Abstract
New molecular markers are constantly increasing our knowledge of developmental processes. In this review article we have attempted to summarize the keystones of lymphoid tissue development in embryonic and pathological conditions. During embryonic lymph node development in the mouse, cells from the anterior cardinal vein start to bud and sprout, forming a lymph sac at defined sites. The protrusion of mesenchymal tissue into the lymph sacs forms the environment, where so-called ‘lymphoid tissue inducer cells’ and ‘mesenchymal organizer cells’ meet and interact. Defects of molecules involved in the recruitment and signalling cascades of these cells lead to primary immunodeficiency diseases. A comparison of molecules involved in the development of secondary lymphoid organs and tertiary lymphoid organs, e.g. in autoimmune diseases, shows that the same molecules are involved in both processes. This has led to the hypothesis that the development of tertiary lymphoid organs is a recapitulation of embryonic lymphoid tissue development at ectopic sites.
Keywords: alymphoplasia, lymph node development, secondary lymphoid organs, tertiary lymphoid organs
Introduction
Lymph nodes (LNs), as well as Peyer’s patches (PPs), tonsils, or in rodents nasal-associated lymphoid tissue (NALT), and the white pulp of the spleen are called secondary lymphoid organs (SLOs). They are located at strategic sites of the body, enabling a quick and effective immune response. Lymphocytes enter LNs via the blood or afferent lymphatics and are organized in T- and B-cell areas. Antigens enter the LNs via afferent lymphatic vessels, and are presented to lymphocytes by dendritic cells (DCs). The interposition of LNs, in the network of the lymphatic vasculature, allows particles to be filtered and the lymph fluid to be screened for foreign agents, which will be presented to lymphocytes in LNs to induce specific immune reactions. The foundation for the co-operation of these two systems is laid in the embryonal development of LN and lymphatic vasculature, as both arise from embryonal lymph sacs. Over the last few years many steps of this developmental cascade have been characterized. Here they are reviewed focusing on the molecular factors that are essential in these physiological processes and in pathological conditions. As most molecules have only been described in the mouse so far, we have tried to connect and extrapolate these data to human diseases as primary immunodeficiencies and the development of tertiary lymphoid organs (TLOs).
Lymphatic endothelial cells evolve out of venous endothelial cells and form lymph sacs at defined sites
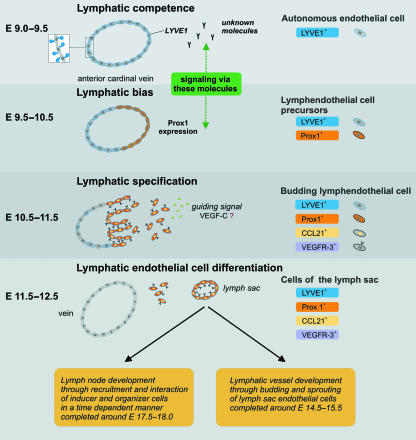
Historically there have been two models describing the development of the lymphatic system. In 1902 and 1909, respectively, Sabin (see Oliver, 2004) proposed a budding and sprouting of endothelial cells from veins into lymph sacs, and Huntington and McClure (see Oliver, 2004) suggested the development of lymph sacs from mesenchyme, followed by venous endothelial sprouting in the lymph sacs. This theory of centripetal development was supported by studies in avian species (Schneider et al. 1999). Later studies using molecular markers for lymphatic endothelial cells (LECs) in the mouse, such as LYVE1, Prox1 and VEGFR-3, supported Sabin’s concept. They showed that endothelial cells (ECs) from the anterior cardinal vein start to express LYVE1 around embryonic day (E) 9.0–9.5 in the mouse (Oliver, 2004). It still remains unclear which gene or the molecule produced is responsible for this expression in vivo and whether LYVE1 expression is essential for the metamorphosis from venous endothelial cell (VEC) to LEC. Studies investigating LYVE1 expression in ECs derived from mouse embryonic stem (ES) cells suggest that this expression is induced via VEGFR-3 signalling (Suzuki et al. 2005). The assumed function of LYVE1 is the hyaluronan transport across the wall of the lymphatic vessel (Jackson et al. 2001). No published data are available that show the necessity of LYVE1 gene activity for ECs to gain lymphatic competence. In his review, Oliver (2004) reported data from a personal communication with N. W. Gale, G. Thurston and G. D. Yancopolous, who found no alteration in the lymphatics in mice with targeted deletion of the LYVE1 gene. To the best of our knowledge, these data remain unpublished. Further studies on LYVE1 knock-out mice might illuminate that question. Oliver called this stage of the cells, as previously argued by Wigle et al. (2002), ‘lymphatic endothelial cell competence’ (Fig. 1). At this critical stage the VECs start to express LYVE1 and gain the ability to react to a specific unknown lymphatic inducing signal (Oliver, 2004). This signal triggers the expression of Prox1 by the cells of one side of the anterior cardinal vein (Fig. 1). This expression occurs around E9.0–10.5, a few hours after LYVE1 expression, and is restricted to a group of cells on one side of the anterior cardinal vein. This cell group later starts to sprout and bud from the vein, attracted by a guidance signal (Wigle & Oliver, 1999) (Fig. 1). It has been documented that VECs of Prox1-deficient mouse embryos stop sprouting and budding from the VECs at E11.5–12.0, they do not attain the phenotype of LECs and as a consequence those mice fail to develop lymph sacs (Wigle et al. 2002). Investigating the expression of specific lymphatic markers VEGFR-3, LYVE1 and CCL21 in LECs of Prox1-deficient embryos showed that Prox1 activity is not only essential for the maintenance of sprouting and budding of the ECs, but that it has an additional function in determining the ‘lymphatic bias’ of the Prox1-positive LECs (Wigle et al. 2002). The concept of ‘lymphatic bias’ was defined by Wigle & Oliver (1999). It describes the developmental stage when the cells start to express Prox1 and LYVE1, which leads to the initiation of lymphangiogenesis. The potency of Prox1 to induce the lymphatic phenotype in conventional VECs was shown in an experimental overexpression of Prox1 in cultured blood ECs. Those cells started to increase the expression of LEC markers (Hong et al. 2002) and decreased the expression of blood endothelial-specific markers (Petrova et al. 2002). This cell reprogramming with Prox1 was also possible in cells that had undergone a different developmental program (Hong et al. 2002). The next stage, the stage of ‘lymphatic specification’, occurs around E10.5–11.5 (Fig. 1). At that point the cells start to sprout from the vein, and also express CCL21 and VEGFR-3. The migration of these cells is disturbed in VEGFC−/– mouse embryos, indicating that VEGF-C, the ligand for VEGFR-3, could be a guidance molecule for these cells. This results in a lack of developing lymph sacs and the death of most embryos at around E15.5–17.5. The potency of VEGF-C to induce migration of Prox1-expressing ECs was shown in vitro (Karkkainen et al. 2004) and large amounts of VEGF-C and VEGFR-3 were found at sites were lymph sacs form (Kukk et al. 1996), further supporting the possible role of VEGF-C in cell guidance.
Fig. 1.
Development of the lymph sacs in the mouse, through budding and sprouting of endothelial cells. The autonomous endothelial cells of the anterior cardinal vein start to express LYVE1 around E9.0–9.5. The cells on one side of the vein follow the lymphatic bias and start to express Prox1. For the budding and sprouting of lymphendothelial cells at E10.5–11.5, the Prox1 expression of the lymphendothelial cell precursors cells is a prerequisite to react to a guiding signal. VEGF-C seems to be involved in this guidance and signalling process. When the lymphendothelial cells start to migrate, they already express most molecules specific for the lymphendothelium such as LYVE1, Prox1, CCL21 and VEGFR-3. Around E11.5–12.5 the lymphatic and the blood vascular system are separated and the lymphatic vessel development and the lymph node development follow different pathways emerging from the lymph sacs. (Modified after Oliver & Alitalo, 2005.)
The formation of the lymph sac is part of the stage of ‘lymphatic endothelial cell differentiation’, at approximately E11.5–12.5 (Fig. 1). At that point the mutual development of the lymphatic vasculature and the LNs stops, and both structures develop independently of each other. Many molecules are involved in the lymph vessel development that evolves through budding and sprouting of the LECs from the lymph sac (Fig. 1). We focus here on the development of lymphoid organs rather than the complicated process of lymphatic vessel development, and refer the interested reader to the recent reviews of Oliver (2004) and Oliver & Alitalo (2005). As shown in Fig. 1 the LNs develop through recruitment and interaction of lymphoid tissue inducer (LTi) cells and lymphoid tissue organizer cells in the LN anlagen (Fig. 1). The lymphotoxin α1β2 (LTα1β2)-expressing LTi cells interact with the corresponding lymphotoxin β receptor (LTβR) on the lymphoid organizer cells. As described in detail below this interaction activates a signalling cascade resulting in the expression of adhesion molecules and homeostatic chemokines. This stimulating microenvironment initiates a positive feedback loop, leading to further cell recruitment and clustering and the development of characteristic lymphoid structures such as high endothelial venules (HEVs).
Multipotent fetal liver cells differentiate into lymphoid tissue inducer cells and accumulate in the lymphoid tissue anlagen
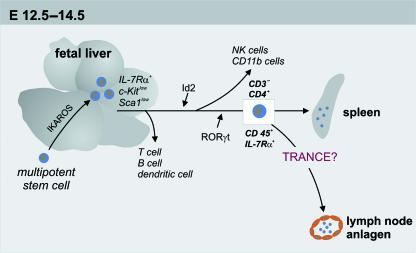
The progenitor of the LTi cells, characterized as IL-7Rα+Sca-1lowc-Kitlow, are initially detected in the fetal liver at E12.5–14.5 (Fig. 2). The stem cell antigen 1, Sca-1, and the membrane receptor for stem cell factor, c-Kit, characterize a special type of hematopoetic stem cell (HSC) that differentiate into a variety of cells such as blood and/or vascular endothelial cells and LTi cells (Okada et al. 1992; Spangrude & Brooks, 1993; Matsui et al. 2004). It seems likely that the transcription factor Ikaros is involved in the generation of the LTi progenitor cells, as it has been proven that Ikaros−/– mice lack T and B lymphocytes, NK cells, DCs, their precursor cells as well as LNs and PPs (Wang et al. 1996; Wu et al. 1997). The IL-7Rα+Sca-1lowc-Kitlow cells are generated in the fetal liver, whereas the differentiation into CD4+CD3−CD45+IL-7Rα+ LTi cells takes place in the periphery at secondary sites (Mebius et al. 1997) (Fig. 2). The differentiation of the progenitor cells into LTi cells seems to require the transcription regulating factors Id2 and RORγt. A loss of one of these factors leads to the lack of the CD4+CD3−CD45+IL-7Rα+ cells at sites where LN anlagen develop. The LN anlage displays the important stage in LN development that is initiated by initial clustering of embryonic cells. As a consequence of missing CD4+CD3−CD45+IL-7Rα+ cells at the defined sites, LNs and secondary lymphoid tissue do not develop (Yokota et al. 1999; Kurebayashi et al. 2000; Sun et al. 2000; Eberl et al. 2004). The exact function of RORγt and Id2 is still under discussion, although it is assumed that these factors are involved in the evolution of CD4+CD3−CD45+IL-7Rα+ cells, and assist in the maintenance and survival of the cells at this stage of the cell cycle. After the differentiation of the IL-7Rα+Sca-1lowc-Kitlow cells into CD4+CD3−CD45+IL-7Rα+ cells, these cells migrate into the potential LN anlagen through extravasation out of the large veins (Yoshida et al. 2002; Eberl et al. 2004). They are detected at about E12.5 around the pharynx, intestine and pericardium (Sun et al. 2000), in the vicinity of vessels (Eberl & Littman, 2003) and in the paraaortic region around 12.5–13.0 days post coitus (dpc) (Yoshida et al. 2002). Another factor that influences the accumulation of CD4+CD3−CD45+IL-7Rα+ cells in the LN anlagen is the TNF-related activation-induced cytokine, TRANCE. The number of CD4+CD3−CD45+IL-7Rα+ cells is remarkably decreased in TRANCE−/– mice compared with wild-type mice and leads to the lack of the peripheral (pLNs) and mesenteric lymph nodes (mLNs) whereas some cervical lymph nodes (cLNs) develop. In those cLNs, B cells fail to form follicles, a defect that has also been observed in the spleen of two-thirds of TRANCE−/– animals. By contrast, the development of PPs and spleen is not affected, reflecting the exclusive function of TRANCE in LN development. Transgenic overexpression of TRANCE in T and B cells increases cell cluster formation and significantly restores all pLNs and mLNs, even though the mLNs of these animals are smaller than those of wild-type mice (Kim et al. 2000). The corresponding receptor for TRANCE, the TRANCER, is located on CD4+CD3−CD45+-, CD4−CD3−CD45+- and on VCAM-1+-expressing cells. This suggests that TRANCE mediates survival or causes differentiation of CD4+CD3−CD45+ cells and regulates the colonization of the LN anlagen through assisting the CD4+CD3−CD45+ LTi cells in forming clusters (Kong et al. 1999; Kim et al. 2000; Cupedo et al. 2004b). It seems as if a threshold of LTi cells is necessary to initiate the clustering of LTi cells and the creation of a positive feedback loop together with the mesenchymal organizer cells (Ansel et al. 2000; Kim et al. 2000).
Fig. 2.
Development from the multipotent stem cell to the lymphoid tissue inducer cell in the mouse. The IL-7Rα+ c-Kitlow Sca1low-expressing cells can be found in the fetal liver around E12.5–14.5. The absence of these cells in Ikaros−/– mice shows the importance of this molecule in the generation of these cells. The multipotent character of these LTi progenitor cells is demonstrated by their ability to develop into lymphoid tissue inducer cells as well as T, B, NK and dendritic cells. For differentiation towards the CD3−CD4−CD45+IL-7Rα+-expressing LTi cells the molecules Id2 and RORγt are required. After differentiation into LTi cells, those cells can be detected at different peripheral sites, e.g. the spleen and the lymph node anlagen. For the recruitment of the inducer cells to the LN anlagen a possible role for TRANCE as a guiding signal is still under discussion.
Lymphoid tissue inducer cells express lymphotoxin α1β2 upon activation
The central role of the LTi cells in the development of LNs and PPs is the expression and presentation of LTα1β2 to the mesenchymal organizer cells and as a consequence of this LTi and organizer cells become organized in cell clusters. This LTα1β2-dependent development is similar to PP development, whereas the spleen and NALT develop independently of LTα1β2 (Fukuyama et al. 2002). As reviewed by Schneider et al. (2004), there are several molecules that regulate LTα1β2 expression. Signalling of TRANCE and signalling via IL-7Rα seems to be important in LN and PP development (Schneider et al. 2004). IL-7Rα triggers LTα1β2 expression on the PP inducer cell (Honda et al. 2001), whereas TRANCE seems to play an exclusive role in LN development, as mentioned above (Kong et al. 1999). LTα1β2 is a membrane-bound heterotrimer consisting of one LTα molecule and two molecules of the LTβ. LTα exists in two additional forms, the soluble LTα3 and the membrane-bound heterodimers LTα2β1. Those TNF family members signal via different receptors. LTα3 shows affinity for TNFR1, TNFR2 and the herpes virus entry mediator (HVEM), LTα1β2 is the substrate for the LTβR, whereas LTα2β1 communicates with the TNFR1 and -2, and conflicting data exist about the ability to signal via the LTβR. A homotrimeric form of LTβ has not been identified thus far. Knock-out studies and studies with functional deactivation of LTα, LTβ and LTβR displayed the crucial function of these molecules in the generation of lymphoid tissue. Signalling via this pathway is essential for the development of LNs and PPs. It does not occur at the same time in the distinct LN and PP developing regions, as reflected by the phenotypes gained from the treatment with soluble LTβR-Ig at different time points of infusion during embryogenesis. Interestingly, mLN development was not affected by this treatment (Rennert et al. 1996; Browning & French, 2002). This suggests different requirements for pLN and mLN development.
Different results were obtained from studies investigating LTα−/– or LTβ−/– mice. The LTα−/– animals lacked all LNs and PPs and had a disorganized splenic microarchitecture (Banks et al. 1995; De Togni et al. 1994), whereas the LTβ−/– mice displayed the same phenotype except that cLNs and mLNs were developed normally (Alimzhanov et al. 1997; Koni et al. 1997; Futterer et al. 1998). The discrepancy between those results suggests an independent role of LTα signalling, for example as the soluble LTα3 (Koni et al. 1997) or a different unknown ligand for the LTβR (Scheu et al. 2002). As mentioned above, LTα3 can signal via the TNFR1, TNFR2 and the HVEM and not via the LTβR which is the target receptor of LTα1β2 and LIGHT (Schneider et al. 2004). LIGHT, a lymphotoxin homologue belonging to the TNF superfamily, is a ligand for the LTβR and HVEM. The LIGHT/LTα-HVEM pathway was proposed to be responsible for the different phenotypes preserved in the different lymphotoxin knockout studies, owing to the high cross-specificity of LIGHT, LTβR, LTα and HVEM (Mauri et al. 1998). This seems unlikely, however, bearing in mind that HVEM−/– mice exhibit no defects in the organogenesis of lymphoid tissues (Wang et al. 2005). Studies investigating LIGHT have suggested that it plays a co-operative role with LTβ in mLN development (Scheu et al. 2002), and it is supposed to have a rescue function through CCL21 expression in LTα−/– mice (Wang et al. 2002).
Organizer cells expressing the lymphotoxin β receptor
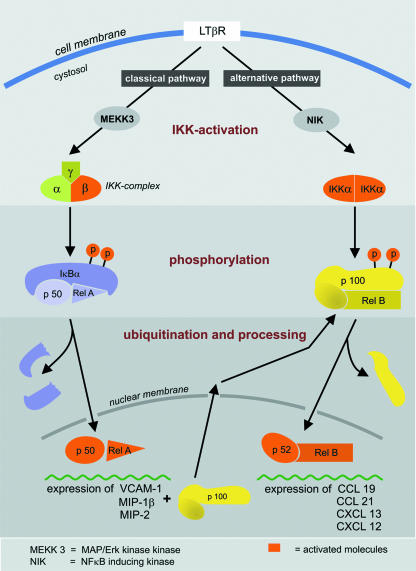
The LTβR, the corresponding receptor for LTα1β2 and LIGHT, is located on the surface of mesenchymal organizer cells and communicates with LTα1β2 on LTi cells during lymphoid organ development. Those LTβR-expressing mesenchymal organizer cells were shown to accumulate in the LN and PP anlagen in the immediate vicinity of LTi cells at E16.5 (Eberl & Littman, 2003; Eberl et al. 2004). The crucial function of the LTβR in lymphoid organ development has been shown in knock-out studies, which revealed a similar phenotype to LTα−/– mice, namely lack of pLNs, PPs and GALT in addition to altered splenic microarchitecture and abberant formation of germinal centres (GCs) and DC networks (Futterer et al. 1998). Signalling from the LTi via the LTβR on the mesenchymal organizer cells leads to the activation of two NF-κB signalling cascades (Fig. 3), leading to the transcription and expression of proinflammatory molecules on the one hand and chemokines and cytokines involved in lymphoid organogenesis on the other hand (Dejardin et al. 2002). As shown in Fig. 3 and reviewed by Weih & Caamano (2003), the classic NF-κB pathway leads to the expression of VCAM-1, MIP-1β and MIP-2 via the p50-RelA signalling cascade and CCL19, CCL21, CXCL12 and CXCL13 are produced after activation of the alternative pathway involving p52 and RelB (Dejardin et al. 2002). The detailed role of CCL19, CCL21, CXCL13 and their corresponding receptors CCR7 and CXCR5 in cell recruitment to the LN anlagen still needs to be determined. It appears that these have similar and partly overlapping functions as guidance molecules that can partly compensate for the function of each other. This makes it difficult to elucidate the particular function of each chemokine and chemokine receptor for the recruiting process of LTis.
Fig. 3.
Activation of NF-κB via the classical and the alternative pathway. Signalling via the LTβR can initiate both NF-κB activation pathways. The classical pathway leads to the expression of the inflammatory molecules VCAM1, MIP-1β and MIP-2, and an increase of p100. The first step in this cascade is the activation of the β unit of the IKK complex. This activated IKKβ complex leads to phosphorylation and ubiquitination of the IκBα, which allows the p50-RelA heterodimer to translocate to the nucleus and bind to the DNA. The alternative pathway follows a similar mechanism: after activation of the IKKα complex, p100 is phosphorylated and processed to p52. The remaining p52-RelB heterodimer induces the expression of the homeostatic cytokines CCL19, CCL21, CXCL13 and CXCL12, after translocation into the nucleus. (Modified from Weih & Caamano, 2003.)
Results from single and combined knock-out studies targeting CCR7, CXCR5 and CXCL13 indicate that chemokine signalling is different for mLN and pLN development (Forster et al. 1996; Ansel et al. 2000; Luther et al. 2003; Ohl et al. 2003) and there are furthermore suggestions that different types of pLNs require different chemokine signalling (Muller et al. 2003). This is consistent with the cellular make-up of the fetal murine LN anlagen recently described: two different organizer populations co-ordinate the distinct development of the mLNs and pLNs (Cupedo et al. 2004b). Those cells differ in the intensity of the expression of ICAM1, VCAM1 and MAdCAM1, leading to a distinct expression of LTβR and chemokine production and as a consequence creating a different chemokine milieu. This may explain the different requirements of mLN and pLN development.
Interactions of the inducer and organizer cell
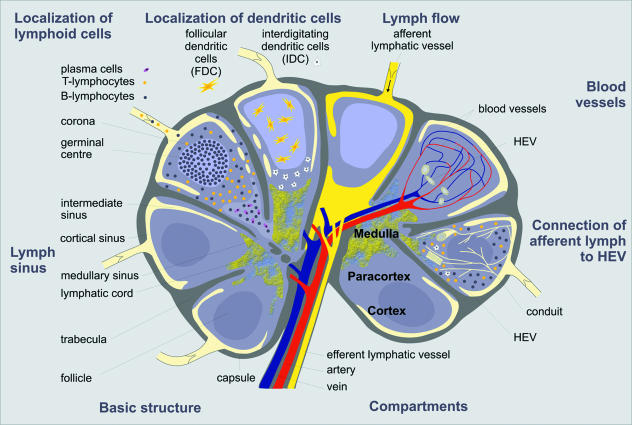
The adhesion of the LTi cell to the mesenchymal organizer cell leads to signalling via the LTβR, resulting in an up-regulation of adhesion molecules and homeostatic chemokines such as CXCL13. This results in the above mentioned positive feedback loop. CXCL13 expression induces further LTα1β2 expression, as shown in the spleen. LTα1β2 signals via the LTβR with the mesenchymal organizer cells. This leads to an increased release of CXCL13 and other chemokines that in turn activate additional functions, for example B-cell attraction and recruitment of further LTα1β2-expressing inducer cells (Ansel et al. 2000; Ansel & Cyster, 2001). The adhesion of the VCAM-1-, ICAM-1- and MAdCAM-1-expressing organizer cell to the activated α4β1 integrin-expressing inducer cell seems to be mediated via VCAM-1, as shown in PP development. Blocking of either α4β1 integrin or VCAM-1 leads to rare or undetectable clustering of inducer and organizer cells. Results from CXCR5−/– knock-out studies indicate that the CXCL13/CXCR5 pathway induces the active α4β1 integrin on the LTi cells (Finke et al. 2002). When a threshold of LTi cells and mesenchymal organizer cells is reached, those cells begin to form cell clusters, allowing a further differentiation of themselves and the surrounding cells. In this stimulating microenvironment blood vessels differentiate into HEVs, which allows blood cells to enter into the developing lymph node. The further organization into different T- and B-cell areas seems to be chemokine dependent. CXCR5-expressing B-cells migrate towards CXCL13, and CCL19 and CCL21 attract T-cells via CCR7. This leads to the development of the characteristic structure of mature LNs, outlined in Fig. 4.
Fig. 4.
Schematic view of a lymph node (LN). The composition of the LN can be divided into different regions. For didactic reasons the distinct functions are displayed in different sections. The cortex consists mainly of B cells organized as primary or secondary follicles. The migration of these cells towards the follicles is mediated by follicular dendritic cells (FDC) also located in the cortical region. T cells migrate to the paracortical region, neighbouring the cortex. They interact with the interdigitating dendritic cells (IDC), also present in the paracortical region. The central region, the medulla, mostly contains plasma cells and B cells. The lymphocytes enter the LN via the afferent lymphatic vessel or through transmigration of the high endothelial venules (HEV). Both systems, the lymph vasculature and the blood vasculature, are connected via a conduit system, and both drain into the efferent lymph vessel via the medullary sinus. (Modified from Pabst, 2004.)
From mice to humans – can data from murine models be transferred?
LN development in mice seems to have been largely defined, as a result of extensive knock-out studies. However, are these observations comparable with the human situation? Patients with alymphoplasia or structural defects of the LNs have been reported (reviewed in Berry, 1970; Kenny & Hitzig, 1979). The majority of these patients have additional immunological defects, which are classified as primary immunodeficiencies. These have been recently summarized by the American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology, including parameters for the diagnosis and advice for treatment (Bonilla et al. 2005). Molecular methods revealed numerous gene defects leading to these malformations (for a detailed review see Cunningham-Rundles & Ponda, 2005), including defects of molecules involved in lymphoid tissue development and lymphocyte maturation. Defects of single molecules involved in this process can lead to distinct severe combined immunodeficiency (SCID) disorders (Rosen et al. 1995). For example, mutations in IL7RA lead to defects in IL7-Rα, which is important for the development of secondary lymphoid tissue, PPs and LNs (Adachi et al. 1998; Luther et al. 2003). This is comparable with data from murine knock-out studies. Roifman et al. (2000) reported three patients with IL7RA mutation who lacked palpable LNs. Defects of molecules involved in the signalling cascade of IL-7Rα, such as Janus kinase 3 (JAK3) and the common γ chain (γc), also result in hypoplasia or lack of LNs as mentioned for all patients suffering from SCID (Russell et al. 1995; Candotti et al. 2002; Buckley, 2004). Thus, the absence of lymphatic tissue is a common feature in a group of patients with SCID (recently reviewed by Buckley, 2004), but can we conclude a defect in LN development from this data?
Although only few gene defects have been defined in humans so far, which are comparable with data in gene-manipulated mice, alymphoplasia seems to be a common feature and more individual cases with other mutations will probably be found in future. This will clarify our understanding of these diseases and broaden the spectrum of treatment.
Tertiary lymphoid organs
Besides the well-known primary and secondary lymphoid organs, structures called ‘tertiary lymphoid organs’ (TLOs) have been described (Picker & Butcher, 1992). These structures closely resemble SLOs, but are observed only in chronically inflamed tissue, as caused by chronic graft rejection, and autoimmune and infectious diseases (for reviews see Aloisi & Pujol-Borrell, 2006; Drayton et al. 2006). It has been shown that chemokines and signalling via members of the TNF family are important steps in the development of such structures (Ruddle, 1999; Fan et al. 2000; Chen et al. 2002b; Grant et al. 2002; Luther et al. 2002; Salomonsson et al. 2002; Manzo et al. 2005). Interestingly, these molecules are the same molecules that are important in the development of SLOs, namely CCL19, CCL21, CXCL13 and LTα1β2 (for reviews see Ruddle, 1999; Cupedo & Mebius, 2003). CCL21 has been shown to induce tissue-dependent lymphocyte recruitment, which might be one reason for the selective occurrence of tertiary lymphoid structures in some organs (Chen et al. 2002a,b). A study investigating the correlation of CCL21+ microvessels with naïve T-cell accumulation in rheumatoid arthritis, ulcerative colitis and psoriasis patients showed a spatial and quantitative correlation of accumulated cells with CCL21+ vessels (Weninger et al. 2003). Keeping in mind that naïve CD4+ T cells can express LTα1β2 upon activation, the CCL21 recruitment might be an early step in tertiary lymphoid tissue development (Gramaglia et al. 1999; Ohshima et al. 1999). Another candidate to be considered as an early step molecule for lymphoid neogenesis is CXCL13. Ectopic expression of CXCL13 stimulates de novo formation of lymphoid aggregates and also has the ability to induce LTα1β2 expression and signalling (Luther et al. 2000). The overlapping function of those two molecules makes it hard to clarify which chemokine initiates the signalling cascade that leads to LTα1β2 expression in the developing TLO. Given our knowledge of LN development, it is possible to envisage that there might be different requirements in distinct tissues, as described for the development of pLNs and mLNs (Forster et al. 1996; Luther et al. 2003; Muller et al. 2003; Ohl et al. 2003; Cupedo et al. 2004b). Furthermore, there might be an intertwining of signalling molecules as described in LN development that makes it difficult to elucidate the initiation molecule (Luther et al. 2003).
As both pathways – organogenesis and TLO development – lead to LTα1β2 expression on cells, and this TNF family member has a crucial role in SLO development, it is assumed that it has the same crucial role in TLO development (Kratz et al. 1996; Ruddle, 1999; Drayton et al. 2003). The ligation of LTα1β2 to the LTβR leads to the activation of two distinct NF-κB cascades in LN development. Activation of the classical pathway leads to the expression of VCAM-1 and inflammatory chemokines. The production of the homeostatic chemokines CCL19, CCL21 and CXCL13 is initiated after activation of the alternative pathway (Dejardin et al. 2002) (Fig. 3). Induction of the NF-κB cascade during acute inflammation results in the expression of inflammatory cytokines and adhesion molecules, via the classical pathway (Fig. 3). As suggested by Cupedo & Mebius (2003), prolonged stimulation of the LTβR might induce more and more stromal cells to use the alternative pathway upon stimulation. This leads to the production and accumulation of the homeostatic cytokines CCL19, CCL21 and CXCL13, and as a result of this a micromilieu stimulating lymphoid organization is created. In this micromilieu migrated cells might become organized in lymphoid aggregates via a positive feedback loop, as described for the development of SLOs (Ansel et al. 2000).
The function of primary and secondary lymphoid organs seems largely defined, but it remains obscure what function the tertiary lymphoid tissue may have. The GCs of the lymphoid aggregates described in autoimmune diseases generate autoreactive antibodies in some cases (Aloisi & Pujol-Borrell, 2006). As recently described, the local production of antibodies in tertiary lymphoid structures is also involved in chronic rejection. These antibodies are directed against the MHC I molecules of the donor, causing a chronic rejection of the graft (Thaunat et al. 2005). These findings suggest that there may be an active immune reaction initially. If the locally accumulated cells cannot cope with the antigen, they will start to recruit more cells to the inflamed site. The accumulation of cells in the stimulating micromilieu will lead to a recapitulation of the embryonic pathways, as previously suggested (Kratz et al. 1996; Drayton et al. 2003; Baddoura et al. 2005; Eberl, 2005). If this is the case, we would anticipate that these lymphoid aggregates gain access to lymph vessels, as known for SLOs. LYVE1 expression has been shown to occur in ectopic induced lymphoid structures, and de novo lymphangiogenesis has been described in human kidney transplants (Cupedo et al. 2004a; Kerjaschki et al. 2004, 2006). This shows the functionality of the TLOs, the integration of this tissue into the lymphatic system and provides a further similarity between TLOs and SLOs.
Concluding remarks
The aim of this review was to summarize the events during physiological and pathological lymphoid tissue development. Lymphoid tissue development has been of growing interest since it was suggested that mechanisms involved in embryonal lymphoid organ development likewise lead to the development of TLOs. Besides autoimmune and chronic inflammatory diseases, recently published data indicate that TLO development also occurs in chronic transplant rejection. Knowledge of the basic signalling cascades, genes and molecules involved in these processes might open up new horizons in the treatment of diseases where TLO development has been described. Not only is the inhibition of TLO development or local antibody formation of further interest, but so too might the stimulation of local antibody production be of increasing relevance with respect to therapies against tumour cells.
The basic mechanisms of SLO and TLO development should first be elucidated, and the comparability of these mechanisms in mouse and humans should be clarified.
Acknowledgments
We would like to thank Dr Tom Cupedo, Rotterdam, for helpful discussions and Sheila Fryk for help with the English text.
References
- Adachi S, Yoshida H, Honda K, et al. Essential role of IL-7 receptor α in the formation of Peyer’s patch anlage. Int Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Cyster JG. Chemokines in lymphopoiesis and lymphoid organ development. Curr Opin Immunol. 2001;13:172–179. doi: 10.1016/s0952-7915(00)00201-6. [DOI] [PubMed] [Google Scholar]
- Baddoura FK, Nasr IW, Wrobel B, et al. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5:510–516. doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, et al. Lymphotoxin-α-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- Berry CL. Histopathological findings in the combined immunity–deficiency syndrome. J Clin Pathol. 1970;23:193–202. doi: 10.1136/jcp.23.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94:S1–S63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- Browning JL, French LE. Visualization of lymphotoxin-β and lymphotoxin-β receptor expression in mouse embryos. J Immunol. 2002;168:5079–5087. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- Candotti F, Notarangelo L, Visconti R, O’Shea J. Molecular aspects of primary immunodeficiencies: lessons from cytokine and other signaling pathways. J Clin Invest. 2002;109:1261–1269. doi: 10.1172/JCI15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Leach MW, Chen Y, et al. Central nervous system inflammation and neurological disease in transgenic mice expressing the CC chemokine CCL21 in oligodendrocytes. J Immunol. 2002a;168:1009–1017. doi: 10.4049/jimmunol.168.3.1009. [DOI] [PubMed] [Google Scholar]
- Chen SC, Vassileva G, Kinsley D, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002b;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Ponda PP. Molecular defects in T- and B-cell primary immunodeficiency diseases. Nat Rev Immunol. 2005;5:880–892. doi: 10.1038/nri1713. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Mebius RE. Role of chemokines in the development of secondary and tertiary lymphoid tissues. Semin Immunol. 2003;15:243–248. doi: 10.1016/j.smim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004a;21:655–667. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Vondenhoff MF, Heeregrave EJ, et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004b;173:2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, et al. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT αβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. The role of the nuclear hormone receptor RORγt in the development of lymph nodes and Peyer’s patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, et al. An essential function for the nuclear receptor RORγ (t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- Finke D, Acha-Orbea H, Mattis A, Lipp M, Kraehenbuhl J. CD4+CD3− cells induce Peyer’s patch development: role of α4β1 integrin activation by CXCR5. Immunity. 2002;17:363–373. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Fukuyama S, Hiroi T, Yokota Y, et al. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3 (−) CD4 (+) CD45 (+) cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gramaglia I, Mauri DN, Miner KT, Ware CF, Croft M. Lymphotoxin αβ is expressed on recently activated naive and Th1-like CD4 cells but is down-regulated by IL-4 during Th2 differentiation. J Immunol. 1999;162:1333–1338. [PubMed] [Google Scholar]
- Grant AJ, Goddard S, Ahmed-Choudhury J, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol. 2002;160:1445–1455. doi: 10.1016/S0002-9440(10)62570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Nakano H, Yoshida H, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Kenny AB, Hitzig WH. Bone marrow transplantation for severe combined immunodeficiency disease. Reported from 1968 to 1977. Eur J Pediatr. 1979;131:155–177. doi: 10.1007/BF00538940. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Regele HM, Moosberger I, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- Kim D, Mebius RE, MacMicking JD, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, et al. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukk E, Lymboussaki A, Taira S, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- Kurebayashi S, Ueda E, Sakaue M, et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo A, Paoletti S, Carulli M, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35:1347–1359. doi: 10.1002/eji.200425830. [DOI] [PubMed] [Google Scholar]
- Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–18607. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–135. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Ohl L, Henning G, Krautwald S, et al. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197:1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y, Yang LP, Avice MN, et al. Naive human CD4+ T cells are a major source of lymphotoxin α. J Immunol. 1999;162:3790–3794. [PubMed] [Google Scholar]
- Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- Oliver G. Lymphatic vasculature development. Nat Rev Immunol. 2004;4:35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- Pabst R. Lymphknoten. In: Drenckhahn D, editor. Anatomie, Makroskopische Anatomie, Histologie, Embryologie, Zellbiologie. Vol. 2. München: Elsevier: Urban und Fischer; 2004. pp. 156–159. [Google Scholar]
- Petrova TV, Makinen T, Makela TP, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roifman CM, Zhang J, Chitayat D, Sharfe N. A partial deficiency of interleukin-7R α is sufficient to abrogate T-cell development and cause severe combined immunodeficiency. Blood. 2000;96:2803–2807. [PubMed] [Google Scholar]
- Rosen FS, Cooper MD, Wedgwood RJ. The primary immunodeficiencies. N Engl J Med. 1995;333:431–440. doi: 10.1056/NEJM199508173330707. [DOI] [PubMed] [Google Scholar]
- Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- Russell SM, Tayebi N, Nakajima H, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Larsson P, Tengner P, et al. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren’s syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- Scheu S, Alferink J, Potzel T, et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Othman-Hassan K, Christ B, Wilting J. Lymphangioblasts in the avian wing bud. Dev Dyn. 1999;216:311–319. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<311::AID-DVDY1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Brooks DM. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993;82:3327–3332. [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Watabe T, Kato M, Miyazawa K, Miyazono K. Roles of vascular endothelial growth factor receptor 3 signaling in differentiation of mouse embryonic stem cell-derived vascular progenitor cells into endothelial cells. Blood. 2005;105:2372–2379. doi: 10.1182/blood-2004-07-2547. [DOI] [PubMed] [Google Scholar]
- Thaunat O, Field AC, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102:14723–14728. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Nichogiannopoulou A, Wu L, et al. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Foster A, Chin R, et al. The complementation of lymphotoxin deficiency with LIGHT, a newly discovered TNF family member, for the restoration of secondary lymphoid structure and function. Eur J Immunol. 2002;32:1969–1979. doi: 10.1002/1521-4141(200207)32:7<1969::AID-IMMU1969>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wang Y, Subudhi SK, Anders RA, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-êB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Weninger W, Carlsen HS, Goodarzi M, et al. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7:483–492. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Naito A, Inoue J, et al. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer’s patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]