated islets, and even apparently well-preserved islets could present irregular outlines, without a capsule being present. Clefts extending from the surface into the core of the islets were observed dividing the parenchyma (Fig. 4). Silver impregnation of the reticular and connective tissue fibres, normally arranged around the vessels, demonstrated an absence of argyrophilia within isolated islets with respect to native islets, indicating a loss of residual connective tissue fibres.
Fig 4.
LM, semithin section, H&E stain, 500× (scale bar = 40 µm). An apparently well-preserved islet with irregular outlines and absence of the capsule. On the left side, clefts tend to divide the parenchyma and some peripheral cells appear swollen and pale stained.
Peripheral cells could present with an irregular free surface and multiple vacuoles, and sometimes appeared turgid and pale stained. We observed that central islet cells often appeared to be more regular in shape and size (Fig. 4).
A relatively large part of the endocrine tissue (26% of the measured surface area) was composed of fragments of variable shapes and sizes. They generally had irregular outlines and appeared as small, round or oblong clusters of cells free from residual connective tissue fibres.
Scanning electron microscopy
The material observed by SEM showed different degrees of purification. Sometimes the islets appeared to be included in variably sized masses of exocrine tissue, vessels and soft tissue. Pure islets appeared as round or oval masses of tissue averaging 150–200 µm in diameter. They did not show connective capsules and there was only a residual discontinuous network of a few thin fibres of connective tissue surrounding the islets (Fig. 5). The appearance of cell cords was clearly visible on the surface of the islets. When the tissue was entirely free of residual capsular fibres, furrows of variable depth divided the cell cords, breaking the islet into small fragments. The plasma membranes of a small number of superficial cells appeared porous and discontinuous, revealing the cell interior (Fig. 6).
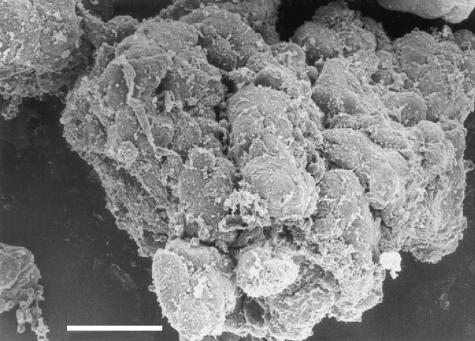
Fig 5.
SEM, scale bar = 25 µm. The surface of an isolated islet showing the cordonal organization of the tissue; a largely incomplete layer of residual connective tissue fibres covers the islet.
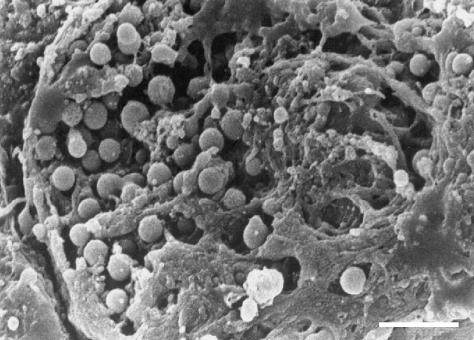
Fig 6.
SEM, scale bar = 2 µm. The apical membrane of this superficial cell appears broken and collapsed. Many small vescicles are visible inside the cell, just under the residual membrane. The vescicles are similar to secretory granules in shape and size (about 0.5 µm or less).
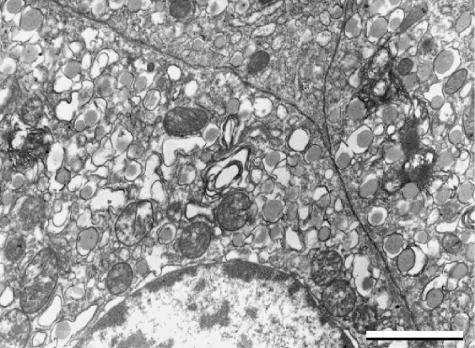
Transmission electron microscopy
Endocrine cells were polyhedral in shape, and maintained their normal relationship to adjacent cells. The plasma membrane was generally well preserved in the inner cells of the islet, but at times the most peripheral cells showed large fissures and breakage of the superficial plasmalemma (Fig. 7). Tight junctions were occasionally visible between β cells. The cytoplasm possessed vacuoles and a large number of small secretory granules, ranging in diameter from 200 to 500 nm. The granules contained a pale, electron-dense core filling the limiting membrane, or a more electron-dense core surrounded by a large clear halo. Both types of granules could be present in the same cell, and were recognized as characteristic of β cells. Owing to the possible loss of cells in the most peripheral layer, and due also to the damaged aspect of some superficial cells, we could not identify granules typical of α and δ cells with certainty.
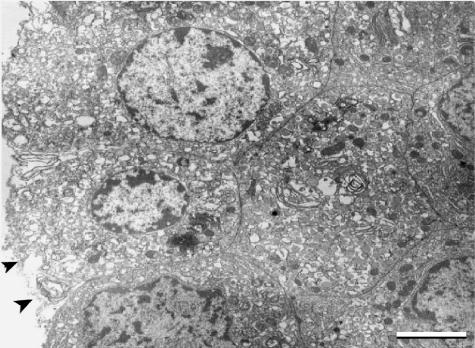
Fig 7.
TEM (×4000), scale bar = 2,5 µm. Endocrine cells maintain their polyhedral shape and their spatial relationship to adjacent cells. The plasma membrane of these peripheral cells is interrupted (arrowheads).
In the cells showing signs of more serious damage, mitochondria were swollen, with fragmented cristae; multilamellar bodies could be found in the cytoplasm (Fig. 8). This kind of injury could often be observed also in both acinar and ductal cells of exocrine tissue.
Fig 8.
TEM (×12 500), scale bar = 1 µm. This cell shows signs of significant damage, consisting of swelling of the mitochondria with fragmented cristae, vacuolization and fusion of the granules and initial formation of multilamellar bodies.
Morphometry
The profile diameter (d) ranged from 59 to 417 µm for in situ islets, and from 34 to 225 µm for the isolated islets (Fig. 9a). The average profile diameter of the isolated islets was 99 ± 48 µm, and was significantly lower (P < 0.001) than the average diameter of the intrapancreatic islets (153 ± 58 µm; see Table 3). The profile section of the isolated islets showed a mean axial ratio of 1.258, vs. 1.215 in native islets, indicating that islets are ellipsoids that can be roughly considered as spheroids. This finding enabled us to calculate the mean true diameter (D), corresponding to 116.28 µm for isolated islets and 209.34 µm for intrapancreatic islets.
Fig 9.
(a) An in situ islet used for morphometric analysis. The profile of the islet is clearly shown by the thin capsule that appears evident with silver impregnation for reticular and collagen tissue fibres (Gomori's stain). The major diameter (A), the major right angle diameter (B) and the surface area are automatically calculated and reported on a database. (b) Digitized image. Following the thin connective tissue septa that divide the parenchyma, visible in Fig. 9a, the cell cords can be delimited. They became more evident by increasing the contrast, if necessary. The measurements were performed as described above.
Table 3.
Comparison between mean morphometric data measured from isolated and in situ islets
| Isolated islets | Intrapancreatic islets | P | |
|---|---|---|---|
| Profile diameter (µm) | 99.12 ± 48.40 | 153.02 ± 58.51 | < 0.001 |
| Major diameter (µm) | 111.63 ± 55.32 | 169.28 ± 66.47 | < 0.001 |
| Minor diameter (µm) | 88.67 ± 44.21 | 139.27 ± 54.91 | < 0.001 |
| Surface area (µm2) | 8199.67 ± 7489.30 | 18990.76 ± 14623.86 | < 0.001 |
The size frequency distribution of the profile diameter of isolated islets showed a bimodal distribution (Fig. 10). The first peak occurred within 90 µm, corresponding approximately to the size of the smallest intrapancreatic islets. However, the percentage of isolated islets forming the first peak was significantly higher than the percentage of intrapancreatic islets of equivalent size. We assumed that the percentage of isolated islets included in the first peak was likely to contain various fragments of larger islets. The second peak (isolated islets) occurred between 130 and 160 µm, and corresponded (with a slight shift toward smaller sizes) to the more frequent size of native islets. These data indicated a general loss of islet mass during the isolation process, resulting in the presence of a high proportion of fragments.
Fig 10.
Size frequency distribution of the mean profile diameter of intact and isolated islets, expressed as a percentage.
The size of the islet fragments was compared with the size of cell cords within in situ islets. A cord was measured as a double or multiple line of cells lying between the thin septa containing small vessels visible in histological preparations (Fig. 9b). We found no significant differences either in mean surface area or in mean diameters (Table 4). From these data we concluded that the dimensions of fragments from the isolated islets are similar to those of cell cords within the intrapancreatic islets.
Table 4.
Comparison between mean morphometric data measured from fragments of islets and islet cell cords
| Fragments of islets | Islet cords | P | |
|---|---|---|---|
| Profile diameter (µm) | 63.14 ± 21.95 | 63.99 ± 12.71 | ns |
| Major diameter (µm) | 70.65 ± 23.10 | 75.86 ± 16.85 | ns |
| Minor diameter (µm) | 57.04 ± 22.04 | 54.96 ± 13.86 | ns |
| Surface area (µm2) | 2694.87 ± 1894.53 | 2564.43 ± 971.58 | ns |
ns, not significant.
Discussion
The presence of exocrine tissue within purified final islet preparations has been regularly reported in the literature (Gray et al. 1984; Schwartz & Traverso, 1984; Ricordi et al. 1990), and we were able to confirm this here. Even in highly purified islet preparations, the percentage of acinar, non-parenchymal and ductal components was reported to range between 20% and 70% (Sever et al. 1992). Although not the case in our present experiment, the effect of exocrine contamination has been found to impair the implantation of islets in rat renal subcapsular space (Gray et al. 1988) and to be related to late graft failure (Downing et al. 1986).
Because some of the material examined did not present evident damage, our observations focused on modifications of the islets after the isolation and purification processes. Two major factors were expected to cause potential cell damage: hypoxia and enzymatic/mechanical trauma related to the experimental procedures.
It has been reported that islet cells are more resistant than acinar tissue to hypoxia, trauma and nutritional deficiency (Baumgartner et al. 1980; Schwartz & Traverso, 1984). Furthermore, it has been demonstrated in isolated islets that a decrease in ATP concentration and ATP/AMP ratio was related to the time of incubation within the isolation procedure (El-Naggar et al. 1993).
TEM observations showed minimal to moderate alterations of cell ultrastructure, presumably due to prolonged hypoxia during the isolation and purification procedures (Sever et al. 1992; Tahir et al. 1992; Pai et al. 1993). However, the nuclei and membranes were substantially intact, with the exception of some of the most peripheral cells. The pleomorphism of the granules in the isolated islets was consistent with the presence of both immature and more mature granules described in normal pancreatic tissue (Bani Sacchi & Bani, 1985). Therefore, our results demonstrated that after islet isolation most of the cells were structurally intact and could be considered to be functioning satisfactorily (Millard et al. 1984; Tahir et al. 1992). The capacity of the islets to reverse diabetes after transplantation supported this observation.
Major relevance should be attributed to the structural changes to the islets as a result of the traumatic isolation procedure. Two main alterations were recognized: fragmentation of the islets and injuries to single cells. The normal structure of the pancreatic islets appeared as a network of anastomosing cords of cells arranged around capillaries (Brelje et al. 1989). According to El-Naggar et al. (1993), isolated islets showed a general decrease in their dimensions with respect to islets within the normal pancreas. As demonstrated, the mean diameter and the surface area of cut surfaces in isolated islets were significantly lower than those of in situ islets, clearly showing that there is loss of tissue during the isolation process. Moreover, islet fragments constituted a significant proportion of the endocrine tissue present in the samples. Morphometric analysis revealed that most of the fragments were comparable in appearance and size to the endocrine cell cords that constitute the normal structure of the islet parenchyma.
Our SEM observations showed a different degree of islet purification from that of collagen fibres. However, we observed that in numerous islets the surface was entirely free from the thin connective capsule that surrounds the islets within the normal pancreas. Furthermore, large furrows extended into the parenchyma, deeply dividing the different cell cords. LM observations failed to detect within isolated islets the connective tissue and reticular fibres that, by contrast, were clearly evident in native islets, around the capillary network. This finding was in accordance with earlier data (Wang et al. 1999) showing that there was a decrease in integrin expression and destruction of basal membranes in isolated islets. Furthermore, enzymatic activity of the collagenase preparation was seen around and even within the isolated islets (van Suylichem et al. 1992).
These observations demonstrated that collagenase digestion on the surface of the islets, and within them, was the most important factor in the fragmentation of the parenchyma (Schwartz & Traverso, 1984; Linetsky et al. 1997), with the detachment and loss of a variable number of cells, generally joined in clusters resembling the cell cords of the native islets. Moreover, the presence of tight junctions after isolation, although they could be absent in islets in situ, has been interpreted as a mechanism for sealing and protecting the islet microdomains, rather than being involved in islet cell function (in't Veld et al. 1984). Therefore, their presence does not appear to indicate a decreased likelihood of detachment of single cells or clusters.
The distribution of cellular constituents within rat islets is typically represented with α, δ and pancreatic polypeptide (PP) cells peripherally arranged around a central core of β cells (Brelje et al. 1989). Our observations showed more serious damage in the peripheral cells of islets and fragments up to the breakage of the plasma membrane, presumably due to the mechanical trauma associated with the isolation procedure. TEM observations could not always detect non-β cells in isolated islets. This was not surprising given that the polymorphism of the granules, mostly in injured peripheral cells, often made it impossible to identify different cell types following isolation. Similar difficulties were also reported by Tahir et al. (1992). Nevertheless, our observations supported a loss of non-β cells in isolated islets. Furthermore, it was demonstrated that in isolated islets the percentage volumes and numbers of α, δ and PP cells were lower than those of the respective cells within native islets (El-Naggar et al. 1993). All these observations indicated that most of the heavily injured cells came from the periphery and corresponded to non-β cells.
These findings are important because of the interactions among cells for the regulation of hormonal secretion. Several studies have pointed to the importance of these interactions: direct cell–cell communications occur through gap junctions (Meda et al. 1979, 1983; Meda, 1996); by contrast, cells can indirectly communicate through the paracrine and endocrine action of hormones (Jörns et al. 1988; Kawai et al. 1995). Both types of intercellular communication play an important role in the regulation of secretory function (Pipeleers et al. 1982; Hopcroft et al. 1985; Jörns, 1994), and quantitative variation of the normal percentage of cellular populations may lead to alterations of the normal secretory function of β cells (Trimble et al. 1982).
In our experiment we observed a successful engraftment, and long-term stable glycaemic control was achieved in all animals. This was evident especially in animals transplanted with larger and better-preserved islets (ratios of 1.6 and 1.2). In animals receiving the 1.0 ratio islets (smaller and more fragmented), we see a drop in blood glucose level (probably owing to post-transplant β-cell loss and acute insulin release), followed by signs of islet functionality and an overall delay in controlling hyperglycaemia as compared with the other two groups. The engrafted mass was still sufficient to treat high blood glucose levels, but the average blood glucose level is higher in ratio 1.0 recipients then in ratio 1.2 and 1.6 recipients.
The effect of an adequate islet mass to reverse diabetes has been emphasized (Keymeulen et al. 1992; Hering & Ricordi, 1999). However, it was also demonstrated that if a given mass of β cells can restore basal glycaemia in streptozotocin-treated rats, a 2.5-fold major mass of cells was necessary to correct other parameters of glucose homeostasis (Keymeulen et al. 1992). Other studies have pointed out that modifications of the secretory dynamics occurred in transplanted islets, suggesting that the transplantation influenced the balance between the stimulatory and inhibitory influence of glucose on the cell's secretory machinery (Shi & Täljedal, 1996).
During IPGTT the ectopically transplanted islets were challenged by a glucose bolus. A normal IPGTT result would show an immediate increase in glucose level that clears within 30–45 min post-infusion, returning to baseline (time point 0) by 120 min. Following islet transplantation with a functional islet graft, it is common to see (in both animals and patients) a reasonable but not physiological response to the injected glucose. In our study all animals showed abnormal responses to glucose challenge, with the worst response from animals receiving the 1.2 and 1.0 ratio islets.
This finding indicates that even when transplanting an equal mass of islets, sufficient to reverse diabetes, the integrity of the islets is crucial for the optimal functionality of the graft and optimal metabolic control: even if not normal, metabolic control improved in line with islet ratio (from 1.0 to 1.6).
In conclusion, structural alterations of the whole islets were a common finding after the isolation process. Experimental and clinical data currently confirm that ‘a critical functional mass’ is necessary to reverse diabetes in a patient. It was evident that structural and quantitative changes occur during the isolation and purification processes, leading to an alteration of both the physiological secretory cell function and the metabolic regulation, which was more evident when smaller and fragmented islets were transplanted. This may in part explain the resultant incomplete metabolic control reported in previous studies (Keymeulen et al. 1992; Shi & Täljedal, 1996) and the recurrence of hyperglycaemia (Alejandro et al. 1986; Hiller et al. 1991; Ricordi et al. 1992; Hering et al. 1994; Hering & Ricordi, 1999; Ryan et al. 2005) often reported in short- and long-term islet transplant studies.
Acknowledgments
This work was partially supported by the Italian Consiglio Nazionale delle Ricerche (CNR).
References
- Alejandro R, Cutfield RG, Shienvold FL, et al. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986;78:1339–1348. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca I, Feurle GE, Klempa I, Ziegler A, Schusdziarra V. Morphometry and function of islet cells after different forms of drainage at pancreatic transplantation in rats. Eur Surg Res. 1990;22:151–159. doi: 10.1159/000129096. [DOI] [PubMed] [Google Scholar]
- Bani Sacchi T, Bani D. New views on the identification of the various cell types in the pancreatic islet of the rat. Acta Anat. 1985;122:1–17. doi: 10.1159/000145977. [DOI] [PubMed] [Google Scholar]
- Baumgartner D, Sutherland DE, Heil JE, et al. Cold storage of segmental canine pancreatic grafts for 24 hours. J Surg Res. 1980;29:248–257. doi: 10.1016/0022-4804(80)90168-7. [DOI] [PubMed] [Google Scholar]
- Brelje T, Scharp DW, Sorenson RL. Three-dimensional imaging of intact isolated islets of Langerhans with confocal microscopy. Diabetes. 1989;38:808–814. doi: 10.2337/diab.38.6.808. [DOI] [PubMed] [Google Scholar]
- Bretzel RG, Hering BJ, Schultz AO, Geier C, Federlin F. International islet transplant registry report. Yearbook of Cell and Tissue Transplantation. 1996:153–160. [Google Scholar]
- CITR. Collaborative Islet Transplant Registry Annual Report. 2005 http://spitfire.emmes.com/study/isl/
- Delaby J, Campana M, Laborie C, Assan R. Reproducible high yields of rat islets of Langerhans. Diabetes Metab Rev. 1989;15:123–127. [PubMed] [Google Scholar]
- Downing R, Morrissey S, Kiske D, Scharp DM. Does the purity of intraportal islet isografts affect their endocrine function? J Surg Res. 1986;41:41–46. doi: 10.1016/0022-4804(86)90006-5. [DOI] [PubMed] [Google Scholar]
- El-Naggar M, Elayat A, Ardawi M, Tahir M. Isolated pancreatic islets of the rat: an immunohistochemical and morfometric study. Anat Rec. 1993;237:489–497. doi: 10.1002/ar.1092370408. [DOI] [PubMed] [Google Scholar]
- Gray DW, McShane P, Grant A, Morris PJ. A method for isolation of islets of Langerhans from the human pancreas. Diabetes. 1984;33:1055–1061. doi: 10.2337/diab.33.11.1055. [DOI] [PubMed] [Google Scholar]
- Gray DW, Sutton R, McShane P, Peters M, Morris PJ. Exocrine contamination impairs implantation of pancreatic islets transplanted beneath the kidney capsule. J Surg Res. 1988;45:432–442. doi: 10.1016/0022-4804(88)90193-x. [DOI] [PubMed] [Google Scholar]
- Hering BJ, Browatzki CC, Schultz AO, Bretzel RG, Federlin K. Islet transplant registry report on adult and fetal islet allografts. Transplant Proc. 1994;26:565–568. [PubMed] [Google Scholar]
- Hering B, Ricordi C. Islet Transplantation for patients with type I Diabetes. Graft. 1999;2:12–27. [Google Scholar]
- Hiller WF, Klempnauer J, Luck R, Steiniger B. Progressive deterioration of endocrine function after intraportal but not kidney subcapsular rat islet transplantation. Diabetes. 1991;40:134–140. doi: 10.2337/diab.40.1.134. [DOI] [PubMed] [Google Scholar]
- Hopcroft DW, Mason DR, Scott RS. Structure–function relationships in pancreatic islets: support for intraislet modulation of insulin secretion. Endocrinology. 1985;117:2073–2080. doi: 10.1210/endo-117-5-2073. [DOI] [PubMed] [Google Scholar]
- Jörns A. Immunocytochemical and ultra-structural heterogeneities of normal and glibenclamide stimulated pancreatic beta cells in the rat. Virchows Arch. 1994;25:305–313. doi: 10.1007/BF00196154. [DOI] [PubMed] [Google Scholar]
- Jörns A, Barklage E, Grube D. Heterogeneities of the islets in the rabbit pancreas and the problem of ‘paracrine’ regulation of islet cells. Anat Embryol. 1988;178:297–307. doi: 10.1007/BF00698661. [DOI] [PubMed] [Google Scholar]
- Kawai K, Yokota C, Ohashi S, Watanabe Y, Yamashita K. Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia. 1995;38:274–276. doi: 10.1007/BF00400630. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Teng H, Vetri M, Gorus F, In't Veld P, Pipeleers DG. Effect of donor islet mass on metabolic normalization in streptozotocin-diabetic rats. Diabetologia. 1992;35:719–724. doi: 10.1007/BF00429090. [DOI] [PubMed] [Google Scholar]
- Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46:1120–1123. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- Meda P, Perrelet A, Orci L. Increase of gap junctions between pancreatic B-cells during stimulation of insulin secretion. J Cell Biol. 1979;82:441–448. doi: 10.1083/jcb.82.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda P, Denef JF, Perrelet A, Orci L. Nonrandom distribution of gap junctions between pancreatic beta-cells. Am J Physiol. 1980;238:C114–C119. doi: 10.1152/ajpcell.1980.238.3.C114. [DOI] [PubMed] [Google Scholar]
- Meda P, Michaels RL, Halban PA, Orci L, Sheridan JD. In vivo modulation of gap junctions and dye coupling between b-cells of the intact pancreatic islet. Diabetes. 1983;32:858–868. doi: 10.2337/diab.32.9.858. [DOI] [PubMed] [Google Scholar]
- Meda P. The role of gap junction membrane channels in secretion and hormonal action. J Bioenerg Biomembr. 1996;28:369–377. doi: 10.1007/BF02110113. [DOI] [PubMed] [Google Scholar]
- Millard PR, Reece-Smith H, Smart YC, McShane P, Morris PJ. Observations on rat collagenase-separated islets and long-term composite islet allografts. Br Exp Pathol. 1984;65:745–751. [PMC free article] [PubMed] [Google Scholar]
- Pai GM, Slavin BG, Tung P, et al. Morphologic basis for loss of regulated insulin secretion by isolated rat pancreatic islets. Anat Rec. 1993;237:498–505. doi: 10.1002/ar.1092370409. [DOI] [PubMed] [Google Scholar]
- Pipeleers D, in't Veld P, Maes E, Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc Natl Acad Sci USA. 1982;79:7322–7325. doi: 10.1073/pnas.79.23.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastellini C, Shapiro R, Corry R, Fung JJ, Starzl TE, Rao AS. Treatment of isolated pancreatic islets to reverse pancreatectomy-induced and insulin dependent type I diabetes in humans: a 6 year experience. Transplant Proc. 1997;29:746–747. doi: 10.1016/s0041-1345(96)00449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in mam and large animals. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Tzakis AG, Carroll PB, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 1992;53:407–414. doi: 10.1097/00007890-199202010-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricordi C, Rastellini C. Methods in pancreatic islets separation. In: Ricordi C, editor. Methods in Cell Transplantation. Austin, TX: R.G. Landes; 1995. pp. 433–438. [Google Scholar]
- Ryan EA, Lakey RT, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton Protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Paty BW, Senior PA, et al. five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, Traverso LW. Morphological changes in pancreatic fragments prepared for transplantation by collagenase treatment. Transplantation. 1984;38:273–280. doi: 10.1097/00007890-198409000-00015. [DOI] [PubMed] [Google Scholar]
- Sever CE, Demetris AJ, Zeng J, et al. Composition of human islet cell preparations for transplantation. Acta Diabetol. 1992;28:233–238. doi: 10.1007/BF00779005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type I diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- Shi CL, Täljedal IB. Dynamics of glucose-induced insulin release from mouse islets transplanted under the kidney capsule. Transplantation. 1996;62:1312–1318. doi: 10.1097/00007890-199611150-00024. [DOI] [PubMed] [Google Scholar]
- van Suylichem PT, Wolters GH, van Schilfgaarde R. Peri-insular presence of collagenase during islet isolation procedures. J Surg Res. 1992;53:502–509. doi: 10.1016/0022-4804(92)90097-j. [DOI] [PubMed] [Google Scholar]
- Tahir M, Elayat AA, Jalalah S, El-Naggar MM. Isolated pancreatic islets of the rat: an ultra-structural study. Acta Anat. 1992;145:93–100. doi: 10.1159/000147348. [DOI] [PubMed] [Google Scholar]
- Trimble ER, Halban PA, Wollheim CB, Renold AE. Functional differences between rat islets of ventral and dorsal pancreatic origin. J Clin Invest. 1982;69:405–413. doi: 10.1172/JCI110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- in't Veld PA, Pipeleers DG, Gepts W. Evidence against the presence of tight junctions in normal endocrine pancreas. Diabetes. 1984;33:101–104. [PubMed] [Google Scholar]
- Wang RN, Paraskevas S, Rosemberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem. 1999;47:499–506. doi: 10.1177/002215549904700408. [DOI] [PubMed] [Google Scholar]
- Warnock GL, Ellis D, Rayotte RV, Dawidson I, Baekkeskov S, Egebjerg J. Studies of the isolation and viability of human islets of Langerhans. Transplantation. 1988;45:957–963. doi: 10.1097/00007890-198805000-00024. [DOI] [PubMed] [Google Scholar]
- Williams MA. Quantitative methods in biology. In: Glauert AM, editor. Practical Methods in Electron Microscopy. North-Holland: Amsterdam; 1977. pp. 48–62. [Google Scholar]