Abstract
Primates have shoulders adapted to a wide range of locomotor functions from terrestrial pronograde quadrupedalism to highly arboreal suspensory behaviours. The shape of the scapula tightly follows these functional differences. Previous analyses of primate postcrania, including the scapula, indicate that quadrupedal monkeys are less variable than non-quadrupeds. It was previously suggested that this difference was due to a relationship between the strength of stabilizing selection and the functional demands of the upper limb. Here it is shown that intraspecific scapular shape variance is highly correlated with the degree of committed quadrupedalism. Primates that engage in frequent suspensory behaviours (e.g. apes and ateline monkeys) average twice the amount of shape variance as quadrupeds (e.g. Old World monkeys and Saimiri). Because this difference in intraspecific shape variance is apparent in infants and does not increase or decrease appreciably over ontogeny, it is not likely that differences in postnatal growth, neuromuscular control or environmental factors such as habitat structure/composition are the primary contributors to differences in adult shape variance. Instead variance in embryonic factors that affect the shape/size of the scapula or epigenetic factors associated with muscle attachments are more likely candidates. In particular, the heterogeneous functional demands of the non-quadrupedal shoulder probably reduce the stringency of stabilizing selection, resulting in the persistence into adulthood of increased amounts of embryonically generated scapular shape variance.
Keywords: atelines, cercopithecoids, geometric morphometrics, hominoids, shoulder morphology, stabilizing selection
Introduction
The scapula is a thin, compact bone that serves as the bony attachment site for numerous muscles between the head, neck and forelimb (Ashton & Oxnard, 1963; Larson, 1993). Given its central role in forelimb use, it is not surprising that the morphology of the scapula is highly variable between species and its shape is largely associated with functional demands of the forelimb (Oxnard, 1967, 1977; Ashton et al. 1976; Larson, 1993, 1995). In primates, morphological differences between species can be discriminated along a continuum from committed terrestrial quadrupeds (e.g. baboons) to highly arboreal and suspensory non-quadrupeds (e.g. gibbons) (Fig. 1) (Oxnard, 1967, 1977; Ashton et al. 1976). These interspecific shape differences reflect the much different ways in which the shoulder is used at the extremes of these two groups: the quadruped scapula functions mostly in retractive–propulsive movements within a restricted parasagittal plane, whereas the non-quadruped scapula permits greater mobility and use of the forelimb above the head in suspensory behaviours (e.g. brachiation, hanging, vertical climbing) (Shea, 1986; Hunt, 1991; Larson, 1993, 1995).
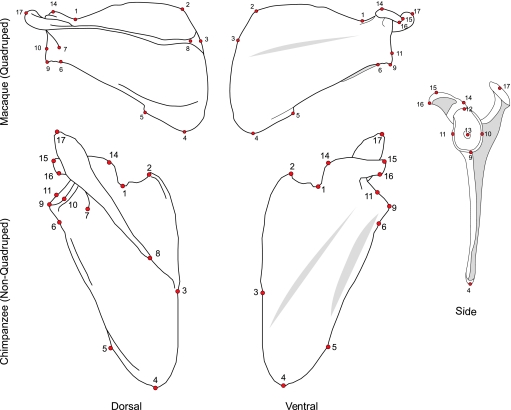
Fig. 1.
The scapula of a typical committed terrestrial quadruped (top, macaque) and an arboreal suspensory non-quadruped (bottom, chimpanzee) in dorsal and ventral view. The side view of a generalized primate scapula is shown on the far right. The typical quadrupedal scapula is longer from vertebral border to glenoid and shorter from superior to inferior angles. In non-quadrupeds such as apes and ateline monkeys this pattern is largely reversed. Note the differences in the shape of the blade, and the orientation and size of the spine that discriminate the extremes of these two groups. Location of landmarks are shown on both specimens: (1) suprascapular notch; (2) superior angle; (3) point on the vertebral margin where the long axis of the scapular spine and the vertebral border meet; (4) inferior angle; (5) teres major fossa; (6) infraglenoid tubercle; (7) spinoglenoid notch; (8) medial extent of the trapezius attachment along the scapular spine; (9) inferior-most point on glenoid fossa; (10) greatest width of the glenoid fossa (lateral); (11) greatest width of the glenoid fossa (medial); (12) superior-most point on glenoid fossa; (13) shallowest point (maximal curvature) of the glenoid fossa; (14) coracoid prominence; (15) distal-most tip of the coracoid process (superior); (16) distal-most tip of the coracoid process (inferior); (17) distal-most point of the acromion.
There is evidence that forelimb function not only plays a major role in scapular shape but also helps pattern intraspecific scapular variation. In particular, scapular shape variation in quadrupedal primates appears to be more canalized than in primates that utilize suspensory behaviours. This evidence comes from several indirect sources. In an interspecific analysis of forelimb and trunk characters, non-quadrupedal apes were found to be more variable than quadrupeds (Larson, 1998; Young, 2003). Re-analysis of these data suggest that many postcranial features, including the scapula, also exhibit greater within-species variance in non-quadrupeds than in quadrupeds. Other postcranial traits such as total vertebrae formulae are also less variable in quadrupedal Old World monkeys than in non-quadrupedal apes (Pilbeam, 2004). Specific to the scapula, Young (2004) found support for differences in covariance structure between non-quadruped apes and quadruped monkeys mostly due to higher correlations among quadruped scapular traits. Higher integration is often associated with constraints on variation (Wagner & Altenberg, 1996), so differences in covariance structure suggest that variance in scapular shape should be lower in quadrupeds than in non-quadrupeds. These studies indicate that the postcrania of suspensory apes may exhibit increased intraspecific variation because of reduced functional integration.
The mechanisms modulating variance in scapular shape are unclear but stabilizing selection is a likely candidate given that traits that are most important in terms of fitness are often the most canalized (Waddington, 1942). In other words, functional differences between non-quadrupeds and quadrupeds may affect the relative intensity of stabilizing selection to remove extreme variants. There is evidence to support this hypothesis. Quadrupedal primate locomotion is kinematically consistent, movements are relatively restricted, and locomotion is used in both terrestrial and arboreal contexts (Fleagle, 1998). By contrast, non-quadrupedal primates use their forelimb less consistently (i.e. discrete functions comprise a smaller proportion of the overall repertoire), and are less restricted in movement, presumably as an adaptation to the complexities of tropical forest canopy structure (Erikson, 1963; Cant et al. 2001, 2003). Given these differences, it is arguable that the primary selective force on the shoulder of a committed terrestrial quadrupedal primate is locomotor efficiency whereas in arboreal non-quadrupedal primates it is flexibility of usage. If stabilizing selection does drive population variance, then a prediction of this hypothesis is that quadrupedal shoulders should exhibit a stronger tendency towards the mean form (i.e. reduced variance and stronger canalization of shape), whereas in non-quadrupeds variants are more likely to persist (i.e. increased variance or weaker canalization of shape).
A further question pertinent to this research is when and how intraspecific scapular shape variance originates. Multiple developmental processes acting at different points during ontogeny can create variance. Prenatal factors may include variance in intrinsic genetic factors affecting shape/size, epigenetic factors such as variance in muscle attachments, variance of in utero neuromuscular activity (e.g. Carter et al. 1998; Nowlan & Prendergast, 2005), or variance in maternal effects (e.g. Atchley & Hall, 1991). Because studying embryogenesis is difficult in primates, the question of how they might differ and the relative impact of these effects remains largely unanswered. Postnatal factors impacting scapular variance, such as epigenetic or environmental factors, are easier to measure and are better documented. For example, it is known that patterns of muscle contraction relate to strains on bone (Herring & Teng, 2000), and these strains could lead to differential growth in areas closest to peak strains (Carter et al. 1998; Herring et al. 2002; Nowlan & Prendergast, 2005). Variation in the response of bone to strains may therefore play a role in producing shape variance. Specifically, three factors may interact with bone growth to produce variance in bone shape. First, variance in muscle attachment location or size may affect placement or the strength of peak strains generated during activity. Unfortunately, although differences are known between species (Ashton & Oxnard, 1963), patterns of within-species variance in the locations of muscle attachments across primates are not as well documented. Second, increases in neuromuscular control during ontogeny (e.g. due to greater co-ordination) may reduce variance by normalizing strains to directions that are preferred or optimal (Zelditch et al. 2004a). If this were the case, scapular variance would decrease during ontogeny and become localized to those areas most frequently used. Third, factors associated with complexity of the environment may affect differences in shape variation independent of muscular or neuromuscular variation. For example, terrestrial quadrupedal primates live in more open environments whereas non-quadrupedal primates typically live in closed and forested environments with complex canopy structure (Fleagle, 1998). Use of more or less heterogeneous environments during ontogeny might produce consequent differences in adult variance.
In this paper, intraspecific variance in scapular shape is compared across anthropoid primates to test the hypothesis that canalization of shape varies in relation to the function of the forelimb. A significant relationship between these variables would support a role of stabilizing selection in modulating variance. An alternative hypothesis is that intraspecific shape variance either does not vary across primates or it is phylogenetically structured. In the former case canalization would be similar across primates regardless of functional or phylogenetic affiliation. In the latter case shape variance would be predicted to be similar in more closely related species. The inclusion of a broad range of taxa including distantly related species that are convergent in function (e.g. atelines and apes) would help to discriminate between these hypotheses.
A second goal of this paper is to examine what factors contribute to intraspecific shape variance and when they act. Ideally, such research would investigate both pre- and postnatal time periods and involve pedigreed animals to partition variance attributable to genetic or environmental factors. Unfortunately, this type of study is not feasible across a wide range of primates or functional categories. However, using the pattern of ontogenetic changes in variance can yield important clues as to when most variance is created and thus which factors might play the largest role. For example, if variance is largely attributable to postnatal factors then one would predict that variance should be equal in infants across all functional categories. Alternatively, differences during the earliest stage of postnatal ontogeny would implicate earlier embryonic events or factors intrinsic to the formation of the scapula itself (e.g. variance in genetic factors associated with bone shape or size). If increased neuromuscular control leads to reduction in variance, then variance should decrease over ontogeny (Zelditch et al. 2004a) while no reduction would indicate either that increasing postnatal neuromuscular control does not reduce variance or that it balances increases in variance generated from other sources. Increasing variance over ontogeny would indicate that environmental components have a large effect on shape variance, particularly if shape variance is initially equal across primates during infancy. For both neuromuscular and environmental variance, differences in the slope would indicate the ability of different primate developmental systems to canalize this variance. If variance is consistent across ontogeny, this would suggest either that postnatal factors contribute only a small portion to variance or that all primates have similar ability to withstand postnatal perturbations to development.
Ultimately, establishing whether and how function interacts with shape over ontogeny to produce variance will yield information pertinent to the evolution of canalization such as the relative importance of stabilizing selection. An ontogenetic study will help to discriminate when the majority of variance is created and thus will help determine which factors play the largest role in generating or reducing variance and when they have their largest effect. Of broader importance, this study will contribute to our understanding of the relative role of various factors in creating phenotypic variance and the evolution of mechanisms involved in modulating canalization.
Materials and methods
Sample
The sample consists of the scapula of 901 individuals from the following institutions: the Museum of Comparative Zoology (Cambridge, Massachusetts), the American Museum of Natural History (New York), the National Museum of Natural History (Washington, DC), the Neil Tappen Collection at the University of Minnesota (Minneapolis, Minnesota), the Field Museum of Natural History (Chicago, Illinois), the Cleveland Museum of Natural History (Cleveland, Ohio), the University of Zürich-Irchel (Zürich, Switzerland), the Zooligische Staatssammlung (Münich, Germany), the Musée Royale de l’Afrique Centrale (Tervuren, Belgium), the Powell-Cotton Museum (Birchington-Kent, UK), the Kyoto University Primate Research Institute (Inuyama, Japan) and the Japan Monkey Center (Inuyama, Japan). The sample comprised the following species (Table 1): Pan troglodytes (common chimpanzee), Pan paniscus (pygmy chimpanzee or bonobo), Gorilla gorilla (lowland gorilla), Pongo pygmaeus pygmaeus (Bornean orangutan), Symphalangus syndactylus (siamang), Hylobates lar (white-handed gibbon), Macaca fasicularis (rhesus macaque), Papio anubis (anubis baboon), Cercocebus albigena (sooty mangabey), Cercopithecus aethiops (vervet monkey), Presbytis cristatus (langur), Nasalis larvatus (proboscis monkey), Colobus guereza (abyssinian colobus), Ateles geoffroyi (spider monkey), Alouatta belzebuth (howler monkey), Lagothrix lagothrica (wooly monkey) and Saimiri sciureus (squirrel monkey). The sample consists of both males and females. Age was determined by the eruption sequence of the molars from associated crania (i.e. m1 = infant, m2 = juvenile, m3 = adult). The majority of the sample was composed of wild-caught individuals. When possible, population heterogeneity was minimized by choosing individuals from the same geographical localities.
Table 1.
Species and sample size organized by ontogenetic stage
| Species | Infant | Juvenile | Adult |
|---|---|---|---|
| Alouatta belzebuth | – | – | 21 |
| Ateles geoffroyi | – | – | 21 |
| Cercocebus albigena | – | 15 | 33 |
| Cercopithecus aethiops | 7 | 14 | 28 |
| Colobus guereza | – | 6 | 21 |
| Gorilla gorilla | 14 | 30 | 109 |
| Hylobates lar | – | 23 | 59 |
| Lagothrix lagothrica | – | – | 27 |
| Macaca fasicularis | 8 | 16 | 22 |
| Nasalis larvatus | – | – | 39 |
| Pan paniscus | 7 | – | 23 |
| Pan troglodytes | 24 | 44 | 78 |
| Papio anubis | – | 15 | 9 |
| Pongo pygmaeus | – | 17 | 31 |
| Presbytis cristatus | 11 | 7 | 43 |
| Saimiri sciureus | – | 21 | 16 |
| Symphalangus syndactylus | – | – | 42 |
| Total | 71 | 208 | 622 |
Landmarks
Three-dimensional coordinate data were recorded for 17 landmarks using a Microscribe-3Dx digitizer (Immersion Corp., San Jose, CA, USA) (Fig. 1). Landmark definition is complicated by the fact that the scapula has few sutures or easily identified bony junctions, and tissue boundaries (e.g. mesodermal vs. neural crest) are cryptic (Matsuoka et al. 2005). For this reason, landmarks were chosen either to reflect previously published diagnostic features of the scapula or defined in reference to geometric properties (Oxnard, 1967, 1977; Ashton et al. 1976; Larson, 1993, 1995). The latter are known as ‘Type III’ or ‘mathematical’ landmarks (Dryden & Mardia, 1998). The majority of landmarks, including Type III, correspond directly to the endpoints of linear measurements used in previous analyses of the scapula (e.g. Oxnard, 1967; Larson, 1995). A subset representing ∼15% of the sample was digitized on three occasions in order to assess precision. Observer-based error was both low and comparable between infants and adults, and between large and small species.
Geometric morphometrics
Landmark data were aligned using a generalized least-squares Procrustes superimposition algorithm (GLS) as implemented in NTSYS-PC (Rohlf, 2002) and IMP (Sheets, 2004a). GLS results in a new set of landmarks (Procrustes coordinates) with information concerning scale, position and orientation removed. Geometric scale is defined by centroid size (CS) or the sum of the square root of the squared distance between each landmark and the centroid (mean x, y, z) of an individual specimen's landmarks. To control for the effect of size heterogeneity on the estimation of variance, Procrustes coordinates were regressed on CS in 3DStand (Sheets, 2004b) and the residuals were used in subsequent analyses (Zelditch et al. 2004b). Procrustes distance, or the distance of an individual specimen from the mean configuration, was calculated as the square root of the sum of the squared deviations of a specimen's Procrustes coordinates from the grand mean of each landmark coordinate.
Analyses
Shape variance
Canalization is a property of an organism that ensures similarity of phenotypic expression by buffering development against both environmental and genetic perturbations (Waddington, 1942; Hallgrímsson et al. 2002). Operationally, canalization can be measured as the among-individual variance of a population. Comparisons of variance in two populations (e.g. quadrupeds vs. non-quadrupeds or infants vs. adults) can be used to assess the relative degree of canalization. Here shape variance is used as the measure of canalization. Shape variance for each species’ age class was calculated as:
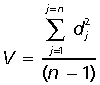 |
where V is the population shape variance, dj is the Procrustes distance of individual j, and n is the sample size (Zelditch et al. 2004a,b). This value is identical to the trace of the variance–covariance matrix of the Procrustes data. Standard errors and confidence limits of the shape variance statistic were estimated by resampling (Manly, 1997). Each population was resampled with replacement 1000 times and a new shape variance was calculated using the software program PopTools (Hood, 2003).
Ontogenetic trends in shape variance were assessed by computing the significance of differences between groups and age classes via the t-test for shape variance:
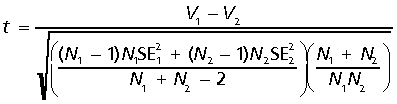 |
where V is the shape variance, SE is the standard error of the shape variance and N is the sample size (Zelditch et al. 2004b). To test whether shape variance was correlated with function, shape variance was regressed against a quadrupedalism index (described below).
Shape covariance
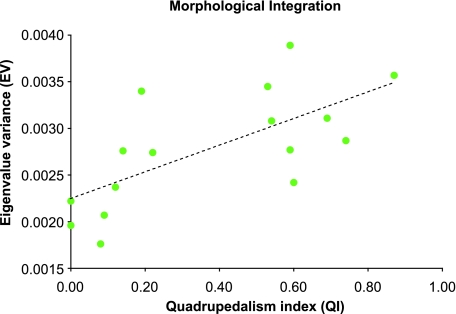
Morphological integration, which is assessed by analysis of covariance structure, has been linked to constraints on the production of variance (Wagner & Altenberg, 1996; Chernoff & Magwene, 1999). Consequently, high covariance between scapular traits could be correlated with differences in shape variance. Previous comparisons of primate scapular integration found differences between suspensory apes and two quadrupedal Old World monkeys (Young, 2004). In this paper the degree of morphological integration was computed by calculating the eigenvalue variance (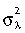 or EV) from adult covariance matrices (Wagner, 1984, 1990; Van Valen, 2005). In Wagner (1984) eigenvalue variance was derived from a correlation matrix, and thus was directly comparable across species. This is not true of eigenvalue variance calculated from covariance matrices (i.e. the statistic is scale dependent) so eigenvalues were standardized by the total shape variance (the trace of the variance–covariance matrix). Eigenvalue variance measures multivariate tightness, i.e. whether shape variance can be explained by a small number of principal components (ellipsoid or high EV), or whether variance is more evenly distributed across principal components (spheroid or low EV). The former case would be considered more integrated and the latter less integrated. EV was calculated using code written in R (R Development Core Team, 2004). Eigenvalue variance was regressed against both QI (see below) and shape variance. If integration acts as a constraint on variance in the scapula, then EV should be significantly correlated with QI and shape variance. Papio was not included in this analysis because adult sample size was too low to estimate covariance structure accurately.
or EV) from adult covariance matrices (Wagner, 1984, 1990; Van Valen, 2005). In Wagner (1984) eigenvalue variance was derived from a correlation matrix, and thus was directly comparable across species. This is not true of eigenvalue variance calculated from covariance matrices (i.e. the statistic is scale dependent) so eigenvalues were standardized by the total shape variance (the trace of the variance–covariance matrix). Eigenvalue variance measures multivariate tightness, i.e. whether shape variance can be explained by a small number of principal components (ellipsoid or high EV), or whether variance is more evenly distributed across principal components (spheroid or low EV). The former case would be considered more integrated and the latter less integrated. EV was calculated using code written in R (R Development Core Team, 2004). Eigenvalue variance was regressed against both QI (see below) and shape variance. If integration acts as a constraint on variance in the scapula, then EV should be significantly correlated with QI and shape variance. Papio was not included in this analysis because adult sample size was too low to estimate covariance structure accurately.
Function
There are multiple potential selective factors affecting shape of the scapula, but here it is assumed that locomotion plays a primary role. That is, as quadrupedalism decreases as a component of overall locomotor uses, energetic efficiency is reduced as a selective factor and is replaced by other considerations (e.g. climbing efficiency, arm mobility, mortality risk avoidance, mechanical advantage). Comparisons of locomotor function involving the scapula are complicated by the fact that categories can be defined in multiple ways. Here an index of quadrupedalism (QI) was derived from the percentage of time spent using quadrupedal locomotor behaviours in arboreal contexts with values derived from published data (Rose, 1979; Fleagle & Mittermeier, 1980; Fleagle, 1980; Susman, 1984; Tuttle & Watts, 1985; Cant, 1988; Boinski, 1989; Hunt, 1991; Gebo, 1992; Doran, 1993a,b; Gebo & Chapman, 1995; Gebo, 1996; Cant et al. 2001, 2003). Because of the paucity of data comparing locomotion between ontogenetic stages this index was based on adults and so it is assumed that ontogenetic change in locomotor behaviour is minimal (although see Doran, 1997).
This index was considered to be the simplest and most direct way of capturing overall differences in locomotion between primates while avoiding complications due to different methodologies. Quadrupedalism is relatively easy to define, most observers can agree on its definition and this behaviour is frequently scored in the literature. Here it is assumed that suspensory and other non-quadrupedal forelimb-dominated behaviours (e.g. vertical climbing) that are hypothesized to have a large effect on shape variance occur only in arboreal contexts. It is assumed that when quadrupedalism is practised more frequently in an arboreal context, it is more likely that the species in question is a committed quadruped in other contexts. Using arboreal quadrupedalism also helps to reduce any conflation of the compromised quadrupedalism practised by suspensory apes when terrestrial (e.g. chimpanzees, gorillas) with that of committed terrestrial quadrupeds (e.g. baboons) (Fleagle, 1998). For example, in terrestrial contexts chimpanzees are frequently quadrupedal, but this locomotion is compromised and comparatively inefficient (Hunt, 1991). This suggests that some aspect of selection for a suspensory scapula outweighs the inefficiencies this morphology imposes on quadrupedal locomotion (Pontzer & Wrangham, 2004). These compromises in quadrupedal efficiency are likely to affect other suspensory primates as well.
Phylogeny
A phylogenetic distance matrix was calculated from aligned γ-globin sequence data (12 118 bp) using the HKY-85 model (Page & Goodman, 2001). Computation of the distance matrix was performed in paup* (Swofford, 2002). The γ-globin gene has been sequenced in all the species sampled here except Symphalangus, and the phylogenetic tree is congruent with analyses from all major primate clades. The phylogenetic distance matrix was compared with a shape variance difference matrix using a matrix correlation and Mantel's test to assess significance.
Results
Shape variance, standard errors, 95% confidence intervals, QI and EV are reported in Table 2. QI was largely bimodal, with one group < 0.25 (Ateles, Hylobates, Gorilla, Lagothrix, Pan paniscus, Pan troglodytes, Pongo and Sympalangus) and another > 0.50 (Alouatta, Cercocebus, Cercopithecus, Colobus, Macaca, Nasalis, Papio, Presbytis and Saimiri). This result reflects that the species compared were either substantially quadrupedal or non-quadrupedal within arboreal contexts. For this reason, the former group was combined and designated ‘non-quadrupedal’ and the latter group was combined and designated ‘quadrupedal’.
Table 2.
Shape variance (V), standard error (SE), 95% confidence intervals, quadrupedalism index (QI) and eigenvalue variance (EV)
| Infant | Juvenile | Adult | QI | EV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | V | SE | 95% CI | V | SE | 95% CI | V | SE | 95% CI | ||
| Alouatta belzebuth | – | – | – | – | – | – | 0.0044 | 0.0004 | 0.0034–0.0050 | 0.59 | 0.0039 |
| Ateles geoffroyi | – | – | – | – | – | – | 0.0059 | 0.0004 | 0.0048–0.0065 | 0.19 | 0.0034 |
| Cercocebus albigena | – | – | – | 0.0022 | 0.0002 | 0.0018–0.0023 | 0.0026 | 0.0002 | 0.0022–0.0029 | 0.54 | 0.0028 |
| Cercopithecus aethiops | 0.0052 | 0.0011 | 0.0025–0.0065 | 0.0045 | 0.0006 | 0.0031–0.0053 | 0.0036 | 0.0003 | 0.0029–0.0040 | 0.59 | 0.0031 |
| Colobus guereza | – | – | – | 0.0031 | 0.0007 | 0.0017–0.0045 | 0.0040 | 0.0003 | 0.0031–0.0044 | 0.53 | 0.0028 |
| Gorilla gorilla | 0.0071 | 0.0007 | 0.0052–0.0077 | 0.0057 | 0.0004 | 0.0048–0.0065 | 0.0074 | 0.0003 | 0.0066–0.0077 | 0.09 | 0.0035 |
| Hylobates lar | – | – | – | 0.0064 | 0.0008 | 0.0045–0.0077 | 0.0070 | 0.0005 | 0.0059–0.0080 | 0.00 | 0.0022 |
| Lagothrix lagothrica | – | – | – | – | – | – | 0.0061 | 0.0005 | 0.0050–0.0069 | 0.22 | 0.0021 |
| Macaca fasicularis | 0.0027 | 0.0004 | 0.0014–0.0031 | 0.0036 | 0.0005 | 0.0024–0.0044 | 0.0034 | 0.0003 | 0.0027–0.0039 | 0.74 | 0.0027 |
| Nasalis larvatus | – | – | – | – | – | – | 0.0037 | 0.0003 | 0.0030–0.0043 | 0.60 | 0.0029 |
| Pan paniscus | 0.0075 | 0.0015 | 0.0048–0.0100 | – | – | – | 0.0050 | 0.0006 | 0.0038–0.0060 | 0.14 | 0.0024 |
| Pan troglodytes | 0.0076 | 0.0007 | 0.0059–0.0085 | 0.0081 | 0.0006 | 0.0067–0.0093 | 0.0072 | 0.0003 | 0.0065–0.0077 | 0.08 | 0.0018 |
| Papio anubis | – | – | – | 0.0023 | 0.0002 | 0.0017–0.0026 | 0.0034 | 0.0004 | 0.0022–0.0039 | 0.68 | – |
| Pongo pygmaeus | – | – | – | 0.0076 | 0.0010 | 0.0055–0.0091 | 0.0071 | 0.0005 | 0.0059–0.0079 | 0.12 | 0.0024 |
| Presbytis cristatus | 0.0036 | 0.0004 | 0.0025–0.0040 | 0.0028 | 0.0004 | 0.0015–0.0032 | 0.0035 | 0.0003 | 0.0029–0.0040 | 0.69 | 0.0031 |
| Saimiri sciureus | – | – | – | 0.0039 | 0.0004 | 0.0029–0.0045 | 0.0033 | 0.0003 | 0.0025–0.0036 | 0.87 | 0.0036 |
| Symphalangus syndactylus | – | – | – | – | – | – | 0.0060 | 0.0003 | 0.0052–0.0065 | 0.00 | 0.0020 |
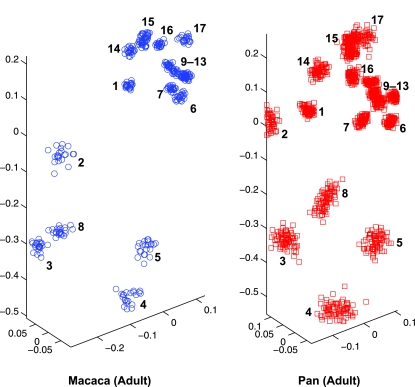
Standard errors for shape variance were comparable across species. The highest standard errors were associated with small sample sizes in infants (e.g. Cercopithecus) or with juveniles of non-quadrupeds (e.g. Hylobates, Pan troglodytes and Pongo), but these were still small relative to observed shape variance. In all species, the landmarks that appeared to be the most variable in location were numbers 2–5, which are associated with the shape of the scapular blade (Fig. 2). Landmarks exhibiting less variability in location were numbers 9–13, which were associated with the glenoid, the articulation point of the scapula and the humerus. Non-quadrupeds appeared to be more variable across all regions of the scapula.
Fig. 2.
Three-dimensional scatterplots of Procrustes-aligned landmark data for adult Macaca fasicularis (left, blue) and Pan troglodytes (right, red). Macaques exhibit significantly less shape variance at this ontogenetic stage (V = 0.0034) and earlier stages as compared with chimpanzees (V = 0.0072) (t = 6.221, P = 0.000).
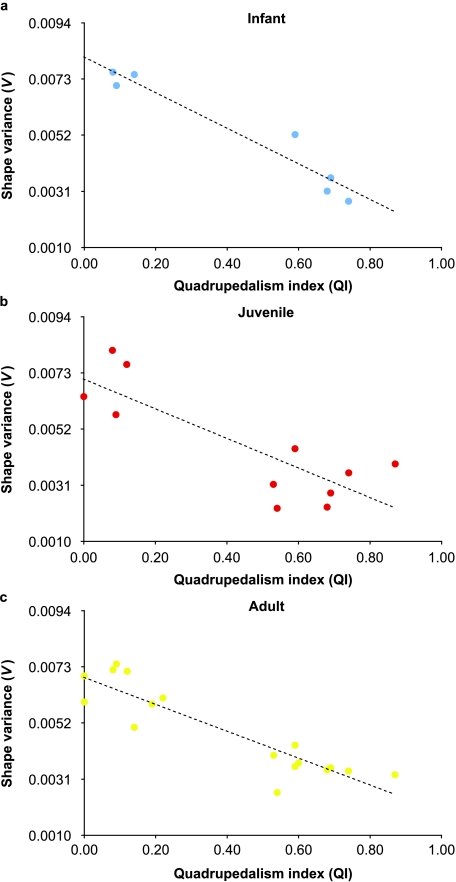
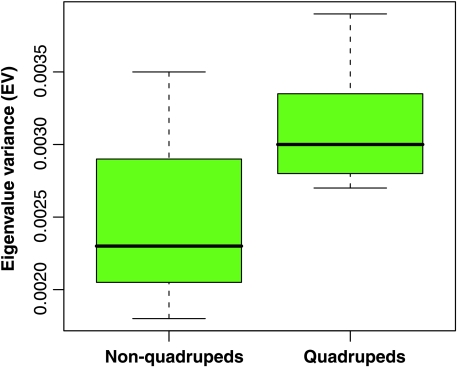
Shape variance was significantly correlated with QI in infants (r2 = 0.933, d.f. = 5, P = 0.000) (Fig. 3a), juveniles (r2 = 0.683, d.f. = 9, P = 0.002) (Fig. 3b) and adults (r2 = 0.815, d.f. = 15, P = 0.000) (Fig. 3c). However, these high correlations were dependent on the large differences between quadrupeds and non-quadrupeds as groups. Correlations of QI and shape variance within groups were not significant (e.g. adult quadrupeds: r2 = 0.041; adult non-quadrupeds: r2 = 0.118). This suggests that differences between functional groups in variance are not graded but discrete. Addition of primate species that fill this functional gap would help to clarify this relationship. Regardless, when combined into groups, there were significant differences between quadrupeds and non-quadrupeds in shape variance across all ontogenetic stages (Fig. 4). Intraspecific shape variance in non-quadrupeds averaged twice that of quadrupeds, and this difference was present from infancy. The correlation between adult shape variance and phylogenetic distance was not significant (rm = 0.120, P = 0.091), which indicates the relationship of canalization to function was not phylogenetically structured. EV was significantly correlated with QI in adults (r2 = 0.464, d.f. = 14, P = 0.004) (Fig. 5) and with shape variance (r2 = 0.422, d.f. = 14, P = 0.006). As a group, quadrupeds were significantly more integrated than non-quadrupeds [determined by resampling within species and recalculating mean EV within groups (1000 replicates, P = 0.000)] (Fig. 6).
Fig. 3.
Bivariate plot illustrating the relationship of locomotor function (QI) with shape variance in: (a) infants (r2 = 0.933, d.f. = 5, P = 0.000), (b) juveniles (r2 = 0.683, d.f. = 9, P = 0.002), and (c) adults (r2 = 0.815, d.f. = 15, P = 0.000). Dashed lines represent regressions through the data.
Fig. 4.
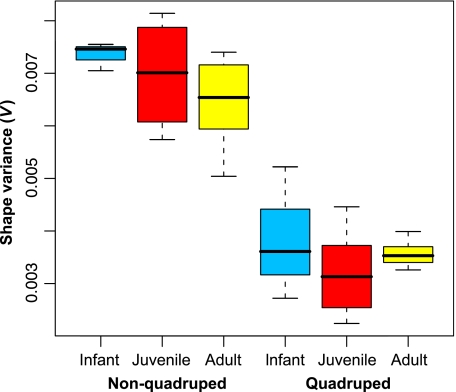
Boxplot showing the distribution of shape variance when species are combined into two groups comprised of non-quadrupeds (QI < 0.25: Ateles, Hylobates, Gorilla, Lagothrix, Pan paniscus, Pan troglodytes, Pongo and Symphalangus) and quadrupeds (QI > 0.50: Alouatta, Cercocebus, Cercopithecus, Colobus, Macaca, Nasalis, Papio, Presbytis and Saimiri) for three ontogenetic stages (infant = m1, juvenile = m2, and adult = m3). All shape variances were calculated from Procrustes data standardized by centroid size to minimize overestimates due to ontogenetic size heterogeneity. Shape variance is higher in non-quadrupeds in all ontogenetic stages.
Fig. 5.
Bivariate plot showing the relationship of the quadrupedalism index (QI) to the morphological integration index (EV, eigenvalue variance standardized by within-species shape variance) (r2 = 0.464, d.f. = 14, P = 0.004). Dashed line represents a regression through the data.
Fig. 6.
Boxplot comparing the distribution of the integration index (EV, eigenvalue variance standardized by within-species variance) in non-quadrupeds and quadrupeds. The mean EV of non-quadrupeds is significantly lower than that of quadrupeds (1000 resampled replicates, P = 0.000).
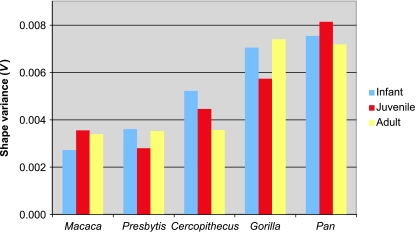
In the five species for which there were data available from all three ontogenetic stages (Macaca, Presbytis, Cercopithecus, Gorilla, Pan troglodytes), there was no consistent trend to shape variance over time (Fig. 7). This was also true of species for which there were only data on juvenile and adult shape variance (Table 2). In some species infant shape variance was higher than in adults, whereas in others adult shape variance was higher than earlier stages. However, in only one species was this difference significant (Gorilla adult > juvenile: t = 2.46, d.f. = 137, P = 0.004). When species were combined into functional categories there did seem to be a slight trend on average for reductions in variance between infant and adults within both groups, although this trend was not statistically significant (Fig. 4). Consequently, there was no relationship between shape variance, ontogeny and QI except for the previously noted difference between quadrupeds and non-quadrupeds.
Fig. 7.
Bar graph showing ontogenetic trends in shape variance for the species in which data are available for all stages (blue: infant; red: juvenile; yellow: adult).
Discussion
These results demonstrate that function, specifically whether a primate species is predominantly quadrupedal or non-quadrupedal, is correlated with the amount of intraspecific scapular shape variance. In other words, quadrupedal primates are significantly more canalized in scapular shape than non-quadrupeds. In non-quadrupedal primates intraspecific shape variance is double that in quadrupedal primates across all ontogenetic stages. Alternative explanations, such as phylogenetic relatedness, are not supported by this analysis. Phylogenetically distant taxa that have similar locomotor profiles such as Lagothrix and Pongo (non-quadrupeds) or Saimiri and Macaca (quadrupeds) are more similar to each other than they are to more closely related species (e.g. Lagothrix and Alouatta are significantly different despite both being atelines).
Intraspecific shape variance is correlated with differences in morphological integration between adults. On average the quadruped scapula is more integrated than that of non-quadrupeds. It is often difficult to compare integration results between analyses because different methods can produce contradictory results or results that are difficult to interpret (e.g. see Discussion in Chernoff & Magwene, 1999). However, there are commonalities between this analysis and Young (2004). In Young (2004), correlation structure was found to be similar across catarrhines, and differences between apes and Old World monkeys were attributed to strengthening of correlations in the latter (quadrupedal) outgroup. Results from the analysis here suggest there is a significant difference across primates in scapula integration that is consistent with this interpretation. It remains to be tested whether integration differs between functionally associated elements of the postcranium. Re-analysis of macaque (quadruped) and gibbon (non-quadruped) data from Young & Hallgrímsson (2005) indicates that gibbons also exhibit higher humeral shape variation, which would be consistent with this hypothesis.
There is no consistent pattern to changes in variance during ontogeny. Some species exhibit increases in variance over ontogeny, while others decrease. These differences in stage-specific shape variance are generally not significant. A conservative interpretation of this result is that there are no discernible upward or downward trends in shape variance in these primates. As a consequence, shape variance must be produced early in development and subsequently remains constant into adulthood. When averaged across functional groups, there is again no significant difference between infants, juveniles and adults, although there is a slight trend towards decreases.
Because intraspecific shape variance is high before postnatal differences in such factors as environmental variance or neuromuscular control are hypothesized to have an effect, and variance does not increase or decrease appreciably during postnatal growth, the most parsimonious explanation for these data is that variance in shape is produced prenatally and is maintained at a constant level into adulthood. No decrease in shape variance indicates that increasing neuromuscular control during postnatal ontogeny does not affect variance enough to explain differences between functional groups. The flat trend line also suggests that postnatal environmental factors probably do not play a role in the observed differences between adults in shape variance. It is not possible to interpret from these results whether this result is because neither of these factors has an effect on variance, or because they balance each other over ontogeny. Zelditch et al. (2004b) contend that in the absence of developmental regulation one would expect ontogenetic increases in variance. Consequently, a scenario in which some mechanism such as increasing neuromuscular control balances factors that would increase variance is plausible. Further study is required to discriminate these alternative possibilities.
If differences in shape variance are not due to postnatal factors, then what prenatal factors might be implicated? Possibilities include variance in intrinsic genetic factors associated with bone shape, epigenetic factors such as variance in muscle attachments that affect bone shape, variance of in utero neuromuscular activity that co-ordinate these epigenetic effects (Carter et al. 1997; Zelditch et al. 2004b; Nowlan & Prendergast, 2005), or variance in maternal effects such as uterine environment (Atchley & Hall, 1991). It is difficult to test for differences in maternal effects, but this would also seem to be an unlikely candidate given there is no a priori expectation that this effect should have such a strong differential expression associated with function, and there is no phylogenetic correlation with these results. In addition, neuromuscular firing in utero is unlikely to be more co-ordinated in either of these groups, although random movements could play a role if there were differences in the variance of muscular attachments (Zelditch et al. 2004b; Nowlan & Prendergast, 2005).
Epigenetic factors such as how muscle and bone interact in utero may also play a significant role in producing shape and consequently shape variance. Matsuoka et al. (2005) recently demonstrated that mesodermal or neural crest-derived cell populations of the shoulder are associated with muscle attachments that precisely match their tissue origins. Whereas muscle patterns are highly conserved, the bone these muscles attach to has ‘morphed’ around this scaffold over evolutionary time. They argue from this scaffold model that bones are essentially epiphenomena of muscle attachments. This model suggests that variance in muscle attachment sites or perhaps even size could in turn be relatable to variance in the patterning or location of muscles.
Intrinsic genetic factors are hypothesized to play a role in the shape or size of bone by affecting underlying developmental processes such as the number of cells in mesenchymal condensations, time of the initiation of condensation, rate of cell proliferation, rate of apoptosis or number of mitotically active cells (Atchley & Hall, 1991). Interestingly, the shape of the primate scapula changes little over ontogeny (Young, 2002), which indicates that most aspects of species-specific scapular shape are determined during embryogenesis and subsequent growth is largely a process of changing the size of the scapula rather than shape. This earlier finding parallels those found here for shape variance, suggesting that the same processes that generate embryonic scapular shape could potentially produce phenotypic variance. In both adult scapular shape and shape variance, these embryonic events appear to have long-term residual effects. In quadrupeds, elimination of the extremes of adult variance would select for those individuals whose embryonic processes were more canalized, while in non-quadrupeds greater variability in the developmental system would be tolerated. Although not within the scope of this paper, this hypothesis could be tested by comparing population variance in these developmental processes and the downstream effects they have on phenotypic variance. Increasing knowledge about the genetic regulatory mechanisms involved in inducing and patterning scapular morphology will be critical in this regard (e.g. Timmons et al. 1994; Pellegrini et al. 2001; Pröls et al. 2004; Kuijper et al. 2005; Matsuoka et al. 2005).
What mechanism canalizes variance in the scapula? Previously, a case was made for a role of stabilizing selection because traits that are the most important in terms of fitness are often the most canalized. That is, stabilizing selection removes extremes, leading to a greater tendency towards the mean phenotype. Traits under strong stabilizing selection would exhibit lower phenotypic variance and those under weaker selection would exhibit relatively higher variance (Salazar-Ciudad, 2006). Consequently, differences in the strength of stabilizing selection are likely to contribute to differences in population variance. This would seem to fit the case here because quadrupeds exhibit a stronger canalization of scapular shape, whereas non-quadrupedal scapular shape is more weakly canalized. This difference is relatable to a functional difference likely to affect fitness in these two groups.
By using QI as a proxy for function, locomotor function or performance was assumed to be the main factor affecting the strength of stabilizing selection. If QI has a quantitatively continuous relationship with selection one would predict a strong linear relationship between quadrupedalism and shape variance, as was demonstrated. However, this correlation was largely driven by a discontinuity between the two groups, with no correlation within locomotor groups. Consequently, QI may differentiate these two groups, but it does not necessarily represent the only or primary selective factor acting on intraspecific variance in these two groups. Indeed, it is possible that selective factors acting on the shape of the scapula may change as suspensory behaviours increase in frequency.
For example, for a suspensory primate safety factors may play a more important role than efficiency (Pontzer & Wrangham, 2004). Unlike quadrupedal primates, chimpanzees have many aboreal positional adaptations despite the fact they incur significant costs during terrestrial locomotion. However, inefficiencies may be tolerated because these same adaptations also may reduce mortality risks associated with falling (Pontzer & Wrangham, 2004). In quadrupeds, falling may not be as significant a factor on fitness (e.g. due to smaller body sizes or other adaptations outside the scapula that minimize this risk), allowing selective factors such as locomotor efficiency to dominate scapular morphology. If selection for a high safety factor has a different effect on variance than do purely locomotor factors, then the same differences in variance observed here could result.
One way to test this scenario would be to examine shape variance in obligate cursors (where performance and/or efficiency is presumably even more important a selective factor) to see if shape variance is even further reduced. If so, then this would suggest that locomotor efficiency plays a large role in the strength of stabilizing selection on scapula shape. However, if variance were similar in cursors and quadrupedal primates, then this would indicate that selection for a reduction in the arm's role in locomotion has removed efficiency as a primary selective factor. Comparison of other mammal groups in which there are functional differences between forelimb use (e.g. within carnivores: climbing bears vs. cursorial cheetah) would be predicted to show similar differences in shape variance. Further study linking differences in intraspecific shape variance to either energetics or other important contributors to fitness (e.g. climbing efficiency) will help to clarify what functional differences drive these observed differences in stabilizing selection between quadrupedal and non-quadrupedal primates.
Conclusions
The primate scapula exhibits significantly lower intraspecific shape variance in quadrupeds compared with non-quadrupeds. The fact that this difference in canalization of shape is patent even in infants, combined with a lack of an ontogenetic trend in this measure, indicates that differences in shape variance must arise prenatally and are not significantly impacted by external postnatal factors. Possible candidate factors for differences in shape variance include variance in intrinsic genetic factors associated with bone shape, variance in extrinsic factors such as muscle attachment location or size, or other related epigenetic factors acting during embryogenesis. Stabilizing selection probably plays a role in allowing this prenatally generated variance to persist in non-quadrupeds by acting on developmental processes underlying variation in shape and size of the scapula. This analysis suggests that to understand evolutionary history of selection on scapular shape better will require a more detailed understanding of how intrinsic genetic factors act during embryogenesis to pattern the shape of the shoulder. In addition, a prediction is that other postcranial traits related to forelimb function would be similarly less canalized in non-quadrupeds than in quadrupeds, and that these differences arise during embryogenesis rather than postnatal ontogeny.
Acknowledgments
I would like to thank D. Pilbeam, D. Lieberman, M. Ruvolo and B. Hallgrímsson for intellectual support of this work. The following individuals kindly provided access to specimens in their care: M. Rutzmoser and J. Chupasko (Museum of Comparative Zoology), B. Randall (American Museum of Natural History), L. Gordon (National Museum of Natural History), M. Tappen (Neil Tappen Collection at the University of Minnesota), M. Schulenberg (Field Museum of Natural History), L. Jellema (Cleveland Museum of Natural History), K. Isler (University of Zürich-Irchel), O. Röhrer-Ertl (Zooligische Staatssammlung), W. Van Neer (Musée Royale de l’Afrique Centrale), J. Harrison (Powell-Cotton Museum) and H. Gunji (Kyoto University Primate Research Institute and the Japan Monkey Center). S. Page provided the aligned γ-globin sequence data. The American School of Prehistoric Research, the Cora du Bois Charitable Trust and the Alberta Ingenuity Fund provided funding for this research.
References
- Ashton EH, Oxnard CE. The musculature of the primate shoulder. Trans Zool Soc Lond. 1963;29:554–650. [Google Scholar]
- Ashton EH, Flinn RM, Oxnard CE, Spence TF. The adaptive and classificatory significance of certain quantitative features of the forelimb in primates. J Zool (Lond) 1976;179:515–556. [Google Scholar]
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Boinski S. The positional behavior and substrate use of squirrel monkeys: ecological implications. J Hum Evol. 1989;18:659–677. [Google Scholar]
- Cant JGH. Positional behavior of long-tailed macaques (Macaca fasicularis) in Northern Sumatra. Am J Phys Anthropol. 1988;76:29–37. doi: 10.1002/ajpa.1330760104. [DOI] [PubMed] [Google Scholar]
- Cant JGH, Youlatos D, Rose MD. Locomotor behavior of Lagothrix lagothrica and Ateles belzebuth in Yasuní National Park, Ecuador: general patterns and nonsuspensory modes. J Hum Evol. 2001;41:141–166. doi: 10.1006/jhev.2001.0485. [DOI] [PubMed] [Google Scholar]
- Cant JGH, Youlatos D, Rose MD. Suspensory locomotion of Lagothrix lagothrica and Ateles belzebuth in Yasuní National Park, Ecuador. J Hum Evol. 2003;44:685–700. doi: 10.1016/s0047-2484(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Carter DR, Mikic B, Padian K. Epigenetic mechanical factors in the evolution of long bone epiphyses. Zool J Linn Soc. 1998;123:163–178. [Google Scholar]
- Chernoff B, Magwene P. Morphological integration: 40 years later. In: Olson EC, Miller RL, editors. Morphological Integration. Chicago: University of Chicago Press; 1999. pp. 319–353. [Google Scholar]
- Doran DM. Comparative locomotor behavior of chimpanzees and bonobos: the influence of morphology on locomotion. Am J Phys Anthropol. 1993a;91:83–98. doi: 10.1002/ajpa.1330910106. [DOI] [PubMed] [Google Scholar]
- Doran DM. Sex differences in adult chimpanzee positional behavior: the influence of body size on locomotion and posture. Am J Phys Anthropol. 1993b;91:99–116. doi: 10.1002/ajpa.1330910107. [DOI] [PubMed] [Google Scholar]
- Doran DM. Ontogeny of locomotion in mountain gorillas and chimpanzees. J Hum Evol. 1997;32:323–344. doi: 10.1006/jhev.1996.0095. [DOI] [PubMed] [Google Scholar]
- Dryden IL, Mardia KV. Statistical Shape Analysis. New York: John Wiley; 1998. [Google Scholar]
- Erikson GE. Brachiation in New World monkeys and in anthropoid apes. Symp Zool Soc Lond. 1963;10:135–164. [Google Scholar]
- Fleagle JG. Locomotion and posture. In: Chivers DJ, editor. Malayan Forest Primates. New York: Plenum Press; 1980. pp. 191–207. [Google Scholar]
- Fleagle JG, Mittermeier RA. Locomotor behavior, body size, and comparative ecology of seven Surinam monkeys. Am J Phys Anthropol. 1980;52:301–314. [Google Scholar]
- Fleagle JG. Primate Adaptation and Evolution. 2. New York: Academic Press; 1998. [Google Scholar]
- Gebo DL. Locomotor and postural behavior in Alouatta palliata and Cebus capucinus. Am J Primatol. 1992;26:277–290. doi: 10.1002/ajp.1350260405. [DOI] [PubMed] [Google Scholar]
- Gebo DL, Chapman CA. Positional behavior in five sympatric Old World monkeys. Am J Phys Anthropol. 1995;97:49–76. doi: 10.1002/ajpa.1330970105. [DOI] [PubMed] [Google Scholar]
- Gebo DL. Climbing, brachiation, and terrestrial quadrupedalism: historical precursors of hominid bipedalism. Am J Phys Anthropol. 1996;101:55–92. doi: 10.1002/(SICI)1096-8644(199609)101:1<55::AID-AJPA5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hallgrímsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological Integration in primate limbs. Yrbk Phys Anthropol. 2002;45:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Teng S. Strain in the braincase and its sutures during function. Am J Phys Anthropol. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Decker JD, Liu Z, Ma T. Temporomandibular joint in miniature pigs: anatomy, cell replication, and relation to loading. Anat Rec. 2002;266:152–166. doi: 10.1002/ar.10049. [DOI] [PubMed] [Google Scholar]
- Hood GM. Poptools, Version 2.5.9. Canberra: CSIRO; 2003. http://www.cse.csiro.au/poptools. [Google Scholar]
- Hunt KD. Positional behavior in the Hominoidea. Int J Primatol. 1991;12:95–118. [Google Scholar]
- Kuijper S, Beverdam A, Kroon C, et al. Genetics of shoulder girdle formation: roles of Tbx15 and aristaless-like genes. Development. 2005;132:1601–1610. doi: 10.1242/dev.01735. [DOI] [PubMed] [Google Scholar]
- Larson SG. Functional morphology of the shoulder in primates. In: Gebo D, editor. Postcranial Adaptation in Nonhuman Primates. DeKalb, Illinois: Northern Illinois University Press; 1993. pp. 45–69. [Google Scholar]
- Larson SG. New characters for the functional interpretation of primate scapulae and proximal humeri. Am J Phys Anthropol. 1995;98:13–35. doi: 10.1002/ajpa.1330980103. [DOI] [PubMed] [Google Scholar]
- Larson SG. Parallel evolution in the hominoid trunk and forelimb. Evol Anthropol. 1998;6:87–99. [Google Scholar]
- Manly B. Randomization, Bootstrap and Monte Carlo Methods in Biology. London: Chapman & Hall; 1997. [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlan NC, Prendergast PJ. Evolution of mechanoregulation of bone growth will lead to non-optimal bone phenotypes. J Theor Biol. 2005;235:408–418. doi: 10.1016/j.jtbi.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Oxnard CE. The functional morphology of the primate shoulder as revealed by comparative anatomical, osteometric and discriminant function techniques. Am J Phys Anthropol. 1967;26:219–240. [Google Scholar]
- Oxnard CE. Morphometric affinities of the human shoulder. Am J Phys Anthropol. 1977;46:367–374. doi: 10.1002/ajpa.1330460215. [DOI] [PubMed] [Google Scholar]
- Page SL, Goodman M. Catarrhine phylogeny: noncoding DNA evidence for a diphyletic origin of the mangabeys and for a human-chimpanzee clade. Mol Phyl Evol. 2001;18:14–25. doi: 10.1006/mpev.2000.0895. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Pantano S, Paola Fumi M, Lucchini F, Forabosco A. Agenesis of the scapula in Emx2 homozygous mutants. Dev Biol. 2001;232:149–156. doi: 10.1006/dbio.2001.0159. [DOI] [PubMed] [Google Scholar]
- Pilbeam D. The anthropoid postcranial axial skeleton: comments on development, variation, and evolution. J Exp Zool (Mol Dev Evol) 2004;302B:241–267. doi: 10.1002/jez.b.22. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Wrangham RW. Climbing and the daily energetic cost of locomotion in wild chimpanzees: implications for hominoid locomotor evolution. J Hum Evol. 2004;46:317–336. doi: 10.1016/j.jhevol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Pröls F, Ehehalt F, Rodriguez-Niedenführ M, He L, Huang R, Christ B. The role of Emx2 during scapula formation. Dev Biol. 2004;275:315–324. doi: 10.1016/j.ydbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2004. http://www.R-project.org. [Google Scholar]
- Rohlf FJ. NTSYS-PC: Numerical Taxonomy and Multivariate Analysis System. Setauket, NY: Exeter Software; 2002. Version 2.1. [Google Scholar]
- Rose MD. Positional behavior of natural populations: some quantitative results of a field study of Colobus guereza and Cercopithecus aethiops. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behavior, and Morphology: Dynamic Interactions in Primates. New York: Gustav Fischer; 1979. pp. 75–94. [Google Scholar]
- Salazar-Ciudad I. Developmental constraints vs variational properties: how pattern formation can help to understand evolution and development. J Exp Zool Mol Dev Evol. 2006;306B:107–125. doi: 10.1002/jez.b.21078. [DOI] [PubMed] [Google Scholar]
- Shea BT. Scapula form and locomotion in chimpanzee evolution. Am J Phys Anthropol. 1986;70:475–488. [Google Scholar]
- Sheets HD. Simple3d. Buffalo: Canisius College; 2004a. [Google Scholar]
- Sheets HD. 3DStand. Buffalo: Canisius College; 2004b. [Google Scholar]
- Susman RL. The locomotor behavior of Pan Paniscus in the Lomako Forest. In: Susman RL, editor. The Pygmy Chimpanzee. New York: Plenum Press; 1984. pp. 369–383. [Google Scholar]
- Swofford DL. PAUP*Phylogenetic Analysis Using Parsimony. Sunderland, MA: Sinauer Associates; 2002. Version 4.0. [Google Scholar]
- Timmons PM, Wallin J, Rigby PW, Balling R. Expression and function of Pax1 during development of the pectoral girdle. Development. 1994;124:1909–1918. doi: 10.1242/dev.120.10.2773. [DOI] [PubMed] [Google Scholar]
- Tuttle RH, Watts DP. The positional behavior and adaptive complexes of Pan gorilla. In: Kondo S, editor. Primate Morphophysiology, Locomotor Analyses, and Human Bipedalism. Tokyo: University of Tokyo Press; 1985. pp. 261–288. [Google Scholar]
- Van Valen L. The statistics of variation. In: Hallgrímsson B, Hall BK, editors. Variation: a Central Concept in Biology. Boston: Elsevier Academic; 2005. pp. 29–47. [Google Scholar]
- Waddington CH. The canalization of development and the inheritance of acquired characters. Nature. 1942;150:563. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidence for a non-random origin of quantitative genetic variation. J Math Biol. 1984;21:77–95. [Google Scholar]
- Wagner GP. A comparative study of morphological integration in Apis mellifera (Insecta, Hymenoptera) Zool Syst Evol Forsch. 1990;28:48–61. [Google Scholar]
- Wagner GP, Altenberg L. Perspective: complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Young NM. Homology and homoplasy in the hominoid postcranium. Harvard University; 2002. PhD dissertation. [Google Scholar]
- Young NM. A reassessment of living hominoid postcranial variability: implications for ape evolution. J Hum Evol. 2003;45:441–464. doi: 10.1016/j.jhevol.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Young NM. Integration and modularity in the hominoid scapula. J Exp Zool Mol Dev Evol. 2004;302B:226–240. doi: 10.1002/jez.b.21003. [DOI] [PubMed] [Google Scholar]
- Young NM, Hallgrímsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution. 2005;59:2691–2704. [PubMed] [Google Scholar]
- Zelditch ML, Lundrigan BL, Garland T. Developmental regulation of skull morphology. I. Ontogenetic dynamics of variance. Evol Dev. 2004a;6:194–206. doi: 10.1111/j.1525-142X.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric Morphometrics for Biologists: a Primer. New York: Academic Press; 2004b. [Google Scholar]